Aluminium (Al) toxicity is one of the most important limiting factors for crop yield in acidic soils. However, the mechanisms that confer Al tolerance remain largely unknown. Based on the global transcriptome analysis of the roots and leaves of two contrasting soybean genotypes, BX10 (Al-tolerant) and BD2 (Al-sensitive) under 0 µM and 50 µM Al3+ treatments, our findings suggest that BX10 can resist Al by secreting additional citrate into the rhizosphere from the roots to chelate Al and by avoiding a JA-mediated defense response that allows resource allocation to maintain leaf growth.
Keywords: Aluminium tolerance, citrate metabolism, jasmonic acid, soybean, transcriptome
Abstract
Aluminium (Al) toxicity is one of the most important limiting factors for crop yield in acidic soils. However, the mechanisms that confer Al tolerance still remain largely unknown. To understand the molecular mechanism that confers different tolerance to Al, we performed global transcriptome analysis to the roots and leaves of two contrasting soybean genotypes, BX10 (Al-tolerant) and BD2 (Al-sensitive) under 0 and 50 μM Al3+ treatments, respectively. Gene Ontology and Kyoto Encyclopedia of Genes and Genomes analyses revealed that the expression levels of the genes involved in lipid/carbohydrate metabolism and jasmonic acid (JA)-mediated signalling pathway were highly induced in the roots and leaves of both soybean genotypes. The gene encoding enzymes, including pyruvate kinase, phosphoenolpyruvate carboxylase, ATP-citrate lyase and glutamate-oxaloacetate transaminase 2, associated with organic acid metabolism were differentially expressed in the BX10 roots. In addition, the genes involved in citrate transport were differentially expressed. Among these genes, FRD3b was down-regulated only in BD2, whereas the other two multidrug and toxic compound extrusion genes were up-regulated in both soybean genotypes. These findings confirmed that BX10 roots secreted more citrate than BD2 to withstand Al stress. The gene encoding enzymes or regulators, such as lipoxygenase, 12-oxophytodienoate reductase, acyl-CoA oxidase and jasmonate ZIM-domain proteins, involved in JA biosynthesis and signalling were preferentially induced in BD2 leaves. This finding suggests that the JA defence response was activated, possibly weakening the growth of aerial parts because of excessive resource consumption and ATP biosynthesis deficiency. Our results suggest that the Al sensitivity in some soybean varieties could be attributed to the low level of citrate metabolism and exudation in the roots and the high level of JA-mediated defence response in the leaves.
Introduction
Aluminium (Al) is one of the major restricting factors for crop production in acidic soils (pH < 5.0) (Foy 1988). Over 50 % of global arable land is identified as acidic soils (Bot et al. 2000). In China, acidic soils constitute about 20 % of the total land area. Al toxicity leads to the inhibition of root growth and subsequently the poor uptake of water and minerals (Kochian 1995).
Different plant species or different genotypes within the same species have evolved special mechanisms to alleviate Al toxicity and survive in high Al environments. Exudation of organic acids, such as citrate, malate and oxalate from roots, is one of the most important mechanisms to chelate Al in rhizosphere (Ma 2000). Some related genes, including aluminium-activated malate transporter (ALMT1) (Delhaize et al. 2004; Sasaki et al. 2004) and multidrug and toxic compound extrusion (MATE) family members (Magalhaes et al. 2007), are responsible for excretion of malate and citrate, respectively. GmALMT1 encodes a malate transporter in soybean and is required for the adaptation to Al toxicity by regulating malate efflux (Liang et al. 2013).
In soybean, the Al-triggered organic acid secretion from roots is required for Al detoxification; differential exudation is observed in different soybean genotypes, that is, the tolerant cultivars generally secrete more organic acids than the sensitive cultivars (Silva et al. 2001; Dong et al. 2004; Liao et al. 2006). However, aerial proportions are inevitably involved in Al stress response because of the continuous translocation and accumulation of Al in the above-ground structures of plant species. Transcriptome analysis showed that rice leaves exhibit specific responses to abiotic stresses (Minh-Thu et al. 2013; Zhou et al. 2016).
Soybean is an important oil-bearing crop worldwide and is largely cultivated in acidic soils. As the first country to domesticate soybeans, China is rich in soybean germplasm resources with large variations of Al tolerance (Bo et al. 2007). However, Al toxicity restrains the growth of soybeans by inhibiting root elongation, reducing root activity and reducing leaf photosynthesis (Liu et al. 2004; Zhang et al. 2007). The mechanisms underlying different Al responses in soybean genotypes with contrasting Al tolerance are not yet fully understood at the molecular level. Furthermore, no studies have investigated the transcriptional response to Al stress in the leaves of soybeans with different Al tolerance. BX10 soybean, which originated from Brazil, is more resistant to Al than BD2 soybean, which originated from Guangdong, China (Xu 2003; Dong et al. 2004). These two soybean genotypes have been intensively studied because of their different Al-tolerance properties (Dong et al. 2004; Li et al. 2012; Yang et al. 2012). The former exhibits lower root growth inhibition than the latter under Al treatment (Dong et al. 2004; Zhen et al. 2009). Although BX10 secretes more citrate than BD2 after exposure to Al stress (Dong et al. 2004; Binbo et al. 2009), the molecular characterizations for organic acid metabolism and transport of these two soybeans should be clarified with further evidence. In this work, a genome-wide transcriptional analysis was performed to discover the differences in organic acid metabolism by coordinately using the roots and leaves as materials. This study aimed to elucidate the possible mechanisms conferring different Al tolerance in two soybean genotypes.
Methods
Plant growth and treatments
Two soybean genotypes (Glycine max), namely, an Al-tolerant cultivar BaXi10 (BX10) that originates from Brazil and an Al-sensitive cultivar BenDi2 (BD2) that comes from Guangdong Province of China, were employed in this study. The plump seeds were subjected to surface sterilization in 0.1 % mercuric chloride for 10 min and then rinsed five times with distilled water. The seeds were soaked in sterilized distilled water overnight and kept at 25 °C in the dark for germination. After 4 days, the seedlings were transplanted into 1/2 Hoagland nutrient solution in the chamber for 1 week under the following conditions: 26 °C/22 °C (day/night) for 16 h photoperiod (a photon flux density of 400 mmol m−2 s−1), 70 % relative humidity. The uniformly grown seedlings were then transferred into proper vessels containing 100 μM CaCl2 (pH 5.0) with either 0 (−Al) or 50 μM AlCl3 (+Al) solutions. Eight samples (four root samples and four leaf samples) were harvested at 48 h, and 0–1 cm root tips were collected, frozen in liquid nitrogen and kept at −80 °C for RNA extraction. The samples were pooled by genotype and treatment [e.g. BX10+Al (root), BX10−Al (root), BD2+Al (root), BD2−Al (root), BX10+Al (leaf), BX10−Al (leaf), BD2+Al (leaf) and BD2−Al (leaf)] to minimize the inter-individual differences.
