Abstract
Background
Attention deficits in young children with autism spectrum disorder (ASD) are not well understood. This study sought to determine: 1) the prevalence of ADHD symptoms in young children with ASD, typical development (TD), and developmental delay (DD) and 2) the association between ADHD symptoms and cognitive and behavioral functioning in children with ASD.
Method
ADHD symptoms, defined according to Aberrant Behavior Checklist (ABC) hyperactivity subscale scores, were compared across children aged 2–5 from a large case-control study with ASD (n=548), TD (n=423), and DD (n=180). Inattention and hyperactivity items within this subscale were also explored. Within the ASD group, linear and logistic regression were used to examine how ADHD symptoms were associated with cognition as assessed by the Mullen Scales of Early Learning and adaptive functioning as assessed by the Vineland Adaptive Behavior Scales.
Results
Mean hyperactivity subscale scores were lowest in children with TD (mean=3.19), higher in children with DD (12.3), and highest in children with ASD (18.2; between-group p<0.001). Among children with ASD, significant associations were observed with higher ADHD symptoms and poorer adaptive and cognitive functioning (adjusted beta for hyperactivity score in association with: Vineland composite = −5.63, p=0.0005; Mullen visual reception scale = −2.94, p=0.02; for the highest vs. lowest quartile of hyperactivity score, odds of lowest quintile of these scores was approximately doubled). Exploratory analyses highlighted associations with inattention-related items specifically.
Conclusion
These results suggest ADHD symptoms may play a key role in the functioning of young children with ASD.
Keywords: ASD, ADHD, inattention, comorbidity, adaptive functioning
Introduction
Under the Diagnostic and Statistical Manual (DSM)-IV (AmericanPsychiatricAssociation 1994) and International Classification of Disease (ICD)-10 (WorldHealthOrganization 1992), a comorbid diagnosis of attention deficit hyperactivity disorder (ADHD) and an autism spectrum disorder (ASD) was not permitted; however, given research findings of symptom overlap and clinically-impairing inattention and hyperactivity deficits in children with ASD, the DSM-5 now allows for the diagnosis of both ASD and ADHD in the same individuals (AmericanPsychiatricAssociation 2013). Prevalence estimates for ADHD comorbid with ASD vary widely, ranging from 2–78% (Lee and Ousley 2006; Hanson et al. 2013). However, most studies find that at least 30–40% of individuals with ASD exhibit clinically significant ADHD symptoms (Taurines et al. 2012; Matson et al. 2013). A high prevalence of attentional issues also has been reported for children with other cognitive and developmental disorders including fragile X syndrome and dyslexia (Varvara et al. 2014; Wheeler et al. 2014), but in general, the comorbidity of ADHD symptoms with other developmental disorders has not been well studied, especially in young children.
To date, studies of individuals with ASD and ADHD symptoms suggest there is greater social and cognitive impairment, poorer outcomes, and higher rates of internalizing and externalizing problems in children with comorbid ADHD and ASD than in those with ASD alone (Holtmann et al. 2007; Rao and Landa 2014; Visser et al. 2016). However, most prior studies have had modest sample sizes, lacked consideration of a range of potentially confounding factors, and have focused on children of school-age or older (Ehlers et al. 1997; Geurts et al. 2004; Corbett and Constantine 2006; Lundervold et al. 2012; Jang et al. 2013; Rao and Landa 2014). The few studies focusing on young children have been based on clinically-ascertained samples (Visser et al. 2016), which likely have limited generalizability given noted skewed severity distributions in such samples (Rao and Landa 2014). Only two studies to date have examined ADHD symptoms in children under age 5 with ASD drawn from community-based samples, and both were extremely small (Leyfer et al. 2006; Rao and Landa 2014). In addition, these studies, and the majority of prior work in the literature including that conducted in individuals of older ages, have not included a non-ASD developmental disorder control group, which limits the ability to draw inferences about how ADHD symptoms differentially impact functioning in ASD versus development more broadly.
The clinical implications of this overlap of ADHD symptoms in young children with ASD are large. For example, work from the National Survey of Children’s Health found that approximately 20% of nearly 1,500 children aged 2–17 with parent-reported ASD were initially given a diagnosis of ADHD, and were diagnosed with ASD three years later than children without ADHD concerns (Miodovnik et al. 2015). Studies also indicate differences in response to treatments based on presence/absence of ADHD symptoms in children with ASD (Antshel et al. 2011; Cortese et al. 2012; Pearson et al. 2012; Simonoff et al. 2013). Finally, concerns about attention problems, in both those with ASD and their siblings, that persist into middle childhood and school settings also underscore the importance of developing a better understanding of how the two disorders manifest alone and together in childhood (Miller et al. 2015). Taken together, these findings suggest it is imperative to better understand the manifestations ADHD symptoms in early development if we are to ensure accurate early diagnosis, appropriate treatment, and optimal school outcomes.
In order to advance understanding of the manifestations of ADHD symptoms and their association with cognitive and adaptive functioning in early childhood in those with ASD and in those with other forms of developmental delay, we conducted the first study of ADHD symptoms in a large population-based case-control community sample of over 1,100 young children ages 2 to 5 years with confirmed ASD, developmental delay (DD) without ASD, or typical development. Our study goals were to 1) determine the prevalence of inattention and hyperactivity symptoms in young children with ASD, DD, and typical development (TD) and 2) among children with ASD, examine whether these symptoms were associated with cognitive ability level and adaptive functioning. Based on the extant literature, we hypothesized that inattention and hyperactivity would be significantly more common in the ASD and DD groups as compared with TD children, and that they further would be associated with poorer functioning as measured by Mullen Scales of Early Learning and Vineland Adaptive Behavior Scale scores in children with ASD.
Methods
Study population
Participants are part of CHARGE (CHildhood Autism Risk from Genetics and the Environment), an on-going, large, population-based case-control study being conducted in California; CHARGE study design and data collection details have been previously described (Hertz-Picciotto 2006). Briefly, children with ASD and developmental delay (DD) were identified through the California Department of Developmental Services (DDS) and from referrals, and controls through state birth files. Controls were frequency-matched on age, sex, and broad geographic area to cases. DDS diagnoses of ASD were confirmed on the Autism Diagnostic Observation Schedule (ADOS) and Autism Diagnostic Interview-Revised (ADI-R) by clinicians at the UC Davis MIND (Medical Investigations of Neurodevelopmental Disorders) Institute or the UCLA Neuropsychiatric Institute who have achieved and maintained research reliability. Eligibility criteria were: 1) aged 24–60 months at recruitment; 2) born in California; 3) living with a biologic parent who speaks English or Spanish; 4) residing in the catchment area of ~1.5 hours drive to the research clinic. The average age of CHARGE children at enrollment is 3.66 years (standard deviation=0.8) and 79% of probands are male. Population controls were sampled at 4:1 males to females to match ASD sex-ratio estimates.
Over 1,600 probands had been enrolled at the time this analysis was initiated. All children were clinically evaluated to confirm community diagnoses. Children with ASD were defined as those who met criteria on the communication or social interaction domain of the ADI-R and were within 2 points of meeting criteria in the other domain, had onset before 36 months, and met the ASD cut-off for social and communication totals of the ADOS. This diagnostic definition was based on operationalizing DSM criteria and combining information from multiple sources to diagnose ASD (Risi et al. 2006). In the children recruited with developmental delay or as general population controls, the Social Communication Questionnaire (SCQ) was used to screen for autism spectrum symptoms, with a cut-off score of 15. For the current analyses, controls, designated ‘typically developing’ (TD), were defined as children recruited from the general population who scored 70 or higher on the Mullen Scales of Early Learning (MSEL), 70 or higher on the Vineland Adaptive Behavior Scales (VABS), and 14 or lower on the SCQ. Developmental delay (DD) controls were defined according to a score of <70 on either the MSEL or the VABS, with the other score ≤76 (a value within one half of one standard deviation of the cut-off score of 70), and a score of 14 or lower on SCQ or clinician judgment. For these analyses, we included only those children meeting these clinical cut-offs (excluded n=180 due to missing or incomplete assessments, n=81 children enrolled as autism not confirmed, and n=36 due to atypical development not meeting diagnostic cut-off criteria). Further, individuals who did not complete the ABC (n=97) were excluded from analyses, as were individuals missing items on the ABC Hyperactivity subscale and derived subdomains (discussed below; n=2), leaving 1,151 children for analysis.
