Abstract
BACKGROUND
Fludarabine and clofarabine are purine nucleoside analogues with established clinical activity in patients with acute myeloid leukemia (AML).
METHODS
Herein, the authors evaluated the efficacy and safety of idarubicin and cytarabine with either clofarabine (CIA) or fludarabine (FIA) in adults with newly diagnosed AML. Adults with newly diagnosed AML who were deemed suitable for intensive chemotherapy were randomized using a Bayesian adaptive design to receive CIA (106 patients) or FIA (76 patients). Patients received induction with idarubicin and cytarabine, plus either clofarabine or fludarabine. Responding patients could receive up to 6 cycles of consolidation therapy. Outcomes were compared with a historical cohort of patients who received idarubicin and cytarabine.
RESULTS
The complete remission/complete remission without platelet recovery rate was similar among patients in the CIA and FIA arms (80% and 82%, respectively). The median event-free survival was 13 months and 12 months, respectively (P = .91), and the median overall survival was 24 months and not reached, respectively (P = .23), in the 2 treatment arms. CIA was associated with more adverse events, particularly transaminase elevation, hyperbilirubinemia, and rash. Early mortality was similar in the 2 arms (60-day mortality rate of 4% for CIA vs 1% for FIA; P = .32). In an exploratory analysis of patients aged <50 years, FIA was found to be associated with improved survival compared with idarubicin and cytarabine (2-year event-free survival rate: 58% vs 30% [P = .05] and 2-year overall survival rate: 72% vs 36% [P = .009]).
CONCLUSIONS
CIA and FIA have similar efficacy in younger patients with newly diagnosed AML, although FIA is associated with a better toxicity profile.
Keywords: acute myeloid leukemia, clofarabine, fludarabine, induction, nucleoside analog
INTRODUCTION
The combination of cytarabine and an anthracycline has been the standard induction regimen for patients with acute myeloid leukemia (AML) for >40 years.1 Several studies have evaluated the addition of a third agent to standard induction chemotherapy.2 Although many efforts to improve AML therapy have been unsuccessful, the addition of nucleoside analogues to cytarabine-anthracycline regimens has shown promising results.3,4 The addition of deoxyadenosine analogues such as cladribine, clofarabine, and fludarabine to cytarabine increases intracellular levels of cytarabine triphosphate (Ara-CTP), which is the active antileukemic metabolite of cytarabine.5–10 This mechanism provides a rationale for the combination of nucleoside analogues with standard AML chemotherapy.
In a randomized study of cytarabine with or without clofarabine in older patients with recurrent/refractory AML, the combination regimen was found to be associated with significantly higher overall response rates and longer event-free survival (EFS), but no difference in overall survival (OS) was noted, perhaps because of the excess toxicities from clofarabine used at a dose of 40 mg/m2 daily for 5 days.11 Several reports also have suggested that triplet induction therapy, with a nucleoside analog in combination with an anthracycline and cytarabine, improves outcomes in patients with AML. In 2 sequential studies from Poland, the addition of cladribine to standard AML induction therapy was found to be associated with higher complete remission (CR) rates and longer OS.3,12 In the AML15 trial, the intensive fludarabine-containing regimen of fludarabine, high-dose cytarabine, granulocyte colony-stimulating factor, and idarubicin (FLAG-IDA) resulted in improved outcomes in younger patients with newly diagnosed AML.4 Frontline therapy in older patients with AML comparing clofarabine with or without cytarabine also had demonstrated that the addition of cytarabine can improve outcomes.13
The relative benefit of different nucleoside analogues in the treatment of patients with AML is not well established. We designed a randomized phase 2 trial to evaluate the efficacy and safety of clofarabine or fludarabine in combination with anthracycline-based and cytarabine-based induction chemotherapy for patients with newly diagnosed AML.
MATERIALS AND METHODS
Patients
Adults with newly diagnosed AML (excluding acute promyelocytic leukemia) or higher-risk myelodysplastic syndromes who were deemed suitable for intensive chemotherapy14 were eligible for this randomized phase 2 study. Patients were required to have a Eastern Cooperative Oncology Group performance status of 0 to 3 and adequate cardiac, renal, and hepatic function, including a left ventricular ejection fraction ≥40%, creatinine ≤3 mg/dL, total bilirubin ≤2.5 mg/dL, alanine transaminase ≤3 times the institutional upper limit of normal, and aspartate transaminase ≤5 times the institutional upper limit of normal. The current study was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center and was registered at ClinicalTrials.gov (Clinical-Trials.gov identifier NCT01289457). All patients provided informed consent according to institutional guidelines and the Declaration of Helsinki.
