Abstract
After an initial exposure, subjects can develop test-taking/learning strategies called the “test sophistication effect. Cirrhotics wth prior overt hepatic encephalopathy (OHE) could have persistent learning impairments.
Aim
To define learning/test-sophistication on EncephalApp (downloadable Application) in OHE patients compared to no-OHE patients and controls cross-sectionally and longitudinally.
Methods
The EncephalApp Stroop App consists of 2 sections; the easier “Off” run assesses psychomotor speed while the difficult “On” run assesses cognitive flexibility. Cross-sectional Analysis: Cirrhotic outpatients with/without controlled OHE and healthy controls underwent EncephalApp testing, which requires 5 “off” and 5 “on” runs. We studied the difference in time required between completing trial 1 compared with trial 5 (delta 1–5) in the both the “On” and “Off” runs in controls, all cirrhotics; and between prior OHE/no-OHE cirrhotics. Longitudinal Analyses: Two groups of cirrhotics were studied; one was administered EncephalApp, ≥ 2 weeks apart and the second before, and 6 months post-liver transplantation.
Results
89 controls and 230 cirrhotics (85 prior OHE, MELD 11) with similar age (64 vs 61, p=0.9) were included. Cirrhotic patients had impaired EncephalApp total times and impaired learning on the On runs compared to controls. OHE patients had worse EncephalApp times and learning with the On runs compared to no-OHE patients, which persisted in the longitudinal cohort. No differences in learning were seen in the Off runs. After transplant there was restoration of learning capability with the On runs in the OHE patients.
Conclusion
Cognitive flexibility tested by the EncephalApp On runs improves over time in healthy controls and no-OHE but not prior OHE. Psychomotor speed remains similar over time. The learning Impairment manifested by cirrhotics with OHE is restored post-transplant.
Keywords: Cirrhosis, Cognitive flexibility, Psychomotor speed, Dementia, Longitudinal
Introduction
Patients with hepatic encephalopathy (HE) have impaired daily functioning, which limits their independence(1). HE forms a spectrum of cognitive alterations divided into covert (CHE) and overt Hepatic Encephalopathy (OHE)(2). These deficits in working memory, response inhibition and psychomotor speed are associated with psychosocial impairments(3). In OHE, there is experimental, autopsy and clinical evidence of persistent cognitive impairment related to learning using tests of response inhibition (inhibitory control test ICT) as well as on the psychometric hepatic encephalopathy score (PHES) in limited number of patients(4–8). This persistent cognitive impairment is observed despite standard of care therapy and normal mentation, and could further worsen the ability of these patients to function independently.
The EncephalApp Stroop based on the original Stroop effect, has been validated to define cognitive impairment in cirrhosis(9–11). It consists of five separate runs of an easier “Off” state that evaluates psychomotor speed and 5 runs of the more difficult “On” state that assesses cognitive flexibility, inhibition of a dominant response, and dealing with interference(12). In the Off runs the subject is required to identify as quickly as possible the color of ink used to create the hashtag. Therefore, these trials specifically assess psychomotor speed. In contrast, in the On runs the subject sees a word written in a discordant ink color. For example, the subject is asked to disregard reading the written word, but instead select their response based on the color of ink used to spell the word. The brain easily understands the meaning of words as a result of habitual reading. Color recognition is not an automatic process. Therefore, cognitive interference occurs when the word reading and color naming pathways are activated simultaneously. In the On trial run, the pathway (color recognition) that leads to the response (identify ink color, not the written word) is the weaker of the two pathways(13). Therefore, like the Off trial, the On trial has a psychomotor speed component, but uniquely demands response inhibition and cognitive flexibility. Brain imaging techniques have shown two main brain regions involved in performing the Stroop task. The anterior cingulate cortex allows for the allocation of attentional resources and the selection of an appropriate response. The dorsolateral prefrontal cortex creates the appropriate rules for the brain to accomplish response inhibition(14, 15). These cognitive functions are critical for daily function and their course after development of OHE and post-transplant need further elucidation. Studies evaluating learning associated with test familiarity have shown that the magnitude of carryover effects associated with test familiarity is related to the disorder being assessed (16, 17). For example, in one study (18) the extent of practice effects was associated with an individual’s level of intelligence. Those with higher IQ scores at baseline testing showed greater improvement with subsequent test administrations. It may be the case that cirrhotic subjects with a prior history of OHE will suffer specific neuropathologic changes that serve to reduce brain reserve, making them less able to benefit from test familiarity. While the current study is not designed to clarify neuropathologic processes