Abstract
Actinobacteria of the acI lineage are the most abundant microbes in freshwater systems, but there are so far no pure living cultures of these organisms, possibly because of metabolic dependencies on other microbes. This, in turn, has hampered an in-depth assessment of the genomic basis for their success in the environment. Here we present genomes from 16 axenic cultures of acI Actinobacteria. The isolates were not only of minute cell size, but also among the most streamlined free-living microbes, with extremely small genome sizes (1.2–1.4 Mbp) and low genomic GC content. Genome reduction in these bacteria might have led to auxotrophy for various vitamins, amino acids and reduced sulphur sources, thus creating dependencies to co-occurring organisms (the ‘Black Queen’ hypothesis). Genome analyses, moreover, revealed a surprising degree of inter- and intraspecific diversity in metabolic pathways, especially of carbohydrate transport and metabolism, and mainly encoded in genomic islands. The striking genotype microdiversification of acI Actinobacteria might explain their global success in highly dynamic freshwater environments with complex seasonal patterns of allochthonous and autochthonous carbon sources. We propose a new order within Actinobacteria (‘Candidatus Nanopelagicales’) with two new genera (‘Candidatus Nanopelagicus’ and ‘Candidatus Planktophila’) and nine new species.
Introduction
The pelagic zone of oceans and fresh waters is numerically dominated by microbes with cell volumes <0.1 μm3. The genomes of such oligotrophic ‘ultramicrobacteria’ are considered to be ‘streamlined’, that is, <1.5 Mbp, with highly conserved core-genomes, few pseudogenes, low ratios of intergenic spacer DNA to coding DNA, reduced numbers of paralogues and a low genomic GC content (Giovannoni et al., 2014; Luo et al., 2015). Streamlining theory proposed that gene loss reflects an evolutionary adaptation to the competition for limiting resources under oligotrophic conditions (Giovannoni et al., 2014). However, genome reduction might also lead to metabolic dependencies on co-occurring microorganisms, as outlined by the ‘Black Queen Hypothesis’ (Morris et al., 2012), possibly explaining why few taxa with this lifestyle have so far been isolated.
Actinobacteria of the acI lineage are among the most successful bacteria in lacustrine waters where they may constitute >50% of all microbes (Newton et al., 2011). They are distributed ubiquitously in a wide range of freshwater habitats of different trophic states and varying pH, oxygen and salinity levels (Newton et al., 2007, 2011). There are no stable pure cultures of acI Actinobacteria that has been explained by a high degree of metabolic interconnectedness and dependencies on co-occurring microbes (Garcia et al., 2015). A strain from this lineage in a mixed enrichment culture has been tentatively described as ‘Ca. Planktophila limnetica’ (Jezbera et al., 2009). Cultivation-independent methods and in situ experiments indicate slower growth rates and lower competitiveness of acI Actinobacteria than copiotrophic bacteria under elevated nutrient and substrate regimes (Burkert et al., 2003; Neuenschwander et al., 2015), but also proposed a reduced vulnerability to bacterivorous protists (Šimek et al., 2014). Co-occurring phytoplankton blooms (Eckert et al., 2012), and also allochthonous dissolved organic matter from terrestrial sources (Pérez and Sommaruga, 2006), are likely providers of carbohydrates and other carbon sources for acI Actinobacteria (Buck et al., 2009; Salcher et al., 2013).
So far, 4 complete genomes of transient cultures (Kang et al., 2017), 10 single-cell amplified genomes (SAGs; Ghylin et al., 2014) and several metagenome assembled genomes (MAGs; Ghai et al., 2014; Garcia et al., 2015; Bendall et al., 2016) provided first insights into the metabolic potential of different acI lineages. However, the low number of complete genomes as well as the intrinsic incompleteness of SAGs and the mixed genotype origin of MAGs limits their usefulness for comparative population genomics and for understanding the metabolic distinctions within sympatric ecotypes.
Here, we report on 16 complete high-quality genomes of freshwater acI Actinobacteria from monoclonal cultures that were obtained from a single site, Lake Zurich, Switzerland. All but one strain were isolated in spring in two consecutive years, thus representing sympatric genotypes. Genome analysis suggests a clear split into two related, but distinct genera within the acI lineage. Our data set allows for a first comparative genomic analysis of representatives from two closely related genera on a (sub)species level and, moreover, provides first insights into diversification and potential niche separation within co-occurring acI populations. We also describe a clear temporal separation between the two genera in Lake Zurich, and we assess the global occurrence of the studied genotypes in all currently available freshwater metagenomes.
Materials and methods
Sampling of Lake Zurich, Switzerland
Lake Zurich is a large, deep (136 m), prealpine, oligo-mesotrophic lake that is characterized by persistent annual blooms of the toxic cyanobacterium Planktothrix rubescens (Posch et al., 2012). Sampling was conducted every second week in 2012 and 2013 (n=37) at the deepest part of the lake (47.30N, 8.58E). Vertical profiles of temperature, conductivity, turbidity, oxygen and chlorophyll a content were recorded with a YSI multiprobe (Yellow Springs Instruments, model 6600, Yellow Springs, OH, USA) and a bbe fluoroprobe (TS-16-12, bbe Moldaenke GmbH, Schwentinental, Germany) calibrated to distinguish between different phytoplankton groups and P. rubescens according to the fluorescent spectra of their pigments (Beutler et al., 2002). Water samples were taken from 0, 5, 10, 20, 30, 40, 60, 80 and 100 m depth, and additionally from the depth of recorded chlorophyll a maxima (n=348). Then, 40 ml water was fixed with formaldehyde (2% final concentration) for estimating prokaryotic abundances via flow cytometry and stored at 4 °C until measurements. Next, 5–10 ml was fixed with paraformaldehyde (pH 7.4, 2% final concentration) for 1–2 h, filtered onto white polycarbonate filters (0.22 μm pore size, 47 mm diameter, Millipore, Billerica, MA, USA) and stored at −20 °C for CARD-FISH (fluorescence in situ hybridization followed by catalysed reporter deposition) analyses. Two litres of water from 5 m depth for the preparation of media and the isolation of Actinobacteria was transported to the laboratory within 15 min. Chemical parameters were determined at monthly intervals by standard techniques by the Zurich Water Supply Company.
Isolation of planktonic Actinobacteria
Planktonic Actinobacteria were isolated as part of a dilution-to-extinction isolation campaign during 2010–2013 (Salcher et al., 2015). Filtered (0.2 μm pore-sized polysulfone filters, Millipore) and autoclaved lake water amended with different types of carbon and nutrient sources served as medium (Supplementary Table S1) and prefiltered water (0.45 μm pore-sized polycarbonate filters, Millipore) served as inoculum. The final inoculum size ranged between 1 and 10 cells per well of 24-well plates (Thermo Scientific, Waltham, MA, USA) filled with 2 ml of medium. Growth in individual wells was checked microscopically after 2–3 months of incubation at close to in situ temperature in a dark/light cycle (16:8 h). Dense cultures (that is, >105–106 cells per ml) were split in 500 μl aliquots that were (1) propagated to fresh medium, (2) amended with glycerol (30% final concentration) and stored at −80 °C, and (3) pelleted by centrifugation (30 min, 16 000 g) and used for PCR and 16S rRNA sequencing as well as whole-genome amplification and genome sequencing. For more details on the 46 isolation campaigns see Salcher et al. (2015). Sizing of the 16 strains was done with a Zeiss AxioImagerM.1 microscope (Zeiss, Oberkochen, Germany) after 4’,6-diamidino-2-phenylindole (DAPI) staining, using the software LUCIA (Laboratory Imaging, Prague, Czech Republic) according to a previously published workflow (Posch et al., 2009).
Whole-genome amplification and sequencing of isolates
The REPLI-g single cell kit (Qiagen, Venlo, The Netherlands) was used for multiple displacement amplification (MDA). All preamplification steps were performed in a particle-free environment dedicated to MDA. Fresh PCR-clean pipet tips were used for each MDA session and reaction tubes and PCR plates were ultraviolet treated before usage. MDA was conducted according to the manufacturer’s protocol with the following modifications: lysates for 6 to 8 replicate MDA reactions were produced in a 0.5 ml reaction tube (8 μl samples containing ∼4000–40 000 cells, 6 μl each of the reagents D2 and stop) and subsequently distributed (2.5 μl each) among wells of 96-well plates each containing 10 μl MDA reaction mix and SYBR I green (0.2 × final concentration). Amplification was conducted in a CFX96 (Bio-Rad, Hercules, CA, USA) at 30 °C with fluorescence measurements every 6 min until stationary phase was reached, followed by heat inactivation at 60 °C for 3 min.
