Abstract
Introduction
In Essential Tremor (ET), tremor characteristics and the impairment caused by tremor may vary from task to task. A variability of tremor frequency between postural and kinetic tasks has been proposed in ET, suggesting either multiple central oscillating networks, or peripheral or proprioceptive feedback-mechanisms. This electrophysiological study aimed to assess tremor frequencies and amplitudes in tasks involving postural and kinetic tremor, and compare findings within and across tasks, to delineate physiological differences underlying individually affected manual tasks in ET.
Methods
40 ET patients were included in the study. Tremor was characterized clinically, as well as electrophysiologically using accelerometry and digitizing tablet tasks. Tremor amplitude measures and frequencies were extracted for tasks involving kinetic (digital spiral drawing, handwriting), as well as postural tremor. Tremor was compared between and within tasks.
Results
Digital spiral tremor frequencies were significantly higher compared to postural tremor frequencies, as measured by accelerometry, with a mean difference of > 2 Hz (p<0.001). Within-task variability of repeated digital spirals revealed a significant amplitude reduction over time in both hands (p<0.001), with an up to 32% reduction compared to the first spiral.
Conclusion
ET exhibited a frequency variability, which was dependent on activation condition, suggesting neurophysiologically distinct pathways between postural and kinetic tremor. The reduction of tremor amplitudes observed in repeated digital spiral drawing may be explained by a learning effect or adaptation, and should be considered as non-random factor of variability when using spirals in ET to assess effects of interventions.
Keywords: Essential Tremor, spiral analysis, accelerometry, neurophysiology, learning effect
Introduction
ET is one of the most common movement disorders with a prevalence of up to 5% in the elderly population [1–3]. The mechanisms underlying ET remain far from well understood and no specific pathophysiological correlate has been determined [4, 5]. Commonly studied brain regions in this context involve the cerebellum, the inferior olive, and the locus coeruleus [6].
Physiological parameters characterizing tremor are amplitude and frequency. The tremor frequency in ET patients typically lies between 4 and 12 Hz and is largely constant within a patient and decreases with age. The amplitude, however, can vary significantly intra-individually and may be reduced by medication and mental relaxation [7–9].
Due to a lack of disease-specific biological markers, the diagnosis of ET often proves difficult. The diagnosis is a based on clinical criteria, and no objective diagnostic method exists. However, electrophysiological studies are often helpful in the differential diagnosis of ET and are capable to objectively track ET symptoms over time, e.g. before and after an intervention [10]. Electrophysiological methods used in the assessment and quantification of tremor include accelerometry, electromyography (EMG) and digital spiral analysis [11].
Tremor impairment in spiral drawing and handwriting were found to correlate well with the overall functional disability caused by ET [12]. To objectively assess spiral tremor, digitizing tablets have been used to assess frequency and amplitude. This method allows objective tremor intensity quantification in tasks, which are relevant for patients’ daily life involving penmanship. Measurements are objective and small variations in amplitude and frequency can be detected [13–15].
Short-time frequency variations have been found to be uncommon in ET, whereas the tremor amplitude may vary significantly during the same day. Using accelerometry, differences in the frequency of postural tremor and kinetic manual tasks have been described, but whether this variation is due to a peripheral or central mechanism remains unclear [12]. The current thinking of the neurophysiological correlate of ET is based on hypotheses involving a tremor-origin from a central nervous oscillator or oscillating network [6, 16]. Variation of frequency between different tasks suggests that different networks may be activated for different motor tasks, and somatosensory input alters the central generation of tremor.
Impairment is related to the amplitude rather than by the frequency of ET [17]. It is crucial to quantify how reduction in tremor amplitude relates to functional benefit following a treatment intervention [12]. As the measurement of postural tremor alone does not adequately measure patients’ impairment, spiral drawing may present a valid alternative surrogate for functionally relevant kinetic tremor. However, manual tasks may be subject to several other influences such as random variability, adaptation behaviors, and learning effects, and therefore potentially confound changes observed after therapeutic interventions. To assess the clinical meaningfulness of changes in tremor amplitudes across tasks, these non-treatment effects need to be quantified before a task can be applied to measure effects of treatment interventions.
The objective of this study was therefore to assess the electrophysiological characteristics of postural and action tremor. Specifically, the goal was to investigate how tremor frequencies vary between conditions of postural and kinetic tremor to delineate potential physiological differences underlying individual affected manual tasks in ET. Furthermore, this study aimed to assess the impact of repeated task-performance on motor tasks affected by kinetic tremor, such as spiral drawing and handwriting.