associated with learning deficits in cirrhotics subjects, a recent study using measures of functional brain activation (19) demonstrated alteration in cortical structures necessary for good performance on the EncephalApp task (i.e., frontal/parietal regions). In a multi-center study (20) examining cognitive function in children following liver transplantation (LT), children who had received LT prior to 5 years of age displayed twice the rate of intellectual delay and three times the rate of learning disability compared to the general population. Most clinicians believe that the majority of the clinical features recognized as HE are reversed by successful liver transplantation (21). However, Campagna et al., found an incomplete reversal of certain cognitive defects in patients who had bouts of overt HE before they underwent liver transplantation. Interestingly, while abnormal electroencephalographic (EEG) patterns normalized after transplantation, previously abnormal cognition domains did not return to expected baseline in patients with a pre-transplant history of overt HE (22). We hypothesized that both psychomotor speed and cognitive flexibility will be impaired in patients with prior OHE on maximal treatment tested by the improvement in the 5th run compared to the 1st run on the “On” and “Off” states of EncephalApp compared to patients without OHE and healthy controls
Experimental Procedures
Study Population
We prospectively enrolled cirrhotic patients of all etiologies from our outpatient clinics. Cirrhosis was diagnosed using liver biopsy, Fibroscan, radiological evidence of cirrhosis, endoscopic evidence of varices in patients with chronic liver disease or evidence of frank decompensation. All patients required a Mini Mental status exam of >25 to qualify for the study. Data recorded were years of education (high school equivalent was 12 years), demographics and cirrhosis complications including OHE and treatment. Subjects were excluded if they were actively using psychotropic drugs or were actively abusing psychoactive substances including alcohol, were color blind or unable to understand English. Subjects with a history of OHE were on standard of care with lactulose and/or rifaximin and were adherent on these medications based on active questioning, chart review and pharmacy dispensing records. None of the patients without prior OHE were on lactulose or rifaximin. We enrolled age-matched healthy controls without any chronic diseases as well. Two additional patient groups were recruited for longitudinal studies; one group was tested at least two weeks apart with EncephalApp and another group was tested before and 6 months after liver transplant.
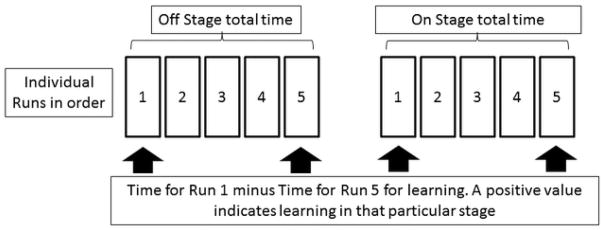
Encephalapp Testing: Potential subjects who were comfortable with the tablet or smartphone and were not color-blind, were tested based on the Encephalapp Stroop test by a trained provider. After appropriate instructions and a trial run, patients were tested on the app. A total of 5 runs were done in the Off state and then 5 more runs were attempted on the On state. Total stage times were recorded for all groups. Standard EncephalApp metrics are times taken for five successful Off stage runs (OffTime), for 5 successful On stage runs (OnTime), total time taken (OffTime+OnTime), extra time in On stage (OnTime minus OffTime), and number of runs needed to complete 5 Off and 5 On runs successfully. Learning was defined by the change in times from run 1 to run 5 was compared between groups (Figure 1). CHE was assessed based on US norms from the a multi-center North American experience(23) now available at http://www.encephalapp.com/test1.html.
Figure 1.
Design of individual runs of EncephalApp Stroop and study result calculations
Statistical analysis
Statistical analysis was performed using t-tests, Wilcoxon rank tests and Chi-square test as appropriate for patients. Variables compared were etiology of cirrhosis, demographics, years of schooling and education level, cirrhosis severity median run times on the “Off” and “On” states. Patients tested twice were analyzed using paired t-tests or Wilcoxon paired rank sum tests.
The study was conducted at the Virginia Commonwealth University and McGuire Veterans Center in Richmond, VA and the study protocol was approved by the Institutional Review Boards at both centers.
Results
Demographics and disease details
We enrolled a total of 230 cirrhotic patients and 89 controls for the cross-sectional study. The controls had similar age (59.1±9.9 vs. 60.5±8.3, p=0.26) and education status (14.8±2.6 vs. 14.0±2.4, p=0.07) but lower proportion of men (52 vs 67 men, p<0.001) compared to the cirrhotic patients. Of the enrolled cirrhotics, 145 had never had any documented OHE episodes and were not on any therapy. The remaining 85 cirrhotics had 1 or more episodes of OHE before enrollment and were alert and oriented at enrollment. Of the 85 OHE patients, 23 had their last episode within 6 months, 22 had it between 6 months and one year while 40 patients had experienced OHE more than one year ago. Fifteen patients were on lactulose only, 47 were on lactulose and rifaximin while 23 patients were not on regular OHE treatment. OHE patients had similar demographics compared to no-OHE but were significantly more likely to have alcoholic disease and MELD score and a lower prevalence of hepatitis C (Table 1).