All MDA products were screened by 16S rDNA PCR amplification and Sanger sequencing. PCR was conducted with GoTaq green Master Mix (Promega, Madison, WI, USA) and the primers GM1f and GM3r (Muyzer et al., 1993, 1995) according to the manufacturer’s protocol. PCR products were purified with a GenElute PCR Cleanup Kit (Sigma-Aldrich, St Louis, MO, USA) and sequenced with BigDye v 3.1 and the primer GM1f on an ABI 3730 Genetic Analyzer (Applied Biosystems, Waltham, MA, USA). MDA products that resulted in clean sequences were used to construct 550-bp libraries with the TruSeq PCR-Free LT Sample Preparation Kit (Illumina, San Diego, CA, USA) according to the manufacturer’s protocol. Paired-end sequences (2 × 150 bp or 2 × 250 bp) were generated on an Illumina MiSeq instrument (for details see Supplementary Table S2). Raw reads have been deposited to The National Center for Biotechnology Information (NCBI; BioProject PRJNA327031).
Genome assembly and annotation
Adapter sequences and Illumina sequencing artefacts were removed with Trimmomatic (Bolger et al., 2014) and Prinseq-lite (Schmieder and Edwards 2011). MDA-derived coverage peaks were removed with kmernorm (https://sourceforge.net/projects/kmernorm). Assembly was conducted with SPAdes (Bankevich et al., 2012) or A5-miseq (Tritt et al., 2012; Coil et al., 2014) and different software combinations were compared for each genome and best assemblies were selected (Supplementary Table S2). Reads were subsequently mapped to the resulting assemblies with Bowtie2 (version 2.1.0; Langmead and Salzberg, 2012) in order to identify potential assembly errors. Regions containing low coverage (⩽10 fold), ambiguities or anomalies in the mapping were verified by PCR and Sanger sequencing. A total of 350 primers were designed with Primer3 (version 2.3.4; Untergasser et al., 2012) in Geneious 7 and 9 (www.geneious.com) and used for producing 268 additional Sanger sequences as described above.
All genomes were annotated with the NCBI microbial genome annotation pipeline and BlastKOALA (Kanehisa et al., 2016) and inconsistencies between the two methods and hypothetical proteins manually curated with NCBI CDD (Marchler-Bauer et al., 2015). Pathways were reconstructed by using BlastKOALA, the KEGG (Kyoto Encyclopedia of Genes and Genomes) Mapper tool (http://www.kegg.jp/kegg/mapper.html) and by manually inspecting KEGG maps. Transporters were identified based on KEGG BlastKOALA annotations.
The average nucleotide identity and average amino acid identity between strains and other Actinobacteria was calculated according to previously published algorithms (Goris et al., 2007; Rodriguez-R and Konstantinidis, 2014), and the percentages of conserved proteins were computed via all-vs-all comparisons (>50% identity with a coverage of >50%) following the method described in Qin et al. (2014). The core- and pan-genomes were computed using all-vs-all comparisons of all proteins for each genome using BLASTP. A cutoff of 50% identity and 50% coverage was used to define an orthologue.
The annotated genome sequences have been deposited in GenBank under accession numbers CP016768– CP016783 (BioProject PRJNA327031).
Genomic and single gene/protein tree reconstructions
Phylogenomic trees of Actinobacteria were constructed by identifying conserved proteins on the basis of COG (Clusters of Orthologous Groups of proteins) annotations (Tatusov et al., 2001). Proteins were concatenated and aligned using Kalign (Lassmann and Sonnhammer, 2005), and maximum likelihood trees (100 bootstraps) were constructed with FastTree2 (Price et al., 2010). Three different trees with a different number of genomes were computed: (1) our 16 genomes and >100 complete genomes affiliated to all orders of Actinobacteria (Sen et al., 2014), with Staphylococcus aureus and Listeria monocytogenes used as outgroup (48 concatenated proteins); (2) our genomes, other complete genomes (Kang et al., 2017) as well as SAGs (Ghylin et al., 2014) (only those with an estimated completeness of >70%) and MAGs (Ghai et al., 2014) affiliated to ‘Ca. Nanopelagicales’, with Rhodoluna lacicola MWH-Ta8 (Hahn et al., 2014) and ‘Ca. Aquiluna sp.’ IMCC13023 (Kang et al., 2012) used as outgroup (87 concatenated proteins); and (3) for a fine-scale analysis of branching patterns only complete genomes with R. lacicola MWH-Ta8 (Hahn et al., 2014) and ‘Ca. Aquiluna sp.’ IMCC13023 (Kang et al., 2012) used as outgroup (462 concatenated proteins).
Genes encoding 16S and 23S rRNA were aligned with the SINA web aligner (www.arb-silva.de/aligner/), imported to ARB (Ludwig et al., 2004) using the SILVA database SSU Ref 123 or LSU Ref 123 (Pruesse et al., 2007), manually checked, and bootstrapped maximum likelihood trees (GTR-GAMMA model, 100 bootstraps) were calculated with RAxML (Stamatakis et al., 2005). R. lacicola MWH-Ta8 (Hahn et al., 2014) and ‘Ca. Aquiluna sp.’ IMCC13023 (Kang et al., 2012) served as outgroup. Protein sequences of rhodopsins were aligned with Kalign (Lassmann and Sonnhammer, 2005) and a maximum likelihood tree (100 bootstraps) was constructed with FastTree2 (Price et al., 2010). Xanthorhodopsin sequences of Salinibacter ruber and Thermus aquaticus were used as outgroup.
Design and application of novel specific 23S rDNA probes for actinobacterial lineages
CARD-FISH with fluorescein-labelled tyramides was conducted as previously described (Sekar et al., 2003) with a probe specific for the whole acI lineage of Actinobacteria (AcI-852; Warnecke et al., 2005) and two novel probes targeting ‘Ca. Nanopelagicus’ (sublineages acI-B1) and ‘Ca. Planktophila vernalis’ (sublineage acI-A7; see Supplementary Table S3 for details). Probe design was based on 23S rRNA genes extracted from the genomes and additional publically available sequences. Sequences were aligned with the SINA web aligner (www.arb-silva.de/aligner/), imported to ARB (Ludwig et al., 2004) using the SILVA database LSU Ref 123 (Pruesse et al., 2007) and manually checked. A bootstrapped maximum likelihood tree (GTR-GAMMA model) of 23S rDNA sequences (Supplementary Figure S10) served as backbone for probe design with the ARB tools probe_design and probe_check. The resulting probes Npel-23S-2669 and Pver-23S-1420 with their corresponding competitor and helper oligonucleotides (Supplementary Table S6) were tested with different formamide concentrations to achieve stringent hybridization conditions. CARD-FISH-stained samples were analysed by fully automated high-throughput microscopy (Zeder and Pernthaler, 2009). Images were analysed with the freely available image analysis software ACMEtool (technobiology.ch), and interfering autofluorescent cyanobacteria or debris particle were individually excluded from hybridized cells. At least 10 high-quality images or >1000 DAPI-stained bacteria were analysed per sample. Cell sizes of CARD-FISH-stained ‘Ca. Nanopelagicus’ and ‘Ca. Planktophila vernalis’ were measured from two different samples (18 July 2012 and 15 May 2013; 5 m depth) with the software LUCIA (Laboratory Imaging) following a previously described workflow (Posch et al., 2009). At least 87 individual cells per sample were subjected to image analysis.
Total numbers of heterotrophic prokaryotes and picocyanobacteria were determined by an inFlux V-GS cell sorter (Becton Dickinson, Franklin Lakes, NJ, USA) equipped with a ultraviolet light (355 nm) laser. Subsamples of 1 ml were stained with DAPI (1 μg ml−1 final concentration), and scatter plots of DAPI fluorescence vs 90° light scatter were analysed with an in-house software (J Villiger, unpublished).
Recruitment from metagenomes
Publically available metagenomes (n=126) were used for fragment recruitment (see Supplementary Table S7 for sampling sites, dates and accession numbers). rRNA sequences were masked in both genomes and metagenomes to avoid biases, and metagenomic reads were queried against the genomes using BLASTN (Altschul et al., 1997) (length ⩾50 bp, identity ⩾95%, e-value ⩽1e−5). These hits were used to compute RPKG values (number of reads recruited per kb of genome per Gb of metagenome) that provide a normalized value that is comparable across different metagenomes. Reads from three different time points (spring, summer, autumn) from Lake Mendota were also mapped on genomes without masking rRNA sequences and with identity values ⩾80% for a graphical overview of seasonal differences of different genotypes (Supplementary Figure S13).
Comparative genomics and identification of genomic islands (GIs)
Whole-genome alignments were performed by comparison of all proteins from all genomes with each other using BLASTP. Stretches of genes without any hits to other genomes were manually examined to define GIs.