Root length measurement
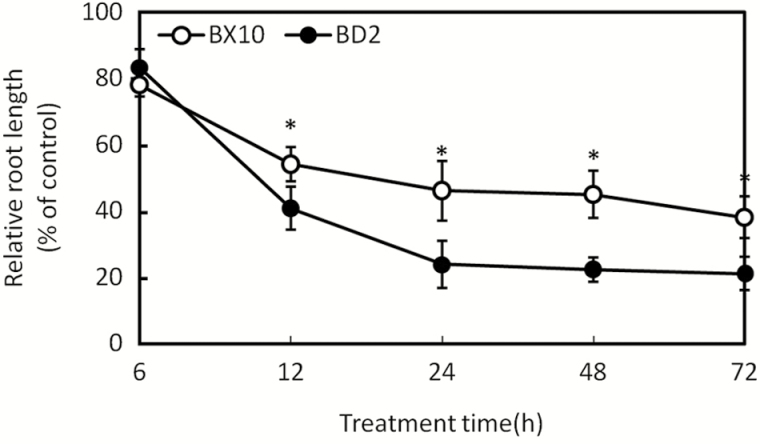
Root length was measured by inserting the root into a transparent plastic tube and observing its appearance until 72 h. Relative root length (RRL) is the mean of 100 × (net growth in a treatment solution)/(net growth in the control) (Ryan et al. 1995). Two-tailed t-test was performed to determine the significance between the RRL of two genotypes at the same treatment time.
RNA isolation, library construction and high-throughput sequencing
Total RNA was isolated by using Trizol reagent (Takara, Dalian, China). The mRNAs were purified by using Dynabeads Oligo(dT)25 mRNA isolation beads (Thermo Fisher Scientific Inc., USA). mRNA fragmentation was conducted by physical and chemical approaches, and the mRNA fragments of about 155 bp in length were collected. The obtained mRNAs were immediately reverse transcribed into first-strand cDNA and then used for second-strand cDNA synthesis. After the end reparation and 3′ A-tailing processes, the double-strand cDNAs were ligated to special adaptors. The products were purified using AMPureXP beads (NEB, USA), and each library was normalized by adjusting the cDNA concentration to 10 nM before subjecting to high-throughput sequencing on an Illumina HiSeq 2000 sequencer at Personalbio Co., Ltd (Shanghai, China).
Analysis of high-throughput sequencing data
The raw reads were filtered using FastQC package to discard contaminant sequences and low quality reads (phred quality score < 30 and read length < 50 bp). The obtained clean reads were mapped to reference genome (Glyma1.0) using Bowtie/Tophat program (http://tophat.cbcb.umd.edu/). BLASTp searches (E-value < 1e-5) were conducted for the following databases: Ensembl, JGI, Kyoto Encyclopedia of Genes and Genomes (KEGG) and eggNOG to find the corresponding transcripts in soybean genome and acquire the annotations of these transcripts. Expression abundance was normalized by using reads per kilo bases per million reads (RPKM).
Differential expression analysis, and Gene Ontology and KEGG enrichment analysis
DEGSeq was utilized to identify the differentially expressed genes (DEGs) in each pairwise comparison (Wang et al. 2010). False discovery rate (FDR) < 0.05, and log2 ratio of each gene expression > 1 or < −1 (+Al/−Al) were used as the threshold to identify the significance of the difference for each gene expression. Functional annotation was performed against Gene Ontology (GO) (http://geneontology.org/) database. KEGG pathway enrichment analysis was conducted against the KEGG database by using the differentially expressed transcripts with KEGG ORTHOLOGY (KO) accession numbers.
Real-time PCR validation
In brief, 1.0 μg of total RNA was reversely transcribed using GoScript™ Reverse Transcription System (Promega, USA). The first-stand cDNA was used as template for real-time PCR with SYBR Fast qPCR Mix (Takara, Dalian, China). GAPDH mRNA was used as an internal control to normalize each gene expression. PCR was conducted on ABI 7500 Real-Time PCR System. Data were acquired with SDS software v2.0 (Applied Biosystems, USA). The 2−ΔΔCt method was used to calculate the relative expression of the each gene (Livak and Schmittgen 2001). Two-tailed t-test was performed to compare the differences in the expression of paired samples. Three biological replicates were used, and the means were considered significantly different when P < 0.05.
Results
Morphological responses of two soybean genotypes under Al stress
Root elongation inhibition is a typical response to Al toxicity in plants (Foy 1988). We determined RRL of two soybean genotypes exposed to Al3+ and contained solution at different time intervals. Under stress Al treatments, the growth of BD2 roots was greatly inhibited at different time periods after Al stress [see Supporting Information—Fig. S1]. The two soybean genotypes have significantly different root inhibition rates at 48 h of Al treatment (Fig. 1). These results clearly indicate that BX10 exhibits higher Al tolerance than BD2 in terms of root elongation.
Figure 1.
Time course of RRL of BX10 and BD2 soybeans under 0 or 50 μM Al3+ treatments. Error bars denote mean ± SD (n = 15). Asterisk (*) indicates significant difference at P < 0.05.
Overview of high-throughput sequencing results
Eight libraries from the roots and leaves of BX10 and BD2 soybean genotypes after 48 h treatment were constructed for high-throughput sequencing. Each library contained more than 54 million high-quality 100-bp pair-ended reads. Over 93 % of trimmed reads corresponded to the soybean reference genome (Schmutz et al. 2010). Among which, > 97 % was uniquely mapped, and > 91 % was mapped to genes. These findings suggest the fine quality of the RNA-seq results. Genome annotation was initiated by blasting against a series of open database, which allowed the annotation of 54175 transcripts (Table 1).
Table 1.
Summary of RNA-seq reads mapped to soybean genome.
| Root | Leaf | |||||||
|---|---|---|---|---|---|---|---|---|
| BX10−Al | BX10+Al | BD2−Al | BD2+Al | BX10−Al | BX10+Al | BD2−Al | BD2+Al | |
| Raw reads | 76370154 | 88995142 | 86099746 | 64260978 | 54985492 | 70889914 | 108647888 | 119421598 |
| Trimed reads | 50689428 | 59117704 | 57933170 | 42823052 | 36814596 | 47292610 | 86951872 | 95824802 |
| Mapped reads | 47890719 | 55927457 | 53885648 | 40353198 | 35404671 | 45469878 | 83790325 | 92040829 |
| Unique mapped | 46820673 | 54492541 | 52643212 | 39457219 | 34286520 | 44131138 | 81489112 | 89494217 |
| Multiple mapped | 1070046 | 1434916 | 1242436 | 895979 | 1118151 | 1338740 | 2301213 | 2546612 |
| Mapped to gene | 46303509 | 53896105 | 51959290 | 38971445 | 33951147 | 43699034 | 80767625 | 88715040 |
General effects of Al stress on gene expression in the roots and leaves of soybeans
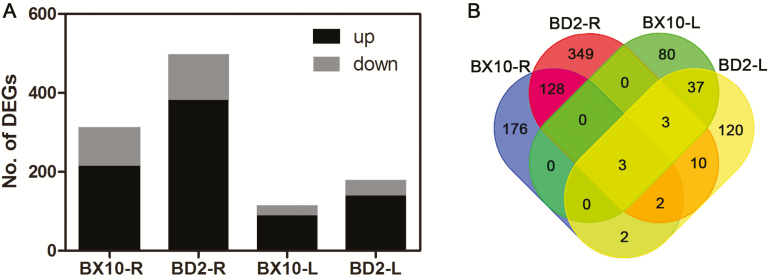
A total of 311 and 495 DEGs were screened in roots, whereas 122 and 176 DEGs were screened in the leaves of BX10 and BD2, respectively [see Supporting Information—Table S1]. Furthermore, the majority of DEGs was up-regulated under 48 h Al stress condition (Fig. 2A). A total of 133 transcripts showed common differential expression in the roots of BX10 and BD2, whereas 43 transcripts showed common differential expression in the leaves of BX10 and BD2 (Fig. 2B). Three transcripts revealed the Al response in the roots and leaves of these two soybean genotypes. Among these transcripts, two ALS3 homologs were up-regulated.
