Abstract
Objective
To study the combination of thermal MRI imaging and novel hypothermic cooling, via an Endorectal Cooling Balloon (ECB) to assess the effective dispersion and temperature drop in pelvic tissue to potentially reduce inflammatory cascade in surgical applications.
Methods
Three male subjects, prior to undergoing robot-assisted radical prostatectomy (RARP), were cooled via an ECB, rendered MRI compatible by for patient safety Prior to ECB hypothermia. MRI studies were performed using a 3T scanner and included T2w anatomical scan for the pelvic structures, followed by a temperature mapping scan. The sequence was performed repeatedly during the cooling experiment while the phase data were collected using an integrated MR-HIFU workstation in real time. Pelvic cooling was instituted with a cooling console located outside the MRI magnet room
Results
The feasibility of pelvic cooling measured a temperature drop of the ECB of 20–25 degrees in real time was achieved after an initial time delay of 10–15′ for the ECB to cool. The anatomic image of the prostate and NVB and demonstrate cooling at this interface of 10–15 degrees, and that cooling extends into the prostate itself ~ 5 degrees, and disperses into the pelvic region as well.
Conclusion
An MRI compatible ECB coupled with Thermal MRI is a feasible method to assess effective hypothermic diffusion and saturation to pelvic structures. By inference hypothermia induced rectal cooling could potentially reduce inflammation, scarring and fistula in RP as well as other urologic tissue procedures of HIFU, ERBT, radioactive seed implants, transurethral microwave therapy, and TURP.
Introduction
New prostate cancer cases are estimated to be >161,000 men per year, representing a major cause of cancer death in men. 1 Radical Prostatectomy (RP) is an important therapeutic intervention in decreasing mortality, but it is not without treatment morbidity. We have recently developed an innovative methodology to cool the lower intra-abdominal region with endo-rectal cooling balloon during radical prostatectomy.
In initial studies, controlled local hypothermia was achieved by use of a novel endorectal cooling balloon (ECB) positioned with the rectum below the prostate, with cold saline irrigation used in closed circulation to maintain hypothermia. Early continence (zero pads) was found to be significantly shorter in the hypothermia group compared to a historical control. 2 Preliminary work has shown that Hypothermia induced RP significantly reduces the morbidity of postoperative urinary continence and sexual function that is common in aged men, greatly improving the tolerance of treatment. Notably, hypothermia provided more improved outcomes the older the age cohorts were, i.e., greatest improvement in elder men >60 years old. Hypothermia does not impact the rate of positive surgical margins, and thus safe for cancer control.3
However, the effective depth and spread of hypothermic cooling within the urogenital tissue is poorly understood. It is important to get accurate temperatures in 3 dimensions and to map the range and depth of the temperature gradients induced by the Hypothermic balloon to confirm if cooling of the nerves within the Neurovascular Bundles (NVBs) is feasible and has been maximally achieved to preserve sexual potency.
Initially we used a thermal camera during open surgery to photograph the cooling emanating from the ECB in the rectum after the prostate has been removed and demonstrated the uro-pelvis above the rectum was cooled to 20°C ±1° by the ECB.4 However measuring the point surface temperatures made by the thermal camera requires open surgery and is truly impractical: due to the bulky size of the camera and great difficulty for reliable and reproducible thermal photography of gradients especially laterally and ventrally. Preliminary studies by Chopra et al.5 in a study of tissue damage generated by heating of a laser induced hyperthermia on focal prostate cancer provided conceptual proof that MRI thermal mapping of the prostate is viable. In our current study, we tested the feasibility of MRI Thermography to map the temperature diffusion of hypothermic cooling via an ECB within the urogenital pelvis.
Methods
The Endorectal Cooling Balloon (ECB) system is designed to apply targeted temperature control to the pelvic anatomy during robot-assisted radical prostatectomy (RARP). The pelvis is cooled transrectally via a closed cycle recirculation of chilled sterile saline using a disposable balloon catheter connected via a circulation set to a control console comprised of a refrigeration system. The heat transfer fluid is circulated in closed-loop fashion through the catheter for the purpose of removing heat from the pelvic space.4 To make the cooling system completely MR compatible several modifications were made including 1. An electrically isolated ECB thermistor removed, 2. Ceramic pressure relief valve substituted for the ECB metal device, 3. RPX cooling console (Phillips Healthcare) was located outside the magnet with 30′ of cooling tubing to reach the subject inside the MRI chamber. As the ECB was positioned in the rectum, a wrap- around MRI coil was placed over the subject’s pelvis for data acquisition.