Results and discussion
Isolation and genome analysis of genome-streamlined freshwater ‘Ca. Nanopelagicales’
Sixteen strains affiliated to acI Actinobacteria were isolated using a dilution-to-extinction method (Salcher et al., 2015) from the pelagial of Lake Zurich, Switzerland (Table 1 and Supplementary Tables S1 and S3). All isolates displayed slow growth, but reached relatively high densities (that is, ∼106 cells per ml) in the initial cultures; they were of conspicuously small size with cell volumes ranging between 0.012 and 0.029 μm3 (Supplementary Figure S1 and Supplementary Table S4). This corresponds to the in situ cell sizes of freshwater Actinobacteria from Lake Zurich (0.012–0.015 μm3, Supplementary Table S3) and other lakes (Posch et al., 2009; Šimek et al., 2014). These microbes are thus among the smallest members of the bacterioplankton, matching the small size of other abundant genome-streamlined bacteria (Supplementary Table S5; Salcher et al., 2011; Ghai et al., 2013; Zhao et al., 2016). Although isolation campaigns were run throughout the growing season (Salcher et al., 2015), only one strain (‘Ca. Planktophila limnetica’ MMS-VB-114) was isolated in summer and all others in spring (Supplementary Tables S1 and S3). Interestingly, each spring isolation campaign yielded a set of different genotypes, with strains affiliated to the same species being isolated multiple times even in different years. This already hints at a high number of sympatric genotypes during spring with remarkably recurrent temporal patterns. All axenic cultures were further inoculated to fresh medium prepared from sterile lake water; however, few strains survived more than three propagations and all attempts to further maintain them in monocultures failed. Therefore, we used subsamples (0.5 ml) from the initial cultures (concentrated via centrifugation and frozen) for whole-genome sequencing after MDA. Assembly of sequence reads resulted in one—three large contigs that could be ordered and fully closed via PCR and Sanger sequencing. Additional 268 Sanger sequences from PCRs with primers designed for low-coverage regions (<10 ×) ensured a very high quality of the final genomes. This clearly contrasts with the fragmented nature of publically available acI SAGs consisting of 22 to 91 scaffolds (Ghylin et al., 2014) and confirms that the use of high number of clonal cells (rather than a single cell) adequately compensates for MDA artefacts (Fitzsimons et al., 2013).
Table 1. Details of the sequenced strains of planktonic ‘Ca. Nanopelagicales’.
| Taxonomy | Tribe a | Strains | Cell volume (μm3) | Genome size (Mbp) | GC content (%) | No. of CDS | Spacer length (bp) | Coding density (%) |
|---|---|---|---|---|---|---|---|---|
| ‘Ca. Planktophila dulcis’ | acI-A1 | MMS-IIA-65 MMS-IA-53 MMS-21-155 | 0.018–0.023 | 1.35–1.37 | 47.9–48.0 | 1344–1361 | 11–12 | 95.7 |
| ‘Ca. Planktophila sulfonica’ | acI-A1 | MMS-IA-56 | 0.026 | 1.34 | 48.6 | 1336 | 12 | 96.0 |
| ‘Ca. Planktophila versatilis’ | acI-A1 | MMS-IIB-76 MMS-IA-79 MMS-IA-105 MMS-IIB-142 | 0.021–0.027 | 1.27–1.33 | 48.2–48.3 | 1258–1329 | 14 | 95.2–95.5 |
| ‘Ca. Planktophila lacus’ | acI-A1 | MMS-IIB-106 MMS-IIB-60 MMS-21-148 | 0.025–0.029 | 1.38–1.46 | 47.5–47.8 | 1368–1438 | 15–16 | 95.3–95.6 |
| ‘Ca. Planktophila limnetica’ | Phila | MMS-VB-114 | 0.016 | 1.33 | 45.0 | 1333 | 10 | 96.0 |
| ‘Ca. Planktophila vernalis’ | acI-A7 | MMS-IIA-15 | 0.020 | 1.36 | 45.7 | 1355 | 10 | 95.7 |
| ‘Ca. Nanopelagicus limnes’ | acI-B1 | MMS-21-122 | 0.018 | 1.24 | 41.5 | 1216 | 11 | 95.7 |
| ‘Ca. Nanopelagicus hibericus’ | acI-B1 | MMS-21-160 | 0.012 | 1.22 | 42.4 | 1211 | 13 | 95.4 |
| ‘Ca. Nanopelagicus abundans’ | acI-B1 | MMS-IIB-91 | 0.020 | 1.16 | 40.2 | 1150 | 14 | 95.3 |
Abbreviation: CDS, coding DNA sequence.
Classified according to ‘tribes’ proposed by Newton et al. (2011).
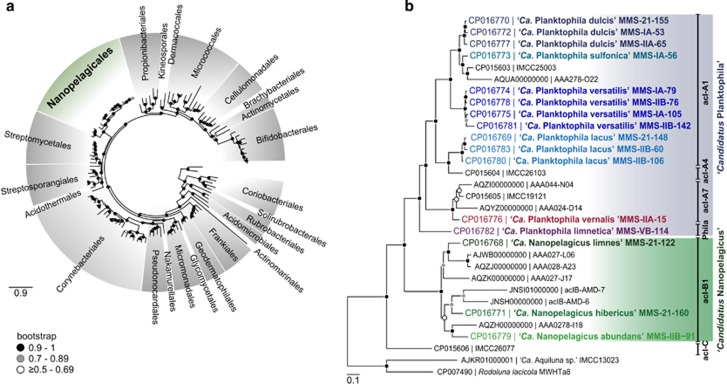
The 16S rRNA gene phylogeny (Supplementary Figure S2) suggested a separation into two genera (acI-A and acI-B; sequence similarity <95%) and four species-like ‘tribes’ (acI-A1, acI-Phila, acI-A7, acI-B1; sequence similarity ⩾97% Newton et al., 2011). However, average nucleotide identities (>95%, Supplementary Figure S3), average amino acid identities (>90%, Supplementary Figure S4) and phylogenomic trees based on conserved concatenated proteins (Figure 1 and Supplementary Figure S5) revealed that the two genera harboured nine different species. The majority of strains (13) were affiliated to the acI-A lineage (Newton et al., 2011), one with a 16S rRNA sequence 100% identical to ‘Ca. Planktophila limnetica’ MWHEgel2-3 (FJ428831, Supplementary Figure S2), the only validly described mixed culture of acI Actinobacteria (Jezbera et al., 2009). Thus, the acI-A lineage strains will be hereafter referred to as ‘Ca. Planktophila’. Another strain (‘Ca. Planktophila vernalis’ MMS-IIA-15) showed high similarity to ‘tribe’ acI-A7. All other ‘Ca. Planktophila’ isolates formed a monophyletic branch and were grouped in four putative species containing 1–4 strains each (Figure 1b and Supplementary Figure S5). Three strains were affiliated to lineage acI-B1 (Newton et al., 2011), all of them different species, and we tentatively assigned them to a novel genus, ‘Ca. Nanopelagicus’. Together, both genera formed a robust sister lineage to the orders Streptomycetales, Streptosporangiales and Acidothermales (bootstrap value: 0.997) in a phylogenomic tree of >100 complete genomes of Actinobacteria (48 conserved proteins, Figure 1a and Supplementary Figure S6 (Sen et al., 2014). We propose a novel order within the phylum Actinobacteria, ‘Ca. Nanopelagicales’ (family ‘Ca. Nanopelagicaceae’), for the taxonomic placement of these microbes. The four strains published by Kang et al. (2017) were also affiliated to ‘Ca. Nanopelagicales’ representing different species within ‘Ca. Planktophila’ or a novel genus (strain IMCC26077; Figure 1b and Supplementary Figures S2–S5).
Figure 1.
Phylogeny of freshwater ‘Ca. Nanopelagicales’. (a) Phylogenomic tree with >100 complete genomes of the phylum Actinobacteria. Forty-eight concatenated conserved proteins were used to generate a maximum likelihood phylogenetic tree. The genomes of Staphylococcus aureus and Listeria monocytogenes were used as outgroup. Bootstrap values are indicated by black (0.9–1), grey (0.7–0.89) or white circles (⩾0.5–0.69) on the nodes. The proposed novel order ‘Ca. Nanopelagicales’ is highlighted in green. (b) Phylogenomic tree of freshwater ‘Ca. Nanopelagicales’. Eighty-seven concatenated conserved proteins were used to generate a maximum likelihood phylogenetic tree. In addition to 20 complete genomes, seven single amplified genomes (prefixed by AAA) and two genomes assembled from metagenomes (prefixed by acIB) were used to create the tree. The genomes of ‘Ca. Aquiluna sp.’ and Rhodoluna lacicola were used as outgroup. Bootstrap values are indicated black (0.9–1), grey (0.7–0.89) or white circles (⩾0.5–0.69) on the nodes.