Patients and Methods
Patients
40 subjects diagnosed with classical ET per MDS consensus criteria by a movement disorder specialist (DH), were included in the study [18]. Patient characteristics and demographics can be found in Table 1. Patients remained on their tremor-medication during the study (n=26).
Table 1.
Patient demographics and clinical characteristics (n=40)
| Age (years, mean ± SD, range) | 64.7 ± 13.7 (26–88) |
| Gender (n) | 24 male; 16 female |
| Handedness (n) | right: 39; left: 1 |
| Positive ET family history (n, %) | 30 (75%) |
| Age at ET onset (years, mean ± SD) | 38.3 ± 19.7 |
| Tremor duration (years, mean ± SD, range) | 27.0 ± 16.7 (3–75) |
| On tremor medication, at time of study (n, %) | 26 (65%) |
| Propranolol | 12 (30.0%) |
| Primidone | 8 (20.0%) |
| Topiramate | 4 (10.0%) |
| Lamotrigine | 1 (2.5%) |
| Herbal essence (passion flower extract) | 1 (2.5%) |
| Subjective rating of alcohol effect | |
| Beneficial effect reported (i.e., tremor reduction) (n, %) | 19 (47.5%) |
| No effect (n, %) | 7 (17.5%) |
| Unknown whether effect or not / does not drink alcohol (n, %) | 14 (35.0%) |
Methods
This study was conducted as an unblinded, single-center non-interventional observation study, approved by the local ethics committee of the Medical University of Vienna. Informed consent was given by all subjects. Baseline characteristics included the assessment for hand-dominance using the “Edinburgh Handedness Inventory” [19], and a clinical rating scale (The Essential Tremor Rating Scale, TETRAS) [20]. To assess objective tremor parameters, tremor accelerometry and digitizing-tablet based assessments (spirals, handwriting) were conducted.
Digitizing tablet
The measurements by digitizing tablet aimed at assessing tasks involving kinetic tremor. The tasks included spiral drawing, handwriting and a digital version of the dot-approximation test, which is part of the TETRAS performance scale. Data was collected and analyzed using the software-platform Neuroglyphics (http://www.neuroglyphics.org/), installed on a Windows-based tablet-PC.
Subjects were asked to draw 10 spirals per hand back to back. To standardize the generated spiral-datasets, 3.75 loops of each spiral were selected electronically for further data processing. For the assessment of handwriting, patients were asked to write continuous cursive letters “e” between two lines across the tablet-PC screen, five lines per hand. For the dot-approximation test, patients were asked to hold the tip of the pen closely over the marked center of the tablet for 10 seconds per hand, without touching the surface. The software registered the pen-tip position over time, either along the trajectory of the spiral or handwriting sample, or in relation to the center for the dot-approximation task. Continuous time-series data including x- and y-positions for each data-point at a sampling rate of 200Hz were converted. Each data sample was numerically integrated and velocity spectra calculated using a Fast-Fourier-Transformation. The tremor frequency was extracted from the spectral tremor peak. A +/−1 Hz area under the curve window, centered at the spectral tremor peak, was calculated as surrogate measure of tremor amplitude [14]. The action-tremor task dataset per patients consisted of ten spirals, five handwriting samples (cursive e-lines), and one dot-approximation sample, with samples collected separately for each hand.
Accelerometry and EMG
For accelerometry, a uniaxial accelerometer mounted at the dorsum of each hand (measuring in the z-axis) was used. Surface EMG electrodes were placed on the M. flexor carpi ulnaris and the M. extensor carpi ulnaris of each arm. The tremor was then measured in the “posture” position with arms stabilized on the arm-rests of a chair and hands extended over the edge of the armrest, parallel to the ground. This position was recorded first without, then with the addition of 500-gram weights, with a recording duration of 30 seconds each. The weighted condition was used to identify the central tremor component. Tremor was analyzed using the commercially available tremor-analysis package ‘CPeak’. Parameters extracted from accelerometry were the peak frequency, Half-Width Power (H–W Power, corresponding to the tremor power under the main spectral frequency peak) [21, 22].