Figure 2.
Screening of DEGs in the roots and leaves of two soybean genotypes. Up-/down-regulated genes (A) and multiple intercomparison (B) were shown. BX10-R and BX10-L denote DEGs in the roots and leaves of BX10, whereas BD2-R and BD2-L represent DEGs in the roots and leaves of BD2, respectively.
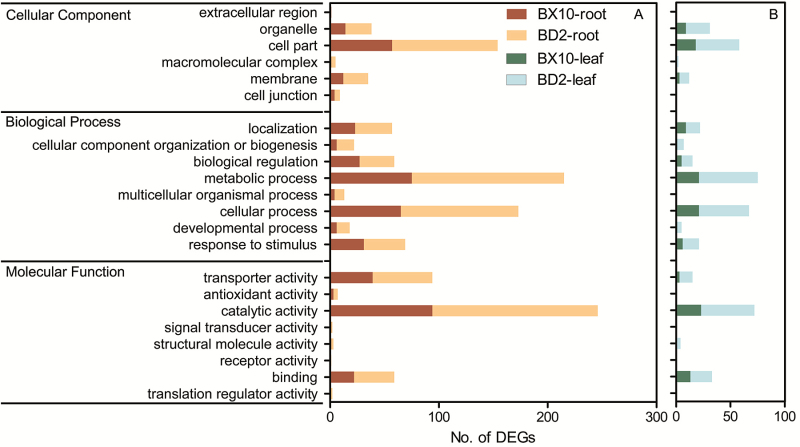
Gene Ontology enrichment analysis was used to explore the functional classifications of DEGs in the roots and leaves of these two soybean genotypes (Fig. 3). In the ‘Molecular function’ category, the genes involved in ‘Catalytic activity’, ‘Transporter activity’ and ‘Binding activity’ were over-represented. In the ‘Biological process’ category, the most represented group were ‘Metabolic process’, ‘Localization’ and ‘Cellular process’. However, the number of DEGs involved in metabolic process in the roots was larger than those in the leaves, confirming that the root is the major target of Al toxicity (Delhaize and Ryan 1995). With regard to the ‘Cellular component’ category, the ‘Cell part’, ‘Organelle’ and ‘Membrane’ were highly enriched.
Figure 3.
GO classification of DEGs in the roots (A) and leaves (B) of two soybean genotypes. ‘Cellular component’, ‘Biological process’ and ‘Molecular function’ categories were shown. Some categories are explained as follows: Binding, interacts selectively and non-covalently with substances, such as DNA, ATP, protein, etc.; Localization, positions a substance or cellular entity and maintains these in those locations; Cell part, cell component; Membrane, plasma or organelle membrane.
The over-represented (P value < 0.05) GO terms, including ‘Cell wall metabolism’ process, were enriched in both sets of DEGs from the two tissues of each soybean. However, some striking differences were observed between the over-represented GO terms of roots and leaves [see Supporting Information—Table S2]. For example, GO terms related to hydrogen peroxide catabolic process and cellular oxidant detoxification processes were preferentially enriched in the roots. By contrast, GO terms related to the regulation of jasmonic acid (JA)-mediated signalling pathway and cellular cation homeostasis were highly enriched in the leaves.
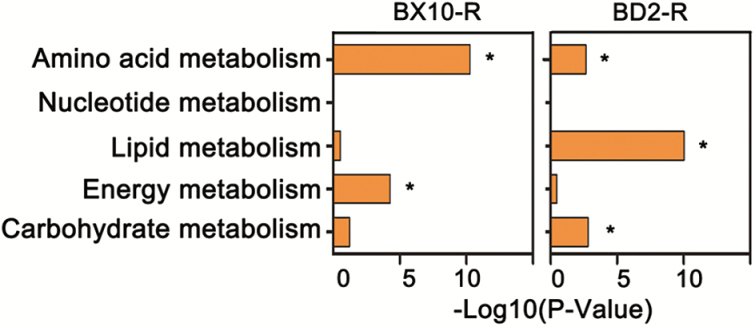
Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis revealed that genes involved in ‘Amino acid metabolism’ pathway were enriched in the roots of both soybean genotypes (Fig. 4). By contrast, the genes involved in ‘Carbohydrate metabolism’ and ‘Lipid metabolism’ pathways were prefereially enriched in the roots of BD2 (Fig. 4).
Figure 4.
KEGG enriched pathways of DEGs in the roots of BX10 and BD2. Asterisk (*) indicates significant difference at P < 0.05.
Effects of Al stress on the expression of genes involved in organic acid metabolism and exudation in roots
The exudation of organic acid from roots is required for Al tolerance in soybeans (Nian et al. 2007; Xu et al. 2010). The genes, including citrate synthase gene (CS) and malate dehydrogenase gene (MDH), directly involved in citrate cycle were not responsive to 48 h Al stress in the roots of these two soybean genotypes. This finding suggests that organic acid biosynthesis is not a rate-limiting step for the exudation of organic acids in the roots of both soybean genotypes, which has been observed in some other plant species, such as triticale (Hayes and Ma 2003), buckwheat (Zhu et al. 2015) and maize roots (Maron et al. 2008). However, the genes that encode pyruvate kinase (PK) and phosphoenolpyruvate carboxylase (PEPC) had up-regulated expression levels. The expression of gene encoding ATP-citrate lyase (ACL) was down-regulated only in BX10. In addition, the expression of gene encoding mitochondrial glutamate-oxaloacetate transaminase 2 (GOT2) was induced in the roots of BX10. However, these four genes showed no difference in BD2 (Table 2). These findings revealed that outside the mitochondrion, different types of organic acid metabolism occurred between the roots of BX10 and BD2.
Table 2.
Expression of genes associated with citrate metabolism and exudation in roots. aPK, pyruvate kinase; PEPC, phosphoenolpyruvate carboxylase; ACL, ATP-citrate lyase; FRD3b, ferric reductase defective3b; MATE, multidrug and toxic compound extrusion; MMC, mitochondrialoxoglutarate/malatecarrierprotein; GOT2, aspartate aminotransferase. bBX10R and BD2R denote the roots of BX10 and BD2, respectively. cFold change, means the ratio of Al treatment vs. control.
| Gene ID | Annotationa | BX10Rb | BX10R | Fold changec | BD2R | BD2R | Fold change |
|---|---|---|---|---|---|---|---|
| −Al | +Al | −Al | +Al | ||||
| Citrate metabolism | |||||||
| glyma10g34490 | PK | 372.26 | 815.17 | 2.19 | 121.83 | 123.83 | 1.02 |
| glyma12g33820 | PEPC | 118.74 | 562.77 | 4.74 | 130.48 | 229.15 | 1.76 |
| glyma08g17010 | ACL | 433.24 | 179.49 | 0.41 | 723.23 | 685.12 | 0.95 |
| Citrate transport | |||||||
| glyma09g15550 | FRD3b | 574.44 | 390.76 | 0.68 | 1996.88 | 734.89 | 0.37 |
| glyma13g27300 | MATE | 217.15 | 13497.02 | 62.15 | 12.10 | 15846.95 | 1309.98 |
| glyma02g31370 | MATE | 3336.45 | 12374.30 | 3.71 | 717.18 | 8632.32 | 12.04 |
| glyma01g02950 | MMC | 502.77 | 955.39 | 1.90 | 475.24 | 1051.99 | 2.21 |
| Amino acid metabolism | |||||||
| glyma01g32360 | GOT2 | 284.54 | 681.49 | 2.40 | 194.42 | 225.67 | 1.16 |
Multidrug and toxic compound extrusion family plays an important role in transporting organic acids during Al stress. A previous genome-wide analysis found 117 MATE transporter genes in soybean, some of which were responsive to Al stress (Liu et al. 2016). In the present study, we screened three MATE genes in the roots of two soybean genotypes (Table 2). Glyma09g15550 (GmFRD3b), an AtFRD3 homolog, was significantly down-regulated only in BD2. However, glyma13g27300 and glyma02g31370 were sharply up-regulated in both soybean genotypes under 48 h Al stress, specifically to a larger extent in BD2.