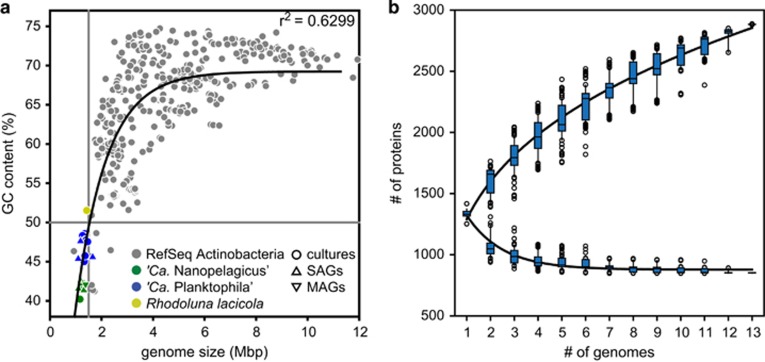
All strains have one circular chromosome of very small size (1.16–1.47 Mbp, Table 1 and Supplementary Table S3) with a low genomic GC content (40.2–48.6%). These are the smallest recorded genome sizes with the lowest GC content of so far completely sequenced genomes of free-living Actinobacteria, except for the pathogen Tropheryma whipplei (Figure 2a). ‘Ca. Nanopelagicus’ genomes (as also estimated from incomplete SAGs (Ghylin et al., 2014) and MAGs (Ghai et al., 2014)) were smaller with lower GC content than ‘Ca. Planktophila’. Summary statistics for all genomes are shown in Table 1 and Supplementary Table S3. As expected, they have very short intergenic spacers (10–16 bp), high coding densities (>95%) and only 2–5 genes encoding sigma factors (Supplementary Figure S7), all typical for streamlined genomes (Giovannoni et al., 2014). The genomes reported here are in the same size range or even smaller than other genome-streamlined freshwater and marine microbes (Supplementary Table S5). All of these organisms are also of very small cell size, have a low genomic GC content and possess rhodopsins (Steindler et al., 2011; Hahn et al., 2014). Rhodopsins in ‘Ca. Nanopelagicales’ genomes were very similar to previously published actinorhodopsins of the acI lineage (Ghai et al., 2014; Ghylin et al., 2014; Kang et al., 2017) with almost identical protein sequences (>99.3% sequence similarity) within the proposed species (Supplementary Figure S8). The rhodopsins are tuned to green light (L at position 105), like most freshwater and coastal marine rhodopsins. All strains encoded genes for β-carotene biosynthesis; however, five strains lacked the enzyme for the final step of retinal biosynthesis (blh; Supplementary Figure S8 and Supplementary Table S8), similar to two ‘Ca. Nanopelagicales’ genomes published by Kang et al. (2017) and Rhodoluna lacicola, whose proton-pumping activity was dependent on exogenous retinal (Keffer et al., 2015). It is likely that energy generated by rhodopsins help ‘Ca. Nanopelagicales’ survive in the oligotrophic conditions of the plankton. The core-genome of the 13 ‘Ca. Planktophila’ strains (Figure 2b) encodes 852 conserved proteins (59–68% of the genome) comparable to that of the SAR11 group (Grote et al., 2012). The size of the pan-genome of ‘Ca. Planktophila’ was estimated to be ca. 2900 genes and power law regression analysis indicated an open pan-genome for this genus, similar to marine SAR11 (Grote et al., 2012).
Figure 2.
Genome streamlining and core-genome conservation in ‘Ca. Nanopelagicales’. (a) Genomic GC content versus genome size for all complete published genomes of Actinobacteria (n=610; data taken from RefSeq). ‘Ca. Nanopelagicales’ and Rhodoluna lacicola are marked in different colours. (b) Pan- and core-genome analysis of ‘Ca. Planktophila’. Regression lines were fitted with two-parameter power law regression (pan-genome) or exponential decay (core-genome).
‘Ca. Nanopelagicales’ are ubiquitous, highly abundant and show seasonal and habitat preferences
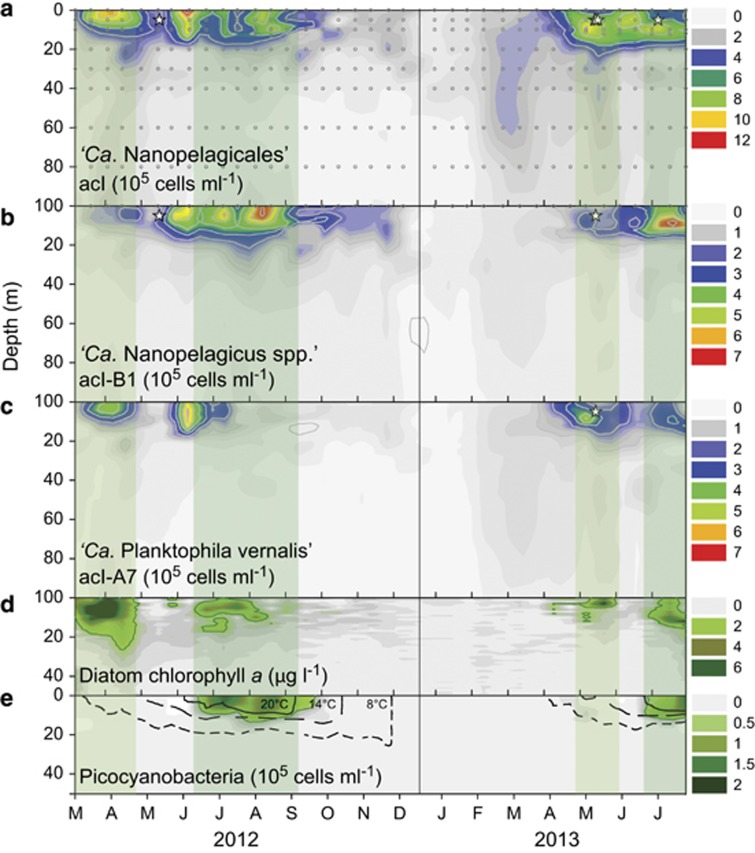
As 16S rRNA was insufficient as a taxonomic marker gene to discriminate isolates at strain levels, we used 23S rRNA sequences for designing specific oligonucleotide probes for ‘Ca. Planktophila vernalis’ and the genus ‘Ca. Nanopelagicus’ (Supplementary Figure S9 and Supplementary Table S3). These probes and the general probe for the order ‘Ca. Nanopelagicales’ (probe AcI-852; Warnecke et al., 2005) were used for studying spatiotemporal occurrence patterns by CARD-FISH in Lake Zurich, the origin of all strains (n=348 from two consecutive years). ‘Ca. Nanopelagicales’ were highly abundant and seasonally variable in the epilimnion (0–20 m depth) forming up to one-third of all microbes during the growing season (Figure 3). Distinct peaks of >106 cells per ml in spring and summer co-occurred with or followed algal and/or picocyanobacterial blooms, in agreement with earlier reports (Allgaier and Grossart, 2006; Salcher, 2014). It has been proposed that these microbes profit from phytoplankton blooms by utilizing carbohydrate-rich algal exudates (Garcia et al., 2013; Salcher et al., 2013; Pérez et al., 2015), besides allochthonous carbon sources (Buck et al., 2009; Pérez and Sommaruga, 2006). Arguably, copiotrophic microbes (for example, Limnohabitans spp., Flavobacterium spp.) react faster to resource pulses and might thus outcompete oligotrophs such as ‘Ca. Nanopelagicales’ (Šimek et al., 2014; Neuenschwander et al., 2015). However, copiotrophs with a medium to large cell size are in the optimal range of size-selective protists and are therefore heavily top-down controlled. Actinobacteria, being of very small size (Supplementary Figure S1 and Supplementary Table S4), are less vulnerable to grazing (Šimek et al., 2013, 2014) and they are less frequently ingested and/or digested by flagellates (Tarao et al., 2009; Šimek et al., 2014). Moreover, ‘Ca. Nanopelagicales’ might also indirectly profit from the trophic cascades that typically follow algal blooms (that is, high numbers of copiotrophs followed by a maximum of protists and phages) as they grow on cell material that is released during bacterivory or viral lysis (Eckert et al., 2013).
Figure 3.
Abundances of ‘Ca. Nanopelagicales’ in Lake Zurich, Switzerland. (a) Abundances of all ‘Ca. Nanopelagicales’ (105 cells per ml) in a 2-year high-resolution sampling campaign. Circles in grey indicate individual samples (n=348). (b) Abundances of ‘Ca. Nanopelagicus spp.’ and (c) ‘Ca. Planktophila vernalis’. Asterisks indicate date and depth of isolation of strains. (d) Profiles of chlorophyll a derived from Diatoms (μg l−1). (e) Profiles of water temperature (lines) and picocyanobacteria (105 cells per ml).
‘Ca. Nanopelagicus’ constituted roughly half of all ‘Ca. Nanopelagicales’ in Lake Zurich. They had distinct maxima of >7 × 105 cells per ml (ca. 20% of all microbes) in summer, but were not abundant in spring (Figure 3). In contrast, ‘Ca. P. vernalis’ were present in high numbers in spring, but less so during periods when ‘Ca. Nanopelagicus’ reached their maxima. Redundancy analysis suggested a clear separation of these two lineages: The abundances of ‘Ca. Nanopelagicus’ were positively related to water temperatures and the occurrence of picocyanobacteria, whereas ‘Ca. P. vernalis’ were more correlated to typical spring conditions, that is, high concentrations of oxygen, dissolved organic carbon, ammonium and chlorophyll a from diatoms and chlorophytes (Supplementary Figures S10 and S11). This niche separation of ‘Ca. Nanopelagicales’ is in line with previous observations within single systems and across different lakes (Newton et al., 2007; Buck et al., 2009).