All imaging was performed on a 3T scanner (Achieva; Philips Medical Systems, Best, The Netherlands) with an integrated Sonalleve (Philips Healthcare, Helsinki, Finland) HIFU (High Intensity Focused Ultrasound) ablation System. The Sonalleve workstation was operated in emulator mode without HIFU hardware to collect and display in real time the thermal mapping data from MR scanner with a slight modification to its software for this study. Written informed consent for our institutional review board approved study (IRB (HS# 2012-8392) was obtained from each of the 3 participating male subjects. MRI was performed for each subject prior to undergoing RARP.
The balloon was inserted rectally by a trained clinician while the subject was placed on the tabletop of the scanner. The ECB was inflated with saline at body temperature. Prior to commencing the cooling experiment with MR thermal mapping, T2-weighted (T2w) images were acquired in axial slices as the anatomical template for color-coded overlay of temperature in the Sonalleve workstation, covering the prostate and surrounding pelvic area and used as the anatomic map. Circulation of chilled sterile saline was initiated from the control console of refrigeration system of ECB after starting the thermal mapping scan and acquiring 2–3 time point data as baseline using a Philips-provided thermal mapping protocol modified for this study: fat-suppressed, multishot, echo planar imaging (EPI), with TR/TE=48/16 (ms), flip-angle at 20° and 2 number of signal average (NSA) in 3-mm thick axial slices. The thermal mapping was performed as dynamic scans, repeating and updating the temperature map in the Sonalleve workstation every 76 sec, until it was stopped by the operator. The study was usually well tolerated by the subjects up to about 1 hour after which a large motion-artifact was typically observed and the thermal mapping was stopped subsequently. The - thermal mapping data based on phase mapping using the Proton Resonance Frequency method (PRF) 6 were also collected along with other MRI images for offline display of temperature map using a custom program written in Matlab 7.8.0 (Mathworks, Natick, USA).
Results
The three subjects consented to MR imaging had ages (69, 51, 61), PrePSA (10.9, 36.3, 5.2), Gleason Scores (7, 9, 6) prostate weight (62, 63, 64 grams), and BMI (24.5, 24.1, 26.1). Time to pad free continence was 22 and 80 days in two subjects, and 224 days to a security pad in the 3rd older subject. Subject tolerability of the MRI was 1–1.5 Hrs.
Thermal MRI Acquisition
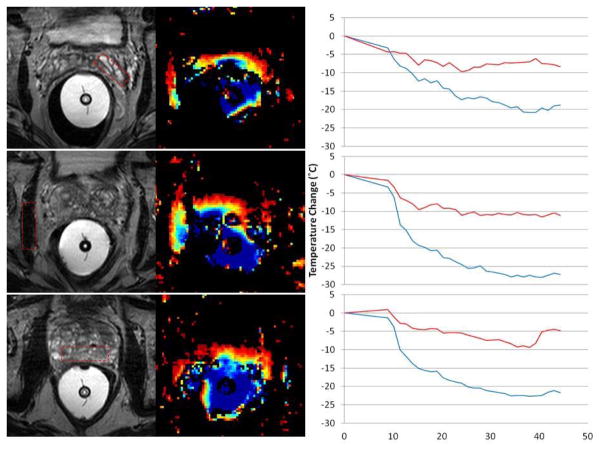
Figure 1ABC matches the Thermal MR image with the anatomic MR image and demonstrates that pelvic cooling measured in real time is feasible. In the color cooling scale the coldest is deep blue at 17–19 °C and red the warmest at 35–37°C. This is presented within 3 specific prostatic regions, near NVB (top), Lateral Muscle of UG diaphragm (middle) and posterior Prostate (bottom). Results at 1 hour after initiation of endorectal cooling demonstrate a temperature drop of 20–25 degrees was measured in the cooling balloon (blue line). Figure 1B middle and bottom (2C) compares the anatomic images of the prostate and NVB and demonstrates cooling at this interface of ~10 degrees (red line) per 20–25 degrees of ECB cooling, cooling extends into the prostate itself ~ 5 degrees, and disperses into the pelvic region as well.