Measures and definition of ADHD symptoms
The Aberrant Behavior Checklist (ABC) (Aman et al. 1985) is a 58-item informant-rated measure designed to assess problem behaviors. Each item is scored from 0 (not at all a problem) to 3 (problem in severe degree). The ABC includes five subscales (irritability, lethargy, stereotypy, hyperactivity, and inappropriate speech), created based on factor analysis (Aman et al. 1985; Aman et al. 1987); higher scores on the ABC subscales indicate greater impairment/higher maladaptive behaviors. The ABC is one of the few available measures that can be used to evaluate ADHD symptoms in young children. The validity of its subscales and use of the ABC in children with ASD and other conditions, as well as in typically-developing populations, has been established, including in children as young as 2 years of age (Marshburn and Aman 1992; Brinkley et al. 2007; Karabekiroglu and Aman 2009; Kaat et al. 2014), as well as in toddlers aged 14–43 months (Karabekiroglu and Aman 2009). In this study, we focused on the hyperactivity subscale of the ABC in order to gain information on ADHD-like symptoms, as it includes 16 items related to inattention, hyperactivity, impulsivity, and defiant behaviors. See Appendix A for a list of ABC hyperactivity scale items.
Exploratory subdomains
To further explore ADHD symptoms and potential differences by attention-deficit subtypes, we separated this hyperactivity subscale of the ABC into 2 subdomains, which we clinically derived based on what type of behavior each item was assessing: hyperactivity/impulsivity (the sum of 10 items from the hyperactivity subscale, for a total score range of 0–30), and inattention (the sum of 3 items from the hyperactivity subscale, for a total score range of 0–9); see Appendix A for items included in these subdomains. These two domains are consistent with the two subdomains in the Diagnostic and Statistical Manual of Mental Disorders: Fifth Edition (American Psychiatric Association, 2013), which differentiates patients with both hyperactivity and inattention from those with inattention alone. The additional 3 items not included in our subdomains assessed defiant behaviors, which are not a central feature of ADHD. We defined ‘high ADHD symptoms’ as the highest quartile of these subscale/subdomains (according to the distribution in the study group for across-groups analysis, and according to the distribution in the ASD group for ASD-only analyses), though we also considered use of other cut-points, such as 1 standard deviation above the mean, however, this type of cut-point did not produce well balanced groups as described below.
Cognitive ability level and adaptive functioning assessments
Instruments for assessment of cognitive and adaptive functioning were, respectively the Mullen Scales of Early Learning (MSEL) ((Mullen 1995) and the Vineland Adaptive Behavior Scales (VABS) (Sparrow 1984). The MSEL is administered to the child by a trained professional, and provides 5 scales of developmental abilities (expressive language, measuring ability to use language; receptive language, measuring ability to follow directions and understand concepts; fine and gross motor skills, measuring skill at using hands and conducting larger movements respectively; and visual reception, measuring ability to process information as received via visual inputs. These 5 subscales can also be combined into an Early Learning Composite score). The VABS is a semi-structured parent/caregiver interview, commonly used in studies of developmental disabilities. Scores on 4 domains (communication, daily living skills, socialization, motor skills), and an optional 5th domain (maladaptive behaviors), are calculated, as well as an overall composite score of level of adaptive functioning. For both the MSEL and VABS, lower scores indicate greater impairment, while higher scores indicate better functioning. We defined ‘low cognitive and behavioral scores’ as the lowest quintile of scores on these measures, though again, we examined other cut-offs.
Statistical analyses
All analyses were conducted using SAS version 9.2 (SAS Institute, 2014). Basic demographic comparisons were conducted across groups using one-way ANOVA and chi-square tests as appropriate. In order to test whether ADHD symptom scores differed by diagnostic group, mean scores on the hyperactivity subscale of the ABC were compared using one-way ANOVA across groups and t-tests for pairwise comparisons. We compared the highest quartile (here based on the full study group distribution) of the ABC hyperactivity subscale by diagnostic group using chi-squared tests. Within the full study group, we also calculated Pearson correlation coefficients for ABC Hyperactivity subscale and subdomain scores and other subscales of the ABC, as well as the VABS and MSEL, to gain additional information on how these symptoms might co-vary with other features.
Among children with ASD, we examined group differences between high and low scores on the ABC hyperactivity subscale (and secondarily our derived sub-domain scores) and basic demographic factors, cognitive ability level, and adaptive functioning. High and low ADHD symptoms were defined here according to the distribution of these scores within the ASD group, as our aim was to examine how the ADHD symptoms were associated with functioning within the ASD group. Quartiles of ADHD symptoms and quintiles of cognitive and behavioral scores were utilized to maximize information from the distribution while balancing sample size, and to examine dose-response effects, explore non-linearity in the relationship with outcomes, and also given inadequate variability for analysis of differences within the ASD group when we examined more clinically-derived cut-points. However, high ADHD symptom scores corresponded to roughly 1–1.5 standard deviations above the mean of the ASD group, while low cognitive/behavioral cut-offs were generally <1 standard deviation below the mean for the ASD group.
Linear regression was used in analyses of hyperactivity symptom scale/subdomain scores and the continuous cognitive and behavioral scores as outcomes to examine continuous associations. Logistic regression was used to obtain odds ratios (OR) and 95% confidence intervals (CI) of the associations between high ADHD symptoms (as predictor variables) and low cognitive and behavioral scores (as outcome variables), defined above. For both parameterizations, adjusted models included variables that could influence associations between these scores: matching factors (child sex, year of birth, regional area of birth), maternal education, health insurance status at delivery (as a measure of socio-economic status), maternal age, maternal smoking during pregnancy (Joelsson et al. 2016), and child age. In addition to adjustment for these potential confounders, for categorical parameterizations we also conducted weighted conditional logistic regression adjusted analyses with Proc Survey Logistic utilizing inverse-probability of participation weights, designed to achieve sample representativeness of the source population and thereby adjust for potential biases from differential participation rates related to sociodemographic factors. Weights were derived from a model fit to population data predicting participation in the study from maternal education level, age, country of birth, insurance status at child’s delivery, and child race/ethnicity, obtained from birth files. Final models of ADHD-related scores predicting cognitive and behavioral outcomes included these study weights, study matching factors, maternal age (in years) and education (in categories of high school or less, some college, college degree, or graduate degree), child age (continuous, in months), selected for greatest impact on results.
Exploratory subdomain analyses
Parallel secondary analyses examined associations with the exploratory subdomains (inattention and hyperactivity/impulsivity), assessing prevalence of highest quartile of these subdomains across study groups and, within the ASD group, examining binary ‘high’ inattention and hyperactivity/impulsivity (vs. remaining quartiles) with cognitive and adaptive functioning as in primary analyses.
Sensitivity analyses
Due to the number of tests performed, we adjusted for multiple comparisons using SAS Proc Multtest (Westfall and Tobias 1999), which obtains corrected p-values with the Hochberg step-up method (Hochberg and Tamhane 1987). We also examined whether removing the youngest children (<3 years old) altered results, given potential difficulty of assessing ADHD symptoms at this age. Finally, we assessed whether adjustment for ADOS comparison score (an indicator of level of autism severity, ranging from 1–10) impacted results of associations with cognitive and behavioral scores by adding this score to adjusted models.