Table 1.
Comparison of EncephalApp between controls and cirrhotic patients
| Run Times (seconds) | Controls (N=89) | Cirrhosis (N=230) | P value |
|---|---|---|---|
| Total Off | 69.9±12.4 | 92.2±26.5 | <0.0001 |
| Total On | 82.6±15.1 | 114.5±44.3 | <0.0001 |
| OffTime+OnTime | 152.4±69.3 | 206.7±69.3 | <0.0001 |
| OnTime minus OffTime | 12.7±7.1 | 22.3±23.0 | <0.0001 |
| Number of runs off state (median) | 5 (5–6) | 5 (5–6) | 0.45 |
| Number of Runs on state (median) | 5 (5–6) | 6 (5–7) | 0.12 |
| Off run 1 minus Off run 5 | −0.06 (−0.129 – 1.06) | −0.15 (−1.23 – 0.95) | 0.94 |
| On run 1 minus On run 5 | 1.37 (0.14 – 2.47) | 0.64 (−1.24 – 2.09) | 0.01 |
Data in mean ± standard deviation unless mentioned otherwise, Comparisons made using t-test and Kruskal-Wallis tests
Cross-sectional standard EncephalApp metrics
All standard metrics were higher (more impaired) in cirrhotics compared to controls and within cirrhosis was significantly higher in those with prior OHE (Tables 1 and 2). There was no difference in the number of runs required between the controls and cirrhotic patients while in OHE patients, there was a higher number of runs required in the On stage. As expected age was correlated with EncephalApp Off and OnTimes (r=0.4, p<0.001 for both) as was MELD score (r=0.2, p=0.02 OffTime and r=0.2, p=0.03, OnTime) and education (r=−0.2, p=0.02 for both). Within the cirrhosis group, no significant differences were found between alcohol/non-alcoholic etiologies, treatment status within OHE group and date of last OHE episode within the cirrhosis groups, were found with respect to standard EncephalApp metrics.
Table 2.
Baseline variable compared between compared between cirrhotic patients with and without prior overt hepatic encephalopathy
| Variable | No overt HE (N=145) | Prior overt HE (N=85) | P value |
|---|---|---|---|
| Age | 60.2±8.5 | 60.9±7.9 | 0.54 |
| Male | 123 | 75 | 0.47 |
| Years of education | 13.3±2.2 | 13.4±2.7 | 0.85 |
| MELD | 11.6±5.8 | 14.7±6.5 | <0.001 |
| Hepatitis C (N) | 77 | 33 | 0.04 |
| Non-alcoholic fatty liver (N) | 30 | 19 | 0.45 |
| Alcoholic etiology (N) | 14 | 23 | 0.01 |
| MHE based on Encephalapp stroop test only(N) | 102 | 77 | <0.001 |
Data in mean ± standard deviation unless mentioned otherwise, Comparisons made using t-test and Kruskal-Wallis tests
In the OHE subgroup 77 (90.5%) tested positive for MHE based solely on the EncephalApp stroop test. The median times for On 1–5 runs was non-significant between both the groups (0.26(−2.42,2.09) vs 1(0.15,2.4), p=0.27). In the No OHE group, 102 (70.3%) tested positive for MHE and on looking at the median run times for the On state 1–5 runs no significant difference was noted (0.70(−0.99,2.27) vs 0.71(−0.65,1.8), p=0.78), On comparing the median run times for the On 1–5 runs between No OHE and OHE groups with a positive MHE test on the EncephalApp no significance difference was noted (0.70(−0.99,2.27) vs 0.26(−2.42,2.09), p=0.26).
Learning on EncephalApp
As shown in tables 1 and 3, there was significantly more learning with On runs in controls compared to cirrhotic patients; and in cirrhotic patients without prior OHE compared to those with prior OHE. This was not seen during the Off stage. When explored further within the OHE group, EncephalApp On run learning effect did not differ as a function of treatment status (none 0.63, lactulose 2.3, Rifaximin 0.45, p=0.36). The recency of the prior OHE episode did not affect On run learning (< 6mths −0.32, 6mth-1yr: −0.35, >1 yr: 1.02, p=0.49).
Table 3.
