Abstract
The aim of this protocol is to generate COPII-coated procollagen I (PC1) carriers in a cell-free reaction. The COPII-coated PC1 carriers were reconstituted from donor membrane, cytosol, purified recombinant COPII proteins, and nucleotides. This protocol describes the preparation of donor membrane and cytosol, the assembly of the reaction, and the isolation and detection of reconstituted COPII-coated carriers. This cell-free reaction can be used to test conditions that stimulate or suppress the packaging of PC1 into COPII-coated carriers.
Keywords: COPII, Collagen, Membrane, Budding, Reconstitution
Background
The coat protein complex II (COPII) plays an essential role in transporting secretory cargos from the endoplasmic reticulum (ER) en route to the Golgi apparatus. The genes required for cargo traffic from the ER were discovered in genetic studies in yeast and the precise roles of the protein products of the genes required for vesicle budding were elucidated with the aid of a cell-free vesicle budding reaction supplemented with purified components ( Novick et al., 1981 ; Kaiser et al., 1990 ; Barlowe et al., 1994 ). A similar reaction was developed to detect the role of COPII in cargo traffic from the ER in cultured mammalian cells ( Kim et al., 2005 ). Mammalian COPII-coated vesicles are approximately 80-100 nm in diameter, which is seemingly too small to accommodate large secretory cargos such as the rigid 300 nm procollagen I (PC1) triple helical rod. Despite the potential size discrepancy, COPII is essential for the secretion of large cargos including PC1 ( Boyadjiev et al., 2006 ). Recently, we reported the existence of bona fide large COPII-coated PC1 carriers, exceeding 300 nm in diameter, in cells evaluated by stochastic optical reconstruction microscopy (STORM), correlated light electron microscopy (CLEM) and live-cell imaging ( Gorur et al., 2017 ). Cell-free COPII budding reactions that successfully reconstituted small COPII vesicles did not allow the detection of large COPII-coated PC1 carriers (Fromme and Schekman, 2005). Therefore, we devised an alternative vesicle budding protocol to allow the detection of PC1 packaged into large COPII vesicles as well as the characterization of both small and large COPII-coated vesicles. Using this new protocol, we showed that the capture of PC1 into large COPII vesicles requires COPII proteins and the GTPase activity of the COPII subunit SAR1 ( Gorur et al., 2017 ).
Materials and Reagents
Falcon®150 mm TC-treated cell culture dish (Corning, Falcon®, catalog number: 353025) or equivalent
BioExpress GeneMate 50 ml centrifuge tubes (BioExpress, Greiner Bio One, catalog number: C-3394-4) or equivalent
-
BioExpress GeneMate racked pipet tips, low retention, 200 μl (BioExpress, catalog number: P-1234-200)
Manufacturer: Biotix, catalog number: P-1234-200CS.
-
BioExpress GeneMate racked pipet tips, low retention, 1,000 μl (BioExpress, catalog number: P-1234-1000)
Manufacturer: Biotix, catalog number: P-1234-1000CS.
BioExpress GeneMate 15 ml centrifuge tubes (BioExpress, Greiner Bio One, catalog number: C-3394-2) or equivalent
Falcon® 100 mm TC-treated cell culture dish (Corning, Falcon®, catalog number: 353003) or equivalent
Amicon® Ultra-15 ml centrifugal filter unit with Ultracel-3K membrane (Merck, catalog number: UFC900324)
Amicon® Ultra-0.5 ml centrifugal filter unit with Ultracel-3K membrane (Merck, catalog number: UFC500324)
Oxygen® 1.5 ml MAXYMmum recoveryTM microcentrifuge tube (low retention) (Corning, Axygen®, catalog number: MCT-150-L-C)
Microscope slides (Fisher Scientific, catalog number: 12-550-343) or equivalent
Microscope cover glass (Fisher Scientific, catalog number: 12-542A) or equivalent
Tube, 7 x 20 mm, thickwall, polycarbonate (Beckman Coulter, catalog number: 343775)
Prot/Elec tips (gel loading tips) (Bio-Rad Laboratories, catalog number: 2239915)
Cell scraper 25 cm (SARSTEDT, catalog number: 83.1830) or equivalent
Corning 1 L filter system 0.22 μm (Corning, catalog number: 431098) or equivalent
Steriflip® 50 ml filter 0.22 μm (Merck, catalog number: SCGP00525) or equivalent
Posi-click 1.7 ml micro-centrifuge tube (Danville Scientific, catalog number: C2170(1001002)) or equivalent
Microfuge tube, polypropylene, 1.5 ml (Beckman Coulter, catalog number: 357448)
Cuvettes (SARSTEDT, catalog number: 67.742)
Immobilon®-P transfer membrane PVDF 0.45 μm (Merck, catalog number: IPVH00010)
-
HT-1080 human fibrosarcoma (ATCC, catalog number: CCL-121) for cytosol preparation
Note: Other fast-growing cell lines that support PC1 secretion may also be used for this purpose.