Treatment
Patients were randomized to receive idarubicin and cytarabine induction chemotherapy in combination with either clofarabine (CIA regimen) or fludarabine (FIA regimen). All patients received idarubicin at a dose of 10 mg/m2 intravenously (iv) daily on days 1 to 3 and cytarabine at a dose of 1 g/m2 iv administered over 2 hours daily on days 1 to 5. Clofarabine and fludarabine were given at doses of 15 mg/m2 and 30 mg/m2, respectively, iv daily on days 1 to 5. Fludarabine and clofarabine were given 4 hours before cytarabine to optimize Ara-CTP formation.6,7,9,15 The clofarabine dosing of the CIA regimen was determined by a preceding phase 1 study of CIA in patients with recurrent/refractory AML (reported separately). Patients with, fms-related tyrosine kinase 3 internal tandem duplications (FLT3-ITD) mutations could receive concomitant sorafenib at a dose of 400 mg orally twice daily continuously at the discretion of the treating physician.16
Patients not achieving CR or CR without platelet recovery (CRp) after 1 course of therapy could receive a second induction course if the treating physician determined this to be in the patient’s best interests. Patients who achieved CR or CRp could continue with up to 6 consolidation courses, as is the standard consolidation approach used at the study institution for idarubicin and cytarabine (IA)-based regimens. For consolidation therapy, patients received idarubicin at a dose of 8 mg/m2 iv daily on days 1 to 2 and cytarabine at a dose of 1 g/m2 iv over 2 hours daily on days 1 to 3. Clofarabine and fludarabine were given at doses of 15 mg/m2 and 30 mg/m2, respectively, iv daily on days 1 to 3. Consolidation cycles were repeated every 4 to 6 weeks depending on the recovery of neutrophil and platelet counts and toxicity. Allogeneic stem cell transplantation (SCT) was performed based on availability of a suitable donor and at the discretion of the treating physician.
Response Definitions
CR, CRp, CR with inadequate count recovery, and partial remission were defined according to International Working Group guidelines for AML.17 EFS was calculated from the time of treatment initiation until treatment failure (defined as lack of response to induction therapy or disease recurrence) or death. OS was calculated from the time of treatment initiation until death. Neither OS nor EFS were censored for allogeneic SCT in the primary analysis.
Minimal Residual Disease
Minimal residual disease (MRD) was assessed by multiparameter flow cytometry performed on the bone marrow at the time of CR or CRp as previously described.18 MRD positivity was defined as a cluster of ≥20 cells demonstrating altered expression of ≥2 antigens. The sensitivity of this MRD assay was 0.01%.
Gene Sequencing
Mutation analysis was performed using a 28-gene panel as previously described.19–21 Genomic DNA was extracted from bone marrow aspirates or peripheral blood. Amplicon-based next-generation sequencing targeting the entire coding regions of a panel of 28 genes associated with myeloid neoplasms was performed using a MiSeq platform (Illumina, San Diego, California). The genes analyzed included ABL1; additional sex combs-like 1, transcriptional regulator (ASXL1); BRAF; DNA methyl-transferase 3 alpha (DNMT3A); epidermal growth factor receptor (EGFR); EZH2; FLT3; GATA1; GATA2; HRAS; isocitrate dehydrogenase 1 (IDH1); IDH2; IKZF2; JAK2; KIT; KRAS; MDM2; MLL; MPL; MYD88; NOTCH1; nucleophosmin/nucleoplasmin family, member 1 (NPM1); NRAS; PTPN11; runt-related transcription factor 1 (RUNX1); TET2; tumor protein P53 (TP53); and WT1. For clinical reporting, a minimum sequencing coverage of ×250 (bidirectional true paired-end sequencing) was required. The analytical sensitivity was established at 5% mutant reads in a background of wild-type reads.
Statistical Analysis
The primary objective of the current phase 2, randomized study was to compare the EFS rates of the CIA and FIA regimens. An adaptive randomization algorithm was used to favor the treatment arm with the better EFS. Initially, 40 patients were randomized equally to the 2 treatment arms. After the completion of the equal randomization, the adaptive randomization algorithm was used to unbalance the randomization probabilities in favor of the better performing treatment arm. A sample size of 200 patients was planned, which could provide 93% power to detect an EFS hazard ratio of 0.625 between the 2 arms at a 1-sided significance level of .1. However, this trial was stopped early after the enrollment of 182 patients when it was determined that the difference between the 2 treatment arms was small.
Secondary objectives compared the CR/CRp rates, MRD negativity rates, OS, and safety profile of the 2 regimens. Differences among variables were evaluated using the chi-square test and Mann-Whitney U test, respectively, for categorical and continuous variables. EFS and OS were calculated with Kaplan-Meier estimates, and survival estimates were compared with the log-rank test. The data cutoff for this analysis was August 1, 2016. The data analyses were performed using SAS statistical software (SAS Institute Inc, Cary, NC).
Outcomes of the CIA and FIA regimens also were compared with our prior regimen of IA. IA induction consisted of idarubicin at a dose of 12 mg/m2 iv on days 1 to 3 and cytarabine at a dose of 1.5 g/m2 iv on days 1 to 4.16,22 Responding patients could then receive allogeneic SCT or consolidation chemotherapy for up to 6 cycles. The consolidation regimen consisted of idarubicin at a dose of 8 mg/m2 iv on days 1 to 2 and cytarabine at a dose of 0.75 g/m2 iv on days 1 to 3. Sorafenib for FLT3-ITD mutations was not allowed as per the IA protocol.