Figure 1. Thermal MRI Acquisition.
Figure 1 matches the Thermal MR image with the anatomic MR image within 3 specific prostatic regions, near NVB (top), Lateral Muscle of UG diaphragm (middle) and posterior Prostate (bottom). Results at 1 hour after initiation of endorectal cooling demonstrate a temperature drop of 20–25 degrees was measured in the cooling balloon (blue line). Middle and bottom (2C) compares the anatomic images of the prostate and NVB and demonstrates cooling at this interface of ~10 degrees (red line) per 20–25 degrees of ECB cooling, cooling extends into the prostate itself ~ 5 degrees, and disperses into the pelvic region as well.
Thermal mapping of the perineum and penile stalk
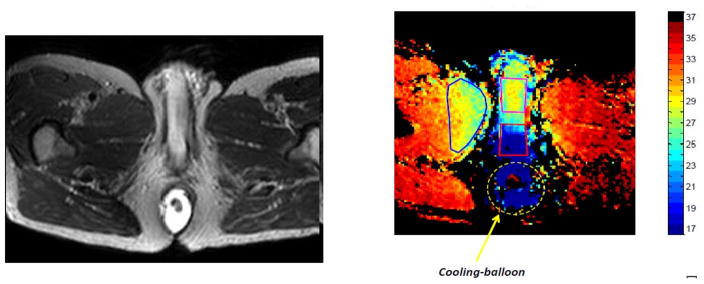
Figure 2 shows the cooling below the penile stalk and surrounding tissues of 10–15 degrees, and penetrated laterally into the muscle and peri-rectal tissue cooling of 8–10°. Likewise the penile stalk was cooled 7–10°, although the ECB study was truncated due to partial expulsion of the ECB.
Figure 2. Thermal mapping of the perineum and penile stalk.
Figure 2 shows the cooling below the penile stalk and surrounding tissues of 10–15 degrees, and penetrated laterally into the muscle and peri-rectal tissue cooling of 8–10°. Likewise the penile stalk was cooled 7–10°, although the ECB study was truncated due to partial expulsion of the ECB.
Discussion
Beyond oncologic control, quality of life (QOL) after radical prostatectomy relies on the return of continence and sexual potency after surgery to their pre-operative ‘normal’ status. Potency rates for patients are dependent on preservation of the Neurovascular Bundles (NVB) during RP, and they potentially can take years to recover, due to the nerve injury and trauma from the surgical procedure. 7 Similarly post RP incontinence is dependent on preservation of the nerves that control the external urethral sphincter, bladder, and urogenital diaphragm, and limiting Inflammation which affects nerves, but also may directly damage the bladder, urethra, and the pelvic floor. Effective strategies to prevent this damage are currently lacking. One stratagem to prevent or minimize such damage is the use of prophylactic local hypothermia with ice or cold irrigation around the nerves and tissues prior to, during, and after intragenic RP injury has occurred. Numerous experimental models of central and peripheral nervous system injury that use of moderate hypothermia (i.e. 28–33°C) support neuroprotective safety in humans, during cardiac, kidney, and brain surgery. 8–14 Morales et al had demonstrated a direct reduction in neutrophils compared to non-cooled controls in RP histology supporting the reduction of inflammation hypothesis. 15
The current study demonstrates the proof of concept that the diffusion of hypothermic cooling via an ECB can be directly measured and quantified within the uropelvis and targeted tissues with MRI. In the figure 1 image of the prostate, Denonvillier’s fascia and NVB and demonstrate cooling at this interface of 10–15 degrees, and into the prostate peripheral zone cooling 10–20 degrees, and central zone at 5–10 degrees of cooling, with bilateral cooling into the pelvic region also.