Statistical Analysis
The study outcome measures were tremor frequency and amplitude-measure differences within and across tasks. Statistical analysis was conducted using SPSS 20. A significance level of p<0.05 was applied for individual comparisons, after correction for multiple comparisons. For test-retest reliabilities and across-task validations, the intraclass correlation coefficients (ICC) were computed. The digital cursive e task and dot approximation were validated against the TETRAS scale and digital spirals with regard to their validity for ratings in tremor severity and impact on patients’ daily lives using Spearman’s test of correlation, as data were not normally distributed. Due to the limited sample size, non-parametric statistics (Wilcoxon Signed Ranks test) were applied to test for differences of tremor frequencies between tasks and methods, and corrected for multiple comparisons. After log-transformation, repeated measures ANOVA was applied to investigate the effect of repeated task performance on tremor amplitude measures. Post-hoc comparisons as well as all other pair-wise comparisons were corrected for multiple testing using the Bonferroni-method. Relative changes of amplitude measures were computed on a patient-basis using the absolute (non-log transformed) data, after normalization to the first spiral.
Results
Task-specific Variability of Frequency
Within-Task-Comparisons: spiral drawing, handwriting
Descriptive summaries of frequencies of each task are listed in Table 2. The spiral tremor frequencies across the 10 trials were in excellent agreement both in the dominant (ICC=0.959) and non-dominant hand (ICC=0.971). In the handwriting task, the tremor frequencies were similarly highly stable across e-lines in the dominant (ICC=0.962) and non-dominant hand (ICC=0.981). Due to this high test-retest reliability of tremor frequencies in the spiral and handwriting tasks, the data from the first spiral and handwriting sample were used for all further frequency comparisons.
Table 2.
Spectral peak tremor frequencies during the digitizing tablet based of action tremor and accelerometry tasks to quantify postural tremor. Spiral drawing was performed 10 times per hand, a cursive e-line was drawn as handwriting sample 5 times per hand. The dot approximation task was performed once per hand. Using accelerometry, dominant and non-dominant hand tremor was recorded concurrently, and each condition (without weight, with the addition of 500 g per hand) was recorded once. Descriptive statistics are given across all subjects (n=40).
| Dominant Hand Peak Frequency (Hz) | Non-Dominant Hand Peak Frequency (Hz) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Digitizing Tablet Tasks | Mean | SD | Median | Minimum | Maximum | Mean | SD | Median | Minimum | Maximum |
| Spiral 1 | 8.94 | 1.44 | 8.75 | 6.32 | 12.11 | 8.82 | 1.50 | 8.74 | 6.30 | 12.11 |
| Spiral 2 | 9.00 | 1.38 | 8.79 | 6.32 | 12.11 | 8.73 | 1.51 | 8.67 | 5.43 | 11.91 |
| Spiral 3 | 9.03 | 1.50 | 9.00 | 6.24 | 12.89 | 8.77 | 1.55 | 8.89 | 6.24 | 12.21 |