EncephalApp Stroop Values in Cirrhosis depending on prior overt HE
| Run Times (seconds) | No Overt HE (n=145) | Prior Overt HE (n=85) | P value |
|---|---|---|---|
| Total Off | 83.2±18.8 | 107.7±30.5 | <0.0001 |
| Total On | 100.9±29.1 | 13.7.7±55.0 | <0.0001 |
| OffTime+OnTime | 185.1±47.9 | 245.4±82.8 | <0.0001 |
| OnTime minus OffTime | 17.7±13.4 | 30.0±32.3 | 0.001 |
| Number of runs off state (median) | 5 (5–6) | 5 (5–6) | 0.82 |
| Number of Runs on state (median) | 6 (5–7) | 6 (5.5–7.5) | 0.04 |
| Off run 1 minus Off run 5 | −0.12 (−1.0 – 0.78) | −0.24 (−1.84 – 1.76) | 0.86 |
| On run 1 minus On run 5 | 0.85 (−0.87 – 2.4) | 0.34 (−2.1 – 1.0) | 0.05 |
Data in mean ± standard deviation unless mentioned otherwise, Comparisons made using t-test and Kruskal-Wallis tests
Longitudinal follow-up
Twenty six cirrhotic outpatients who were a subset of the main cross-sectional cohort, were retested 3±13 weeks apart, of whom 15 had prior OHE controlled on rifaximin, while 11 were had never experienced HE. The clinical course and HE course remained stable during the visits (MELD 1st visit 11.6 vs 12.0 2nd visit, p=0.43). There were no hospitalizations, TIPS (transjugular intra-hepatic portosystemic shunting) placement or episodes of HE in between visits. As shown in Table 4, there was a significant improvement (reduction) in OnTime and OffTime+OnTime indicating overall learning in patients without prior OHE. No significant changes in any other aspect of EncephalApp were seen in these patients or those with prior OHE.
Table 4.
Patients with multiple visits (n=26)
| Run Times (seconds) | Visit 1 | Visit 2 | ||||
|---|---|---|---|---|---|---|
| All | No-OHE | Prior OHE | All | No-OHE | Prior OHE | |
| Total Off | 101.8±34.4 | 81.3±15.4 | 114.1±37.2† | 103.4±36.8 | 82.0±18.5 | 116.5±39.4† |
| Total On | 124.7±54.8 | 100.5±27.2 | 148.7±80.9† | 130.6±69.3 | 90.3±22.2* | 145.4±58.2† |
| OffTime+OnTime | 226.5±87.9 | 184.4±45.0 | 259.6±93.8† | 234.2±104.2 | 170.1±34.5* | 265.0± 118† |
| OnTime minus OffTime | 22.9±24.7 | 18.4±11.8 | 31.3±27.2† | 27.0±37.7 | 9.0±11.8* | 32.2±46.7† |
| Number of runs off state (median) | 5 | 5 | 5 | 5 | 5 | 5 |
| Number of Runs on state (median) | 6 | 6 | 6 | 6 | 6 | 6 |
| Off run 1 minus Off run 5 (median) | −0.31 | 0.11 | −1.54 | 0.10 | −0.23 | 0.43 |
| On run 1 minus On run 5 (median) | 0.78 | 0.76 | 0.80 | 0.10 | 0.51 | −0.15 |
OHE: overt hepatic encephalopathy. Fifteen patients had OHE while 11 patients did not have OHE; this was stable at both time points. Data in mean ± standard deviation unless mentioned otherwise *p<0.05 between visits 1 and 2 (Wilcoxon signed rank sum tests),†p<0.05 OHE vs. no-OHE (Kruskal-Wallis and t-tests). A positive run 1 minus run 5 score indicates lesser time spent in run 5 than run 1 reflecting learning over the test. Patients with prior OHE did not improve (decrease) their times, while those without OHE improved their times. No change in learning capability was seen over time within and between groups.
Pre/post-Liver Transplant
Twenty patients on the deceased LT list were studied pre and 6±2 months post-LT. These patients were stably controlled on tacrolimus post-LT without episodes of rejection, sepsis or hospitalizations within two months of post-LT testing. The mean age pre-LT was 57.2±6.2, fourteen were men with a mean MELD at listing of 17.4±8.5. Twelve patients had pre-LT OHE which was controlled on lactulose and rifaximin. There was a significant improvement in the total EncephalApp scores post-LT compared to the pre-LT performance (table 5). There was also a significant improvement in On 1–5 scores in those with prior OHE compared to their pre-LT baseline. This change was higher than that observed in patients without prior OHE.
Table 5.