There are several limitations in accurately assessing tissue temperature in the uro-pelvis. Due to restrictions and danger of having magnetic items (e.g. metal cooling console) within the proximity of MRI chamber, we adapted our methods to these constraints. Thus this feasibility study is an ‘imperfect facsimile’ of actual therapeutic cooling used for RARP, in that the heat loss sustained with the extended 30′ of cooling tubing versus 10′ used in actual RARP, means that the full extent of hypothermic cooling at the MR coil is not achieved as it is in the surgical theater. This is also supported by thermistor readings which are ≥ 5 degrees cooler in RARP cases than measured by thermal MRI. Patient tolerance was generally 1–1.5 hours, as they were not sedated and instructed to lie still for this time period, with earmuffs to minimize the MRI noise. Although patient volunteers were forthcoming, minimizing patient use with computationally accurate replicas is an attractive alternative.
A method to compensate for ‘in vivo’ MRI under assessment would be to design virtual MRI simulations of deeper and longer cooling within the ECB which would more accurately deploy cooling during radical prostatectomy. The goal is to develop 2D and 3D models by correlating the mathematically predicted temperatures with the experimental derived temperatures using MR. The virtual MR imaging will allow us to create new virtual balloon designs to optimize cooling. Development of accurate MR modeling will allow virtual designs of ECB and other cooling devices, reducing the need for study subjects to undergo MR, except for validation.
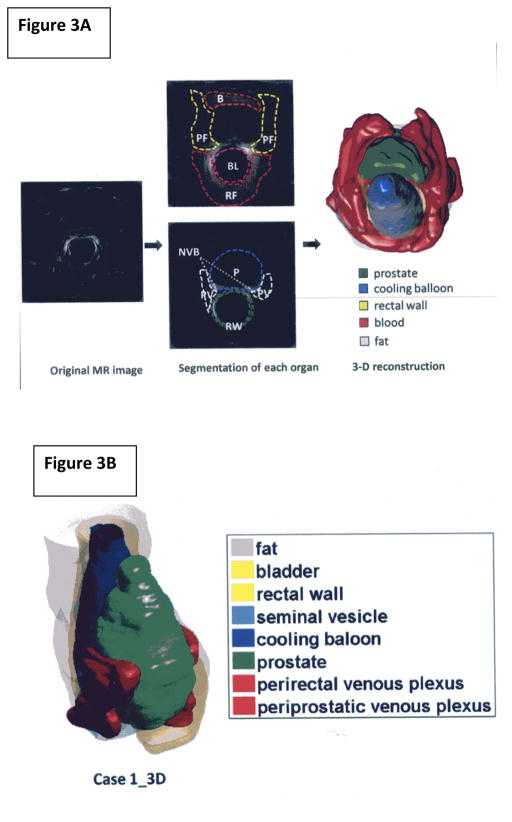
As simulation study by Lin et al 16 addresses the computational issues when building a virtual MRI model of endorectal cooling. Virtual studies are needed to address inherent cooling challenges of variable prostate volumes, venous plexus, ‘cold’ sink effect of blood flow, and tissue thermal properties. The initial simulation work was done using a 2D model to demonstrate the feasibility, and is expandable to more realistic 3D models. Extrapolating from our pilot MR imaging using thermal conductivity and tissue densities, a computational description of the thermal dynamics of hypothermic dispersion over time, can be computed across the pelvic region. Figure 3 A demonstrates feasibility of describing the thermal dynamics during the ECB cooling based on the 2D reconstruction of tissue. Virtual 3-Dimensional MR models can be constructed to conform to different patient prostate sizes, body habitus etc. in the pelvic region (manuscript in preparation), Figure 3B. As noted in our study, the ECB cooling gradient did not fully extend into the neurovascular bundles, and the dorsal half of the ECB tended to be the coldest section. The use of computer-generated modeling allows redesign of more effective ECB models. The iterative testing of numerous virtual devices would greatly benefit in the creation of new more efficient prototypes before being tested in human volunteers, creating a considerable cost and subject savings.
Figure 3.
Figure 3A. Image Segmentation and 3D Reconstruction The tissues in the pelvic region were specified by manually identifying their boundaries manually based on MR images. These can be divided into a few categories: prostate (P), per-prostatic venous plexus (PV), rectum (RW), peri-prostatic (rectal) fatty tissue (PF and RF), seminal vesicle, urinary bladder (B), and endorectal cooling balloon (BL). The manual segmentation of the pelvic anatomy was supervised by an experienced radiologist.