| Spiral 4 | 9.01 | 1.45 | 8.94 | 6.01 | 13.28 | 8.79 | 1.51 | 8.79 | 6.20 | 12.11 |
| Spiral 5 | 9.02 | 1.50 | 8.91 | 6.03 | 12.89 | 8.76 | 1.49 | 8.84 | 6.04 | 12.16 |
| Spiral 6 | 8.94 | 1.34 | 8.98 | 6.12 | 11.82 | 8.74 | 1.48 | 8.72 | 6.25 | 12.16 |
| Spiral 7 | 8.97 | 1.46 | 9.05 | 6.06 | 12.50 | 8.86 | 1.53 | 8.89 | 6.08 | 12.16 |
| Spiral 8 | 9.06 | 1.43 | 9.08 | 6.08 | 12.79 | 8.81 | 1.53 | 8.86 | 5.88 | 12.11 |
| Spiral 9 | 9.01 | 1.44 | 9.18 | 5.75 | 12.70 | 8.82 | 1.59 | 8.84 | 5.15 | 12.21 |
| Spiral 10 | 8.97 | 1.46 | 8.89 | 5.91 | 12.01 | 8.82 | 1.55 | 8.89 | 6.04 | 12.26 |
| Handwriting 1 | 8.65 | 1.48 | 8.81 | 5.15 | 11.57 | 8.77 | 1.68 | 8.72 | 4.42 | 12.21 |
| Handwriting 2 | 8.71 | 1.44 | 8.79 | 5.67 | 11.77 | 8.74 | 1.70 | 8.69 | 4.26 | 12.13 |
| Handwriting 3 | 8.77 | 1.38 | 8.89 | 5.93 | 11.43 | 8.74 | 1.62 | 8.64 | 5.03 | 11.89 |
| Handwriting 4 | 8.77 | 1.46 | 8.69 | 5.63 | 11.79 | 8.71 | 1.71 | 8.74 | 4.09 | 11.96 |
| Handwriting 5 | 8.65 | 1.52 | 8.94 | 4.70 | 11.82 | 8.80 | 1.74 | 8.87 | 4.05 | 11.94 |
| Dot Approximation | 8.37 | 1.50 | 8.35 | 5.23 | 12.11 | 8.90 | 1.44 | 8.84 | 5.71 | 12.26 |
|
| ||||||||||
| Accelerometry Tasks | Mean | SD | Median | Minimum | Maximum | Mean | SD | Median | Minimum | Maximum |
|
| ||||||||||
| Posture / non-weight | 6.18 | 1.05 | 6.03 | 5.00 | 10.27 | 6.39 | 1.15 | 6.14 | 4.53 | 11.13 |
| Posture / weight (500gr) | 6.11 | 1.34 | 5.63 | 4.63 | 10.53 | 6.23 | 1.49 | 5.70 | 4.67 | 10.67 |
Within-Method-Comparisons: digitizing tablet
The comparison of tremor frequencies between digital spirals (first spiral) and handwriting (first e-line) showed no difference in both the dominant and non-dominant hand. The frequencies of the first spiral were slightly higher than during the dot approximation test in dominant hand (p=0.004, corr.), but not in the non-dominant hand. The overall agreement of tremor frequencies between digitizing based methods was high (spiral vs. e-lines ICC=0.783, spiral vs. dot-approximation ICC=0.711, e-lines vs; dot-approximation ICC=0.660).
Between-Method-Comparisons: digitizing tablet vs. accelerometry
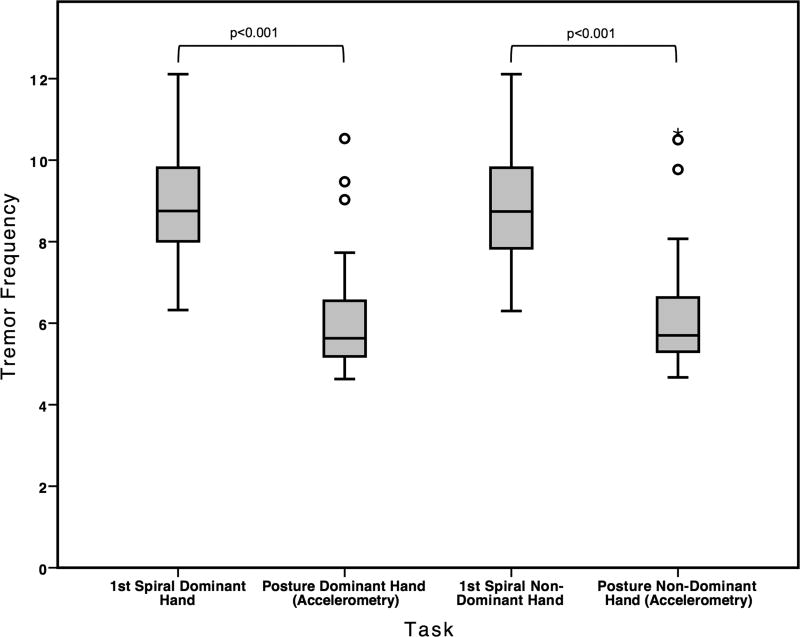
As there was no difference in peak frequencies between accelerometric postural tremor conditions (with, without weights, p=n.s for each hand, see Table 2), the following comparisons are demonstrated using the frequencies derived from the weighted condition, as these represent the central tremor component. All p-values were corrected for multiple pair-wise comparisons using the Bonferroni-method. The mean frequency of the first spiral was significantly higher compared to the central postural tremor frequency (dominant hand: mean difference: 2.83 Hz; p<0.001, non-dominant hand: mean difference: 2.59 Hz; p<0.001, Figure 1). While there was a trend for a correlation between higher postural tremor frequency and smaller increase in frequency from posture to spiral drawing (Spearman’s rho=−0.327, p=0.063), there was no difference in spiral frequencies in patients with a higher (7 Hz or higher) compared to lower postural (< 7 Hz) tremor frequency.
Figure 1.