Results
Full study population results: Basic comparisons across diagnostic groups
In these analyses, 548 ASD cases, 423 TD controls, and 180 DD controls were included. Year of birth and parental age were similar across groups; DD children were more likely to have a Hispanic mother and ASD and DD children were more likely to have lower MSEL and VABS scores (Table 1). Mean hyperactivity subscale scores demonstrated an increase by study group, from TD to DD and from DD to ASD, with significantly higher scores in the ASD group compared to each of the other groups and for DD compared to TD (p<0.0001 from t-tests for all comparisons); these scores (summarized in Supplementary Table 1) are similar to previously reported means and standard deviations for children in these and related groups (Karabekiroglu and Aman 2009).
Table 1.
Basic characteristics of the study population
| ASD Cases n=548 |
TD Controls n=423 |
DD Controls n=180 |
p-value1 | |
|---|---|---|---|---|
| Mean (std) | ||||
|
| ||||
| Demographic factors | ||||
|
| ||||
| Maternal age (years) | 31.0 (5.6) | 30.9 (5.7) | 30.8 (6.5) | 0.87 |
|
| ||||
| Paternal age (years) | 33.6 (6.5) | 33.2 (6.9) | 33.5 (7.5) | 0.65 |
|
| ||||
| Child age (months) | 44.8 (10.1) | 42.9 (9.7) | 46.9 (9.1) | <0.0001 |
|
| ||||
| Cognitive & behavioral scores | ||||
|
| ||||
| ABC hyperactivity Subscale2 | 18.2 (10.6) | 3.19 (4.9) | 12.3 (11.6) | <0.0001 |
|
| ||||
| Hyperactivity/impulsivity Subdomain3 | 10.8 (7.3) | 2.06 (3.4) | 7.71 (7.7) | <0.0001 |
|
| ||||
| Inattention Subdomain4 | 4.02 (2.2) | 0.52 (1.0) | 2.48 (2.3) | <0.0001 |
|
| ||||
| VABS Composite | 64.4 (12.3) | 102.5 (14.8) | 58.0 (8.2) | <0.0001 |
|
| ||||
| VABS Communication | 67.7 (15.8) | 103.8 (13.0) | 62.4 (9.6) | <0.0001 |
|
| ||||
| VABS Daily Living Skills | 66.2 (12.6) | 97.1 (14.6) | 60.9 (9.8) | <0.0001 |
|
| ||||
| VABS Socialization | 68.1 (12.8) | 103.0 (12.8) | 71.1 (11.3) | <0.0001 |
|
| ||||
| VABS Motor skills | 76.3 (17.8) | 104.6 (14.2) | 57.9 (13.4) | <0.0001 |
|
| ||||
| MSEL Expressive language-T score | 26.4 (10.1) | 51.9 (10.9) | 22.8 (5.8) | <0.0001 |
|
| ||||
| MSEL Receptive language-T score | 26.9 (11.4) | 52.1 (11.0) | 23.0 (5.7) | <0.0001 |
|
| ||||
| MSEL Visual reception-T score | 29.8 (13.5) | 57.1 (10.8) | 24.3 (8.1) | <0.0001 |
|
| ||||
| MSEL Fine motor- T score | 28.0 (11.7) | 53.2 (12.5) | 21.4 (4.1) | <0.0001 |
|
| ||||
| MSEL Composite | 61.3 (17.5) | 107.3 (17.2) | 53.1 (7.0) | <0.0001 |
|
| ||||
| n (%) | ||||
|
| ||||
| Demographic factors and basic characteristics | ||||
|
| ||||
| Maternal education level | <0.0001 | |||
| High school or less | 77 (14%) | 61 (14%) | 57 (32%) | |
| Some college | 229 (42%) | 148 (35%) | 62 (34%) | |
| College or graduate degree | 242 (44%) | 213 (50%) | 60 (33%) | |
|
| ||||
| Maternal race | 0.002 | |||
| Caucasian | 325 (59%) | 282 (67%) | 90 (50%) | |
| Hispanic | 137 (25%) | 83 (20%) | 60 (33%) | |
| Other | 86 (16%) | 58 (15%) | 30 (17%) | |
|
| ||||
| Insurance status at delivery | <0.0001 | |||
| Private insurance | 442 (81%) | 350 (83%) | 117 (65%) | |
| Government program | 106 (19%) | 73 (17%) | 63 (35%) | |
|
| ||||
| Male child5 | 470 (86%) | 353 (83%) | 107 (59%) | 0.005 |
P-value shown here from one-way ANOVA for comparison of means across groups; from chi-squared test for comparison of categorical variables.
ABC Hyperactivity Subscale ranges from 0–48. 43 ASD, 9 TD, and 15 DD were missing this subscale due to missing 1 or more ABC items used in creating the score.
Created by summing 10 items from the ABC Hyperactivity Subscale related to hyperactivity; see text for further information.
Created by summing 3 items from the ABC Hyperactivity Subscale related to inattention; see text for further information.
TD controls were sex-matched to ASD cases, but DD controls were not.
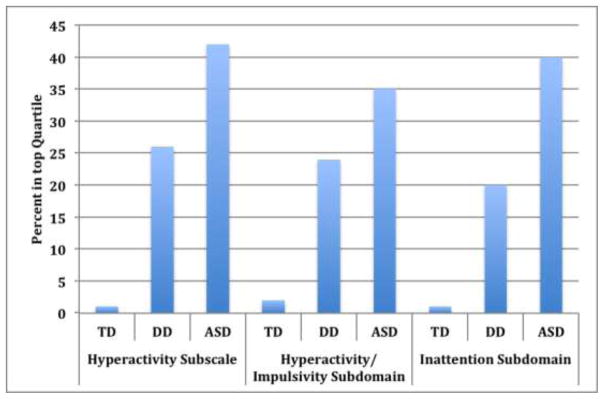
When categorizing ABC scores in highest quartiles for the full study group, ASD cases had significantly higher sub-scale scores (as well as scores on the exploratory sub-domains) relative to other groups; 42% of ASD cases scored in the top quartile of the hyperactivity sub-scale, compared to 26% of DD and 1% of TD (Figure 1). Utilizing standard-deviation cut-offs, rather than quartiles, was not informative for these comparisons, as all cases of ASD fell within the ‘high’ category when defining symptoms in this way. Examining correlations within the full study population, ABC scores for irritability, lethargy, and stereotypy subscales were highly correlated with hyperactivity and inattention subdomain scores across study groups (r~0.5 to 0-.7). Scores on these other ABC scales were generally not correlated with VABS or MSEL scores, though hyperactivity subscale scores were moderately correlated with reduced MSEL expressive language scores within ASD cases (r=.31).
Figure 1. ADHD Symptoms by diagnostic group.
Graph displays the percent of each diagnostic group in the top quartile (indicating greater impairment/symptoms) of the Hyperactivity Subscale of the ABC and the two derived subdomains of the ABC Hyperactivity Subscale. TD=Typically developing controls; DD= developmentally delayed controls; ASD=autism spectrum disorder cases. Quartiles here were defined within the full study group distribution (ASD+DD+TD).
ASD case group results: Associations between ADHD symptoms and cognitive and behavioral scores within children with ASD
Children with ASD and the highest levels of ADHD-like symptoms (as defined here by the highest quartile of ABC hyperactivity scores within the ASD group) differed from children with ASD in remaining quartiles of these scores on a number of basic demographic, behavioral, and cognitive factors (Table 2).
Table 2.