RESULTS
Patient Characteristics
Between August 2011 and June 2016, a total of 182 patients were enrolled (106 in the CIA arm and 76 in the FIA arm) (see Supporting Information Fig. 1). The imbalance of the arms was due to the better performance of CIA during the initial period of the trial. Baseline patient characteristics are summarized in Table 1. Treatment arms were well balanced after randomization, although patients randomized to the CIA arm were slightly older than those in the FIA arm (median age of 53 years vs 49 years, respectively); 9 patients (8%) in the CIA arm and 3 patients (4%) in the FIA arm were aged ≥60 years. Approximately 27% and 25%, respectively, of patients enrolled in the CIA and FIA arms had poor-risk cytogenetics (ie, −5 karyotype, −7 karyotype, or complex karyotype). Two patients in each arm had core-binding factor leukemia. Approximately 20% of patients harbored an FLT3-ITD mutation; of those patients with identified FLT3-ITD mutations, 12 (55%) in the CIA arm and 8 (53%) in the FIA arm received sorafenib. Nearly 60% of patients in each arm were classified as intermediate-2 or adverse risk by European LeukemiaNet (ELN) criteria.23
TABLE 1.
Baseline Patient Characteristics
| Characteristica | CIA N = 106 |
FIA N = 76 |
|---|---|---|
| Age (range), y | 53 (20–66) | 49 (18–66) |
| WBC (range), 109/L | 3.7 (0.6–103.0) | 4.9 (0.5–59.4) |
| Hemoglobin (range), g/dL | 9.5 (7.3–13.1) | 9.1 (7.5–13.1) |
| Platelet count (range), 109/L | 37 (1–1069) | 41 (5–399) |
| BM blasts (range), % | 52 (1–96) | 54 (11–96) |
| Diagnosis, no. (%) | ||
| AML | 103 (97) | 73 (96) |
| High-risk MDS | 3 (3) | 3 (4) |
| Cytogenetics, no. (%) | ||
| Diploid | 48 (45) | 34 (45) |
| −5, −7 and/or complex | 29 (27) | 19 (25) |
| Others | 29 (27) | 23 (30) |
| s-AML/t-AML, no. (%) | 13 (12) | 12 (16) |
| FLT3-ITD mutation, no. (%) | 22/103 (21) | 15/76 (20) |
| ELN risk | ||
| Favorable/intermediate-1 | 43/101 (43) | 29/69 (42) |
| Intermediate-2/adverse | 58/101 (57) | 40/69 (58) |
Abbreviations: AML, acute myeloid leukemia; BM, bone marrow; CIA, clofarabine, idarubicin, and cytarabine; ELN, European LeukemiaNet; FIA, fludarabine, idarubicin, and cytarabine; FLT3, fms-related tyrosine kinase 3; ITD, internal tandem duplications; MDS, myelodysplastic syndrome; s-AML/t-AML, secondary acute myeloid leukemia or therapy-related acute myeloid leukemia; WBC, white blood cell.
Continuous variables are shown as the median (range) and categorical variables are shown as the number (%).
Response Rates
Response rates are shown in Table 2. The composite rate of CR and CRp was similar between the 2 treatment arms (80% for CIA vs 82% for FIA; P = .84). CR was achieved in 72% of patients in the CIA arm and in 74% of patients in the FIA arm. Eight patients in the CIA arm (8%) and 5 patients in the FIA arm (7%) received 2 courses of induction. Of those patients who received 2 induction courses, 6 in the CIA arm and 3 in the FIA arm achieved CR or CRp. MRD negativity rates at remission were found to be higher in the CIA treatment arm (80% vs 65%; P = .07).
TABLE 2.
Response Rates
| Best Response | CIA, No. (%) N = 106 |
FIA, No. (%) N = 76 |
P |
|---|---|---|---|
| CR | 76 (72) | 56 (74) | |
| CRp | 9 (8) | 6 (8) | |
| CR plus CRp | 85 (80) | 60 (82) | .84 |
| CRi | 2 (2) | 0 | |
| PR | 1 (1) | 1 (1) | |
| No response | 15 (14) | 12 (16) | |
| Early death | 3 (3) | 1 (1) | |
| MRD negativity at CR/CRp | 56/70 (80) | 36/55 (65) | .07 |
Abbreviations: CIA, clofarabine, idarubicin, and cytarabine; CR, complete remission; CRi, complete remission with inadequate count recovery; CRp, complete remission without platelet recovery; FIA, fludarabine, idarubicin, and cytarabine; MRD, minimal residual disease; PR, partial remission.
Comprehensive mutation profiling was performed in 84 patients (44 patients in the CIA arm and 40 patients in the FIA arm). Supporting Information Figure 2 shows the relationship between baseline cytogenetics and mutation status and response rates according to treatment arm. The most common mutations identified were NPM1 (29%), FLT3-ITD (27%), RAS (26%), and DNMT3A (20%). Among these 84 patients, a poor-risk mutation profile (ie, defined as a RUNX1, ASXL1, or TP53 mutation or wild-type NPM1 with an FLT3-ITD mutation with an allelic ratio >0.524) was present in 6 patients in the CIA arm (14%) and in 5 patients in the FIA arm (13%). In an exploratory analysis among patients with poor-risk mutations, the CR/CRp rate was 33% and 100%, respectively, for CIA and FIA. Using the entire cohort for which the baseline karyotype was available, the CR/CRp rate for CIA and FIA was 52% and 70%, respectively, for patients with poor-risk cytogenetics.