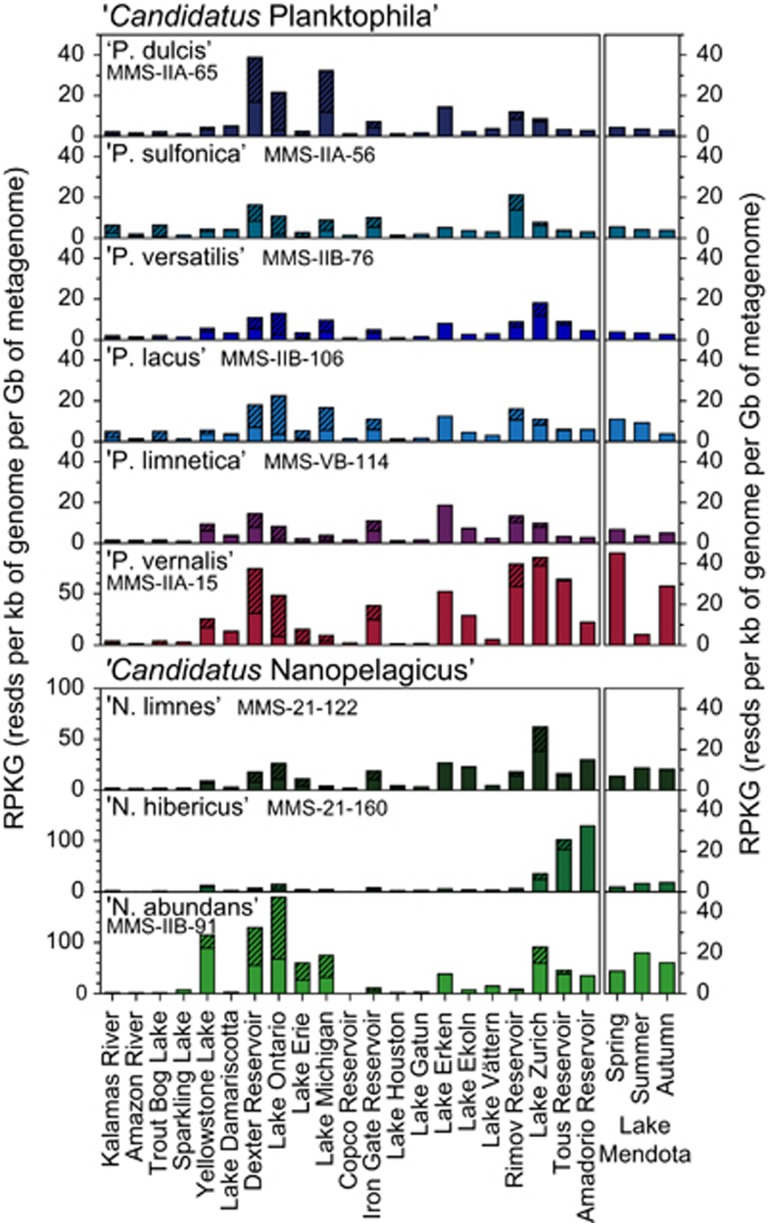
Metagenomic fragment recruitment from freshwater samples (n=130, Supplementary Table S7) showed a ubiquitous distribution for several isolates and very high abundances of all strains in lakes and reservoirs, whereas they were largely absent from the two rivers (Figure 4 and Supplementary Figure S12). ‘Ca. N. abundans’ MMS-IIB-91 was the most abundant genotype in all metagenomes and recruited more in summer samples than in spring, following the same trend as detected with CARD-FISH in Lake Zurich (Figure 3 and Supplementary Figure S13). The opposite was observed for ‘Ca. P. vernalis’ MMS-IIA-15 that was rare in a metagenome from Lake Mendota in summer but frequent in spring and autumn. This particular strain was highly abundant in most metagenomes, whereas other taxa of ‘Ca. Planktophila’ recruited less reads. Generally, the different genotypes were relatively more abundant in lakes of the northern temperate zone than in lakes at lower latitudes (Lake Houston and Lake Gatun). We could also identify a geographic preference of one strain (‘Ca. N. hibericus’ MMS-21-160) that was highly abundant in two Spanish reservoirs. The closest relative of this strain was assembled from a metagenome from Amadorio Reservoir, Spain (acIB-AMD-6; average nucleotide identity: 95%), and displayed a similar recruitment pattern (Supplementary Figure S12; Ghai et al., 2014). The two publically available genomes of Actinobacteria from the luna lineage (Kang et al., 2012; Hahn et al., 2014) recruited <10 times less than any of the ‘Ca. Nanopelagicales’ and, thus, seem to be of less relevance in fresh waters. Fragment recruitments of MAGs and SAGs were in the same range or lower than our genomes with the exception of one highly abundant SAG (AAA024-D14; Ghylin et al., 2014) (Supplementary Figure S12).
Figure 4.
Metagenomic fragment recruitment of ‘Ca. Nanopelagicales’ across diverse freshwater ecosystems. Open bars represent the mean RPKG (number of reads recruited per kb of genome per Gb of metagenome) per ecosystem if multiple metagenomes were available, and shaded bars display the maximum RPKG. The data sets used (n=130) are listed in Supplementary Table S7 and sorted according to habitat (rivers and lakes) and latitude (separately for North America and Europe). The right panel gives an example for seasonal differences in recruitment for Lake Mendota, USA.
High metabolic versatility of ‘Ca. Nanopelagicales’
Metabolic reconstructions suggested an aerobic chemoheterotrophic lifestyle of ‘Ca. Nanopelagicales’ with striking strain-specific differences and multiple auxotrophies. None of the strains encoded genes for assimilatory sulphate reduction (Supplementary Table S8), thus confirming a proposition based on SAGs (Ghylin et al., 2014) and four complete genomes (Kang et al., 2017). This indicates a dependency on exogenous sources of reduced sulphur for growth, as reported for marine ‘Ca. Pelagibacter’ (Tripp et al., 2008). The genomes shared several pathways of the central carbon metabolism (for example, glycolysis, tricarboxylic acid cycle, pentose phosphate pathway; see Supplementary Text and Supplementary Table S7), but differed in gluconeogenesis, carbohydrate metabolism and the biosynthesis of amino acids and cofactors. ‘Ca. Planktophila’ strains were amino acid prototrophs except for ‘Ca. P. vernalis’ (serine auxotroph). All ‘Ca. Nanopelagicus’ strains were auxotrophs for histidine and ornithine. ‘Ca. N. abundans’ MMS-IIB-91 and ‘Ca. N. limnes’ MMS-21-122 also had incomplete pathways for methionine and lysine biosynthesis, and proline biosynthesis, respectively. However, most of these incomplete pathways lacked specific aminotransferases whose functions might be replaced by other predicted aminotransferases. ABC (ATP-binding cassette) transporters for amino acids were annotated for all genomes (Table 2), in agreement with their rapid in situ uptake (Pérez et al., 2010; Salcher et al., 2010; Salcher et al., 2013). Cyanophycinases were present in all isolates (and in SAGs (Ghylin et al., 2014) and MAGs (Ghai et al., 2014)); this might provide additional amino acid sources in ‘Ca. Nanopelagicales’ that tend to co-occur with picocyanobacteria (Figure 3). All strains were prototrophs for vitamins B3 (nicotinamide adenine dinucleotide) and B6 (pyridoxine) and auxotrophs for B1 (thiamine), B5 (pantothenate), B12 (cobalamin; except for ‘Ca. P. limnetica’) and B7 (biotin). Two strains (‘Ca. N. limnes’ and ‘Ca. N. hibericus’) also lacked genes for the biosynthesis of vitamin B2 (riboflavin). This high degree of auxotrophy for various amino acids, vitamins and reduced sulphur sources might explain the unsuccessful long-term cultivation of our isolates. Such a dependence on co-occurring organisms providing essential metabolites seems to be a common feature among genome-streamlined planktonic microbes (Morris et al., 2012; Giovannoni et al., 2014; Garcia et al., 2015).