Effects of Al stress on the expression of genes involved in JA biosynthesis and signalling in leaves
Two genes (glyma08g20200 and glyma13g31280) encoding lipoxygenase (LOX) were only significantly induced in BD2 leaves (Table 3). Similarly, genes encoding 12-oxophytodienoate reductase (OPR) and acyl-CoA oxidase (ACX) were also significantly up-regulated only in BD2 leaves. However, these genes were not induced in BX10 leaves.
Table 3.
Expression of genes associated with JA biosynthesis and signalling pathway. aLOX, lipoxygenase; OPR, 12-oxophytodienoate reductase; ACX, acyl-CoA oxidase; JAZ, jasmonate ZIM-domain protein. bBX10L and BD2L denote the leaves of BX10 and BD2, respectively. cFold change means the ratio of Al treatment vs. control.
| Gene ID | Annotationa | BX10Lb | BX10L | Fold changec | BD2L | BD2L | Fold change |
|---|---|---|---|---|---|---|---|
| −Al | +Al | −Al | +Al | ||||
| JA biosynthesis | |||||||
| glyma08g20200 | LOX | 54.30 | 49.50 | 0.91 | 105.84 | 213.23 | 2.01 |
| glyma13g31280 | LOX | 44.12 | 29.17 | 0.66 | 101.77 | 207.33 | 2.04 |
| glyma13g16950 | OPR | 3.39 | 3.54 | 1.04 | 68.19 | 222.07 | 3.26 |
| glyma05g31390 | ACX | 463.84 | 855.65 | 1.84 | 467.12 | 947.23 | 2.03 |
| JA signalling | |||||||
| glyma15g09980 | JAZ | 3.39 | 18.56 | 5.47 | 29.51 | 166.06 | 5.63 |
| glyma13g17180 | JAZ | 133.49 | 563.07 | 4.22 | 610.62 | 2266.88 | 3.71 |
| glyma17g05540 | JAZ | 99.56 | 158.22 | 1.59 | 374.51 | 838.16 | 2.24 |
| glyma11g04130 | JAZ | 50.91 | 175.02 | 3.44 | 317.52 | 1694.02 | 5.34 |
| glyma01g41290 | JAZ | 71.27 | 214.80 | 3.01 | 582.12 | 2381.84 | 4.09 |
| glyma09g08290 | JAZ | 150.46 | 540.97 | 3.60 | 643.19 | 2097.87 | 3.26 |
| glyma15g19840 | JAZ | 238.71 | 416.33 | 1.74 | 884.38 | 2340.57 | 2.65 |
As the key repressors of JA signalling pathway, jasmonate ZIM-domain proteins (JAZs) belong to the TIFY transcription factor family (Vanholme et al. 2007). We found that seven TIFY genes showed differential expression in both soybean genotypes (Table 3). These seven TIFY genes were all induced in BD2, whereas only four of them were induced in BX10. Moreover, the basal expression levels of these genes in BD2 were significantly higher than that in BX10.
Real-time PCR validation of RNA-seq data
Ten randomly selected genes displaying diverse expression profiles were analysed by real-time PCR to validate the expression pattern of the DEGs. Among these genes, three were unresponsive to Al stress (FDR > 1), whereas the other seven showed differential expression (FDR < 0.05) both in the roots of BX10 and BD2 according to the 2-fold ratio and FDR < 0.05 thresholds. We found similar patterns between mRNA-seq and real-time PCR. Two sets of data revealed a significant correlation (R2 = 0.94) [see Supporting Information—Fig. S2], indicating the reliability of the high-throughput sequencing data.
Discussion
Global responses of genes in different tissues of two soybean genotypes
We investigated the expression profiling of genes in the roots and leaves of two soybean genotypes under Al treatments by using Illumina HiSeq 2000 sequencing platform. Comparative analysis revealed that more genes in the root tips showed responsiveness to Al than those in the leaves in both soybean genotypes. This analysis supports the observation that root is the primary target of Al toxicity (Kollmeier et al. 2000). Moreover, more genes were up-regulated in roots than in leaves, suggesting that the roots might be more active than the leaves in response to Al stress.
Kyoto Encyclopedia of Genes and Genomes analysis revealed that genes involved in amino acid metabolism were mostly up-regulated in the roots of these two soybean genotypes. The expression levels of the genes that encode specific amino acid biosynthesis were induced under various rhizotoxic ion (Al, Cu and Cd) stresses (Zhao et al. 2010). In addition, increasing amino acid accumulation is considered as a hallmark of Al-toxicity alleviation (Wang et al. 2015). Therefore, the activation of amino acid metabolism could be a common mechanism of Al tolerance in these two soybean genotypes.
Kyoto Encyclopedia of Genes and Genomes analysis also revealed that the expression levels of the genes implicated in lipid biosynthesis were up-regulated in the roots of BD2. Maintaining the activities of lipid biosynthesis-associated enzymes and the stability of lipid composition is required for Al resistance in rice (Huynh et al. 2012). One gene encodes GDSL-like lipase and functions as lipid hydrolysis; this gene was strikingly induced in BX10 and BD2 with a larger extent in BD2, which supported the observation that a large decrease in lipid content is correlated with Al sensitivity (Huynh et al. 2012). Therefore, the induction of lipid metabolism-related genes appears to be a passive measure for the turnover of lipid to maintain the stability of membrane lipid composition.
Differential citrate metabolism in the roots of two soybean genotypes
Al-induced citrate and malate exudation are critical for Al resistance in soybeans (Yang and Zheng 2006; Liang et al. 2013). Thus, the induction of genes encoding citrate transporters is required for Al-stimulated citrate efflux (Yang and Zheng 2006). Citrate can be secreted into rhizosphere and/or vascular system for Al chelation. The function of MATE proteins as mediators of citrate transport is conserved in different plant species (Magalhaes et al. 2007; Zhou et al. 2013). Glyma13g27300, a MATE gene that is considered as a candidate gene involved in Al tolerance in soybeans (Liu et al. 2016), was significantly up-regulated in our study. In addition, the down-regulation of another MATE gene, GmFRD3b, received our attention. Previous report revealed that both GmFRD3a and GmFRD3b are involved in Fe deficiency response in soybeans (Rogers et al. 2009; Takanashi et al. 2014). Further study showed that GmFRD3a is up-regulated in response to Al (You et al. 2011). In the present study, we found that GmFRD3a was not induced in these two soybean genotypes, instead, GmFRD3b was down-regulated in the roots of BD2 but not in BX10. In Arabidopsis, AtFRD3 functions in transporting citrate into root vasculature where it forms a complex with iron and then transfers to shoots (Rogers and Guerinot 2002; Green and Rogers 2004; Durrett et al. 2007). Moreover, the overexpression of AtFRD3 contributes to Al tolerance (Durrett et al. 2007). Therefore, the up-regulation of glyma13g27300 and glyma02g31370 in both genotypes and the down-regulation of GmFRD3b in BD2 roots suggest that the enhanced citrate exudation is a common mechanism, whereas the sensitive soybeans increase the supply of citrate for exudation by inhibiting the internal flow of citrate into xylem. We suggest that the decreased expression of GmFRD3b in BD2 may suppress citrate loading into root xylem. As a result, a relatively large amount of toxic Al would be translocated into the aerial portions, accumulate and cause Al toxicity in the leaves.
