Introduction
Pulmonary arterial hypertension (PAH) is an obstructive vasculopathy in which vascular cells proliferate too rapidly and/or are resistant to apoptosis. PAH is also characterized by increases in inflammation, vasoconstriction and fibrosis. Ultimately these vascular changes increase right ventricular afterload leading to heart failure and death.
Pulmonary vascular cells from patients with PAH have adaptations that ensure their high rates of proliferation are not thwarted by energy limitation, mitochondrial-mediated apoptosis or inadequate rates of mitotic fission1. Histological examination reveals the consequences of this proliferative diathesis, including: intimal hyperplasia and plexiform lesions, medial hypertrophy, and adventitial fibrosis. The resulting endothelial dysfunction favours thrombosis and vasoconstriction, the hyperplasia of pulmonary artery smooth muscle cells (PASMC) favors vasoconstriction and the adventitial fibrosis results in stiff, noncompliant arteries.
Two articles in the current edition of Circulation describe a metabolic adaptation that is good for the abnormal cell but bad for the patient, namely a shift in glucose metabolism called the Warburg phenomenon. Otto Warburg, a German Nobel laureate, described cancer’s predilection to support its rapid growth by a reliance on glycolysis, despite an abundance of available oxygen. Warburg metabolism is a failure of two fundamental pathways, glucose metabolism and mitochondrial oxygen sensing.
Glycolysis and glucose oxidation, are normally coupled, meaning they occur in proportion to each other. The final products of glycolysis are ATP and pyruvate. Pyruvate enters the mitochondria via the mitochondrial pyruvate transporter (MTP) and acts as the substrate for pyruvate dehydrogenase (PDH), which regulates glucose oxidation and supplies acetyl CoA to Krebs’ cycle (Figure). Warburg metabolism represents a state of uncoupled glycolysis, in that glycolysis is disproportionately elevated compared to mitochondrial pyruvate utilization and glucose oxidation. Warburg metabolism allows the cell a source of energy (glycolysis) while suppressing mitochondrial apoptosis and stimulating cell cycle progression.
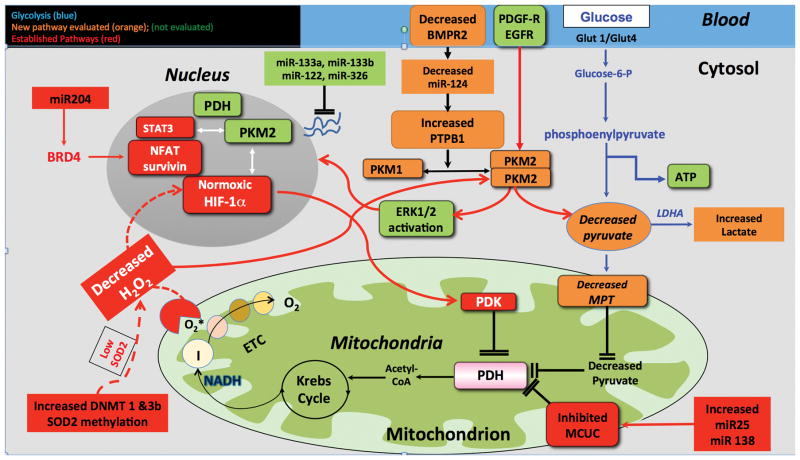
Figure 1. A Growing Cast of Warburg Mediators.
A: The new cast: Decreased miR-124 expression and/or BMPR2 downregulation increases PTBP1 expression which alters PK splicing creating a PKM2/PKM1 ratio increase that favours uncoupled glycolysis (Warburg metabolism). This metabolic shift is associated with endothelial cell and fibroblast proliferation. The nuclear effects of PKM2, as a regulator of histone phosphorylation and potentiator of HIF-1α activation, were not studied. Likewise, PKM2’s ability to promote cell proliferation by activating ERK1/2 was not assessed. An oxidant environment, such as elevated H2O2 levels, inhibits PKM2 activity.
B) The old cast: Increased lung specific activation of DNMT 1 and 3b methylate the SOD2 promoter and reduce SOD2 levels and ETC 1 activity. This impairs oxygen sensing and leads to normoxic HIF-1α activation. HIF-1α increases PDK expression and promotes PASMC proliferation2. In addition, increased expression of miR25 and miR138 decrease function of the mitochondrial calcium uniporter complex (MCUC). This suppresses MCUC function-raises cytosolic calcium and lowers intramitochondrial calcium-causes vasoconstriction and impairs PDH activity. Loss of the MCU function also causes mitochondrial fission. These effects together promote PASMC proliferation and inhibits apoptosis3. PDH also translocates to the nucleus where it regulates histone acetylation. Finally decreases in miR-204 increase expression of bromodomain containing protein 4 (BRD4) that binds to acetylated histone via its tandem bromodomains 4. This activates nuclear factor of activated T cells (NFAT) and survivin.