-
IMR-90 human lung fibroblasts (Coriell Cell Repositories at the National Institute on Aging, Coriell Institute for Medical Research) (Coriell Institute, catalog number: I90-83) for donor membrane preparation
Note: Other cell lines that express endogenous PC1 and prolific at PC1 secretion may be used for this purpose. For this reaction, it is important to use young IMR-90 with cumulative Population Doubling Level (PDL) lower than 37.5, because aged cells secrete significantly less PC1. PDL was calculated using a standard formula: cumulative PDL = initial PDL + 3.32 [log (current cell yield) - log (cell plated)].
Phosphate-buffered saline (PBS, pH 7.4)
cOmpleteTM, EDTA-free, protease inhibitor cocktail tablets (Roche Diagnostics, catalog number: 05056489001)
Bio-beadsTM SM-2 adsorbent media (Bio-Rad Laboratories, catalog number: 1523920)
Bio-Rad protein assay dye reagent concentrate (Bradford) (Bio-Rad Laboratories, catalog number: 5000006)
Liquid nitrogen
0.25% trypsin-EDTA (Thermo Fisher Scientific, GibcoTM, catalog number: 25200056)
HyClone® trypan blue solution (GE Healthcare, HyCloneTM, catalog number: SV30084)
OptiPrepTM density gradient medium (Sigma-Aldrich, catalog number: D1556)
NovexTM WedgeWellTM 4-20% Tris-glycine gel (Thermo Fisher Scientific, InvitrogenTM, catalog number: XP04205BOX)
-
Antibodies
Rabbit anti-PC1 (LF-41) was a gift from L. Fisher (National Institute of Dental and Craniofacial Research, Bethesda, MD), and it was used at 1:5,000
Rabbit anti ribophorin I, ERGIC53, and SEC22B were made in-house and they were used at 1:5,000
Mouse anti HSP47 (Enzo Life Sciences, catalog number: ADI-SPA-470-D), and it was used at 1:5,000
PierceTM ECL 2 Western blotting substrate (Thermo Fisher Scientific, catalog number: 32132)
Bovine serum albumin (BSA) (Sigma-Aldrich, catalog number: A3294-100G)
Life Science Seradigm premium grade fetal bovine serum (FBS) (VWR, catalog number: 1500-500)
DMEM, GlutaMAXTM (Thermo Fisher Scientific, GibcoTM, catalog number: 10566016)
HEPES (Sigma-Aldrich, catalog number: RDD002-1KG)
Potassium hydroxide (KOH)
D-Sorbitol (Sigma-Aldrich, catalog number: S1876-5KG)
Potassium acetate (KoAc) (Fisher Scientific, catalog number: BP364-500)
Magnesium acetate tetrahydrate (MgoAc) (Sigma-Aldrich, catalog number: M0631-500G)
Sodium dodecyl sulfate (SDS) (Avantor Performance Materials, J.T.Baker®, catalog number: 4095-02)
Glycerol (AMRESCO, catalog number: M152-4L)
Bromophenol blue (Bio-Rad Laboratories, catalog number: 1610404)
Glycine (Fisher Scientific, catalog number: BP381-5)
2-Mercaptoethanol (βME) (AMRESCO, catalog number: M131-100ML)
Sodium chloride (NaCl) (Fisher Scientific, catalog number: S271-3)