Table 2. Complete membrane transport modules in ‘Ca. Nanopelagicales’.
| P. dulcis (n=3) | P. sulfonica | P. versatilis (n=4) | P. lacus (n=3) | P. limnetica | P. vernalis | N. limnes | N. hibericus | N. abundans | |
|---|---|---|---|---|---|---|---|---|---|
| Amino acids/peptides | |||||||||
| Branched-chain amino acids | X | X | X | X | X | X | X | X | X |
| Polar amino acids | X | X | X | ||||||
| Glycine betaine/proline | X | X | |||||||
| Glutamate | X | ||||||||
| Peptide/Ni2+ | X | X | X | X | X | X | X | X | |
| Polyamines | |||||||||
| Spermidine/putrescine | X | X | X | X | X | X | X | X | X |
| Spermidine, putrescine | 1 | X | X | X | |||||
| Carbohydrates and alcohols | |||||||||
| Simple sugar | 3 | X | X | X | |||||
| Multiple sugar | X | X | X | X | X | X | |||
| Glucose/mannose | X | X | X | ||||||
| Fructose | X | X | |||||||
| Ribose | X | X | X | X | |||||
| Rhamnose | X | ||||||||
| D-xylose | X | X | X | X | |||||
| Cellobiose | X | 1 | X | ||||||
| N,N'-diacetylchitobiose | 1 | ||||||||
| Raffinose/stachyose/melibiose | X | X | X | X | |||||
| Arabinogalactan oligomer/ maltooligosaccharide | X | ||||||||
| Glycerol | X | ||||||||
| Sorbitol/mannitol | 2 | ||||||||
| α-Glucoside | X | X | X | ||||||
| Nucleotides and coenzymes | |||||||||
| Xanthine/uracil | X | X | X | X | X | X | X | X | X |
| Nicotinamide mononucleotide | X | X | X | X | X | X | X | X | X |
| Biotin | X | X | X | X | X | X | |||
| Riboflavin (putative, riboswitch) | X | X | |||||||
| Thiamine (putative, riboswitch) | X | X | |||||||
| Cobalamine (putative, riboswitch) | X | X | X | X | X | ||||
| Organic acids | |||||||||
| Cyanate | X | X | |||||||
| Benzoate | X | ||||||||
| Sulfonate | X | ||||||||
| Ions | |||||||||
| PO43− | X | X | X | X | X | X | X | X | X |
| H+ | X | X | X | X | X | X | X | X | X |
| NH4+ | X | X | X | X | X | X | X | X | X |
| K+ | X | X | X | X | X | X | X | X | X |
| Fe2+ | X | X | X | X | X | X | X | X | X |
| Ni2+ | 1 | X | X | X | X | ||||
| Zn2+ | 1 | X | X | X | |||||
| Metal ions | 2 | X | 3 | 2 | X | ||||
| Na+ | X | ||||||||
| Co2+/ Ni2+ | X | X | 3 | X | X | ||||
| Fe complex | X | X | X | X | |||||
| K+ | X | ||||||||
| Export | |||||||||
| Osmolytes | X | X | X | X | X | X | X | X | X |
| Teichoic acid | X | X | X | X | X | X | X | X | X |
| Lipopolysaccharide | X | X | |||||||
| Drug metabolites | X | X | X | ||||||
| Lipooligosaccharide | X | ||||||||
Genomes of the same species were merged for a better overview. Numbers indicate the number of genomes within one species that possess specific transporters.
The most striking differences between the strains were related to membrane transporters for low molecular weight organic compounds (Table 2 and Supplementary Table S8). All isolates shared transporters for spermidine/putrescine and the subsequent degradation pathway to succinate, as well as transporters for branched-chain amino acids, xanthine/uracil, nicotinamide adenine dinucleotide and several ions, in line with other ‘Ca. Nanopelagicales’ genomes (Kang et al., 2017). All other membrane transporters (n=32) were only present in some strains, mostly in a species-specific manner. ‘Ca. P. dulcis’ had the highest number of different carbohydrate transporters (ribose, xylose, cellobiose, raffinose/stachyose/melibiose, α-glucoside) and carbohydrate metabolism in general, whereas the three ‘Ca. Nanopelagicus’ strains and ‘Ca. P. limnetica’ had the lowest diversity. Strain-specific differences in transporters were most pronounced in ‘Ca. P. versatilis’. A specialization on carbohydrates and versatile carbon substrate acquisition has also been hypothesized from the analysis of SAGs (Ghylin et al., 2014). Such a metabolic microdiversification of closely related strains might be one reason for the high numbers of ‘Ca. Nanopelagicales’ in fresh waters, where resources are seasonally fluctuating and heterogeneously distributed at the microscale (Stocker, 2012).
High microdiversification of closely related genotypes encoded in GIs
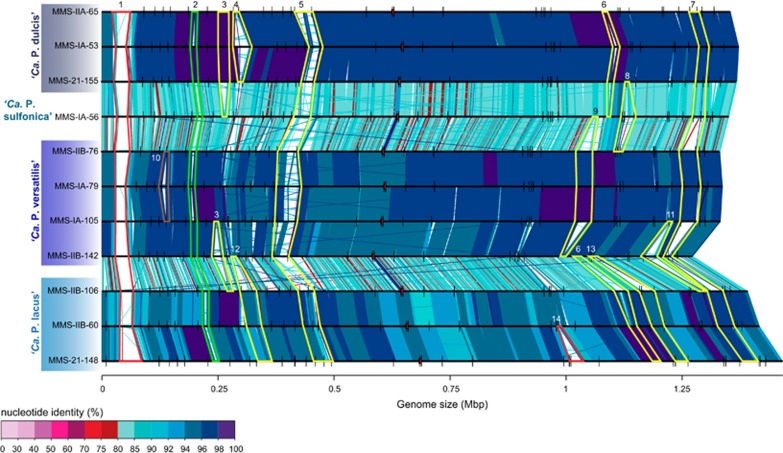
We aligned the genomes of the 11 most closely related strains of ‘Ca. Planktophila’ in a pairwise manner to identify GIs (that is, hypervariable regions; Figure 5 and Supplementary Table S9). Such an analysis can be better conducted with complete genomes and was thus not performed for previously described SAGs and MAGs. Two ‘Ca. Planktophila’ strains (‘Ca. P. vernalis’ MMS-IIA-15 and ‘Ca. P. limnetica’ MMS-VB-114) had large inversions in some parts of their genomes (verified by PCR) that made it difficult to identify GIs (Supplementary Figure S14). We could recognize 14 distinct GIs that were also largely present in metagenomic recruitments (Supplementary Figure S14; that is, metagenomic islands (Rodriguez-Valera et al., 2009)). Most GIs were additive and encoded genes for membrane transporters and/or carbohydrate metabolism (Figure 5 and Supplementary Table S9). These GIs were typically flanked by a transfer RNA and appear to be of importance for metabolic microdiversification, and might be rapidly evolving via horizontal gene transfer (as deduced from their patchy intra- and interspecific distribution; Vergin et al., 2007; Hoetzinger et al., 2017).
Figure 5.
Whole-genome alignment of 11 ‘Ca. Planktophila’ strains. The genomes have been linearized for simplicity and are arranged in the same order as in the phylogenomic tree in Figure 1b. Synteny and different degrees of sequence similarity are indicated by vertical lines connecting the genomes. Ribosomal RNA (rRNA) operons in the individual genomes are displayed as red arrows and transfer RNAs (tRNAs) as short vertical lines. Genomic islands (GIs) have been marked in different colours and numbered (see Supplementary Table S9 for genes encoded in each island). Red: genes encoding mainly cell wall biosynthesis and modifications; green: genes encoding pilus assembly; yellow: genes encoding mainly membrane transport and/or carbohydrate metabolism; brown: unknown function.
One large replacement GI (GI-1) present in all strains featured genes encoding for cell wall biosynthesis and modifications like UDP-glucose 4-epimerase and glycosytransferases. Moreover, one more additive GI contained genes for cell wall biosynthesis (GI-14), and another one (GI-2) for pilus assembly. All of these extracellular structures are potential phage recognition sites; modifications in the cell surface most likely serves as a defence against phage predation by changing the glycotype that is sensitive to a specific set of viruses (Rodriguez-Valera et al., 2009). The three ‘Ca. Nanopelagicus’ strains were relatively distantly related and ‘Ca. N. limnes’ had a large inversion in the genome. Still, we identified eight GIs, of which three possessed genes involved in cell wall biosynthesis and/or modification (Supplementary Figure S15 and Supplementary Table S10). This high frequency of GIs encoding for cell surface properties and their high in situ abundances hints at high infection rates of phages (Knowles et al., 2016). As abundant freshwater viruses (actinophages) were found to infect acI Actinobacteria (Ghai et al., 2017), these microbes might suffer from similarly high phage mortality rates as marine SAR11 (Zhao et al., 2013). A positive feedback loop of a large number of closely related genotypes with high recombination rates was suggested to explain the large population sizes of SAR11 in the oceans despite being highly infected by viruses (‘king-of-the-mountain hypothesis’ Giovannoni et al., 2013). As ‘Ca. Nanopelagicales’ possess multiple GIs with genes encoding potential phage recognition sites (Figure 5, Supplementary Figure S15 and Supplementary Tables S9 and S10), we suggest that a comparable mechanism allows for their high abundances in fresh waters. However, none of the ‘Ca. Nanopelagicales’ genomes encoded genes for the CRISPR-Cas system.