Postremission Therapies
The median number of cycles received in the CIA and FIA arms was 3 (range, 1–8 cycles) and 2 (range, 1–7 cycles), respectively (P = .57). A total of 76 patients in the CIA arm (72%) and 52 patients in the FIA arm (68%) received at least 1 cycle of consolidation therapy. A total of 37 patients in the CIA arm (35%) and 28 patients in the FIA arm (37%) underwent allogeneic SCT in first remission.
Survival Outcomes
The median duration of follow-up for the surviving patients was 27 months (range, 1–58 months). The median EFS for patients who received CIA and FIA was 13 months and 12 months, respectively; the 2-year EFS rate was 44% in both arms (P = .91) (Fig. 1A). The median OS for patients who received CIA and FIA was 24 months and not reached, respectively, and the 2-year OS rates were 51% and 57%, respectively (P = .23) (Fig. 1B). In a sensitivity analysis in which patients were censored at the time of allogeneic SCT, the median OS was 18 months and not reached, respectively, and the 2-year OS rates were 43% and 53%, respectively, in the CIA and FIA arms (P = .17). No differences in EFS or OS were observed according to baseline factors, including cytogenetics, mutations, or ELN risk group.
Figure 1.
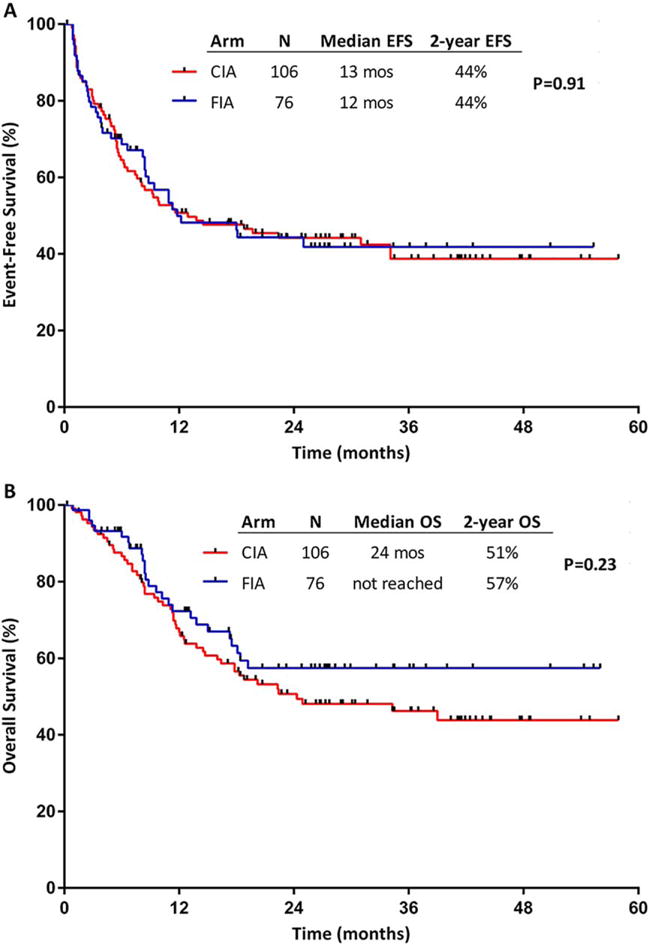
(A) Event-free survival (EFS) and (B) overall survival (OS) by treatment arm. CIA indicates idarubicin and cytarabine with clofarabine; FIA, idarubicin and cytarabine with fludarabine.
Safety and Early Mortality
Table 3 summarizes the nonhematologic adverse events observed in at least 5% of patients, regardless of causality. Treatment with CIA generally was associated with more adverse events compared with FIA, including a higher rate of transaminase elevation (29% vs 4% for all grades; 13% vs 4% for grades ≥3), hyperbilirubinemia (26% vs 9% for all grades; 4% vs 3% for grades ≥3), and rash (29% vs 12% for all grades; 4% vs 3% for grades ≥3). The early mortality rates were similar in both treatment arms. One patient in each arm died within the first 30 days of treatment. Four patients in the CIA arm (4%) and 1 patient in the FIA arm (1%) died within the first 60 days of treatment (P = .32).
TABLE 3.
Nonhematologic Adverse Events (≥5%)a
| CIA N = 106 |
FIA N = 76 |
|||
|---|---|---|---|---|
| Nonhematologic Adverse Event | All Grades No. (%) |
Grade ≥3 No. (%) |
All Grades No. (%) |
Grade ≥3 No. (%) |
| Anorexia | 14 (13) | 3 (3) | 4 (5) | 1 (1) |
| Diarrhea | 18 (17) | 1 (1) | 9 (12) | 0 |
| Elevated ALT/AST | 31 (29) | 14 (13) | 3 (4) | 3 (4) |
| Elevated bilirubin | 28 (26) | 4 (4) | 7 (9) | 2 (3) |
| Fatigue | 17 (16) | 0 | 5 (7) | 1 (1) |
| Febrile neutropenia | 74 (70) | 74 (70) | 49 (64) | 49 (64) |
| Hemorrhage | 8 (8) | 3 (3) | 10 (13) | 3 (4) |
| Infection | 33 (31) | 32 (30) | 24 (32) | 22 (29) |
| Mucositis/stomatitis | 13 (12) | 1 (1) | 5 (7) | 2 (3) |
| Nausea/vomiting | 26 (25) | 2 (2) | 14 (18) | 2 (3) |
| Pain | 30 (28) | 10 (9) | 12 (16) | 4 (5) |
| Rash | 31 (29) | 4 (4) | 9 (12) | 2 (3) |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; CIA, clofarabine, idarubicin, and cytarabine; FIA, fludarabine, idarubicin, and cytarabine.