Tris base (Fisher Scientific, catalog number: BP152-5)
Triton® X-100 (Sigma-Aldrich, catalog number: X100-500ML)
TWEEN® 20 (Sigma-Aldrich, catalog number: P7949-500ML)
Tris-buffered saline (TBS, pH 7.6)
Digitonin (Sigma-Aldrich, catalog number: D141-500MG)
Dimethyl sulfoxide (DSMO) (Sigma-Aldrich, catalog number: D8418-100ML)
Trypsin inhibitor from glycine max (soybean) (Sigma-Aldrich, catalog number: T9003)
Lithium chloride (LiCl) (Sigma-Aldrich, catalog number: 203637)
Creatine phosphate (Sigma-Aldrich, catalog number: 2380-25GM)
Creatine kinase (Roche Diagnostics, catalog number: 10127566001)
Adenosine 5’-triphosphate (ATP) (GE Healthcare, catalog number: 27-1006-01)
GTP 100 mM Li Salt (Sigma-Aldrich, Roche Diagnostics, catalog number: 11140957001)
Methanol (Fisher Scientific, catalog number: A452-4)
Mammalian cell culture medium (see Recipes)
-
Buffer solutions (see Recipes)
B88
B88-0
Sample buffer (5x)
Buffer C
Sample buffer C (1x)
Transfer buffer
HK buffer
TBST
-
Stock solutions (see Recipes)
1 M HEPES pH 7.2
10% SDS
Digitonin stock
Trypsin inhibitor stock
0.5 M LiCl
ATP regeneration system (ATP r.s.)
GTP
Equipment
SorvallTM ST16R centrifuge, TX-200 Swinging Bucket Rotor, 400 ml Round Buckets, 4 x 50 ml, 9 x 15 ml conical adapters (Thermo Fisher Scientific, model: SorvallTM ST 16R, catalog number: 75818382) or equivalent
TLA-55 ultracentrifuge rotor (Beckman Coulter, model: TLA-55, catalog number: 366725)
Centrifuge 5430R, refrigerated with fixed angle rotor FA-45-30-11 (Eppendorf, model: 5430 R, catalog number: 5428000015)
Light microscope with a 16x or 25x objective (any simple or compound light microscope is fine)
S-24-11-AT swinging bucket rotor (Eppendorf, catalog number: 5409715003)
Spectronic Genesys 5 spectrophotometer (Spectronic Instruments) or equivalent
Table top ultracentrifuge, we used Optima MAX-XP, Optima TL, and Optima TL-100 for this experiment (Beckman Coulter, models: OptimatTM MAX-XP, OptimaTM TL, OptimaTM TL-100)
Microman® positive-displacement pipet M250 (Gilson, catalog number: F148505)
Microman® capillary pistons for M250 (Gilson, catalog number: F148114)
TLS-55 swinging bucket ultracentrifuge rotor (Beckman Coulter, model: TLS-55, catalog number: 346936)
TLS-55 adapter, Delrin, for 7 x 20 mm tubes (Beckman Coulter, catalog number: 358615)
Micro tube mixer MT-360 (TOMY SEIKO, model: MT-360)
BioExpress GeneMate GyroMixer XL (BioExpress, GeneMate, catalog number: R-3200-1XL) or equivalent platform rotator
ChemiDocTM MP Imaging System (Bio-Rad Laboratories, model: ChemiDocTM MP)
Small beaker
Software
ImageLab software v4.0
ImageJ
Procedure
Note: All procedures are performed on ice and all centrifugations are performed at 4 °C unless otherwise stated.