Conclusions
We present 16 high-quality genomes from isolates of the most abundant ubiquitously distributed microbes in freshwater systems, the acI lineage of Actinobacteria. They form a new order that we putatively named ‘Ca. Nanopelagicales’. These isolates are of extremely small cell size and possess the smallest genomes of all fully sequenced free-living Actinobacteria. The process of genome streamlining has resulted in a high degree of auxotrophy for various vitamins, amino acids and reduced sulphur. We observed a high inter- and intraspecific diversity in metabolic pathways, especially regarding carbohydrate transport and metabolism, and these features were mainly encoded in genomic islands. This microdiversification of different genotypes might explain their global success in heterogeneous and variable environments such as lakes, where short-lived phytoplankton blooms and sporadic terrestrial influx serve as main carbon sources. We further propose that ‘Ca. Nanopelagicales’ suffer from high phage mortality rates that they balance off with genotype-specific modifications in their cell envelope, resulting in high total population sizes in the environment.
Acknowledgments
We thank Eugen Loher and Thomas Posch for help with sampling of Lake Zurich and Daniel Marty and Katya Pushkareva for help with CARD-FISH. Aharon Oren is acknowledged for etymological advice for correct Latin names for the proposed new taxa. We also thank three anonymous reviewers for supportive and helpful comments. This work was financed by the Czech Science Foundation (CSF Grant Numbers 13–00243S and 17-04828S), by the Swiss National Science Foundation as part of the European Science Foundation EUROCORES Programme EuroEEFG (SNF grant number 31EE30-132771) and by the University Research Priority Program (URPP) ‘Global Change and Biodiversity’.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
The authors declare no conflict of interest.
Supplementary Material
References
- Allgaier M, Grossart H. (2006). Diversity and seasonal dynamics of Actinobacteria populations in four lakes in northeastern Germany. Appl Environ Microbiol 72: 3489–3497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W et al. (1997). Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acid Res 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS et al. (2012). SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19: 455–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall ML, Stevens SLR, Chan L-K, Malfatti S, Schwientek P, Tremblay J et al. (2016). Genome-wide selective sweeps and gene-specific sweeps in natural bacterial populations. ISME J 10: 1589–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beutler M, Wiltshire K, Meyer B, Moldaenke C, Lüring C, Meyerhöfer M et al. (2002). A fluorometric method for the differentiation of algal populations in vivo and in situ. Photosynth Res 72: 39–53. [DOI] [PubMed] [Google Scholar]
- Bolger AM, Lohse M, Usadel B. (2014). Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30: 2114–2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck U, Grossart HP, Amann R, Pernthaler J. (2009). Substrate incorporation patterns of bacterioplankton populations in stratified and mixed waters of a humic lake. Environ Microbiol 11: 1854–1865. [DOI] [PubMed] [Google Scholar]
- Burkert U, Warnecke F, Babenzien D, Zwirnmann E, Pernthaler J. (2003). Members of a readily enriched beta-proteobacterial clade are common in surface waters of a humic lake. Appl Environ Microbiol 69: 6550–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coil D, Jospin G, Darling AE. (2014). A5-miseq: an updated pipeline to assemble microbial genomes from illumina miseq data. arXiv preprint 1401: 5130. [DOI] [PubMed] [Google Scholar]
- Eckert EM, Salcher MM, Posch T, Eugster B, Pernthaler J. (2012). Rapid successions affect microbial N-acetyl-glucosamine uptake patterns during a lacustrine spring phytoplankton bloom. Environ Microbiol 14: 794–806. [DOI] [PubMed] [Google Scholar]
- Eckert EM, Baumgartner M, Huber IM, Pernthaler J. (2013). Grazing resistant freshwater bacteria profit from chitin and cell-wall derived organic carbon. Environ Microbiol 15: 2019–2030. [DOI] [PubMed] [Google Scholar]
- Fitzsimons MS, Novotny M, Lo C-C, Dichosa AEK, Yee-Greenbaum JL, Snook JP et al. (2013). Nearly finished genomes produced using gel microdroplet culturing reveal substantial intraspecies genomic diversity within the human microbiome. Genome Res 23: 878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia SL, McMahon KD, Martinez-Garcia M, Srivastava A, Sczyrba A, Stepanauskas R et al. (2013). Metabolic potential of a single cell belonging to one of the most abundant lineages in freshwater bacterioplankton. ISME J 7: 137–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia SL, Buck M, McMahon KD, Grossart H-P, Eiler A, Warnecke F. (2015). Auxotrophy and intrapopulation complementary in the ‘interactome’ of a cultivated freshwater model community. Mol Ecol 24: 4449–4459. [DOI] [PubMed] [Google Scholar]
- Ghai R, Mizuno CM, Picazo A, Camacho A, Rodriguez-Valera F. (2013). Metagenomics uncovers a new group of low GC and ultra-small marine Actinobacteria. Sci Rep 3: 2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai R, Mizuno CM, Picazo A, Camacho A, Rodriguez-Valera F. (2014). Key roles for freshwater Actinobacteria revealed by deep metagenomic sequencing. Mol Ecol 23: 6073–6090. [DOI] [PubMed] [Google Scholar]
- Ghai R, Mehrshad M, Megumi Mizuno C, Rodriguez-Valera F. (2017). Metagenomic recovery of phage genomes of uncultured freshwater actinobacteria. ISME J 11: 304–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghylin TW, Garcia SL, Moya F, Oyserman BO, Schwientek P, Forest KT et al. (2014). Comparative single-cell genomics reveals potential ecological niches for the freshwater acI Actinobacteria lineage. ISME J 8: 2503–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni S, Temperton B, Zhao Y. (2013). Giovannoni et al. reply. Nature 499: E4–E5. [DOI] [PubMed] [Google Scholar]
- Giovannoni SJ, Cameron Thrash J, Temperton B. (2014). Implications of streamlining theory for microbial ecology. ISME J 8: 1553–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goris J, Konstantinidis KT, Klappenbach JA, Coenye T, Vandamme P, Tiedje JM. (2007). DNA–DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol 57: 81–91. [DOI] [PubMed] [Google Scholar]
- Grote J, Thrash JC, Huggett MJ, Landry ZC, Carini P, Giovannoni SJ et al. (2012). Streamlining and core genome conservation among highly divergent members of the SAR11 clade. MBio 3: pii: e00252-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Schmidt J, Taipale SJ, Doolittle WF, Koll U. (2014). Rhodoluna lacicola gen. nov., sp. nov., a planktonic freshwater bacterium with stream-lined genome. Int J Syst Evol Microbiol 64: 3254–3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoetzinger M, Schmidt J, Jezberová J, Koll U, Hahn MW. (2017). Microdiversification of a pelagic Polynucleobacter species is mainly driven by acquisition of genomic islands from a partially interspecific gene pool. Appl Environ Microbiol 83: pii: e02266-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezbera J, Sharma AK, Brandt U, Doolittle WF, Hahn MW. (2009). 'Candidatus Planktophila limnetica', an actinobacterium representing one of the most numerically important taxa in freshwater bacterioplankton. Int J Syst Evol Microbiol 59: 2864–2869. [DOI] [PubMed] [Google Scholar]
- Kanehisa M, Sato Y, Morishima K. (2016). BlastKOALA and GhostKOALA: KEGG tools for functional characterization of genome and metagenome sequences. J Mol Biol 428: 726–731. [DOI] [PubMed] [Google Scholar]
- Kang I, Lee K, Yang S-J, Choi A, Kang D, Lee YK et al. (2012). Genome sequence of “Candidatus Aquiluna” sp. strain IMCC13023, a marine member of the Actinobacteria isolated from an Arctic fjord. J Bacteriol 194: 3550–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang I, Kim S, Islam MR, Cho J-C. (2017). The first complete genome sequences of the acI lineage, the most abundant freshwater Actinobacteria, obtained by whole-genome-amplification of dilution-to-extinction cultures. Sci Rep 7: 42252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keffer JL, Hahn MW, Maresca JA. (2015). Characterization of an unconventional rhodopsin from the freshwater Actinobacterium Rhodoluna lacicola. J Bacteriol 197: 2704–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles B, Silveira CB, Bailey BA, Barott K, Cantu VA, Cobián-Güemes AG et al. (2016). Lytic to temperate switching of viral communities. Nature 531: 466–470. [DOI] [PubMed] [Google Scholar]