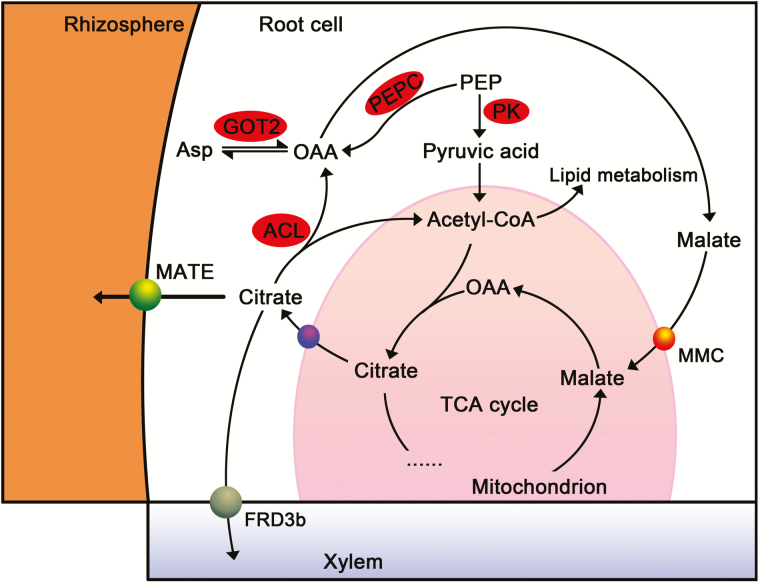
ALS3 is localized to the plasma membrane of phloem, which transports Al into phloem and then moving it away from the sensitive tissues to maintain root growth in Arabidopsis (Larsen et al. 2005). We noticed that two ALS3 genes, glyma03g33290 and glyma10g05420, were highly up-regulated by Al stress in the roots of both soybean genotypes, specifically to a relatively large extent in BD2 [see Supporting Information—Fig. S3]. This finding suggests that the up-regulation of ALS3 is a possible mechanism in response to Al stress in different soybean genotypes. However, the long-distance transfer of Al through the phloem transport system would be more promoted in BD2 than in BX10. Phloem transport consumes energy (Dong and Zhang 1986), which suggests that more ATP may be used by BD2 to redistribute Al away from the root tip. The altered organic acid metabolism in some plant species has been addressed in response to Al stress (de Carvalho Gonçalves et al. 2005; Yang et al. 2011). In BX10, genes encoding PK and PEPC were up-regulated, which suggested that the transformation from phosphoenolpyruvate (PEP) to pyruvic acid and PEP to oxaloacetic acid (OAA) was enhanced (Fig. 5). Increased pyruvic acid and OAA then promote the flow of tricarboxylic acid (TCA) cycle. Additionally, we found that the expression of ACL gene was down-regulated in BX10 (Fig. 5). ACL catalyses the cleavage of citrate into two products: OAA and acetyl-CoA. During this process, OAA can be used for the anaplerotic reaction of TCA cycle while acetyl-CoA is required for lipogenesis (Takeda 1969). Therefore, the decrease in ACL gene expression implicated that the degradation of citrate in cytosol will be suppressed in BX10. By contrast, the dissolution of extramitochondrial citrate was not inhibited in BD2, which suggested that the amount of citrate available for secreting was less in BD2 than that in BX10. Genes involved in lipid metabolism were over-represented in the roots of BD2, which suggested that a large amount of acetyle-CoA would be recruited to facilitate fatty acid biosynthesis. Thus, few acetyle-CoA can be utilized for citrate biosynthesis. This condition can retard the TCA cycle in BD2 roots. In addition, the expression of GOT2, which catalyses the reversible conversion between aspartate and OAA, was up-regulated in BX10 roots. This finding indicated that organic acid metabolism could be favoured by the extra supply of OAA produced from the enhanced amino acid metabolism. Therefore, we deduced that metabolism might be one of the leading causes of Al sensitivity in BD2.
Figure 5.
Schematic network reveals the differences in organic acid metabolism in the roots of two soybean genotypes. Genes differentially expressed in BX10 are highlighted in red oval. Transporters are shown in coloured spheres.
Differential JA biosynthesis and signalling in the leaves of two soybean genotypes
Jasmonic acid is a phytohormone that functions in the signalling of defence response (Howe 2001). JA can be induced by various stimuli (Gao et al. 2004; Glazebrook 2005; Maksymiec et al. 2006; Hess 2010). JA-mediated regulation of defence-associated genes in leaves is an essential part of self-defence mechanism in response to adverse circumstances (Omer et al. 2000; Henkes et al. 2008; Black et al. 2009). The proteins, including LOX, OPR and ACX, in Citrus species were up-regulated in roots under Al stress (Jiang et al. 2015). A latest study reported that JA can enhance the Al-induced root growth inhibition in Arabidopsis (Yang et al. 2017). These findings revealed that in roots, JA signalling was involved in Al stress. However, we observed that the genes, including LOX, OPR and ACX, involved in JA biosynthesis were significantly induced in the leaves of BD2. Increased JA expression can serve as an important signalling molecule to initiate defence response by activating the gene expression levels (Blée 2002). These genes involved in JA biosynthesis were not induced in the leaves of BX10, which suggested that the intensity of Al stress was insufficient to trigger the JA response in BX10.
JAZs are involved in JA signalling transduction pathway as transcription repressors (Pauwels and Goossens 2011). Under stress condition, increased bioactive JA (JA-Ile conjugate) guides the binding of JAZs to SCFCOI1 ubiquitin E3 ligase complex to initiate the 26S proteasome degradation process. After the destruction of JAZs, transcription factors are released from JAZ-mediated repression, and the subsequent activation of JA-responsive genes is allowed (Pauwels and Goossens 2011). We found that seven JAZ genes were induced in the leaves of BD2, whereas three of them were induced in the leaves of BX10. The dissolution and induction of JAZ genes have at least two functions: facilitating the activation of defence genes and attenuating the following JA response, which may be essential for maintaining cellular stability because the long-term stimulation of defence response may result in damage (Thines 2007). In BD2 leaves, the up-regulation of genes involved in JA biosynthesis and the resulting induction of JAZs suggested that JA defence response was activated. Given that long-term stress response requires resource consumption, and the decrease in ATP biosynthesis may impair Al tolerance of plants (Honda et al. 1997; Hamilton et al. 2001), the activated expression of JA-associated genes would inevitably cause growth arrest in leaf, thereby weakening its adaptation to Al toxicity in BD2.