-
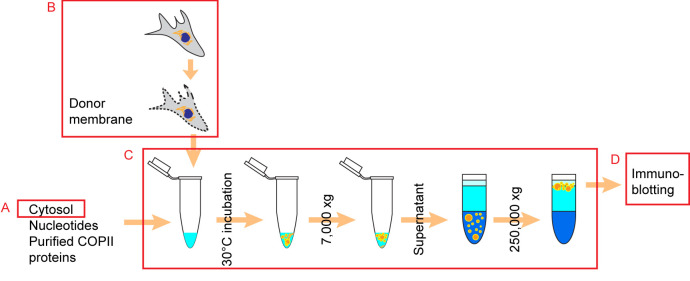
Preparation of cytosol from cultured HT-1080 cells (prepared ahead of time, will be used in the reaction, Figure 1)
-
Culture 20 x 15 cm plates of HT-1080 to 95% confluent in 30 ml culture medium per plate.
Note: HT-1080 was chosen because it supports the secretion of overexpressed PC1, while it does not express PC1 endogenously and thus minimized background. Its fast growth rate also makes it easy to scale-up for cytosol preparation.
Remove media and wash cells with 10 ml PBS/plate, repeat the wash one more time, and remove as much of PBS as possible.
-
Scrape cells on ice and collect with 1 ml B88 buffer/plate (see Recipes) with protease inhibitors (used as suggested by manufacturer: 1 tablet per 50 ml).
Note: This is done 5 plates at a time with 5 ml of B88 buffer. Collect cells from the first plate by resuspending with 5 ml of B88, and then use the same buffer to collect cells from the next 4 plates.
Transfer cell suspension to a 50 ml Falcon tube.
Add digitonin (40 mg/ml; see Recipes) to cell suspension to reach a final concentration of 80 μg/ml. Mix by inverting the tube a few times.
Rotate for 30 min on a platform rotator at 4 °C.
-
During the 30 min incubation, hydrate 4 g Bio-beadsTM (1 g per 5 x 15 cm plates) with 25 ml B88 buffer:
Centrifuge at 300 × g for 5 min at Acceleration Setting (Accel) 9 (default) and Deceleration Setting (Decel) 7 using SorvallTM ST16R centrifuge.
Discard supernatant and wash beads two more times with 25 ml B88 buffer.
After the last wash, discard as much buffer as possible.
Note: To remove buffer between Bio-beadsTM, press a P1000 tip to the bottom of the tube through beads then aspirate buffer with minimal disruption. This step should be immediately followed by the addition of crude cytosol to prevent Bio-beadsTM from drying out.
After the 30 min digitonin incubation, centrifuge the cell suspension at 300 × g for 5 min.
Take the supernatant (crude cytosol) and transfer to wash Bio-beadsTM.
Incubate cytosol with Bio-beadsTM with mild agitation to absorb digitonin from the crude cytosol on a platform rotator at 4 °C overnight.
The next morning, clarify the cytosol-beads mixture at 300 × g for 5 min at Decel 7.
Recover supernatant and aliquot to about 14 x 1.5 ml polypropylene microfuge tubes.
Centrifuge at 135,300 × g for 30 min in TLA-55 rotors at Accel 2 Decel 6.
Collect supernatant conservatively and avoid disturbing sedimented material.
-
Concentrate supernatant (cytosol) by centrifuging in 15 ml Amicon-3k concentrator at 4,000 × g for 4 x 10 min.
Note: Cytosol was centrifuged 4 times for 10 min each time and mixed between each sedimentation to minimize protein precipitation.
-
Further concentrate cytosol using 0.5 ml Amicon-3k concentrators at 14,000 × g for 3 x 10 min in a fixed angle rotor (FA-45-30-11, Eppendorf).
Note: Cytosol was mixed between each sedimentation to minimize protein precipitation.
-
Collect concentrated cytosol and measure protein concentration using Bradford reagents.
Note: The concentration should be between 40-80 mg/ml.
Freeze small aliquots (recommend 1.6 mg/aliquot) in liquid nitrogen and store at -80 °C for future use in budding reaction. Avoid repeated freeze-thaw cycles.
-
-
Preparation of donor membrane (DM) from cultured human cells (prepare on the day of the reaction fresh, Figure 1)
-
Culture 3 x 10 cm plates of IMR-90 to 95% confluent in 10 ml culture medium per plate.