Adverse events were graded according to European LeukemiaNet (ELN) criteria.
Historical Comparison With an IA Regimen
We performed an exploratory analysis to compare the outcomes of patients treated with either CIA or FIA with a historical cohort treated with IA. A total of 92 patients aged <60 years with newly diagnosed AML were treated with IA at the study institution between December 2006 and October 2011. To better balance the CIA/FIA and IA cohorts, the 20 patients with FLT3-ITD mutations who received sorafenib in the CIA/FIA study were excluded from the historical comparison. The baseline characteristics of the combined CIA/FIA cohort (162 patients) and the IA cohort (92 patients) are shown in Supporting Information Table 1. The 2 cohorts were similar with regard to all pretreatment characteristics analyzed, including age, cytogenetics, and ELN risk.
Response rates were found to be similar for patients who received CIA/FIA and those who received IA (CR/CRp rates of 81% vs 79%, respectively; P = .81). A similar percentage of patients in each group underwent allogeneic SCT in first remission (36% vs 34%, respectively; P = .69). The median EFS for patients who received CIA/FIA and IA was 12 months and 9 months, respectively; the 2-year EFS rates were 44% and 35%, respectively (P = .34). The median OS was 25 months and 17 months, and the 2-year OS rates were 51% and 41%, respectively (P = .26). The EFS and OS of the 3 regimens are shown in Figure 2.
Figure 2.
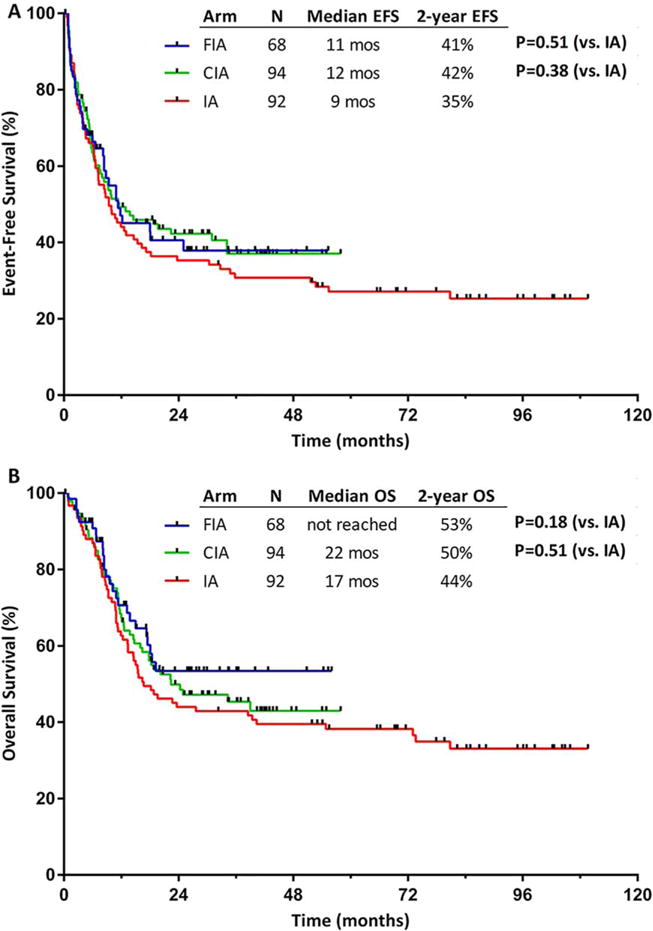
(A) Event-free survival (EFS) and (B) overall survival (OS) for patients treated with idarubicin and cytarabine with clofarabine (CIA), idarubicin and cytarabine with fludarabine (FIA), and an idarubicin and cytarabine (IA) historical cohort.
In an exploratory analysis of patients aged <50 years, a differential impact on survival according to treatment regimen was observed (Figs. 3A and 3B). The median EFS for patients aged <50 years who received FIA (36 patients), CIA (28 patients), and IA (34 patients) was not reached, 10 months, and 9 months, respectively, and the 2-year EFS rate was 58%, 33%, and 30%, respectively (P = .05 for FIA vs IA; and P = .79 for CIA vs IA) (Fig. 3A). The median OS for these younger patients was not reached, 22 months, and 15 months, respectively, and the 2-year OS rate was 72%, 46%, and 36%, respectively (P = .009 for FIA vs IA; and P = .23 for CIA vs IA) (Fig. 3B). The rates of allogeneic SCT for the FIA, CIA, and IA groups were similar (39%, 36%, and 38%, respectively; P = .96).
Figure 3.