Patients pre and post-Transplant (n=20)
| Run Times (seconds) | Pre-transplant | Post-transplant | ||||
|---|---|---|---|---|---|---|
| All | No-OHE | Prior OHE | All | No-OHE | Prior OHE | |
| Total Off | 86.4±27.8 | 76.1±8.3 | 91+34.5 | 73.4±11.3* | 76.9±9.1 | 71.1±12.3* |
| Total On | 96.5±27.2 | 100.7±23.7 | 93.7±30 | 87.1±16.8* | 91.3±3.7 | 84.2±20* |
| OffTime+OnTime | 182.9±44 | 180±34.7 | 184.7±50.7 | 160.5±27.7* | 168.3±19 | 155.3±34* |
| OnTime minus OffTime | 10.1±33.1 | 21.3±14.7 | 2.6±40 | 13.6±7.4 | 14.1±5 | 13.1±8.8 |
| Number of runs off state (median) | 5(5,6) | 5(5,5.75) | 5.5(5,7) | 5(5,5)* | 5(5,5)* | 5(5,5.75) |
| Number of Runs on state (median) | 6(5,6) | 6(5,6.75) | 6(5,6) | 5(5,6.75) | 5(5,5) | 6(5,7) |
| Off run 1 minus Off run 5 (median) | 0.74 | 0.18 | 0.84 | −0.29 | −0.40 | −0.29 |
| On run 1 minus On run 5 (median) | 0.88 | 1.64 | −0.09† | 0.80 | 0.3 | 1.70*† |
OHE: overt hepatic encephalopathy. Twelve patients had OHE while 8 patients did not have OHE pre-transplant. *p<0.05 pre vs post-LT (Wilcoxon signed rank sum test), †p<0.05 OHE vs no-OHE (Kruskal-Wallis test), Data in mean ± standard deviation unless mentioned otherwise A positive run 1 minus run 5 score indicates lesser time spent in run 5 than run 1 reflecting learning over the test. There was an improvement in EncephalApp time metrics after transplant, which was largely related to improvement in those with prior OHE. These patients also improved their learning ability on the On stage of EncephalApp.
Discussion
The study results demonstrate that cognitive flexibility, rather than psychomotor speed, is impaired after OHE development using the EncephalApp. The study has included one of the largest cohorts of cirrhotic outpatients to address the important question of whether maximally treated OHE episodes can result in lasting neuro-cognitive impairment. This is a relevant question because prior studies (24) have demonstrated that the persistence of impairment can lead to a severe burden on patients, caregivers and the healthcare system. A prior diagnosis of OHE and cognitive impairment also modulates the extent of cognitive and brain functional recovery after liver transplant.
While the greater extent of EncephalApp impairment in patients with a prior history of OHE is to be expected, the changes in the On run, rather than the Off run, of the EncephalApp are intriguing. While prior OHE affects both runs of the test, as was reconfirmed by our results, the individual contribution of On and Off runs towards differentiating cognitively impaired and unimpaired subjects is relatively equal. Therefore, the lack of improvement, and actual deterioration over time with the On run, but not the Off runs points towards nuanced changes within the brain after OHE that cannot be picked up by simple addition of the total Off and OnTimes.
The Stroop test, which assesses selective attention, processing speed, and in the On runs, response inhibition, has been studied in a variety of neuro-degenerative disorders(12, 13). Prior studies have demonstrated that response inhibition is impaired in patients with prior OHE using the ICT(4, 6). Additional studies demonstrated that this impaired learning was found over time using the PHES in HE patients(5, 6). This is the first experience using EncephalApp, which extends these findings into a separate, larger population with longitudinal confirmation.
The EncephalApp tests two separate cognitive domains within its Off and On run assessment at the same sitting. The Off run measures psychomotor speed, which is a function of cortical tone and arousal. The On run of the test examines response inhibition, which is primarily a function of lateral pre-frontal cortex integrity (the development of task specific rules for the brain to perform response inhibition) and the anterior cingulate cortex (allocation of attentional resources), and is an excellent measure of overall cognitive flexibility. The results that show that Off run performance remains stable over time both within and between the subject groups regardless of the disease severity; while On run results can improve in non-OHE populations is intriguing. This suggests that practice effects and/or increased familiarity with the Off run trial did not benefit psychomotor speed. Level of achievement and pattern of performance remained constant for controls and cirrhotics subjects at the longitudinal follow-up and pre-post-transplant analyses. Therefore, the benefit derived from task exposure is relatively equal with regard to the total times for OHE patients and cirrhotics greater than no-OHE and controls, respectively. Even when exposed to the EncephalApp longitudinally, the Off time totals improved but without changes in the individual runs or learning. These findings point to a fixed ceiling for psychomotor speed, beyond which there can be little improvement.
In contrast, we found variability in On run performance between trial 1 and trial 5 that can potentially be attributed to improved response inhibition and cognitive flexibility due to increasing familiarity with the task requirements (i.e., a learning effect). This was seen in healthy controls as well as in cirrhotic groups without prior OHE; but was not found in groups with prior OHE. Interestingly, repeated exposure to this test over time neither improved the total EncephalApp metrics nor the On stage learning in prior OHE patients before liver transplant. This lack of learning was not a function of the treatments employed, etiology of cirrhosis, nor timing of the last HE episode. Therefore, the OHE patients’ inability to demonstrate a learning effect from prior task stimuli exposure during the EncephalApp On runs may represent a harbinger of a dementing process in cirrhotic patients. This poor correlation with OHE episode characteristics replicates a prior study in which the development of OHE, rather than relevant medical phenomenology was associated with poor survival(25). Therefore, reaching the OHE stage may mark a major milestone, not only with regard to overall clinical endpoints, but perhaps also in terms of higher cortical dysfunction despite our current standard of care therapy.