Note: Use young cells under PDL 37.5. Older cells are significantly less efficient at secreting PC1. IMR-90 was used to prepare donor membrane because it is the most efficient at PC1 secretion of all cell lines that we tested. Other cell lines such as sv-IMR90 and U-2OS may also be used to prepare donor membrane with lower budding efficiency (Gorur et al., 2017).
Aspirate media from 3 x 10 cm plates of IMR-90.
Wash each plate with 10 ml PBS.
Add 0.5 ml 0.25% trypsin to each plate and incubate at RT for 5 min.
Collect cells from each plate with 6 ml PBS buffer into 2 x 15 ml tubes.
Add 25 μl 10 mg/ml trypsin inhibitor (see Recipes) to each tube and mix well.
Centrifuge at 300 × g for 5 min.
-
Discard supernatant and resuspend each cell pellet in 1 ml B88 buffer with low retention tips.
Note: Dislodge the cell pellet by gently tapping it prior to the addition of buffer. Use low retention tips for all future steps.
Add B88 buffer to each tube so that the final volume in each 15 ml tube is 6 ml.
Add 3 μl 40 mg/ml digitonin to each tube so that final concentration is 20 μg/ml.
Mix well and incubate on ice for 5 min.
Add 8 ml B88 buffer to each tube and centrifuge at 300 × g for 5 min.
Discard supernatant and resuspend each cell pellet in 1 ml B88 buffer and transfer to 2 x 1.5 ml low retention microcentrifuge tubes.
-
Mix 3 μl of trypan blue and 3 μl of cells on a glass slide, carefully lay a cover slip over the sample, then check percentage of permeabilized cells under a light microscope with a 16x or 25x objective.
Note: 100% of cells should be permeabilized at this stage. Blue nuclei and clear to light brown ER surrounding each blue nucleus should be observed.
-
Centrifuge at 300 × g for 5 min in a swinging bucket rotor (S-24-11-AT).
Note: Perform all subsequent centrifugations in a swinging bucket rotor for maximum recovery.
Discard supernatant and resuspend each pellet in 1 ml B88 buffer containing 0.5 M LiCl (see Recipes) in B88.
Incubate on ice for 5 min, then centrifuge at 300 × g for 5 min.
-
Discard supernatant and resuspend each pellet in 1 ml B88 buffer.
Note: Dislodge the cell pellet by gently tapping the tube prior to the addition of buffer.
Incubate on ice for 5 min, then centrifuge at 300 × g for 5 min.
Discard supernatant and resuspend each pellet in 1 ml B88-0 buffer (see Recipes).
Centrifuge at 300 × g for 5 min.
Discard supernatant and resuspend both pellets in 200 μl B88-0. This is a working stock of DM.
-
Determine the concentration of DM in the working stock:
Make a 1 to 50 dilution of DM in B88-0.
-
Blank with B88-0 and measure the optical density of diluted DM sample at a wavelength of 600 nm (OD600) using a spectrophotometer. The reading is the ‘OD600’ of the diluted sample.
Note: OD600 is used as a unit of concentration in this protocol.
-
Calculate the concentration of the working stock by multiplying the OD600 of the diluted sample with 50.
Note: If the OD600 of the working stock is above 2, then there will be enough of DM for 8 x 100 μl budding reactions with the final OD600 of 0.5.
-
Calculate the volume of working stock to be added to each budding reaction using the equation:
C1V1 = C2V2
where, C is the concentration of DM in OD600 and V is the volume in μl.
-
-
Reconstitution of COPII coated vesicles
-
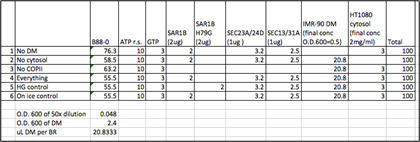
In low retention tubes, assemble budding reactions by adding ingredients from left to right in Table 1. Each 100 μl reaction contains ATP regeneration system (1 mM ATP, 40 mM creatine phosphate, 0.2 mg/ml creatine phosphokinase), 3 mM GTP, 20 ng/μl SAR1B, 10 ng/μl SEC23A/24D, 10 ng/μl SEC13/31A, 2 μg/μl cytosol, and OD600 of 0.5 for DM.