Comparison of frequencies (Hz) of spiral and accelerometric posture (central component, after addition of 500 g weight) for the dominant and non-dominant hand.
A similar difference in frequencies between postural tremor during accelerometry and kinetic tremor during handwriting using the cursive e-task was observed, with frequencies being significantly higher during handwriting than during posture (dominant hand: mean difference: 2.54 Hz; p<0.001; non-dominant hand: mean difference: 2.54 Hz; p<0.001).
Similarly, the tremor during the dot-approximation test exhibited a higher frequency than in accelerometric posture in both the dominant (mean difference: 2.26 Hz; p<0.001) and the non-dominant hand (mean difference: 2.67 Hz; p<0.001). The results remained similarly statistical significant for comparisons of spirals, handwriting, and dot-approximation with tremor frequencies extracted from the non-weighted postural tremor condition (see Supplementary Table).
Task-specific Variability of Tremor Amplitude
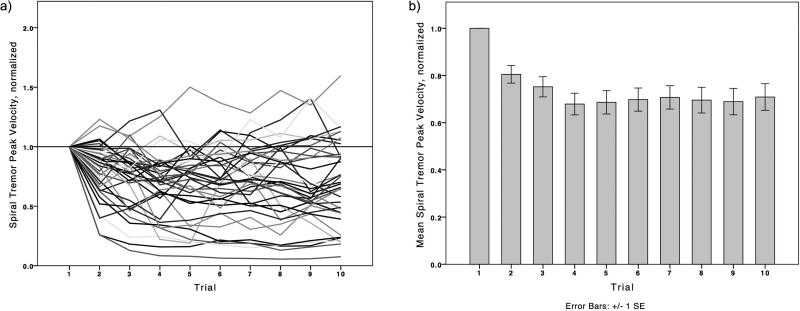
A significant change of the spiral tremor peak velocity, as measure of the tremor amplitude, (dominant hand: p<0.001; non-dominant hand: p<0.001) could be demonstrated across trials (see Figures 2). Post-hoc comparisons showed a significant reduction of tremor after the second spiral dominant hand spiral (p=0.002), and remained significant compared to the first spiral across all spirals to the tenth spiral (p=0.001).
Figure 2.
Normalized absolute tremor velocities of the dominant hand at the spectral frequency peak, across 10 trials. Normalized to the first spiral (value = 1). Values < 1 represent reduced tremor severity, > 1 indicate worsening of tremor; a) individual traces indicating each patient, b) mean ± SE
The average reduction of the absolute dominant spiral tremor amplitude scores was up to 32% (Figure 2b) compared to the first spiral, with individual tremor reductions ranging up to 94% (Figure 2a). Pairwise comparisons suggested a stabilization of the amplitude in the dominant hand after the fourth repetition.
During the handwriting-task, the dominant hand tremor amplitude measures were different across trials (p<0.001). Similar to the spiral tremor findings, post-hoc comparisons demonstrated a significant reduction of tremor amplitude measures with the second repetition (p=0.034), through the fifth repetition (p=0.022), compared to the first attempt. The average reduction of the absolute amplitude measures of subsequent e-lines of the dominant hand were up to 18% below that of the first, suggesting a reduction of tremor amplitudes after repetition (and Supplemental Figures 1–3).
Validity of digital e-lines and dot approximation test
Tremor severity scores obtained using dominant-hand digital cursive e-lines showed a significant correlation with the TETRAS performance scale (Spearman’s rho=0.53, p=0.001) as well as digital spirals (rho=0.85, p<0.001). Similarly, tremor amplitudes during the dot approximation test showed significant correlations with the TETRAS performance scale (rho=0.69, p<0.001) and digital spirals (rho=0.74, p<0.001). Correlations between the rating scale and both transducer-based methods followed a logarithmic relationship.
Discussion
Our study aimed to investigate the changes in tremor characteristics in different tasks in ET, as well as changes of tremor within specific tasks upon repeated performance.
First, this study demonstrated that digitizing-based tasks of tremor during handwriting can be applied in tasks beyond the “classical” drawing of digital spirals. In order to evaluate tremor characteristics in different tasks, this study extends the application of digitizing tablet based tremor assessment to a handwriting-task using cursive e-lines as well as the dot approximation task, an item of isometric pointing using a pen, which is included as item in the TETRAS performance scale. The results presented here provide validation of these tasks using the gold standard of TETRAS as well as digital spiral analysis, suggesting that digital cursive e-lines and the digital dot approximation test are both valid methods for quantifying ET amplitudes. The finding of highly reliable frequencies within a specific task after repetition confirms prior observations that tremor frequencies remain constant within a task, supporting a hypothesis that tremor within the same task is likely generated by a single, specific central oscillating mechanism [7, 23, 24].