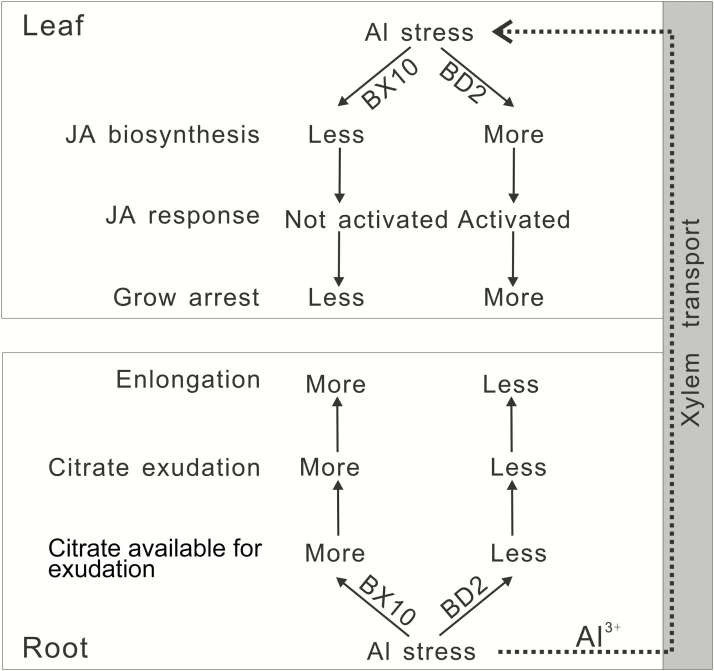
Synergistic effect between roots and leaves in response to Al stress
Growth correlation between the below-ground and above-ground structures of plants is a common physiological phenomenon. We deduced that a unique cooperation scenario occurred between the roots and leaves of the two soybeans in response to Al stress (Fig. 6). Citrate biosynthesis might not be activated in the roots of BD2, which is supported by the previous findings that citrate exudation is lower in BD2 than in BX10 (Dong et al. 2004). Although the citrate transport activity was higher in BD2 roots, this phenomenon is considered as a compensation measure to resolve the shortage of citrate biosynthesis. The internal transport of citrate into xylem might be inhibited in the roots of BD2. Therefore, Al could not be sufficiently chelated by citrate in its root vasculature. As a consequence, a large amount of toxic Al would escape and finally accumulate in the leaves of BD2. Excessive Al may lead to JA biosynthesis via the induction of related genes. High accumulation of JA activates JA response, which results in the growth inhibition of leaves (Ueda et al. 1995; Ulloa et al. 2002; Liu et al. 2010), because JA defence response allows resource diversion (Zavala and Baldwin 2006) and leads to ATP biosynthesis deficiency (Ruiz-May et al. 2011).
Figure 6.
Proposed model for Al-induced citrate metabolism and secretion in the roots, and JA biosynthesis and signalling in the leaves of BX10 and BD2. In BX10, genes involved in citrate metabolism and exudation were induced in the roots. This finding suggested that more citrate could be secreted into rhizosphere for Al chelation, which is very essential for alleviating the Al toxicity in roots. Toxic Al3+ was continuously translocated through apoplastic and/or symplastic pathways. Genes involved in JA biosynthesis and signalling were highly induced in the leaves because much more Al3+ were accumulated in the leaves of sensitive soybean genotype. This finding indicated that JA-mediated defence response was activated, which could lead to resource and energy expenditure and growth arrest of leaves. These conditions are signs of Al toxicity.
Conclusions
We investigated the molecular mechanisms of different Al tolerance in two contrasting soybean genotypes through the global transcriptome analysis of the roots and leaves. Our RNA-seq data reveal that the genes involved in citrate metabolism and secretion are preferentially expressed in the roots of BX10. The genes implicated in JA biosynthesis and signalling are highly induced in the leaves of BD2. These findings suggest that on one hand, BX10 can secrete additional citrate into rhizosphere from the roots to chelate Al. On the other hand, BX10 can avoid JA-mediated defence response that allows resource allocation to maintain leaf growth. Our results provide new insights into the understanding of the molecular mechanisms of Al tolerance in different tissues of soybeans.
Supporting Information
The following additional information is available in the online version of this article—
Table S1. Differentially expressed genes in each category.
Table S2. Over-represented GO terms in roots and leaves.
Figure S1. Time course of root length of BX10 and BD2 soybeans under 0 or 50 μM Al3+ treatments. Error bars denote mean ± SD (n = 15).
Figure S2. Correlation analysis of real-time PCR data and mRNA-seq data. The ratio of each gene expression (log2) in the mRNA-seq data was calculated and plotted against the ratio calculated in the real-time PCR data.
Figure S3. The expression patterns of ALS3 genes in the roots of two soybean genotypes.
Sources of Funding
This research was supported by the National Key Research and Development Program of China (2016YFD0101005), the grants from the National Natural Science Foundation of China (NSFC) (31271744, 31372140), the Program for Changjiang Scholars and Innovative Research Team in University (IRT_14R27) and the Important National Science & Technology Specific Project (2016ZX08011-003, 2014ZX08011-003).
Contributions by the Authors
C.-Y.T., J.-L.Q. and Y.-H.Y. designed research; S.-C.H., S.-J.C., Y.-M.G., Y.-J.J., D.-Q.H. and J.C. performed research; S.-C.H., G.-H.L., R.-W.Y., C.-Y.T., J.-L.Q. and Y.-H.Y. analysed data; S.-C.H., C.-Y.T., J.-L.Q. and Y.-H.Y. wrote the paper. All authors have read and approved the final version of the manuscript.
Conflicts of Interest
None declared.