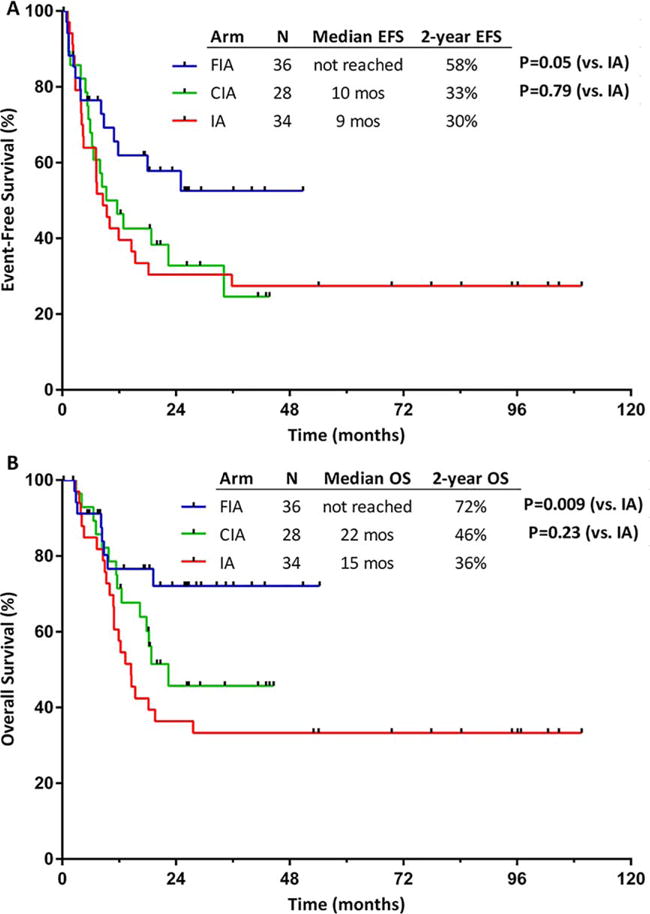
(A) Event-free survival (EFS) and (B) overall survival (OS) among patients aged <50 years treated with idarubicin and cytarabine with clofarabine (CIA), idarubicin and cytarabine with fludarabine (FIA), and an idarubicin and cytarabine (IA) historical cohort.
DISCUSSION
The current randomized phase 2 study was designed to evaluate the efficacy and safety of anthracycline and cytarabine induction-consolidation therapy in combination with clofarabine or fludarabine, both of which have been shown to synergize with cytarabine to potentiate its anti-leukemic activity.10 The 2 nucleoside analog-containing regimens, CIA and FIA, resulted in similar response rates and EFS, although FIA was found to have a better toxicity profile and a trend toward improved OS, an effect that persisted when patients were censored at the time of allogeneic SCT. When compared with a historical cohort of patients who received IA, FIA resulted in improved outcomes for patients aged <50 years, with 2-year EFS and OS rates of 58% and 72%, respectively. These results suggest that the incorporation of fludarabine into AML regimens may improve outcomes in younger patients.
With standard anthracycline and cytarabine induction chemotherapy for patients aged ≤60 years, cure can be achieved in approximately 35% to 40% of patients.25 Intensification of induction chemotherapy using higher doses of anthracyclines or cytarabine improved survival in younger patients and those without high-risk karyotypes.26,27 The addition of nucleoside analogues also has been associated with superior outcomes in some studies.3,4,28 In one large randomized study, the addition of clofarabine to induction chemotherapy in younger patients with AML reduced the probability of disease recurrence but was associated with improved survival only in patients with intermediate-risk disease.29 In the current study, the superior OS observed with FIA compared with our historical IA cohort suggests that the addition of a nucleoside analog may improve outcomes in patients aged <50 years.
The findings of the current study differ in some ways from the results of the prospective Polish Adult Leukemia Group study, which found that the addition of fludarabine to standard induction “3 + 7” chemotherapy (no high-dose cytarabine induction) did not improve survival in younger patients with AML.3 It is interesting to note that the Polish study incorporated fludarabine only in the induction course and combined it with relatively lower doses of cytarabine (200 mg/m2 iv continuous infusion for 7 days), both of which could account for the differences in the results between these 2 studies. It also is unclear whether this study sequenced the fludarabine before cytarabine, which is the optimal method of delivery to maximize Ara-CTP production.6,7,9,15
Although the CIA and FIA regimens use higher doses of cytarabine with the induction course compared with what is used in standard “3 + 7” induction, the cytarabine doses in the CIA/FIA consolidation courses are relatively lower (3 g/m2 for 6 cycles in the CIA/FIA regimens vs 18 g/m2 for 4 cycles with high-dose cytarabine consolidation). The importance of higher doses of cytarabine in consolidation therapy recently has been illustrated in the SWOG S1203 trial, in which no differences in outcomes were observed between the 7 + 3 course and IA, possibly due to the lower doses of cytarabine used with IA consolidation courses (cytarabine at a dose of 2.25 g/m2 per consolidation vs 18 g/m2 per consolidation).30 Because one of the primary rationales for the incorporation of a nucleoside analog into AML regimens is through potentiation of cytarabine activity, it is possible that incremental improvements in outcomes would be obtained with intensification of CIA and FIA using higher doses of cytarabine during consolidation. For example, in the AML15 trial, the FLAG-IDA regimen combined fludarabine and higher doses of cytarabine during induction than were used in the current study (2 g/m2 for 5 days); this was followed by intermediate-dose (1 g/m2) or high-dose (3 g/m2) cytarabine consolidation for a total cumulative cytarabine dose of up to 56 g/m2 (vs 23 g/m2 with CIA/FIA).4 Among patients who were able to receive 4 courses of chemotherapy (ie, 2 courses of FLAG-IDA and 2 courses of cytarabine consolidation), outcomes were excellent, with an 8-year OS rate of 95% in patients with favorable-risk AML and 63% in those with intermediate-risk AML. Younger patients are more likely to experience the benefit of such intensified regimens, and future studies of the more intense cytarabine AML regimens should be considered in this younger, fitter population to reduce interpatient heterogeneity that may obscure signals of clinical benefit.