Basic characteristics of the ASD group (n=548) by high ADHD symptoms, according to ABC hyperactivity subscale scores
| ASD Cases with ‘ehigh ADHD symptoms’1 n= 126 |
ASD Cases with ‘lower ADHD symptoms’2 n= 379 |
P-value3 | |
|---|---|---|---|
| Mean (std) | |||
|
| |||
| Maternal age (years) | 29.4 (5.4) | 31.9 (5.4) | <0.0001 |
|
| |||
| Paternal age (years) | 32.0 (5.8) | 34.3 (6.5) | 0.0006 |
|
| |||
| Child age (months) | 45.5 (10.2) | 44.3 (10.0) | 0.26 |
|
| |||
| Cognitive & behavioral scores | |||
|
| |||
| ABC Hyperactivity Subscale4 | 32.8 (5.6) | 13.3 (6.7) | <0.0001 |
|
| |||
| Hyperactivity/impulsivity Subdomain5 | 20.4 (4.6) | 7.73 (4.9) | <0.0001 |
|
| |||
| Inattention Subdomain6 | 6.35 (1.7) | 3.24 (1.9) | <0.0001 |
|
| |||
| VABS Composite | 60.9 (10.8) | 65.9 (12.6) | <0.0001 |
|
| |||
| VABS Communication | 62.7 (12.8) | 69.6 (15.5) | <0.0001 |
|
| |||
| VABS Daily Living Skills | 63.1 (10.8) | 67.1 (11.4) | 0.0008 |
|
| |||
| VABS Socialization | 64.7 (11.3) | 69.3 (12.3) | 0.0002 |
|
| |||
| VABS Motor skills | 73.0 (17.1) | 77.7 (17.3) | 0.01 |
|
| |||
| MSEL Expressive language- T score | 24.9 (8.2) | 27.2 (10.8) | 0.02 |
|
| |||
| MSEL Receptive language- T score | 24.4 (8.3) | 27.9 (12.2) | 0.0003 |
|
| |||
| MSEL Visual reception- T score | 26.2 (11.0) | 31.1 (14.2) | <0.0001 |
|
| |||
| MSEL Fine motor- T score | 25.9 (9.6) | 28.5 (12.0) | 0.01 |
|
| |||
| MSEL Composite | 57.1 (13.5) | 62.8 (18.7) | 0.0003 |
|
| |||
| N (%) | |||
|
| |||
| Maternal education level | <0.0001 | ||
| High school or less | 31 (25%) | 35 (9%) | |
| Some college | 55 (44%) | 153 (40%) | |
| College degree | 24 (19%) | 124 (33%) | |
| Graduate degree | 16 (13%) | 67 (18%) | |
|
| |||
| Maternal race | |||
| Caucasian | 68 (54%) | 235 (62%) | 0.11 |
| Hispanic | 31 (25%) | 91 (24%) | |
| Other7 | 27 (21%) | 53 (14%) | |
|
| |||
| Insurance status at delivery | |||
| Private insurance | 91 (72%) | 322 (85%) | 0.001 |
| Government program | 35 (28%) | 57 (15%) | |
|
| |||
| Male child | 105 (83%) | 326 (86%) | 0.46 |
|
| |||
| Firstborn child | 60 (48%) | 166 (44%) | 0.40 |
|
| |||
| Multiple birth | 8 (6%) | 18 (5%) | 0.47 |
|
| |||
| Low birth weight (<2500g) | 10 (8%) | 31 (8%) | 0.92 |
“High ADHD symptoms” here defined as the highest quartile of ABC Hyperactivity subscale;
“Lower” defined as the remaining quartiles of the ABC Hyperactivity subscale combined.
P-values from t-tests for continuous variables and Chi-square tests for categorical variables. Other notes as for Table 1:
ABC Hyperactivity Subscale ranges from 0–48.
Created by summing 10 items from the ABC Hyperactivity Subscale related to hyperactivity; see text for further information.
Created by summing 3 items from the ABC Hyperactivity Subscale related to inattention; see text for further information.
Includes Black, Asian, and ‘Other’; difference here driven by Asian and ‘Other.’
In adjusted analyses examining ADHD symptom subscale and subdomains in association with continuous cognitive/behavioral scores in children with ASD, on average VABS and MSEL scores were lower by approximately 3–5 points in those ASD cases with higher ADHD symptoms (Table 3). For the exploratory derived subdomains, all scales demonstrated significant associations with high inattention, with the exception of VABS daily living; fewer significant associations were noted for the hyperactivity/impulsivity subdomain, though most VABS scales demonstrated significant associations with high hyperactivity/impulsivity.
Table 3.
Linear regression results of high ADHD symptoms predicting cognitive and behavioral scores within the ASD group
| ABC hyperactivity subscale | Hyperactivity/impulsivity sub-domain | Inattention sub-domain | ||||
|---|---|---|---|---|---|---|
| Beta (std error) | p-value | Beta (std error) | p-value | Beta (std error) | p-value | |
| VABS | ||||||
| Composite | −4.55 (1.7) | 0.006 | −2.94 (1.6) | 0.06 | −3.67 (1.7) | 0.03 |
| Communication | −5.63 (1.6) | 0.0005 | −4.42 (1.5) | 0.004 | −5.54 (1.6) | 0.0005 |
| Daily living | −3.90 (1.7) | 0.02 | −2.40 (1.6) | 0.13 | −2.31 (1.8) | 0.20 |
| Socialization | −4.19 (1.4) | 0.003 | −3.71 (1.4) | 0.006 | −4.21(1.4) | 0.002 |
| Motor skills | −3.95 (2.5) | 0.09 | −0.12 (2.5) | 0.96 | −4.11 (2.3) | 0.08 |
| MSEL | ||||||
| Composite | −2.91 (1.5) | 0.05 | −0.42 (1.5) | 0.79 | −4.71 (1.4) | 0.0008 |
| Expressive language | −0.79 (0.9) | 0.39 | 0.56 (0.9) | 0.61 | −2.27 (0.8) | 0.005 |
| Receptive language | −1.80 (0.93) | 0.05 | −0.83 (1.0) | 0.39 | −2.63 (0.9) | 0.002 |
| Visual reception | −2.94 (1.2) | 0.02 | 0.07 (1.4) | 0.96 | −4.06 (1.1) | 0.0003 |
| Fine motor | −1.32 (1.2) | 0.25 | −0.07 (1.2) | 0.95 | −2.73 (1.1) | 0.02 |
Results of linear regression models, with high ADHD symptoms (highest quartile of ABC Hyperactivity domain, and the Hyperactivity/impulsivity and Inattention subdomains) used as predictor variables and cognitive and behavioral scores (VABS and MSEL subscales) used as the continuous outcomes. Models are adjusted for study weights, matching factors, maternal age, and maternal education, and child age. Negative beta estimates indicate the high ABC categories are associated with a decrease in VABS/MSEL scores (greater impairment/lower functioning); positive estimates indicate an increase in VABS/MSEL scores (less impairment/higher functioning).