It is also important to note that despite these pre-transplant changes, OHE patients whose post-transplant course was stable, significantly improved on the overall EncephalApp metrics, as well as regained their capacity to learn. This is important because it reflects the potential reversibility of at least some aspects of neuropathology associated with successful transplant, despite the confounding factors of calcineurin therapy. Reversibility of this cognitive deficit post-transplant is an important observation that points towards its liver-associated origin. Prior studies have shown OHE patients have a variable outcome post-transplant(22, 26). A recent study demonstrated that a simple pre-transplant OHE diagnosis was not a powerful predictor of post-transplant cognitive impairment on multi-modal MR imaging(27, 28). Therefore, the restoration of this learning ability points towards a hopeful illness course after transplant in these patients.
While we did not specifically examine the mechanisms accounting for changes in cognitive flexibility and response inhibition in OHE patients, prior studies have examined an altered gut-liver-brain axis with dysbiosis, sarcopenia, as well as structural and functional brain changes as contributors(29). Indeed, in an fMRI study examining the functional basis of cognitive change after liver transplantation Ahluwalia et al., found robust activation in areas within the dorsolateral prefrontal cortex and anterior cingulate cortex(27). These brain regions are hypothesized to underlie successful Stroop test performance. Regardless of the specific mechanism of action, the data demonstrate the neurobehavioral decline associated with an OHE diagnosis(i.e., learning capability) is to some extent reversible. These data speak to a remarkable resiliency of brain function. Since our focus was on evaluation of OHE patients’ recovery, the longitudinal studies had adequate numbers of OHE patients based on prior experiences, however the patients without OHE may be lower than required to definitively make the conclusions longitudinally(4, 27). However, prior data and cross-sectional analyses would not necessarily predict changes in no-OHE patients over time.
The clinical implications of these findings include recognizing the executive dysfunction in this patient population and the development of specific communication and treatment strategies to minimize their impact on patient care. For example, involving caregivers in the decision-making process, simplifying complicated dietary and medical advice, and potentially including Apps or other educational tools may prove efficacious. In addition, counseling that some these cognitive deficits may be reversible with transplant may improve treatment compliance and the patient’s emotional function.
We conclude that despite current maximal treatment and normal mentation, learning, but not psychomotor speed assessed by the EncephalApp, is impaired in patients with overt hepatic encephalopathy. Patients with prior overt hepatic encephalopathy are able to regain their learning ability after successful liver transplant. Clinicians should keep these learning deficits in mind when communicating with patients with overt hepatic encephalopathy to potentially improve both patient care and their understanding of the disease process.
Acknowledgments
Grants and Financial Support: Partly supported by VA Merit Review grant 1I0CX001076 and RO1DK089713 to JSB
Abbreviations
- HE
hepatic encephalopathy
- OHE
overt hepatic encephalopathy
- no-OHE
patients without prior overt hepatic encephalopathy
- CHE
covert hepatic encephalopathy
- ICT
inhibitory control test
- PHES
psychometric hepatic encephalopathy score
- TIPS
transjugular intra-hepatic portosystemic shunting
- LT
liver transplant
- MRI
magnetic resonance imaging
Footnotes
Conflicts of Interest: None for any author
References
- 1.Vilstrup H, Amodio P, Bajaj J, Cordoba J, Ferenci P, Mullen KD, Weissenborn K, Wong P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology. 2014;60(2):715–35. doi: 10.1002/hep.27210. [DOI] [PubMed] [Google Scholar]