Note: Add B88-0, nucleotides (an ATP r.s. and GTP, see Recipes) and recombinant COPII proteins first. Mix well by pipetting and briefly centrifuge to collect liquid at the bottom of each tube. Then add DM and mix by pipetting up and down gently until homogenous. Add cytosol last and mix by gentle pipetting.
Incubate reactions at 30 °C (or on ice as a negative control) for 1 h.
Isolate COPII carriers of PC1 with the following centrifugation steps (Figure 1).
Centrifuge at 7,000 × g for 10 min.
-
Place 50 μl of 60% OptiPrepTM at the bottom of a 7 x 20 mm ultracentrifugation tube with positive-displacement pipet Microman® M250.
Note: The OptiPrepTM gradient purchased from Sigma-Aldrich is a 60% (w/v) solution.
-
Recover 85 μl 7,000 × g supernatant and mix with the 60% OptiPrepTM in a 7 x 20 mm ultracentrifugation tube until homogenous, resulting in a 22.2% (w/v) OptiPrepTM mixture.
Note: Avoid introducing bubbles.
-
Overlay with 100 μl 18% (w/v) OptiPrepTM in B88 by slowly pipetting against the wall using a gel-loading tip.
Important Note: An interphase should be observed between 18% OptiPrepTM and the 22.2% 7,000 x g supernatant OptiPrepTM mixture at this stage.
-
Overlay with 10 μl B88 by pipetting slowly using a gel-loading tip.
Important Note: An interphase should be observed between B88 and 18% OptiPrepTM in B88.
Centrifuge at 55,000 rpm (or 258,488 × g) for 90 min at the Acceleration Setting (Accel) 2 and Decel 6 in TLS-55 rotors and adaptors for 7 x 20 mm tubes.
Collect lipid vesicles from the top of the flotation gradient immediately after the centrifugation is over.
-
-
Immunoblot (Western blot)
Perform standard immunoblotting procedure by following the notes below:
Add DM to 20 μl 1x sample buffer C (see Recipes) to reach a final concentration of 0.1 OD600/μl DM, then mix vigorously using a micro-tube mixer at max speed for 10 min at RT.
Add 5x sample buffer (see Recipes) to float fractions.
Incubate both DM sample and floated samples at 65 °C for 10 min.
Load the desired amount of DM sample and the entire floated fractions onto a 15 wedged well 4-20% gradient gel.
Run sample at constant 25 mA until dye runs out of the gel (about 75 min) at RT.
-
Transfer protein onto a PVDF membrane at constant 0.15 A for 16 h at 4 °C.
Important Note: The transfer condition was optimized for PC1.Conditions may vary for other proteins of interest.
Block with 5% non-fat milk (w/v) in TBST (see Recipes) for 30 min at RT.
-
Incubate with primary antibodies at RT for 2.5 h.
Important Note: The temperature and duration were optimized for the rabbit anti PC1 antibody LF-41, which was used at 1:5,000 in 1% BSA (w/v) TBST. Each aliquot can be stored at 4 °C and reused up to 7 times without compromising the detection. An ER marker (ribophorin I) is included as a negative control, whereas regular COPII cargos (ERGIC53 and SEC22B) are used as positive controls.
Wash with TBST 3 x 5 min.
Incubate with secondary antibodies conjugated with HRP at RT for 1 h.
Wash with TBST 3 x 5 min.
Develop with the HRP substrate ECL plus and image on a ChemiDocTM Imaging System with ImageLab software v4.0.
Figure 1. Schematic overview of the experimental procedure.
The preparations of cytosol and donor membrane for the budding reaction were described in Procedure A and Procedure B, respectively. The assembly of budding reactions and isolation of vesicles from budding reactions were described in Procedure C. Packaging efficiency of COPII cargos were assessed by immunoblotting as described in Procedure D (Republished from Gorur et al., 2017 with modifications).