Literature Cited
- Binbo M, Shen C, Zhiwei Y. 2009. Effect of aluminum on synthesis and secretion of citrate in soybean roots. Ecology & Environmental Sciences 18:1037–1041. [Google Scholar]
- Black CA, Karban R, Godfrey LD, Granett J, Chaney WE. 2009. Jasmonic acid: a vaccine against leafminers (Diptera: Agromyzidae) in Celery. Environmental Entomology 32:1196–1202. [Google Scholar]
- Blée E. 2002. Impact of phyto-oxylipins in plant defense. Trends in Plant Science 7:315–322. [DOI] [PubMed] [Google Scholar]
- Bo QI, Zhao TJ, Gai JY. 2007. Characterization of variation and identification of elite accessions of aluminum toxin tolerance soybean germplasm in China. Soybean Science 26:813–819. [Google Scholar]
- Bot AJ, Nachtergaele FO, Young A. 2000. Land resource potential and constraints at regional and country levels. Rome: Food and Agricultural Organization of the United Nations. [Google Scholar]
- de Carvalho Gonçalves JF, Cambraia J, Mosquim PR, Araújo EF. 2005. Aluminum effect on organic acid production and accumulation in Sorghum. Journal of Plant Nutrition 28:507–520. [Google Scholar]
- Delhaize E, Ryan PR. 1995. Aluminum toxicity and tolerance in plants. Plant Physiology 107:315–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR, Hebb DM, Yamamoto Y, Sasaki T, Matsumoto H. 2004. Engineering high-level aluminum tolerance in barley with the ALMT1 gene. Proceedings of the National Academy of Sciences of the United States of America 101:15249–15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D, Peng X, Yan X. 2004. Organic acid exudation induced by phosphorus deficiency and/or aluminium toxicity in two contrasting soybean genotypes. Physiologia Plantarum 122:190–199. [Google Scholar]
- Dong WY, Zhang WC. 1986. Ultrastructural localization of adenosine triphosphatase activity in the phloem of garlic scape. Journal of Integrative Plant Biology 28:441–443. [Google Scholar]
- Durrett TP, Gassmann W, Rogers EE. 2007. The FRD3-mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiology 144:197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foy CD. 1988. Plant adaptation to acid, aluminum-toxic soils. Communications in Soil Science and Plant Analysis 19:959–987. [Google Scholar]
- Gao XP, Wang XF, Lu YF, Zhang LY, Shen YY, Liang Z, Zhang DP. 2004. Jasmonic acid is involved in the water-stress-induced betaine accumulation in pear leaves. Plant Cell & Environment 27:497–507. [DOI] [PubMed] [Google Scholar]
- Glazebrook J. 2005. Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annual Review of Phytopathology 43:205–227. [DOI] [PubMed] [Google Scholar]
- Green LS, Rogers EE. 2004. FRD3 controls iron localization in Arabidopsis. Plant Physiology 136:2523–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton CA, Good AG, Taylor GJ. 2001. Induction of vacuolar ATPase and mitochondrial ATP synthase by aluminum in an aluminum-resistant cultivar of wheat. Plant Physiology 125:2068–2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JE, Ma JF. 2003. Al-induced efflux of organic acid anions is poorly associated with internal organic acid metabolism in triticale roots. Journal of Experimental Botany 54:1753–1759. [DOI] [PubMed] [Google Scholar]
- Henkes GJ, Thorpe MR, Minchin PE, Schurr U, Röse US. 2008. Jasmonic acid treatment to part of the root system is consistent with simulated leaf herbivory, diverting recently assimilated carbon towards untreated roots within an hour. Plant, Cell & Environment 31:1229–1236. [DOI] [PubMed] [Google Scholar]
- Hess HH. 2010. Jasmonic acid and ethylene modulate local responses to wounding and simulated herbivory in Nicotiana attenuata leaves. Plant Physiology 153:785–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M, Ito K, Hara T. 1997. Effect of physiological activities on aluminum uptake in carrot (Daucus carota L.) cells in suspension culture. Soil Science & Plant Nutrition 43:361–368. [Google Scholar]
- Howe GA. 2001. Cyclopentenone signals for plant defense: remodeling the jasmonic acid response. Proceedings of the National Academy of Sciences of the United States of America 98:12317–12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh VB, Repellin A, Zuily-Fodil Y, Pham-Thi AT. 2012. Aluminum stress response in rice: effects on membrane lipid composition and expression of lipid biosynthesis genes. Physiologia Plantarum 146:272–284. [DOI] [PubMed] [Google Scholar]
- Jiang HX, Yang LT, Qi YP, Lu YB, Huang ZR, Chen LS. 2015. Root itraq protein profile analysis of two citrus species differing in aluminum-tolerance in response to long-term aluminum-toxicity. BMC Genomics 16:949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochian LV. 1995. Cellular mechanisms of aluminum toxicity and resistance in plants. Annual Review of Plant Physiology and Plant Molecular Biology 46:237–260. [Google Scholar]
- Kollmeier M, Felle HH, Horst WJ. 2000. Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basipetal auxin flow involved in inhibition of root elongation by aluminum?Plant Physiology 122:945–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen PB, Geisler MJB, Jones CA, Williams KM, Cancel JD. 2005. ALS3 encodes a phloem-localized ABC transporter-like protein that is required for aluminum tolerance in Arabidopsis. Plant Journal 41:353–363. [DOI] [PubMed] [Google Scholar]
- Li Y, Yang T, Zhang P, Zou A, Peng X, Wang L, Yang R, Qi J, Yang Y. 2012. Differential responses of the diazotrophic community to aluminum-tolerant and aluminum-sensitive soybean genotypes in acidic soil. European Journal of Soil Biology 53:76–85. [Google Scholar]
- Liang C, Piñeros MA, Tian J, Yao Z, Sun L, Liu J, Shaff J, Coluccio A, Kochian LV, Liao H. 2013. Low pH, aluminum, and phosphorus coordinately regulate malate exudation through GmALMT1 to improve soybean adaptation to acid soils. Plant Physiology 161:1347–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H, Wan H, Shaff J, Wang X, Yan X, Kochian LV. 2006. Phosphorus and aluminum interactions in soybean in relation to aluminum tolerance. Exudation of specific organic acids from different regions of the intact root system. Plant Physiology 141:674–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Jiang H, Ye S, Chen WP, Liang W, Xu Y, Sun B, Sun J, Wang Q, Cohen JD, Li C. 2010. The Arabidopsis P450 protein CYP82C2 modulates jasmonate-induced root growth inhibition, defense gene expression and indole glucosinolate biosynthesis. Cell Research 20:539–552. [DOI] [PubMed] [Google Scholar]
- Liu J, Li Y, Wang W, Gai J, Li Y. 2016. Genome-wide analysis of MATE transporters and expression patterns of a subgroup of MATE genes in response to aluminum toxicity in soybean. BMC Genomics 17:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Yang YS, Xu G, Zhu S. 2004. The effect of aluminum stress on morphological and physiological characteristics of soybean root of seedling. Chinese Journal of Oil Crop Scieves 26:49–54. [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25:402–408. [DOI] [PubMed] [Google Scholar]
- Ma JF. 2000. Role of organic acids in detoxification of aluminum in higher plants. Plant & Cell Physiology 41:383–390. [DOI] [PubMed] [Google Scholar]
- Magalhaes JV, Liu J, Guimarães CT, Lana UG, Alves VM, Wang YH, Schaffert RE, Hoekenga OA, Piñeros MA, Shaff JE, Klein PE, Carneiro NP, Coelho CM, Trick HN, Kochian LV. 2007. A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nature Genetics 39:1156–1161. [DOI] [PubMed] [Google Scholar]
- Maksymiec W, Wianowska D, Dawidowicz AL, Radkiewicz S, Mardarowicz M, Krupa Z. 2006. The level of jasmonic acid in Arabidopsis thaliana and Phaseolus coccineus plants under heavy metal stress. Journal of Plant Physiology 162:1338–1346. [DOI] [PubMed] [Google Scholar]