- 2.Bajaj JS, Cordoba J, Mullen KD, Amodio P, Shawcross DL, Butterworth RF, Morgan MY, Nitrogen M International Society for Hepatic E. Review article: the design of clinical trials in hepatic encephalopathy--an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Alimentary pharmacology & therapeutics. 2011;33(7):739–47. doi: 10.1111/j.1365-2036.2011.04590.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. Journal of hepatology. 2001;34(5):768–73. doi: 10.1016/s0168-8278(01)00026-5. Epub 2001/07/04. doi:S0168-8278(01)00026-5 [pii] [DOI] [PubMed] [Google Scholar]
- 4.Bajaj JS, Schubert CM, Heuman DM, Wade JB, Gibson DP, Topaz A, Saeian K, Hafeezullah M, Bell DE, Sterling RK, Stravitz RT, Luketic V, White MB, Sanyal AJ. Persistence of cognitive impairment after resolution of overt hepatic encephalopathy. Gastroenterology. 2010;138(7):2332–40. doi: 10.1053/j.gastro.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riggio O, Ridola L, Pasquale C, Nardelli S, Pentassuglio I, Moscucci F, Merli M. Evidence of persistent cognitive impairment after resolution of overt hepatic encephalopathy. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2011;9(2):181–3. doi: 10.1016/j.cgh.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Nardelli S, Allampati S, Riggio O, Mullen KD, Prakash R, Gioia S, Unser A, White MB, Fagan AC, Wade JB, Farcomeni A, Gavis EA, Bajaj JS. Hepatic Encephalopathy Is Associated with Persistent Learning Impairments Despite Adequate Medical Treatment: A Multicenter, International Study. Digestive diseases and sciences. 2017;62(3):794–800. doi: 10.1007/s10620-016-4425-6. [DOI] [PubMed] [Google Scholar]
- 7.Matsusue E, Kinoshita T, Ohama E, Ogawa T. Cerebral cortical and white matter lesions in chronic hepatic encephalopathy: MR-pathologic correlations. AJNR Am J Neuroradiol. 2005;26(2):347–51. [PMC free article] [PubMed] [Google Scholar]
- 8.Gorg B, Qvartskhava N, Bidmon HJ, Palomero-Gallagher N, Kircheis G, Zilles K, Haussinger D. Oxidative stress markers in the brain of patients with cirrhosis and hepatic encephalopathy. Hepatology. 2010;52(1):256–65. doi: 10.1002/hep.23656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18(6):643–62. [Google Scholar]
- 10.Bajaj JS, Heuman DM, Sterling RK, Sanyal AJ, Siddiqui M, Matherly S, Luketic V, Stravitz RT, Fuchs M, Thacker LR, Gilles H, White MB, Unser A, Hovermale J, Gavis E, Noble NA, Wade JB. Validation of EncephalApp, Smartphone-Based Stroop Test, for the Diagnosis of Covert Hepatic Encephalopathy. Clinical gastroenterology and hepatology: the official clinical practice journal of the American Gastroenterological Association. 2015;13(10):1828–35e1. doi: 10.1016/j.cgh.2014.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bajaj JS, Thacker LR, Heuman DM, Fuchs M, Sterling RK, Sanyal AJ, Puri P, Siddiqui MS, Stravitz RT, Bouneva I, Luketic V, Noble N, White MB, Monteith P, Unser A, Wade JB. The Stroop smartphone application is a short and valid method to screen for minimal hepatic encephalopathy. Hepatology. 2013;58(3):1122–32. doi: 10.1002/hep.26309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wecker NS, Kramer JH, Wisniewski A, Delis DC, Kaplan E. Age effects on executive ability. Neuropsychology. 2000;14(3):409–14. doi: 10.1037//0894-4105.14.3.409. [DOI] [PubMed] [Google Scholar]
- 13.Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: a parallel distributed processing account of the Stroop effect. Psychol Rev. 1990;97(3):332–61. doi: 10.1037/0033-295x.97.3.332. [DOI] [PubMed] [Google Scholar]
- 14.Zhang LJ, Yang G, Yin J, Liu Y, Qi J. Neural mechanism of cognitive control impairment in patients with hepatic cirrhosis: a functional magnetic resonance imaging study. Acta Radiol. 2007;48(5):577–87. doi: 10.1080/02841850701308378. [DOI] [PubMed] [Google Scholar]
- 15.Banich MT, Milham MP, Atchley R, Cohen NJ, Webb A, Wszalek T, Kramer AF, Liang ZP, Wright A, Shenker J, Magin R. fMri studies of Stroop tasks reveal unique roles of anterior and posterior brain systems in attentional selection. J Cogn Neurosci. 2000;12(6):988–1000. doi: 10.1162/08989290051137521. [DOI] [PubMed] [Google Scholar]
- 16.Basso MR, Bornstein RA, Lang JM. Practice effects on commonly used measures of executive function across twelve months. Clin Neuropsychol. 1999;13(3):283–92. doi: 10.1076/clin.13.3.283.1743. [DOI] [PubMed] [Google Scholar]
- 17.Lemay S, Bedard MA, Rouleau I, Tremblay PL. Practice effect and test-retest reliability of attentional and executive tests in middle-aged to elderly subjects. Clin Neuropsychol. 2004;18(2):284–302. doi: 10.1080/13854040490501718. [DOI] [PubMed] [Google Scholar]