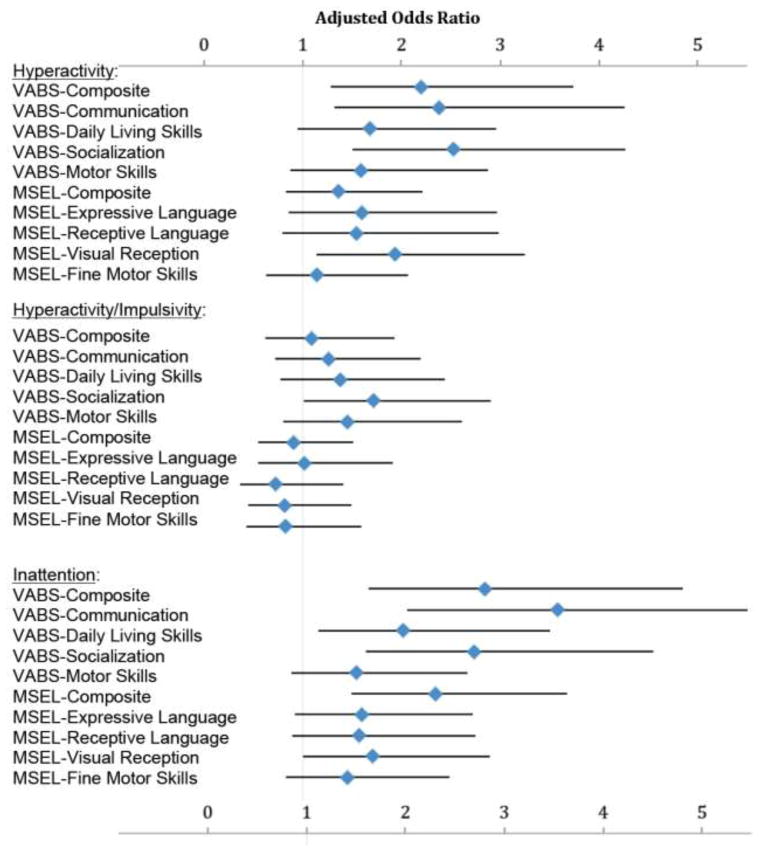
In adjusted analyses of categorized ADHD symptoms and cognitive/behavioral scores, the general pattern was similar, again showing greater cognitive and behavioral deficits among those with more ADHD symptoms (Figure 2). In particular, ASD children with high ADHD symptoms (again, scores in the highest quartile of the ABC hyperactivity subscale) were over twice as likely to have composite scores in the lowest quintile of the VABS, relative to those with lower ADHD symptoms (those in remaining quartiles of the ABC hyperactivity subscale) - (AOR=2.20, 95% CI 1.29–3.74; similar, and significant associations were also observed for the VABS communication and socialization subscales). In addition, children with high ADHD symptoms also had nearly double the odds of poor visual reception as defined by the lowest quintile of scores on the MSEL (AOR= 1.93, 95% CI 1.14–3.25).
Figure 2. High ADHD symptoms as predictors of low congnitive and behavioral scores within the ASD group.
Results of weighted logistic regression, adjusted for study matching factors (child sex, year of birth, and regional catchment area), maternal age, maternal education, child age in months, and weighted to account for selection into study using inverse-probability weights. Odds ratios (OR) represent odds of low (defined as the lowest quintile) VABS or MSEL subscale score in association with high (defined as highest quartile) ADHD symptoms (ABC hyperactivity subscale, hyperactivity/impulsivity subdomain, and inattention subdomain) within the ASD group (n=548). Vertical grey line indicates OR null value of 1; horizontal error bars indicate 95% confidence intervals. Point estimates and confidence intervals from these analyses and models with alternate covariate adjustment can be found in Supplementary Table 1, Model 3 column estimates.
When exploring use of the derived subdomains, associations with cognitive and behavioral scores appeared stronger for the inattention subdomain than for the hyperactivity/impulsivity subdomain. Individuals with high inattention subdomain scores were significantly more likely to have low VABS scores for all subscales other than motor skills, as well as low MSEL expressive composite scores, and language and visual reception subscale scores. The strongest associations were seen for high inattention and low scores for VABS communication (AOR=3.58, 95% CI 2.06, 6.21), VABS composite (AOR=2.85, 95% CI 1.67, 4.85), VABS socialization (AOR=2.73, 95% CI 1.65, 4.55), and MSEL composite (AOR=2.34, 95% CI 1.50, 3.67), and high overall ABC hyperactivity Subscale and VABS socialization (AOR=2.53, 95% CI 1.51, 4.26; Supplementary Table 2; mean scores for each of these categories are also provided in the previously referenced Supplementary Table 1). In contrast, there were no differences, comparing the high vs. low hyperactivity/impulsivity groups, on the MSEL composite or any MSEL subscale.
Sensitivity analyses
After correcting for multiple testing, most of the associations remained significant (Supplementary Table 3). Likewise, results of analyses excluding children <3 years of age were similar to primary analyses including these younger children (Supplementary Table 4); significant associations with greater likelihood of low cognitive and behavioral scores persisted. Further, the distribution of ADHD symptoms was the same by age strata (<3 years, 3 years and older, 4 years and older). Adjustment for ADOS comparison score in final models did not materially change results (data not shown).
Discussion
In this large population-based case-control study, we found a high prevalence of ADHD symptoms as measured by the ABC in preschool-aged children with ASD. There was a clear difference in hyperactivity subscale items across diagnostic groups, and significant associations between these ADHD symptoms and higher levels of other cognitive/behavioral deficits in children with ASD.
The first aim of this work was to determine the prevalence of ADHD symptoms across our diagnostic groups. Approximately 40% of children with ASD fell in the top quartile of the hyperactivity subscale, as well as within our exploratory hyperactivity/impulsivity and inattention subdomains, based on the distribution in the full study sample. The DD group had significantly fewer of these symptoms than the ASD group, though both the ASD and DD groups had significantly more ADHD symptoms than the TD group. These findings suggest that ADHD symptoms may be related to developmental delays more broadly, but could be more prominent in those with ASD-specific delays. Further work with larger numbers of DD cases is required to comparatively examine the second aim of our study (how ADHD symptoms impact functioning) in other developmental disorders besides ASD, as we did not have the statistical precision to conduct such analyses here.
Another striking result in our study was the consistency of associations between presence of ADHD symptoms and evidence of greater cognitive/behavioral impairments in children with ASD. Though not statistically significant for all subscales, higher overall ADHD symptoms corresponded to poorer adaptive functioning, and cognitive abilities, a finding also previously reported for older children (Yerys et al. 2009). Though based on only three items from the ABC, and therefore requiring conservative interpretation, our results suggested that presence of inattention may figure prominently into associations between the ABC hyperactivity subscale and the cognitive and behavioral scores: inattention sub-domain scores were significantly associated with nearly all of the VABS subscales, and with the MSEL composite and all subscale scores, while the hyperactivity/impulsivity subdomain did demonstrate few significant associations. Relatively less impairment with hyperactive-impulsive features of ADHD has also been noted in prior work; a study examining ADHD subtypes and co-occurring psychiatric symptoms in children with ASD reported the least impairment for those with the Hyperactive-Impulsive type (Gadow et al. 2006). Future studies, that more rigorously assess ADHD subtypes, symptoms, and attention deficits should examine these associations between inattention in young children with ASD and related deficits.
Other research has similarly suggested the role of attention deficits in ASD (Gillberg and Fernell 2014; Leitner 2014); in particular, Rao and Landa noted lower cognitive functioning, more severe social impairment, and stronger delays in adaptive functioning in children with ASD and ADHD (Rao and Landa 2014), while a study by Jarrold and colleagues suggested deficits in attention may disrupt social functioning in ASD (Jarrold et al. 2013). Work in atypically developing toddlers aged 18–37 months suggested ASD symptom severity was positively and significantly associated with inattention/impulsivity (Tureck et al. 2013). Follow-up studies of educational attainment for children with combined ASD and ADHD symptoms are needed to determine the impact of such symptoms on academic achievement and other crucial outcomes. That the deficits in cognitive and adaptive behaviors appear in pre-school children is perhaps most concerning, and suggests this subset of children among those with ASD may be at a disadvantage long before they begin school. Our secondary analyses examining low cognitive and behavioral scores in association with high ADHD symptoms in children with TD and DD suggested these associations could be unique to ASD or different for ASD than for DD, although analyses in children with DD, despite being larger than any studies to date, may have lacked sufficient statistical power to detect group differences. Further work should explore how these symptoms may differentially impact functioning in ASD vs. other developmental conditions.