Our study furthermore demonstrated a clear distinction between postural and kinetic tremor frequencies, with frequency differences exceeding 2 Hz. If considered a central effect, a central tremor oscillator in ET – as network or single oscillator - may be influenced by somatosensory inputs. Alternatively, distinct motor pathways of posture vs. kinetic movement may be recruited in a differential fashion, each being susceptible for the generation of pathological oscillations in ET, and activated dependent on the type of voluntary movement. This may be supported by evidence from thalamic recordings, which suggests that neuronal activation patterns in intentional tremor are distinct from postural tremor in ET [25]. Alternatively, a peripheral correlate of frequency differences between postural and kinetic tremor has been suggested before [17], with a tendency for lower postural frequencies to increase, and higher postural frequencies being reduced during kinetic tasks, termed “constriction” of tremor frequencies during writing and drawing. While there was a trend towards larger increases in the lower postural tremor frequency ranges, our study could not confirm the hypothesis of frequency constriction, but rather demonstrated a general frequency-increase during writing. Given the high correlations of different tasks involving kinetic tremor, and an absence of a correlation between kinetic and postural tremor tasks, our data rather support a hypothesis of a central mechanism with different neurophysiological mechanisms of tremor during posture and kinetic movement.
The assessment of tremor amplitudes within tasks provides information about variability and test-retest reliability in ET. In manual tasks for the assessment of kinetic tremor, repeated performance may impact the accuracy of a task. The question therefore arose whether also tremor “improved” during repeated performance. In this respect, we found a significant decline of tremor amplitude during spiral drawing and the cursive e-task and, thus, an improvement of tremor after repetition in both tasks. We hypothesize that this ‘improvement’ without any treatment intervention could be explained by an effect due to repeated task performance, e.g. due to learning and adaptation. This represents a non-random component of test-retest variability of handwriting tasks.
This fact has to be taken into account when measuring effects of treatment interventions on Archimedes spirals or the cursive e-task in ET. While usually only voluntary movements are subject to a learning effect as opposed to involuntary movements such as tremor in ET, our study showed a decline in tremor amplitude. One explanation may be a stress-related enhanced physiologic tremor component, which lessens after repetition and with increased proficiency in task performance.
The reductions of tremor severity measures in handwriting and drawing tasks during to repeated task performance were more than 30%, with the maximum reduction achieved with the fourth spiral and beyond. Following the Weber-Fechner law of psychophysics, which underlies the logarithmic relation between absolute measures of tremor amplitude and visual rating scales [26], our observed change due to repeated performance would roughly relate up to a 1-point change in a 0–4 point visual rating scale, and should be considered as non-random source for test-retest variability of spiral samples when defining clinically meaningful changes of interventions.
In the light of previous evidence of a reduced motor learning ability in ET patients [27], a question to be answered in a larger study is whether a potential learning effect is less developed in patients with more pronounced cerebellar abnormalities, as often seen in ET.
As to our study’s limitations, patients were continuing their tremor medication, therefore we cannot infer whether medication such as propranolol or primidone had a confounding effect on adaptation. One additional limitation is that our methods did not allow us to differentiate between central and peripheral components of tremor in digitizing-tablet based tasks. However, the high trial-to-trial consistency of tremor frequencies during handwriting tasks would be in line with a predominant central tremor mechanism driving the main component of kinetic tremor. Furthermore, as manual tasks were performed repetitively back-to-back, more data is needed to investigate the duration and persistence of any learning effect.
In summary, while isolated measures of tremor frequencies or amplitudes might be of limited applicability in the diagnostic and quantitative assessment of tremor, the variability of frequency and amplitudes may provide clues about the neurophysiological mechanisms of action tremors. They furthermore inform practitioners as well as clinical trialists about the performance of tremor-measures across time and can help to define clinical meaningful changes in tremor amplitudes.
Supplementary Material
Highlights.
Frequencies during kinetic tremor significantly differ from postural tremor in ET.
This frequency-shift is suggestive of distinct tremor generation mechanisms.