Figure 3B. 3-Dimensional reconstruction the ECB within the prostatic and NVB urogenital region.
Success beyond radical prostatectomy could potentially reduce tissue damage from other procedures such as external beam radiation, HIFU and in OB/GYN surgery. Complications such as urethral scarring, urethral stenosis, bladder neck contracture (BNC) and urorectal fistula (URF) can be issues after both radiation for prostate cancer and thermal technologies for treating BPH. 17–18 BNC and urethral stenosis are more extensive after primary HIFU, radiotherapy, and cryotherapy than after RP. 17 Patients that have prior radiotherapy and now undergoing a salvage procedure such as HIFU are at 10 times risk of URF. 17 Rectal cooling may help to decrease thermal injury and protect the already irradiated tissue from further damage.
Urethral structures can occur anywhere along the urethra after TURP. There is a higher incidence with the bipolar technology which is more often employed. 18 Bladder neck stenosis or contracture is a late complication of TURP about 1–9% of cases and is seen with smaller glands. Even in laser ablation treatments and transurethral microwave therapy (TUMT) the biggest complication remains to be urethral scarring/stenosis. TUMT has a 10% rate of prostate urethral scarring despite being less invasive then TURP. 18 There is certainly a role in rectal cooling that can provide intraoperative protection of the urethra during the treatment to reduce incidence of scarring.
That cooling is shown to impact the prostate central and peripheral zones implies that these zones could be protected from potential inflammation from TURP and focal thermal ablation in the transition zone. However, this initial series has yet to show if the transition and fibromuscular stroma zone can be impacted by rectal cooling. Limited reduction of inflammation after IMRT may also be possible within the prostatic peripheral and central zones. Effective cooling below the penile stalk within the urethral bulb, corpus spongiosum, and bulbourethral gland was feasible (Figure 2). Laterally cooling > 15 °C extends to the external and internal rectal sphincters and levator ani.
A recent report of a randomized control trial of hypothermia using the current ECB did not demonstrate a benefit in earlier return to continence. 19 However the study was confounded by variable outcomes by the participating surgeons which resulted in under powering of the study. Interestingly, even with small numbers the hypothermic RCT found that men saw a benefit in potency in one of three trial sites. 20 The prostate MR image may provide the individual anatomy and tissue components for thermal modeling to be used to achieve optimized cooling effects using ECB in men in urological treatments. Further studies should clarify in vivo which tissues are susceptible or impervious to pelvic cooling.
Conclusion
An MRI compatible ECB coupled with Thermal MRI is a feasible method to assess effective hypothermic diffusion and saturation to pelvic structures. By inference hypothermia induced rectal cooling could potentially reduce inflammation, scarring and fistula in RP as well as other urologic tissue procedures of HIFU, ERBT, radioactive seed implants, transurethral microwave therapy, and TURP be measured by MR thermometry.
Références
- 1.Siegel R, Miller K, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Finley D, Osann K, Skarecky D, Ahlering TE. Hypothermic nerve-sparing radical prostatectomy: rationale, feasibility, and effect on early continence. Urology. 2009;73:691–6. doi: 10.1016/j.urology.2008.09.085. [DOI] [PubMed] [Google Scholar]
- 3.Finley D, Chang A, Morales B, Osann K, Skarecky D, Ahlering TE. Impact of Regional Hypothermia on Urinary Continence and Potency following Robot-assisted Radical Prostatectomy. J Endourology. 2010;24:1111–1116. doi: 10.1089/end.2010.0122. [DOI] [PubMed] [Google Scholar]