These findings suggest the importance of improving attention in young children with ASD. While applied behavior analytic approaches (Lovaas et al. 1967; Vismara and Rogers 2010) used in early intervention programs work with children to improve their attentional focus, building attentional skills is typically not considered the primary aim of such treatments. Rather, they may use procedures that facilitate attention in the service of improving language and social behavior. Early intervention programs that specifically target increasing attention may also be beneficial. Mechanisms that may underlie patterns observed in our study may be related to commonalities between brain regions and neural systems implicated in ADHD and in ASD involved in the regulation of attention and behavior, as supported in the literature (Solomon et al. 2009; Dichter 2012; Schweitzer et al. 2012).
Our study’s strengths include a relatively large sample size of over 500 ASD cases, clinical confirmation of all diagnoses through rigorous gold-standard measures and procedures, novel approach to focus on preschool age, and use of multiple statistical procedures to achieve thorough adjustment for confounding, selection bias, and multiple comparisons. However, a number of limitations should be noted. The ABC hyperactivity items overlap with hyperactivity/impulsivity and inattention measures on most ADHD measures, however, it was not designed as an ADHD measure and does not fully capture ADHD. We recognize that for an ADHD diagnosis a clinical interview would be required and thus we can only report on symptoms rather than diagnosis. The focus on symptoms, however, rather than diagnostic categories, is consistent with the Research Domain Criteria (RDoC) approach (Insel et al. 2010). In addition, our subdomain items do not fully capture ADHD subtypes and should be considered preliminary. Our results must therefore be interpreted within the context of the ABC subscale items used, rather than as ADHD specifically. However, the ABC was designed to measure behavioral deficits in this age group, and the hyperactivity subscale has been used in previous work as a measure of ADHD symptoms in ASD (Arnold et al. 2006; Troost et al. 2006; Charnsil 2011). Studies of ABC scores in children with ADHD (Aman et al. 2002; Aman et al. 2004; Hillard et al. 2013) have also supported the validity of this subscale in capturing ADHD symptoms. We also used only mother-report ABC scores, and did not have other informant reports available, though studies reporting high inter-rater reliability for the ABC (Aman et al. 1985; Miller et al. 2004) suggest the use of maternal report alone may be sufficient to capture ADHD symptoms.
Though we hypothesize that ADHD symptoms may be influencing other behaviors within ASD and modeled our analyses of associations with cognitive/behavioral scores according to this hypothesis, a key point to note is that our analyses cannot provide information on the directionality of these associations. Specifically, it is not clear at this time whether the presence of greater ADHD symptoms impairs cognitive and behavioral functioning, or rather, if features of poorer cognitive and behavioral skills lead to greater ADHD symptoms. Research suggests that the inability to regulate attention, including determining which information to focus on and dividing attention between competing events and social cues, impairs social functioning (Kofler et al. 2011). Our results are also consistent with findings of poorer academic achievement and level of functioning for individuals with comorbid ADHD and ASD (Pingault et al. 2011; Cooper et al. 2014; Rao and Landa 2014), and suggest the need for targeted treatment strategies for individuals with ASD who exhibit attention deficits. Effective strategies may improve their academic functioning, which could be more negatively affected than for those with ASD who do not exhibit attention impairments. Notably, ASD symptoms in children diagnosed with ADHD have likewise been associated with increased deficits (Cooper et al. 2014).
Children in our study were 2–5 years old, which is younger than the typical age at ADHD diagnosis, but an appropriate age for targeting interventions. Our goal was to examine inattention and hyperactivity/impulsivity as symptoms and their cognitive/behavioral correlates within young children who have ASD, rather than to determine ADHD diagnoses within this young group. Sensitivity analyses did not suggest results were driven by biased reporting for young children; rather, measures of association were very similar (and in some cases stronger) when excluding children <36 months of age. At least one other investigation has used the ABC in very young samples; a clinical study of toddlers aged 14–43 months found high validity of the ABC with the Autism Behavior Checklist and the Child Behavior Checklist (Karabekiroglu and Aman 2009). An additional feature of our analysis was that we created our own subdomains within this subscale, which may or may not directly map onto ADHD subtypes. Other work has suggested increased validity of ADHD diagnoses in children with ASD when breaking ADHD factors into clinically-relevant subparts (Lecavalier et al. 2011), and further support for use of subtypes derives from reports showing greater relative importance of inattention symptoms as compared to hyperactivity (Lecavalier et al. 2011; Kaat et al. 2014). Our study confirms this literature in young children with ASD.
The stability of ADHD symptoms at this young age in the ASD population is unknown, and measurement of these behaviors at this age can be challenging. Though ADHD symptoms do tend to change with development (Cherkasova et al. 2013), there is also evidence for symptom stability. A recent analysis following-up infant siblings at risk for ASD at school-age (5.5–9 years) supported persistence of clinical concerns for ADHD and other issues (Miller et al. 2015). Persistence of significant ADHD symptoms and impairment in social and academic contexts in comparison to control children has been reported in children 4 to 6 years of age to three years later (Lahey et al. 2004). Further, a large, multi-site study including children aged 3 to 5.5 years, the Preschool ADHD Treatment Study (PATS) (Kollins et al. 2006) reported that 89% (160/180) of the initial participants still met criteria for ADHD diagnosis six years later (Riddle et al. 2013). Continued work on the developmental progression of these symptoms and how they impact outcomes in the ASD population is needed, particularly in light of evidence that ADHD symptoms are associated with poorer academic functioning, increased behavioral and mental health issues, and reduced social functioning, and that when these conditions are comorbid, alternative or additional treatment strategies may be required (Antshel et al. 2011; Cooper et al. 2014).
Implications
Our results suggest significant cognitive and behavioral impairments for those individuals with ASD and the greatest attention and hyperactivity deficits, and that such issues may be present from a very young age. Our exploratory analysis of subdomains of symptoms suggests that inattention may play a strong role in functioning within those with ASD; thus, developing treatments that improve attentional functioning in ASD could be an important clinical consideration. Subsequent research should explore if the inattentive symptoms seen in ASD are qualitatively and neutrally distinct from the inattentive symptoms seen in ADHD. Finally, future work also should examine the impact of ADHD symptoms and subtypes using standardized ADHD assessments in young children with ASD, and explore how these associations influence longer-term trajectories.
Supplementary Material
Highlights.
Attention deficits are common in children with autism and other developmental delays, but how these symptoms impact other areas of functioning, particularly in very young children with these disorders, in not well understood.
This is the largest population-based study examining the impact of attention deficits/ADHD-related symptoms in young children with autism spectrum disorder on cognitive and behavioral scores.
Our results, which demonstrated significant associations between higher levels of attention deficits in children with ASD and lower scores on cognitive and behavioral assessments, suggest ADHD symptoms may play a key role in the functioning of young children with ASD.
Our results also confirm the high prevalence of attention deficits in children aged 2–5 with ASD, and to a lesser extent, children with developmental delay without autism, relative to typically developing children of this age range.
Findings here also suggest the need for better understanding of the manifestations ADHD symptoms in early development to ensure accurate early diagnosis, appropriate treatment, and optimal school outcomes for children with these conditions.
Acknowledgments
This work was supported by the following grants: National Institute of Environmental Health Sciences R01 ES015359, National Institute of Environmental Health Sciences P01 ES11269, Environmental Protection Agency STAR #R829388 & R833292, and National Institute of Mental Health 5-T32 MH073124, National Institutes of Child Health and Human Development and the MIND Institute Intellectual and Developmental Disabilities Research Center, U54 HD079125), as well as the MIND Institute. The authors have no financial relationships relevant to this article or conflicts of interest to disclose. We also acknowledge the Whiteley Center, which provided facilities for a scholarly retreat wherein work on this manuscript was advanced.