ET amplitudes diminish with repeated spiral drawing, suggesting a learning effect.
Tablet-PC tasks of writing and pointing are valid methods capturing tremor.
Acknowledgments
Funding Sources:
The study was supported by university funds of the Department of Neurology, Medical University of Vienna
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s Roles
| Nicole Schuhmayer | 1 A, B, C; 2 A, B; 3 A |
| Corinna Weber | 1 C; 2 C; 3 B |
| Markus Kieler | 1 B, C; 2 C; 3 B |
| Bernhard Voller | 1 C; 2 C; 3 B |
| Walter Pirker | 1 C; 2 C; 3 B |
| Eduard Auff | 1 C; 2 C; 3 B |
| Dietrich Haubenberger | 1 A, B, C; 2 A, B, C; 3 B |
Conflict of Interest concerning the research related to the manuscript:
There is no conflict of interest in any of the authors.
| Walter Pirker | Advisory Boards: AbbVie, Boehringer Ingelheim, Grünenthal; Honoraria: Boehringer Ingelheim, AOP Orphan, AbbVie, Actavis, Tewa-Ratiopharm; Travel Grants: Boehringer Ingelheim, AbbVie; Personal compensation (for scientific workshops, lecturing): Austrian Neurological Association (ÖGN); Employment: City of Vienna |
All other authors have nothing to disclose.
References
- 1.Benito-Leon J, Bermejo-Pareja F, Morales JM, Vega S, Molina JA. Prevalence of essential tremor in three elderly populations of central Spain. Mov Disord. 2003;18(4):389–394. doi: 10.1002/mds.10376. [DOI] [PubMed] [Google Scholar]
- 2.Dogu O, Sevim S, Camdeviren H, Sasmaz T, Bugdayci R, Aral M, Kaleagasi H, Un S, Louis ED. Prevalence of essential tremor: door-to-door neurologic exams in Mersin Province. Turkey, Neurology. 2003;61(12):1804–1806. doi: 10.1212/01.wnl.0000099075.19951.8c. [DOI] [PubMed] [Google Scholar]
- 3.Wenning GK, Kiechl S, Seppi K, Muller J, Hogl B, Saletu M, Rungger G, Gasperi A, Willeit J, Poewe W. Prevalence of movement disorders in men and women aged 50–89 years (Bruneck Study cohort): a population-based study. The Lancet. Neurology. 2005;4(12):815–820. doi: 10.1016/S1474-4422(05)70226-X. [DOI] [PubMed] [Google Scholar]
- 4.Bain PG, Findley LJ, Thompson PD, Gresty MA, Rothwell JC, Harding AE, Marsden CD. A study of hereditary essential tremor. Brain. 1994;117(Pt 4):805–824. doi: 10.1093/brain/117.4.805. [DOI] [PubMed] [Google Scholar]
- 5.Louis ED. Re-thinking the biology of essential tremor: from models to morphology. Parkinsonism Relat Disord. 2014;20(Suppl 1):S88–93. doi: 10.1016/S1353-8020(13)70023-3. [DOI] [PubMed] [Google Scholar]
- 6.Helmich RC, Toni I, Deuschl G, Bloem BR. The pathophysiology of essential tremor and Parkinson's tremor. Curr Neurol Neurosci Rep. 2013;13(9):378. doi: 10.1007/s11910-013-0378-8. [DOI] [PubMed] [Google Scholar]
- 7.Elble RJ. Physiologic and essential tremor. Neurology. 1986;36(2):225–231. doi: 10.1212/wnl.36.2.225. [DOI] [PubMed] [Google Scholar]
- 8.Kenney C, Diamond A, Mejia N, Davidson A, Hunter C, Jankovic J. Distinguishing psychogenic and essential tremor. J Neurol Sci. 2007;263(1–2):94–99. doi: 10.1016/j.jns.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Koller WC, Biary NM. Volitional control of involuntary movements. Mov Disord. 1989;4(2):153–156. doi: 10.1002/mds.870040207. [DOI] [PubMed] [Google Scholar]
- 10.Louis ED. Clinical practice. Essential tremor. N Engl J Med. 2001;345(12):887–891. doi: 10.1056/NEJMcp010928. [DOI] [PubMed] [Google Scholar]