Table 1. Sample calculation of cell-free PC1 budding reactions.
Each row represents a single cell-free reaction. The total volume of each reaction is 100 μl. Each column represents a component of the reaction, and the volume added to the reaction in μl.
Data analysis
Export immunoblot images from ImageLab software v4.0 as .tif files.
Use ImageJ to process immunoblot images (rotating, cropping, adjusting brightness and contrast when necessary).
Prepare figures with Adobe Illustrator® or equivalent software (Figure 2).
Figure 2. COPII is required to package PC1 into reconstituted vesicles.
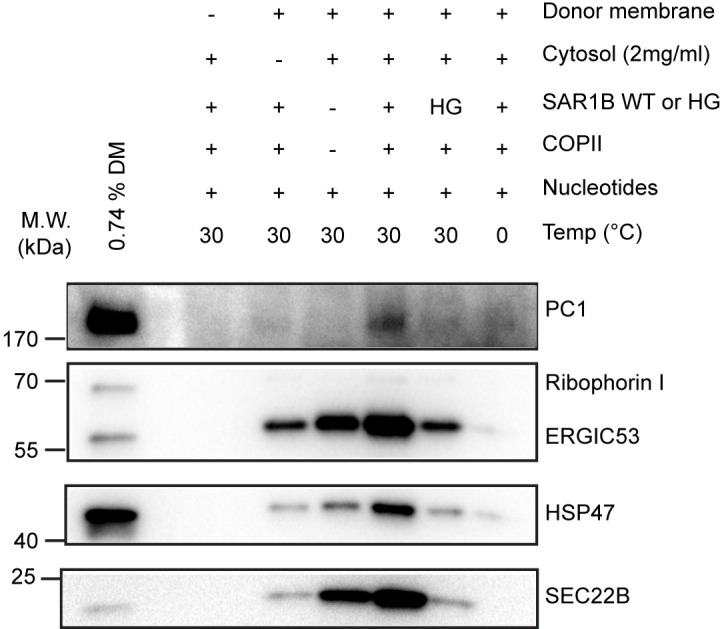
Budding requirements of PC1 and HSP47 (a collagen-specific chaperone) were assessed under different incubation conditions described in Table 1. The top fraction after flotation was taken from each sample and analyzed by immunoblotting. Ribophorin I is an ER resident protein that serves as a negative control. ERGIC53 and SEC22B are found in conventional COPII vesicles and serve as positive controls. (Republished from Gorur et al., 2017 )
Recipes
-
Mammalian cell culture medium
Add 10% FBS to DMEM
-
Buffer solutions
-
B88 (1 L)
Add 20 ml 1 M HEPES pH 7.2 buffer to 0.5 L ddH2O
Dissolve 45.54 g sorbitol, 14.72 g KoAc, and 1 g MgoAc
Adjust volume to 1 L
Filter the buffer
Store at 4 °C
-
B88-0 (50 ml)
Add 1 ml 1 M HEPES pH 7.2 buffer to 30 ml ddH2O
Dissolve 2.28 g sorbitol and 0.7 g KoAc
Adjust volume to 50 ml
Filter the buffer
Store at 4 °C
-
5x sample buffer (10 ml)
Dissolve 1 g of SDS, 5 mg bromophenol blue in a small beaker with 2 ml 1 M Tris pH 6.8 with constant stirring
Mix in 3 ml glycerol
Adjust volume to 10 ml with ddH2O
Aliquot to 200 μl/tube and store at -20 °C
Add 10 μl βME to an aliquot fresh before use
-
Buffer C (1 ml)
Add 10 μl of 1 M Tris pH 7.6, 20 μl of 5 M NaCl, 100 μl of Triton® X-100, and 100 μl 10% SDS
Add water to 1 ml, mix well and store at 4 °C
-
Sample buffer C (1x)
Dilute 5x sample buffer to 1x with buffer C
-
Transfer buffer
Dissolve 6.06 g Tris and 28.8 g glycine in 1.5 L water
Add 1 ml 10% SDS
Add ddH2O to 2 L
Store at 4 °C, use within 2 weeks
Note: For best and consistent result, do not reuse this transfer buffer.