Footnotes
Conflict of interest: The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aman MG, Armstrong S, Buican B, et al. Four-year follow-up of children with low intelligence and ADHD: a replication. Res Dev Disabil. 2002;23(2):119–134. doi: 10.1016/s0891-4222(02)00090-2. [DOI] [PubMed] [Google Scholar]
- Aman MG, Binder C, Turgay A. Risperidone effects in the presence/absence of psychostimulant medicine in children with ADHD, other disruptive behavior disorders, and subaverage IQ. J Child Adolesc Psychopharmacol. 2004;14(2):243–254. doi: 10.1089/1044546041649020. [DOI] [PubMed] [Google Scholar]
- Aman MG, Richmond G, Stewart AW, et al. The aberrant behavior checklist: factor structure and the effect of subject variables in American and New Zealand facilities. Am J Ment Defic. 1987;91(6):570–578. [PubMed] [Google Scholar]
- Aman MG, Singh NN, Stewart AW, et al. The aberrant behavior checklist: a behavior rating scale for the assessment of treatment effects. Am J Ment Defic. 1985;89(5):485–491. [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- American Psychiatric Association, editor. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Association; 2013. [Google Scholar]
- Antshel KM, Polacek C, McMahon M, et al. Comorbid ADHD and anxiety affect social skills group intervention treatment efficacy in children with autism spectrum disorders. J Dev Behav Pediatr. 2011;32(6):439–446. doi: 10.1097/DBP.0b013e318222355d. [DOI] [PubMed] [Google Scholar]
- Arnold LE, Aman MG, Cook AM, et al. Atomoxetine for hyperactivity in autism spectrum disorders: placebo-controlled crossover pilot trial. J Am Acad Child Adolesc Psychiatry. 2006;45(10):1196–1205. doi: 10.1097/01.chi.0000231976.28719.2a. [DOI] [PubMed] [Google Scholar]
- Brinkley J, Nations L, Abramson RK, et al. Factor analysis of the aberrant behavior checklist in individuals with autism spectrum disorders. J Autism Dev Disord. 2007;37(10):1949–1959. doi: 10.1007/s10803-006-0327-3. [DOI] [PubMed] [Google Scholar]
- Charnsil C. Efficacy of atomoxetine in children with severe autistic disorders and symptoms of ADHD: an open-label study. J Atten Disord. 2011;15(8):684–689. doi: 10.1177/1087054710376907. [DOI] [PubMed] [Google Scholar]
- Cherkasova M, Sulla EM, Dalena KL, et al. Developmental course of attention deficit hyperactivity disorder and its predictors. J Can Acad Child Adolesc Psychiatry. 2013;22(1):47–54. [PMC free article] [PubMed] [Google Scholar]
- Cooper M, Martin J, Langley K, et al. Autistic traits in children with ADHD index clinical and cognitive problems. Eur Child Adolesc Psychiatry. 2014;23(1):23–34. doi: 10.1007/s00787-013-0398-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbett BA, Constantine LJ. Autism and attention deficit hyperactivity disorder: assessing attention and response control with the integrated visual and auditory continuous performance test. Child Neuropsychol. 2006;12(4–5):335–348. doi: 10.1080/09297040500350938. [DOI] [PubMed] [Google Scholar]
- Cortese S, Castelnau P, Morcillo C, et al. Psychostimulants for ADHD-like symptoms in individuals with autism spectrum disorders. Expert Rev Neurother. 2012;12(4):461–473. doi: 10.1586/ern.12.23. [DOI] [PubMed] [Google Scholar]
- Dichter GS. Functional magnetic resonance imaging of autism spectrum disorders. Dialogues Clin Neurosci. 2012;14(3):319–351. doi: 10.31887/DCNS.2012.14.3/gdichter. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers S, Nyden A, Gillberg C, et al. Asperger syndrome, autism and attention disorders: a comparative study of the cognitive profiles of 120 children. J Child Psychol Psychiatry. 1997;38(2):207–217. doi: 10.1111/j.1469-7610.1997.tb01855.x. [DOI] [PubMed] [Google Scholar]
- Gadow KD, DeVincent CJ, Pomeroy J. ADHD symptom subtypes in children with pervasive developmental disorder. J Autism Dev Disord. 2006;36(2):271–283. doi: 10.1007/s10803-005-0060-3. [DOI] [PubMed] [Google Scholar]
- Geurts HM, Verte S, Oosterlaan J, et al. How specific are executive functioning deficits in attention deficit hyperactivity disorder and autism? J Child Psychol Psychiatry. 2004;45(4):836–854. doi: 10.1111/j.1469-7610.2004.00276.x. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Fernell E. Autism Plus Versus Autism Pure. J Autism Dev Disord. 2014 doi: 10.1007/s10803-014-2163-1. [DOI] [PubMed] [Google Scholar]
- Hanson E, Cerban CM, Slater Bm, Caccamo LM, et al. Brief report: prevalence of attention deficit/hyperactivity disorder among individuals with an autism spectrum disorder. 2013 doi: 10.1007/s10803-012-1677-7. (1573–3432 (Electronic)) [DOI] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect. 2006;114(7):1119–1125. doi: 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillard B, El-Baz AS, Sears L, et al. Neurofeedback training aimed to improve focused attention and alertness in children with ADHD: a study of relative power of EEG rhythms using custom-made software application. Clin EEG Neurosci. 2013;44(3):193–202. doi: 10.1177/1550059412458262. [DOI] [PubMed] [Google Scholar]
- Hochberg Y, Tamhane AC. Multiple Comparison Procedures. New York: Wiley; 1987. [Google Scholar]
- Holtmann M, Bolte F, Poustka F. Attention deficit hyperactivity disorder symptoms in pervasive developmental disorders: association with autistic behavior domains and coexisting psychopathology. Psychopathology. 2007;40(3):172–177. doi: 10.1159/000100007. [DOI] [PubMed] [Google Scholar]
- Insel T, Cuthbert B, Garvey M, et al. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am J Psychiatry. 2010;167(7):748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Jang J, Matson JL, Williams LW, et al. Rates of comorbid symptoms in children with ASD, ADHD, and comorbid ASD and ADHD. Res Dev Disabil. 2013;34(8):2369–2378. doi: 10.1016/j.ridd.2013.04.021. [DOI] [PubMed] [Google Scholar]
- Jarrold W, Mundy P, Gwaltney M, et al. Social attention in a virtual public speaking task in higher functioning children with autism. Autism Res. 2013;6(5):393–410. doi: 10.1002/aur.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joelsson P, Chudal R, Talati A, et al. Prenatal smoking exposure and neuropsychiatric comorbidity of ADHD: a finnish nationwide population-based cohort study. BMC Psychiatry. 2016;16:306. doi: 10.1186/s12888-016-1007-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaat AJ, Lecavalier L, Aman MG. Validity of the aberrant behavior checklist in children with autism spectrum disorder. J Autism Dev Disord. 2014;44(5):1103–1116. doi: 10.1007/s10803-013-1970-0. [DOI] [PubMed] [Google Scholar]
- Karabekiroglu K, Aman MG. Validity of the aberrant behavior checklist in a clinical sample of toddlers. Child Psychiatry Hum Dev. 2009;40(1):99–110. doi: 10.1007/s10578-008-0108-7. [DOI] [PubMed] [Google Scholar]
- Kofler MJ, Rapport MD, Bolden J, et al. Working memory deficits and social problems in children with ADHD. J Abnorm Child Psychol. 2011;39(6):805–817. doi: 10.1007/s10802-011-9492-8. [DOI] [PubMed] [Google Scholar]
- Kollins S, Greenhill L, Swanson J, et al. Rationale, design, and methods of the Preschool ADHD Treatment Study (PATS) J Am Acad Child Adolesc Psychiatry. 2006;45(11):1275–1283. doi: 10.1097/01.chi.0000235074.86919.dc. [DOI] [PubMed] [Google Scholar]