- Maron LG, Kirst M, Mao C, Milner MJ, Menossi M, Kochian LV. 2008. Transcriptional profiling of aluminum toxicity and tolerance responses in maize roots. The New Phytologist 179:116–128. [DOI] [PubMed] [Google Scholar]
- Minh-Thu PT, Hwang DJ, Jeon JS, Nahm BH, Kim YK. 2013. Transcriptome analysis of leaf and root of rice seedling to acute dehydration. Rice 6:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nian H, Yang Z, Huang H, Yan X, Matsumoto H. 2007. Citrate secretion induced by aluminum stress may not be a key mechanism responsible for differential aluminum tolerance of some soybean genotypes. Journal of Plant Nutrition 27:2047–2066. [Google Scholar]
- Omer AD, Thaler JS, Granett J, Karban R. 2000. Jasmonic acid induced resistance in grapevines to a root and leaf feeder. Journal of Economic Entomology 93:840–845. [DOI] [PubMed] [Google Scholar]
- Pauwels L, Goossens A. 2011. The JAZ proteins: a crucial interface in the jasmonate signaling cascade. The Plant Cell 23:3089–3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EE, Guerinot ML. 2002. FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. The Plant Cell 14:1787–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers EE, Wu X, Stacey G, Nguyen HT. 2009. Two MATE proteins play a role in iron efficiency in soybean. Journal of Plant Physiology 166:1453–1459. [DOI] [PubMed] [Google Scholar]
- Ruiz-May E, De-la-Peña C, Galaz-Ávalos RM, Lei Z, Watson BS, Sumner LW, Loyola-Vargas VM. 2011. Methyl jasmonate induces ATP biosynthesis deficiency and accumulation of proteins related to secondary metabolism in Catharanthus roseus (L.) G. hairy roots. Plant & Cell Physiology 52:1401–1421. [DOI] [PubMed] [Google Scholar]
- Ryan P, Delhaize E, Randall P. 1995. Malate efflux from root apices and tolerance to aluminium are highly correlated in wheat. Functional Plant Biology 22:531–536. [Google Scholar]
- Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H. 2004. A wheat gene encoding an aluminum-activated malate transporter. Plant Journal 37:645–653. [DOI] [PubMed] [Google Scholar]
- Schmutz J, Cannon SB, Schlueter J, Ma J, Mitros T, Nelson W, Hyten DL, Song Q, Thelen JJ, Cheng J, Xu D, Hellsten U, May GD, Yu Y, Sakurai T, Umezawa T, Bhattacharyya MK, Sandhu D, Valliyodan B, Lindquist E, Peto M, Grant D, Shu S, Goodstein D, Barry K, Futrell-Griggs M, Abernathy B, Du J, Tian Z, Zhu L, Gill N, Joshi T, Libault M, Sethuraman A, Zhang XC, Shinozaki K, Nguyen HT, Wing RA, Cregan P, Specht J, Grimwood J, Rokhsar D, Stacey G, Shoemaker RC, Jackson SA. 2010. Genome sequence of the palaeopolyploid soybean. Nature 463:178–183. [DOI] [PubMed] [Google Scholar]
- Silva IR, Smyth TJ, Raper CD, Carter TE, Rufty TW. 2001. Differential aluminum tolerance in soybean: an evaluation of the role of organic acids. Physiologia Plantarum 112:200–210. [DOI] [PubMed] [Google Scholar]
- Takanashi K, Shitan N, Yazaki K. 2014. The multidrug and toxic compound extrusion (MATE) family in plants. Plant Tissue Culture Letters 31:417–430. [Google Scholar]
- Takeda Y. 1969. ATP citrate lyase. Seikagaku the Journal of Japanese Biochemical Society 41:631–643. [PubMed] [Google Scholar]
- Thines B. 2007. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 448:661–665. [DOI] [PubMed] [Google Scholar]
- Ueda J, Miyamoto K, Kamisaka S. 1995. Inhibition of the synthesis of cell wall polysaccharides in oat coleoptile segments by jasmonic acid: relevance to its growth inhibition. Journal of Plant Growth Regulation 14:69–76. [Google Scholar]
- Ulloa RM, Raíces M, MacIntosh GC, Maldonado S, Téllez-Iñón MT. 2002. Jasmonic acid affects plant morphology and calcium-dependent protein kinase expression and activity in Solanum tuberosum. Physiologia Plantarum 115:417–427. [DOI] [PubMed] [Google Scholar]
- Vanholme B, Grunewald W, Bateman A, Kohchi T, Gheysen G. 2007. The tify family previously known as ZIM. Trends in Plant Science 12:239–244. [DOI] [PubMed] [Google Scholar]
- Wang L, Feng Z, Wang X, Wang X, Zhang X. 2010. DEGseq: an R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26:136. [DOI] [PubMed] [Google Scholar]
- Wang LQ, Yang LT, Guo P, Zhou XX, Ye X, Chen EJ, Chen LS. 2015. Leaf cdna-AFLP analysis reveals novel mechanisms for boron-induced alleviation of aluminum-toxicity in citrus grandis seedlings. Ecotoxicology and Environmental Safety 120:349–359. [DOI] [PubMed] [Google Scholar]
- Xu Q, Luo C, Liao H, Yan X, Nian H. 2003. Study on the response of soybean varieties to p deficiency. Soybean Science 22:108–114. [Google Scholar]
- Xu M, You J, Hou N, Zhang H, Chen G, Yang Z. 2010. Mitochondrial enzymes and citrate transporter contribute to the aluminium-induced citrate secretion from soybean (Glycine max) roots. Functional Plant Biology 77:886–892. [Google Scholar]
- Yang ZB, He C, Ma Y, Herde M, Ding Z. 2017. Jasmonic acid enhances al-induced root growth inhibition. Plant Physiology 173:1420–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LT, Jiang HX, Tang N, Chen LS. 2011. Mechanisms of aluminum-tolerance in two species of citrus: secretion of organic acid anions and immobilization of aluminum by phosphorus in roots. Plant Science 180:521–530. [DOI] [PubMed] [Google Scholar]
- Yang T, Liu G, Li Y, Zhu S, Zou A, Qi J, Yang Y. 2012. Rhizosphere microbial communities and organic acids secreted by aluminum-tolerant and aluminum-sensitive soybean in acid soil. Biology & Fertility of Soils 48:97–108. [Google Scholar]
- Yang JL, Zheng SJ. 2006. Citrate transporters play a critical role in aluminium-stimulated citrate efflux in rice bean (Vigna umbellata) roots. Annals of Botany 97:579–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You J, Zhang H, Liu N, Gao L, Kong L, Yang Z. 2011. Transcriptomic responses to aluminum stress in soybean roots. Genome 54:923–933. [DOI] [PubMed] [Google Scholar]
- Zavala JA, Baldwin IT. 2006. Jasmonic acid signalling and herbivore resistance traits constrain regrowth after herbivore attack in Nicotiana attenuata. Plant, Cell & Environment 29:1751–1760. [DOI] [PubMed] [Google Scholar]
- Zhang XB, Liu P, Yang YS, Xu GD. 2007. Effect of Al in soil on photosynthesis and related morphological and physiological characteristics of two soybean genotypes. Botanical Studies 48:435–444. [Google Scholar]
- Zhao CR, Sawaki Y, Sakurai N, Shibata D, Koyama H. 2010. Transcriptomic profiling of major carbon and amino acid metabolism in the roots of Arabidopsis thaliana treated with various rhizotoxic ions. Soil Science & Plant Nutrition 56:150–162. [Google Scholar]
- Zhen Y, Miao L, Su J, Liu S, Yin Y, Wang S, Pang Y, Shen H, Tian D, Qi J, Yang Y. 2009. Differential responses of anti-oxidative enzymes to aluminum stress in tolerant and sensitive soybean genotypes. Journal of Plant Nutrition 32:1255–1270. [Google Scholar]
- Zhou G, Delhaize E, Zhou M, Ryan PR. 2013. The barley MATE gene, HvAACT1, increases citrate efflux and Al3+ tolerance when expressed in wheat and barley. Annals of Botany 112:603–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Yang P, Cui F, Zhang F, Luo X, Xie J. 2016. Transcriptome analysis of salt stress responsiveness in the seedlings of Dongxiang wild rice (Oryza rufipogon Griff.). PLoS One. doi:10.1371/journal.pone.0146242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Wang H, Zhu Y, Zou J, Zhao FJ, Huang CF. 2015. Genome-wide transcriptomic and phylogenetic analyses reveal distinct aluminum-tolerance mechanisms in the aluminum-accumulating species buckwheat (Fagopyrum tataricum). BMC Plant Biology 15:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.