The results of the current study found no difference in the primary outcome of EFS for the entire study cohort, although younger patients (ie, those aged <50 years) who received FIA were found to have superior survival in an exploratory analysis. Higher rates of treatment-related toxicity were observed in the clofarabine-containing arm, particularly transaminase elevation, hyperbilirubinemia, and rash, all of which are established adverse events associated with clofarabine.31 In light of the better toxicity profile of FIA as well as the suggestion of the superior efficacy of this regimen in patients aged <50 years, the findings of the current study argue for the incorporation of fludarabine (rather than clofarabine) in future nucleoside analog-containing investigational AML regimens.
In younger patients with newly diagnosed AML, the use of CIA and FIA resulted in similar response rates and survival, with a CR/CRp rate of approximately 80% and a median EFS of approximately 1 year. Use of FIA was associated with a better toxicity profile compared with CIA, and in patients aged <50 years, FIA was found to be associated with improved OS compared with a historical cohort of patients treated with IA alone. Intensification of cytarabine in the FIA regimen may further improve outcomes in these younger patients and should be evaluated in future randomized trials.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
Supported by The University of Texas MD Anderson Cancer Center Support Grant CA016672.
Footnotes
Additional supporting information may be found in the online version of this article.
CONFLICT OF INTEREST DISCLOSURES
The authors made no disclosures.
AUTHOR CONTRIBUTIONS
Elias Jabbour and Hagop Kantarjian designed the study, treated patients, and wrote the article. Nicholas J. Short collected and analyzed the data and wrote the article. Farhad Ravandi, Guillermo Garcia-Manero, Naveen Pemmaraju, Naval G. Daver, Gautam Borthakur, Nitin Jain, Marina Konopleva, Zeev Estrov, Tapan M. Kadia, William G. Wierda, Courtney D. DiNardo, Susan M. O’Brien, and Jorge E. Cortes treated patients. William Plunkett and Varsha Gandhi provided laboratory rationale for the combination. Xuelin Huang, Lianchun Xiao, and Koji Sasaki provided statistical analysis. Mark Brandt collected and analyzed the data. All authors reviewed and approved the article.
References
- 1.Yates J, Glidewell O, Wiernik P, et al. Cytosine arabinoside with daunorubicin or adriamycin for therapy of acute myelocytic leukemia: a CALGB study. Blood. 1982;60:454–462. [PubMed] [Google Scholar]
- 2.Dombret H, Gardin C. An update of current treatments for adult acute myeloid leukemia. Blood. 2016;127:53–61. doi: 10.1182/blood-2015-08-604520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holowiecki J, Grosicki S, Giebel S, et al. Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: a multicenter, randomized phase III study. J Clin Oncol. 2012;30:2441–2448. doi: 10.1200/JCO.2011.37.1286. [DOI] [PubMed] [Google Scholar]
- 4.Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol. 2013;31:3360–3368. doi: 10.1200/JCO.2012.47.4874. [DOI] [PubMed] [Google Scholar]
- 5.Gandhi V, Plunkett W. Modulation of arabinosylnucleoside metabolism by arabinosylnucleotides in human leukemia cells. Cancer Res. 1988;48:329–334. [PubMed] [Google Scholar]
- 6.Seymour JF, Huang P, Plunkett W, Gandhi V. Influence of fludarabine on pharmacokinetics and pharmacodynamics of cytarabine: implications for a continuous infusion schedule. Clin Cancer Res. 1996;2:653–658. [PubMed] [Google Scholar]
- 7.Gandhi V, Estey E, Keating MJ, Plunkett W. Biochemical modulation of arabinosylcytosine for therapy of leukemias. Leuk Lymphoma. 1993;10(suppl):109–114. doi: 10.3109/10428199309149122. [DOI] [PubMed] [Google Scholar]
- 8.Gandhi V, Estey E, Keating MJ, Chucrallah A, Plunkett W. Chlorodeoxyadenosine and arabinosylcytosine in patients with acute myelogenous leukemia: pharmacokinetic, pharmacodynamic, and molecular interactions. Blood. 1996;87:256–264. [PubMed] [Google Scholar]
- 9.Gandhi V, Estey E, Keating MJ, Plunkett W. Fludarabine potentiates metabolism of cytarabine in patients with acute myelogenous leukemia during therapy. J Clin Oncol. 1993;11:116–124. doi: 10.1200/JCO.1993.11.1.116. [DOI] [PubMed] [Google Scholar]
- 10.Robak T. Purine nucleoside analogues in the treatment of myleoid leukemias. Leuk Lymphoma. 2003;44:391–409. doi: 10.1080/1042819021000035608. [DOI] [PubMed] [Google Scholar]