- Lahey BB, Pelham WE, Loney J, et al. Three-year predictive validity of DSM-IV attention deficit hyperactivity disorder in children diagnosed at 4–6 years of age. Am J Psychiatry. 2004;161(11):2014–2020. doi: 10.1176/appi.ajp.161.11.2014. [DOI] [PubMed] [Google Scholar]
- Lecavalier L, Gadow KD, Devincent CJ, et al. Validity of DSM-IV syndromes in preschoolers with autism spectrum disorders. Autism. 2011;15(5):527–543. doi: 10.1177/1362361310391115. [DOI] [PubMed] [Google Scholar]
- Lee DO, Ousley OY. Attention-deficit hyperactivity disorder symptoms in a clinic sample of children and adolescents with pervasive developmental disorders. 2006. [DOI] [PubMed] [Google Scholar]
- Leitner Y. The co-occurrence of autism and attention deficit hyperactivity disorder in children - what do we know? Front Hum Neurosci. 2014;8:268. doi: 10.3389/fnhum.2014.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, et al. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord. 2006;36(7):849–861. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- Lovaas OI, Freitas L, Nelson K, et al. The establishment of imitation and its use for the development of complex behavior in schizophrenic children. Behav Res Ther. 1967;5(3):171–181. doi: 10.1016/0005-7967(67)90032-0. [DOI] [PubMed] [Google Scholar]
- Lundervold AJ, Stickert M, Hysing M, et al. Attention Deficits in Children With Combined Autism and ADHD: A CPT Study. J Atten Disord. 2012 doi: 10.1177/1087054712453168. [DOI] [PubMed] [Google Scholar]
- Marshburn EC, Aman MG. Factor validity and norms for the aberrant behavior checklist in a community sample of children with mental retardation. J Autism Dev Disord. 1992;22(3):357–373. doi: 10.1007/BF01048240. [DOI] [PubMed] [Google Scholar]
- Matson JL, Rieske LW, Williams Rd. The relationship between autism spectrum disorders and attention-deficit/hyperactivity disorder: an overview. 2013 doi: 10.1016/j.ridd.2013.05.021. 1873–3379 (Electronic)) [DOI] [PubMed] [Google Scholar]
- Miller M, Iosif AM, Young GS, et al. School-age outcomes of infants at risk for autism spectrum disorder. Autism Res. 2015 doi: 10.1002/aur.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ML, V, Fee E, Netterville AK. Psychometric properties of ADHD rating scales among children with mental retardation I: reliability. Res Dev Disabil. 2004;25(5):459–476. doi: 10.1016/j.ridd.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Miodovnik A, Harstad E, Sideridis G, et al. Timing of the Diagnosis of Attention-Deficit/Hyperactivity Disorder and Autism Spectrum Disorder. Pediatrics. 2015;136(4):e830–837. doi: 10.1542/peds.2015-1502. [DOI] [PubMed] [Google Scholar]
- Mullen EM. The Mullen Scales of Early Learning. Circle Pines, MN: American Guidance Service Inc; 1995. [Google Scholar]
- Pearson DA, Aman MG, Arnold LE, et al. High concordance of parent and teacher attention-deficit/hyperactivity disorder ratings in medicated and unmedicated children with autism spectrum disorders. J Child Adolesc Psychopharmacol. 2012;22(4):284–291. doi: 10.1089/cap.2011.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pingault JB, Tremblay RE, Vitaro F, et al. Childhood trajectories of inattention and hyperactivity and prediction of educational attainment in early adulthood: a 16-year longitudinal population-based study. Am J Psychiatry. 2011;168(11):1164–1170. doi: 10.1176/appi.ajp.2011.10121732. [DOI] [PubMed] [Google Scholar]
- Rao PA, Landa RJ. Association between severity of behavioral phenotype and comorbid attention deficit hyperactivity disorder symptoms in children with autism spectrum disorders. Autism. 2014;18(3):272–280. doi: 10.1177/1362361312470494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle MA, Yershova K, Lazzaretto D, et al. The Preschool Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS) 6-year follow-up. J Am Acad Child Adolesc Psychiatry. 2013;52(3):264–278. e262. doi: 10.1016/j.jaac.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risi S, Lord C, Gotham K, et al. Combining information from multiple sources in the diagnosis of autism spectrum disorders. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1094–1103. doi: 10.1097/01.chi.0000227880.42780.0e. [DOI] [PubMed] [Google Scholar]
- Schweitzer JB, Fassbender C, Lit L, et al. Attention-deficit/hyperactivity disorder. Handb Clin Neurol. 2012;106:391–405. doi: 10.1016/B978-0-444-52002-9.00022-X. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Taylor E, Baird G, et al. Randomized controlled double-blind trial of optimal dose methylphenidate in children and adolescents with severe attention deficit hyperactivity disorder and intellectual disability. J Child Psychol Psychiatry. 2013;54(5):527–535. doi: 10.1111/j.1469-7610.2012.02569.x. [DOI] [PubMed] [Google Scholar]
- Solomon M, Ozonoff SJ, Ursu S, et al. The neural substrates of cognitive control deficits in autism spectrum disorders. Neuropsychologia. 2009;47(12):2515–2526. doi: 10.1016/j.neuropsychologia.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow SS, Balla DA, Cicchetti DV. Vineland Adaptive Behavior Scales. Bloomington, MN: Pearson Assessments; 1984. [Google Scholar]
- Taurines R, Schwenck C, Westerwald E, et al. ADHD and autism: differential diagnosis or overlapping traits? A selective review. Atten Defic Hyperact Disord. 2012 doi: 10.1007/s12402-012-0086-2. [DOI] [PubMed] [Google Scholar]
- Troost PW, Steenhuis MP, Tuynman-Qua HG, et al. Atomoxetine for attention-deficit/hyperactivity disorder symptoms in children with pervasive developmental disorders: a pilot study. J Child Adolesc Psychopharmacol. 2006;16(5):611–619. doi: 10.1089/cap.2006.16.611. [DOI] [PubMed] [Google Scholar]
- Tureck K, Matson JL, Cervantes P, et al. Autism severity as a predictor of inattention and impulsivity in toddlers. Dev Neurorehabil. 2013 doi: 10.3109/17518423.2013.807884. [DOI] [PubMed] [Google Scholar]
- Varvara P, Varuzza C, Sorrentino AC, et al. Executive functions in developmental dyslexia. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vismara LA, Rogers SJ. Behavioral treatments in autism spectrum disorder: what do we know? Annu Rev Clin Psychol. 2010;6:447–468. doi: 10.1146/annurev.clinpsy.121208.131151. [DOI] [PubMed] [Google Scholar]
- Visser JC, Rommelse NN, Greven CU, et al. Autism spectrum disorder and attention-deficit/hyperactivity disorder in early childhood: A review of unique and shared characteristics and developmental antecedents. Neurosci Biobehav Rev. 2016;65:229–263. doi: 10.1016/j.neubiorev.2016.03.019. [DOI] [PubMed] [Google Scholar]
- Westfall PH, Tobias RD. SUGI. Vol. 24. SAS Institute, Inc; 1999. Multiple comparisons and Multiple Tests using the SAS System. [Google Scholar]
- Wheeler A, Raspa C, Bann M, Bishop E, et al. Anxiety, attention problems, hyperactivity, and the Aberrant Behavior Checklist in fragile X syndrome. 2014 doi: 10.1002/ajmg.a.36232. (1552–4833 (Electronic)) [DOI] [PubMed] [Google Scholar]
- World Health Organization. International Classification of Disease. Geneva: World Health Organization; 1992. [Google Scholar]
- Yerys BE, Wallace GL, Sokoloff JL, et al. Attention deficit/hyperactivity disorder symptoms moderate cognition and behavior in children with autism spectrum disorders. Autism Res. 2009;2(6):322–333. doi: 10.1002/aur.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.