- 18.Axelrod BN, Brines B, Rapport LJ. Estimating Full Scale IQ While Minimizing the Effects of Practice. Assessment. 1997;4(3):221–7. doi: 10.1177/107319119700400302. [DOI] [PubMed] [Google Scholar]
- 19.Ahluwalia V, Wade JB, Heuman DM, Hammeke TA, Sanyal AJ, Sterling RK, Stravitz RT, Luketic V, Siddiqui MS, Puri P, Fuchs M, Lennon MJ, Kraft KA, Gilles H, White MB, Noble NA, Bajaj JS. Enhancement of functional connectivity, working memory and inhibitory control on multi-modal brain MR imaging with Rifaximin in Cirrhosis: implications for the gut-liver-brain axis. Metabolic brain disease. 2014;29(4):1017–25. doi: 10.1007/s11011-014-9507-6. Epub 2014/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorensen LG, Neighbors K, Martz K, Zelko F, Bucuvalas JC, Alonso EM Studies of Pediatric Liver T, Functional Outcomes G. Cognitive and academic outcomes after pediatric liver transplantation: Functional Outcomes Group (FOG) results. Am J Transplant. 2011;11(2):303–11. doi: 10.1111/j.1600-6143.2010.03363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore KA, Mc LJR, Burrows GD. Quality of life and cognitive function of liver transplant patients: a prospective study. Liver Transpl. 2000;6(5):633–42. doi: 10.1053/jlts.2000.9743. [DOI] [PubMed] [Google Scholar]
- 22.Campagna F, Montagnese S, Schiff S, Biancardi A, Mapelli D, Angeli P, Poci C, Cillo U, Merkel C, Gatta A, Amodio P. Cognitive impairment and electroencephalographic alterations before and after liver transplantation: what is reversible? Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2014;20(8):977–86. doi: 10.1002/lt.23909. [DOI] [PubMed] [Google Scholar]
- 23.Allampati S, Duarte-Rojo A, Thacker LR, Patidar KR, White MB, Klair JS, John B, Heuman DM, Wade JB, Flud C, O’Shea R, Gavis EA, Unser AB, Bajaj JS. Diagnosis of Minimal Hepatic Encephalopathy Using Stroop EncephalApp: A Multicenter US-Based, Norm-Based Study. The American journal of gastroenterology. 2016;111(1):78–86. doi: 10.1038/ajg.2015.377. [DOI] [PubMed] [Google Scholar]
- 24.Bajaj JS, Wade JB, Gibson DP, Heuman DM, Thacker LR, Sterling RK, Stravitz RT, Luketic V, Fuchs M, White MB, Bell DE, Gilles H, Morton K, Noble N, Puri P, Sanyal AJ. The multi-dimensional burden of cirrhosis and hepatic encephalopathy on patients and caregivers. The American journal of gastroenterology. 2011;106(9):1646–53. doi: 10.1038/ajg.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucidi C, Ginanni Corradini S, Abraldes JG, Merli M, Tandon P, Ferri F, Parlati L, Lattanzi B, Poli E, Di Gregorio V, Farcomeni A, Riggio O. Hepatic encephalopathy expands the predictivity of model for end-stage liver disease in liver transplant setting: Evidence by means of 2 independent cohorts. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2016;22(10):1333–42. doi: 10.1002/lt.24517. [DOI] [PubMed] [Google Scholar]
- 26.Mattarozzi K, Cretella L, Guarino M, Stracciari A. Minimal hepatic encephalopathy: follow-up 10 years after successful liver transplantation. Transplantation. 2012;93(6):639–43. doi: 10.1097/TP.0b013e318244f734. [DOI] [PubMed] [Google Scholar]
- 27.Ahluwalia V, Wade JB, White MB, Gilles HS, Heuman DM, Fuchs M, Gavis EA, Fagan A, Tinsley F, Ganapathy D, Thacker LR, Sterling RK, Stravitz RT, Puri P, Sanyal AJ, Siddiqui MS, Matherly S, Luketic V, Steinberg J, Moeller FG, Bajaj JS. Liver transplantation significantly improves global functioning and cerebral processing. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2016;22(10):1379–90. doi: 10.1002/lt.24498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajaj JS, Kamath PS. The brain gets its say: Hepatic encephalopathy and its evolving role in transplant priority. Liver transplantation: official publication of the American Association for the Study of Liver Diseases and the International Liver Transplantation Society. 2016;22(10):1319–20. doi: 10.1002/lt.24521. [DOI] [PubMed] [Google Scholar]
- 29.Ahluwalia V, Betrapally NS, Hylemon PB, White MB, Gillevet PM, Unser AB, Fagan A, Daita K, Heuman DM, Zhou H, Sikaroodi M, Bajaj JS. Impaired Gut-Liver-Brain Axis in Patients with Cirrhosis. Scientific reports. 2016;6:26800. doi: 10.1038/srep26800. [DOI] [PMC free article] [PubMed] [Google Scholar]