- 11.Haubenberger D, Abbruzzese G, Bain PG, Bajaj N, Benito-Leon J, Bhatia KP, Deuschl G, Forjaz MJ, Hallett M, Louis ED, Lyons KE, Mestre TA, Raethjen J, Stamelou M, Tan EK, Testa CM, Elble RJ. Transducer-based evaluation of tremor. Mov Disord. 2016;31(9):1327–1336. doi: 10.1002/mds.26671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bain PG, Mally J, Gresty M, Findley LJ. Assessing the impact of essential tremor on upper limb function. Journal of neurology. 1993;241(1):54–61. doi: 10.1007/BF00870673. [DOI] [PubMed] [Google Scholar]
- 13.Elble RJ, Sinha R, Higgins C. Quantification of tremor with a digitizing tablet. J Neurosci Methods. 1990;32(3):193–198. doi: 10.1016/0165-0270(90)90140-b. [DOI] [PubMed] [Google Scholar]
- 14.Haubenberger D, Kalowitz D, Nahab FB, Toro C, Ippolito D, Luckenbaugh DA, Wittevrongel L, Hallett M. Validation of digital spiral analysis as outcome parameter for clinical trials in essential tremor. Mov Disord. 2011;26(11):2073–2080. doi: 10.1002/mds.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pullman SL. Spiral analysis: a new technique for measuring tremor with a digitizing tablet. Mov Disord. 1998;3:85–89. doi: 10.1002/mds.870131315. [DOI] [PubMed] [Google Scholar]
- 16.Hallett M. Overview of human tremor physiology. Mov Disord. 1998;3:43–48. doi: 10.1002/mds.870131308. [DOI] [PubMed] [Google Scholar]
- 17.Elble RJ, Brilliant M, Leffler K, Higgins C. Quantification of essential tremor in writing and drawing. Mov Disord. 1996;11(1):70–78. doi: 10.1002/mds.870110113. [DOI] [PubMed] [Google Scholar]
- 18.Deuschl G, Bain P, Brin M. Consensus statement of the Movement Disorder Society on Tremor. Ad Hoc Scientific Committee. Mov Disord. 1998;3:2–23. doi: 10.1002/mds.870131303. [DOI] [PubMed] [Google Scholar]
- 19.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 20.Elble R, Lewitt PA, Lyons K, Ondo W, Pahwa R, Sethi K, Stover N, Tarsy D, Testa C, Tintner R, Zesiewicz TA. Reliability of The Essential Tremor Rating Assessment Scale (TETRAS) Mov Disord. 2012;27(Suppl. 1):S409. (Suppl. 1) [Google Scholar]
- 21.Raethjen J, Lauk M, Köster B, Fietzek U, Friege L, Timmer J, Lücking CH, Deuschl G. Tremor analysis in two normal cohorts. Clin Neurophysiol. 2004;115(9):2151–2156. doi: 10.1016/j.clinph.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 22.Lauk M, Timmer J, Lucking CH, Honerkamp J, Deuschl G. A software for recording and analysis of human tremor. Comput Methods Programs Biomed. 1999;60(1):65–77. doi: 10.1016/s0169-2607(99)00012-7. [DOI] [PubMed] [Google Scholar]
- 23.Calzetti S, Baratti M, Gresty M, Findley L. Frequency/amplitude characteristics of postural tremor of the hands in a population of patients with bilateral essential tremor: implications for the classification and mechanism of essential tremor. J Neurol Neurosurg Psychiatry. 1987;50(5):561–567. doi: 10.1136/jnnp.50.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elble RJ, Higgins C, Hughes L. Longitudinal study of essential tremor. Neurology. 1992;42(2):441–443. doi: 10.1212/wnl.42.2.441. [DOI] [PubMed] [Google Scholar]
- 25.Zakaria R, Lenz FA, Hua S, Avin BH, Liu CC, Mari Z. Thalamic physiology of intentional essential tremor is more like cerebellar tremor than postural essential tremor. Brain Res. 2013;1529:188–199. doi: 10.1016/j.brainres.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elble RJ, Pullman SL, Matsumoto JY, Raethjen J, Deuschl G, Tintner R, Group TR. Tremor amplitude is logarithmically related to 4- and 5-point tremor rating scales. Brain. 2006;129(Pt 10):2660–2666. doi: 10.1093/brain/awl190. [DOI] [PubMed] [Google Scholar]
- 27.Shill HA, De La Vega FJ, Samanta J, Stacy M. Motor learning in essential tremor. Mov Disord. 2009;24(6):926–928. doi: 10.1002/mds.22479. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.