-
HK buffer
Dissolve 785.2 mg of KoAc in 30 ml ddH2O
Add 1 ml of HEPES, pH 7.2
Adjust volume to 50 ml and store at 4 °C
-
TBST
0.1% TWEEN® 20 (v/v) in 1x TBS
-
-
Stock solutions
-
1 M HEPES pH 7.2
Dissolve 238.3 g of HEPES in 0.5 L ddH2O
Adjust pH to 7.2 with KOH
Add ddH2O to 1 L
Store at RT
-
10% SDS
Dissolve 1 g of SDS in 9 ml of ddH2O
Store at RT
-
Digitonin stock
Dissolve 40 mg digitonin in 1 ml DMSO
Save small aliquots at -20 °C
-
Trypsin inhibitor stock
Dissolve 10 mg trypsin inhibitor in 1 ml B88
Save small aliquots at -20 °C
Avoid repeated freeze-thaws
-
0.5 M LiCl
Dissolve 212 mg LiCl in 10 ml B88
Store at 4 °C
-
ATP regeneration system (ATP r.s.)
Dissolve 2.04 g creatine phosphate, 40 mg creatine kinase, 101.44 mg ATP in 20 ml B88
Store small aliquots at -80 °C
-
GTP
Dilute 400 μl of 100 mM GTP with 3.6 ml HK buffer
Store small aliquots at -80 °C
-
Acknowledgments
We thank the staff at the University of California Berkeley Cell Culture Facility Ann Fisher and Alison Kililea. We also thank past and present members of the Schekman Lab, in particular Liang Ge and Yusong Guo for helpful discussions on optimizing this protocol. RS is supported as an Investigator of the Howard Hughes Medical Institute and the UC Berkeley Miller Institute of Science. LY was supported in part by the Tang family fellowship. This protocol was modified from previous work described in Kim et al., 2005 . The authors declare no conflict of interest or competing interests.
Citation
Readers should cite both the Bio-protocol article and the original research article where this protocol was used.
References
- 1.Barlowe C., Orci L., Yeung T., Hosobuchi M., Hamamoto S., Salama N., Rexach M. F., Ravazzola M., Amherdt M. and Schekman R.(1994). COPII: a membrane coat formed by Sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell 77(6): 895-907. [DOI] [PubMed] [Google Scholar]
- 2.Boyadjiev S. A., Fromme J. C., Ben J., Chong S. S., Nauta C., Hur D. J., Zhang G., Hamamoto S., Schekman R., Ravazzola M., Orci L. and Eyaid W.(2006). Cranio-lenticulo-sutural dysplasia is caused by a SEC23A mutation leading to abnormal endoplasmic-reticulum-to-Golgi trafficking. Nat Genet 38(10): 1192-1197. [DOI] [PubMed] [Google Scholar]
- 3.Fromme J. C. and Schekman R.(2005). COPII-coated vesicles: flexible enough for large cargo? Curr Opin Cell Biol 17(4): 345-352. [DOI] [PubMed] [Google Scholar]
- 4.Gorur A., Yuan L., Kenny S. J., Baba S., Xu K. and Schekman R.(2017). COPII-coated membranes function as transport carriers of intracellular procollagen I. J Cell Biol 216(6): 1745-1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kaiser C. A. and Schekman R.(1990). Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell 61(4): 723-733. [DOI] [PubMed] [Google Scholar]
- 6.Kim J., Hamamoto S., Ravazzola M., Orci L. and Schekman R.(2005). Uncoupled packaging of amyloid precursor protein and presenilin 1 into coat protein complex II vesicles. J Biol Chem 280(9): 7758-7768. [DOI] [PubMed] [Google Scholar]
- 7.Novick P., Ferro S. and Schekman R.(1981). Order of events in the yeast secretory pathway. Cell 25(2): 461-469. [DOI] [PubMed] [Google Scholar]