- 4.Liss M, Skarecky DW, Morales B, Ahlering T. The Application Regional Hypothermia using Transrectal Cooling during Radical Prostatectomy to mitigate surgical and post-surgical inflammatory damage to preserve continence. J Endourology. 2012;12:1553–1557. doi: 10.1089/end.2012.0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chopra R, Tang K, Burtnyk M, et al. Analysis of the spatial and temporal accuracy of heating in the prostate gland using transurethral ultrasound therapy and active MR temperature feedback. Phys Med Biol. 2009;54:2615–2633. doi: 10.1088/0031-9155/54/9/002. [DOI] [PubMed] [Google Scholar]
- 6.Reike V, Pauly K. MR Thermometry. J Magn Res Imag. 2008;27:376–390. doi: 10.1002/jmri.21265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahlering T, Skarecky ‘Science made simple’ Preserving Sexual Function during Robotic Radical Prostatectomy: Evolution of thought regarding Thermal Energy near Nerves. BJUI. 2014;114:131–132. doi: 10.1111/bju.12663. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Olivero W, Wang D, Lanzino G. Cold as a therapeutic agent. Acta Neurochir (Wien) 2006;148:565–70. doi: 10.1007/s00701-006-0747-z. discussion 69–70. [DOI] [PubMed] [Google Scholar]
- 9.Yang XF, Kennedy BR, Lomber SG, Schmidt RE, Rothman SM. Cooling produces minimal neuropathology in neocortex and hippocampus. Neurobiol Dis. 2006;23:637–43. doi: 10.1016/j.nbd.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Kelly C, Creagh T, Grace PA, Bouchier-Hayes D. Regional hypothermia protects against tourniquet neuropathy. Eur J Vasc Surg. 1992;6:288–92. doi: 10.1016/s0950-821x(05)80320-2. [DOI] [PubMed] [Google Scholar]
- 11.Sun X, Tang W, Zheng L. Ultrastructural observation of effect of moderate hypothermia on axonal damage in an animal model of diffuse axonal injury. Chin J Traumatol. 2002;5:355–60. [PubMed] [Google Scholar]
- 12.Zhao H, Wang JQ, Shimohata T, Sun G, Yenari MA, Sapolsky RM, Steinberg GK. Conditions of protection by hypothermia and effects on apoptotic pathways in a rat model of permanent middle cerebral artery occlusion. J Neurosurg. 2007;107:636–41. doi: 10.3171/JNS-07/09/0636. [DOI] [PubMed] [Google Scholar]
- 13.Sahuquillo J, Vilalta A. Cooling the injured brain: how does moderate hypothermia influence the pathophysiology of traumatic brain injury. Curr Pharm Des. 2007;13:2310–22. doi: 10.2174/138161207781368756. [DOI] [PubMed] [Google Scholar]
- 14.Mitsui Y, Schmelzer JD, Zollman PJ, Mitsui M, Kihara M, Low PA. Hypothermic neuroprotection of peripheral nerve of rats from ischemia-reperfusion injury: intraischemic vs. reperfusion hypothermia. Brain Res. 1999;827:63–9. doi: 10.1016/s0006-8993(99)01289-5. [DOI] [PubMed] [Google Scholar]
- 15.Morales B, Tran HL, Carpenter P, Narula N, Skarecky D, Ahlering T. RARP and localized hypothermia’s impact on continence and inflammatory response. J Urol. 2013;189:A849, e349. [Google Scholar]
- 16.Lin Y, Lin WC, Fwu PT, Shih TC, Yeh LR, Su MY, Chen JH. Investigation of factors affecting hypothermic pelvic tissue cooling using bio-heat simulation models based on MRI-segmented anatomic modes. Computer Meth and Programs in Biomedicine. 2015;122:76–88. doi: 10.1016/j.cmpb.2015.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mundy A, Andrich D. Posterior Urethral Complications of the treatment of prostate cancer. BJUI. 2012;110:304–325. doi: 10.1111/j.1464-410X.2011.10864.x. [DOI] [PubMed] [Google Scholar]
- 18.Chen M, Cornea R, Santucci R. Urthral Strictures and Stenosis caused by prostate therapy. Rev Urol. 2016;18:90–102. doi: 10.3909/riu0685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaler K, Ahlering T. Impact of regional hypothermia on urinary continence during RARP: Results from a double-blinded randomized control study. J Urol. 2016;195:e632–633. Abstract, MP 40–17. [Google Scholar]
- 20.Ko Y-H, Osann K, Skarecky D, Morales B, Ahlering T. A randomized control trial on the impact of regional hypothermia: Ad hoc analysis on short term recovery of sexual function after robot-assisted radical prostatectomy (RARP) Eur Urol. 2017;18(3 Suppl) Abstract 1067, In press. [Google Scholar]