- Langmead B, Salzberg SL. (2012). Fast gapped-read alignment with Bowtie 2. Nat Methods 9: 357–U354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann T, Sonnhammer EL. (2005). Kalign – an accurate and fast multiple sequence alignment algorithm. BMC Bioinformatics 6: 298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig W, Strunk O, Westram R, Richter L, Meier H, Yadhukumar et al. (2004). ARB: a software environment for sequence data. Nucleic Acid Res 32: 1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo H, Thompson LR, Stingl U, Hughes AL. (2015). Selection maintains low genomic GC content in marine SAR11 lineages. Mol Biol Evol 32: 2738–2748. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer A, Derbyshire MK, Gonzales NR, Lu S, Chitsaz F, Geer LY et al. (2015). CDD: NCBI's conserved domain database. Nucleic Acid Res 43: D222–D226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JJ, Lenski RE, Zinser ER. (2012). The Black Queen hypothesis: evolution of dependencies through adaptive gene loss. mBio 3: pii: e00036-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyzer G, Dewaal EC, Uitterlinden AG. (1993). Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction-amplified genes coding for 16S rRNA. Appl Environ Microbiol 59: 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyzer G, Teske A, Wirsen CO, Jannasch HW. (1995). Phylogenetic relationships of Thiomicrospira species and their identification in deep-sea hydrothermal vent samples by denaturing gradient gel electrophoresis of 16S rDNA fragments. Arch Microbiol 164: 165–172. [DOI] [PubMed] [Google Scholar]
- Neuenschwander SM, Pernthaler J, Posch T, Salcher MM. (2015). Seasonal growth potential of rare lake water bacteria suggest their disproportional contribution to carbon fluxes. Environ Microbiol 17: 781–795. [DOI] [PubMed] [Google Scholar]
- Newton RJ, Jones SE, Helmus MR, McMahon KD. (2007). Phylogenetic ecology of the freshwater Actinobacteria acI lineage. Appl Environ Microbiol 73: 7169–7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S. (2011). A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol R 75: 14–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez MT, Sommaruga R. (2006). Differential effect of algal- and soil-derived dissolved organic matter on alpine lake bacterial community composition and activity. Limnol Oceanogr 51: 2527–2537. [Google Scholar]
- Pérez MT, Hörtnagl P, Sommaruga R. (2010). Contrasting ability to take up leucine and thymidine among freshwater bacterial groups: implications for bacterial production measurements. Environ Microbiol 12: 74–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez MT, Rofner C, Sommaruga R. (2015). Dissolved organic monomer partitioning among bacterial groups in two oligotrophic lakes. Environ Microbiol Rep 7: 265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posch T, Franzoi J, Prader M, Salcher MM. (2009). New image analysis tool to study biomass and morphotypes of three major bacterioplankton groups in an alpine lake. Aquat Microb Ecol 54: 113–126. [Google Scholar]
- Posch T, Koster O, Salcher MM, Pernthaler J. (2012). Harmful filamentous cyanobacteria favoured by reduced water turnover with lake warming. Nat Clim Change 2: 809–813. [Google Scholar]
- Price MN, Dehal PS, Arkin AP. (2010). FastTree 2 – approximately maximum-likelihood trees for large alignments. PLoS One 5: e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruesse E, Quast C, Knittel K, Fuchs BM, Ludwig W, Peplies J et al. (2007). SILVA: a comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acid Res 35: 7188–7196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Q-L, Xie B-B, Zhang X-Y, Chen X-L, Zhou B-C, Zhou J et al. (2014). A proposed genus boundary for the prokaryotes based on genomic insights. J Bacteriol 196: 2210–2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-R LM, Konstantinidis KT. (2014). Bypassing cultivation to identify bacterial species. ASM Microbe Magazine 9: 111–118. [Google Scholar]
- Rodriguez-Valera F, Martin-Cuadrado A-B, Rodriguez-Brito B, Pasic L, Thingstad TF, Rohwer F et al. (2009). Explaining microbial population genomics through phage predation. Nat Rev Microbiol 7: 828–836. [DOI] [PubMed] [Google Scholar]
- Salcher MM, Pernthaler J, Posch T. (2010). Spatiotemporal distribution and activity patterns of bacteria from three phylogenetic groups in an oligomesotrophic lake. Limnol Oceanogr 55: 846–856. [Google Scholar]
- Salcher MM, Pernthaler J, Posch T. (2011). Seasonal bloom dynamics and ecophysiology of the freshwater sister clade of SAR11 bacteria 'that rule the waves' (LD12). ISME J 5: 1242–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcher MM, Posch T, Pernthaler J. (2013). In situ substrate preferences of abundant bacterioplankton populations in a prealpine freshwater lake. ISME J 7: 896–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcher MM. (2014). Same same but different: ecological niche partitioning of planktonic freshwater prokaryotes. J Limnol 73: 74–87. [Google Scholar]
- Salcher MM, Neuenschwander SM, Posch T, Pernthaler J. (2015). The ecology of pelagic freshwater methylotrophs assessed by a high-resolution monitoring and isolation campaign. ISME J 9: 2442–2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmieder R, Edwards R. (2011). Quality control and preprocessing of metagenomic datasets. Bioinformatics 27: 863–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekar R, Pernthaler A, Pernthaler J, Warnecke F, Posch T, Amann R. (2003). An improved protocol for quantification of freshwater Actinobacteria by fluorescence in situ hybridization. Appl Environ Microbiol 69: 2928–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen A, Daubin V, Abrouk D, Gifford I, Berry AM, Normand P. (2014). Phylogeny of the class Actinobacteria revisited in the light of complete genomes. The orders ‘Frankiales’ and Micrococcales should be split into coherent entities: proposal of Frankiales ord. nov., Geodermatophilales ord. nov., Acidothermales ord. nov. and Nakamurellales ord. nov. Int J Syst Evol Microbiol 64: 3821–3832. [DOI] [PubMed] [Google Scholar]
- Šimek K, Kasalický V, Jezbera J, Horňák K, Nedoma J, Hahn MW et al. (2013). Differential freshwater flagellate community response to bacterial food quality with a focus on Limnohabitans bacteria. ISME J 7: 1519–1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šimek K, Nedoma J, Znachor P, Kasalický V, Jezbera J, Horňák K et al. (2014). A finely tuned symphony of factors modulates the microbial food web of a freshwater reservoir in spring. Limnol Oceanogr 59: 1477–1492. [Google Scholar]
- Stamatakis A, Ludwig T, Meier H. (2005). RAxML-II: a program for sequential, parallel and distributed inference of large phylogenetic. Concurr Comput Pract Exp 17: 1705–1723. [Google Scholar]
- Steindler L, Schwalbach MS, Smith DP, Chan F, Giovannoni SJ. (2011). Energy starved Candidatus Pelagibacter ubique substitutes light-mediated ATP production for endogenous carbon respiration. PLoS One 6: e19725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker R. (2012). Marine microbes see a sea of gradients. Science 338: 628–633. [DOI] [PubMed] [Google Scholar]
- Tarao M, Jezbera J, Hahn MW. (2009). Involvement of cell surface structures in size-independent grazing resistance of freshwater Actinobacteria. Appl Environ Microbiol 75: 4720–4726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatusov RL, Natale DA, Garkavtsev IV, Tatusova TA, Shankavaram UT, Rao BS et al. (2001). The COG database: new developments in phylogenetic classification of proteins from complete genomes. Nucleic Acid Res 29: 22–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp HJ, Kitner JB, Schwalbach MS, Dacey JWH, Wilhelm LJ, Giovannoni SJ. (2008). SAR11 marine bacteria require exogenous reduced sulphur for growth. Nature 452: 741–744. [DOI] [PubMed] [Google Scholar]
- Tritt A, Eisen JA, Facciotti MT, Darling AE. (2012). An integrated pipeline for de novo assembly of microbial genomes. PLoS One 7: e42304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Untergasser A, Cutcutache I, Koressaar T, Ye J, Faircloth BC, Remm M et al. (2012). Primer3-new capabilities and interfaces. Nucleic Acids Res 40: e115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergin KL, Tripp HJ, Wilhelm LJ, Denver DR, Rappe MS, Giovannoni SJ. (2007). High intraspecific recombination rate in a native population of Candidatus Pelagibacter ubique (SAR11). Environ Microbiol 9: 2430–2440. [DOI] [PubMed] [Google Scholar]
- Warnecke F, Sommaruga R, Sekar R, Hofer JS, Pernthaler J. (2005). Abundances, identity, and growth state of Actinobacteria in mountain lakes of different UV transparency. Appl Environ Microbiol 71: 5551–5559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeder M, Pernthaler J. (2009). Multispot live-image autofocusing for high-throughput microscopy of fluorescently stained bacteria. Cytometry Part A 75A: 781–788. [DOI] [PubMed] [Google Scholar]
- Zhao X, Schwartz C, Pierson J, Giovannoni SJ, McIntosh JR, Nicastro D. (2016). Three-dimensional structure of the ultra-oligotrophic marine bacterium Pelagibacter. Appl Environ Microbiol 83: e02807–e02816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Temperton B, Thrash JC, Schwalbach MS, Vergin KL, Landry ZC et al. (2013). Abundant SAR11 viruses in the ocean. Nature 494: 357–360. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.