- 11.Faderl S, Wetzler M, Rizzieri D, et al. Clofarabine plus cytarabine compared with cytarabine alone in older patients with relapsed or refractory acute myelogenous leukemia: results from the CLASSIC I Trial. J Clin Oncol. 2012;30:2492–2499. doi: 10.1200/JCO.2011.37.9743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holowiecki J, Grosicki S, Robak T, et al. Polish Adult Leukemia Group (PALG) Addition of cladribine to daunorubicin and cytarabine increases complete remission rate after a single course of induction treatment in acute myeloid leukemia. Multicenter, phase III study. Leukemia. 2004;18:989–997. doi: 10.1038/sj.leu.2403336. [DOI] [PubMed] [Google Scholar]
- 13.Faderl S, Ravandi F, Huang X, et al. A randomized study of clofarabine versus clofarabine plus low-dose cytarabine as front-line therapy for patients aged 60 years and older with acute myeloid leukemia and high-risk myelodysplastic syndrome. Blood. 2008;112:1638–1645. doi: 10.1182/blood-2007-11-124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090–1098. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 15.Gandhi V, Huang P, Chapman AJ, Chen F, Plunkett W. Incorporation of fludarabine and 1-beta-D-arabinofuranosylcytosine 5′-triphosphates by DNA polymerase alpha: affinity, interaction, and consequences. Clin Cancer Res. 1997;3:1347–1355. [PubMed] [Google Scholar]
- 16.Ravandi F, Cortes JE, Jones D, et al. Phase I/II study of combination therapy with sorafenib, idarubicin, and cytarabine in younger patients with acute myeloid leukemia. J Clin Oncol. 2010;28:1856–1862. doi: 10.1200/JCO.2009.25.4888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheson BD, Bennett JM, Kopecky KJ, et al. International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 18.Ravandi F, Jorgensen JL, O’Brien SM, et al. Minimal residual disease assessed by multi-parameter flow cytometry is highly prognostic in adult patients with acute lymphoblastic leukaemia. Br J Haematol. 2016;172:392–400. doi: 10.1111/bjh.13834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel KP, Ravandi F, Ma D, et al. Acute myeloid leukemia with IDH1 or IDH2 mutation: frequency and clinicopathologic features. Am J Clin Pathol. 2011;135:35–45. doi: 10.1309/AJCPD7NR2RMNQDVF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh RR, Bains A, Patel KP, et al. Detection of high-frequency and novel DNMT3A mutations in acute myeloid leukemia by high-resolution melting curve analysis. J Mol Diagn. 2012;14:336–345. doi: 10.1016/j.jmoldx.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luthra R, Patel KP, Reddy NG, et al. Next-generation sequencing-based multigene mutational screening for acute myeloid leukemia using MiSeq: applicability for diagnostics and disease monitoring. Haematologica. 2014;99:465–473. doi: 10.3324/haematol.2013.093765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-Manero G, Tambaro FP, Bekele NB, et al. Phase II trial of vorinostat with idarubicin and cytarabine for patients with newly diagnosed acute myelogenous leukemia or myelodysplastic syndrome. J Clin Oncol. 2012;30:2204–2210. doi: 10.1200/JCO.2011.38.3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dohner H, Estey EH, Amadori S, et al. European LeukemiaNet Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 24.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373:1136–1152. doi: 10.1056/NEJMra1406184. [DOI] [PubMed] [Google Scholar]
- 26.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009;361:1249–1259. doi: 10.1056/NEJMoa0904544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willemze R, Suciu S, Meloni G, et al. High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: results of the EORTC-GIMEMA AML-12 trial. J Clin Oncol. 2014;32:219–228. doi: 10.1200/JCO.2013.51.8571. [DOI] [PubMed] [Google Scholar]
- 28.Nazha A, Kantarjian H, Ravandi F, et al. Clofarabine, idarubicin, and cytarabine (CIA) as frontline therapy for patients ≤60 years with newly diagnosed acute myeloid leukemia. Am J Hematol. 2013;88:961–966. doi: 10.1002/ajh.23544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lowenberg B, Pabst T, Maertens J, et al. Dutch-Belgian Hemato-Oncology Cooperative Group (HOVON) and Swiss Group for Clinical Cancer Research (SAKK) Therapeutic value of clofarabine in younger and middle-aged (18–65 years) adults with newly diagnosed AML. Blood. 2017;129:1636–1645. doi: 10.1182/blood-2016-10-740613. [DOI] [PubMed] [Google Scholar]
- 30.Garcia-Manero G, Othus M, Pagel JM, et al. SWOG S1203: a randomized phase III study of standard cytarabine plus daunorubicin (7+3) therapy versus idarubicin with high dose cytarabine (IA) with or without vorinostat (IA+V) in younger patients with previously untreated acute myeloid leukemia (AML) Blood. 2016;128:901–901. [Google Scholar]
- 31.Ghanem H, Kantarjian H, Ohanian M, Jabbour E. The role of clofarabine in acute myeloid leukemia. Leuk Lymphoma. 2013;54:688–698. doi: 10.3109/10428194.2012.726722. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