The Warburg phenomenon also results from changes in the function of the cell’s acute and chronic oxygen-sensing systems. In response to rapid reductions in airway oxygen, the adjacent small pulmonary arteries (which are also critically involved in PAH) constrict. This diverts blood to better-oxygenated lung segments and optimizes PO2. Hypoxic pulmonary vasoconstriction (HPV)5 is triggered by dynamic decreases in production of reactive oxygen species (ROS) by the mitochondrial electron transport chain (ETC). This decreases generation of the diffusible redox mediator, H2O2 by mitochondrial superoxide dismutase 2 (SOD2), leading to redox regulation of oxygen-sensitive ion channels and enzymes, culminating in vasoconstriction5. When hypoxia is sustained, transcription factors, notably hypoxia inducible factor (HIF-1α) mediate metabolic adaptation, notably shifting the lung to an energetic reliance on glycolysis. While glycolysis is 8-fold less efficient than glucose oxidation, when upregulated, it generates sufficient ATP to maintain energy homeostasis. In cancer and PAH, epigenetically-mediated changes in gene expression inhibit key genes involved in oxygen-sensing and metabolism pathways despite abundant oxygen, creating a state of pseudohypoxia6.
Epigenetic mechanisms alter gene expression without changing gene sequences. They include, DNA methylation, mediated by DNA methyltransferases (DNMT), histone acetylation and micro RNAs (miRs). miRs are short, noncoding RNAs, which are encoded by intronic DNA. They destabilize target mRNA, leading to decreased translation of a gene product. Lower levels of a miR translate to increased levels of its target mRNA’s product. These epigenetic mechanisms are networked and potentially reversible.
The Warburg metabolic phenotype is generated in PAH PASMC by a network of epigenetic changes that include lung-specific upregulation of DNMT 1 and DNMT3b. The resulting methylation of CpG islands in SOD2’s promoter2 impairs transcription, reduces SOD2 levels and decreases H2O2 production. The resulting redox perturbations cause normoxic activation of HIF-1α HIF-1α upregulates key enzymes that inhibit oxidative metabolism, notably, pyruvate dehydrogenase kinase (PDK). Mitochondrial PDKs phosphorylate and inhibit PDH, which inhibits production of Acetyl-CoA and consequently the cell shifts its source of ATP to uncoupled glycolysis. This pathway is called the DNMT-SOD2-HIF-1α-PDK-PDH pathway2.
In paired Circulation articles, Caruso7 and Zhang8 fill the Warburg stage with a new cast of Warburg players in PA endothelial cells and fibroblasts, respectively. They show that pyruvate production is inhibited by a change in the activity of pyruvate kinase (PK). PK, the final and rate-limiting step in glycolysis, catalyzes phosphate transfer from phosphoenolpyruvate (PEP) to ADP, producing pyruvate and ATP (Figure). The mitochondrial supply of pyruvate is further compromised by downregulation of the MPT. Both groups demonstrate that this pro-glycolytic mechanism is stimulated by upregulation of a heterogeneous nuclear ribonucleoprotein, polypyrimidine tract-binding protein (PTBP1). PTBP1 acts as a repressor and binds to the flanking sequences of exon 9, thus inhibiting the PKM1 isoform. The resulting increase in the PKM2/PKM1 ratio favours uncoupled glycolysis.
Caruso et al used an unbiased systems biology approach to identify miRNA and proteomic profiles of blood outgrowth endothelial cells (BOECs) from patients with idiopathic PAH or heritable PAH, due to mutations of bone morphogenetic protein receptor type 2 (BMPR2). They show that decreased production of micro RNA-124 (miR-124) increases PTBP1 expression. miR-124 regulates PTBP1 in cancer cells9. PTBP1 alters PK gene splicing, increasing PKM2 and decreasing PKM1, leading to increased glycolysis in PA endothelial cells and BOECs from PAH patients and in a rodent PAH model (SU5416-hypoxia). This miR-124/PTBP1/PKM2 pathway is well established in the cancer literature where it promotes glycolysis, increases cell proliferation and causes apoptosis resistance9. Desai et al found the switch from PKM1 to PKM2 in 16 tumor types10.
Caruso’s work offers three additional discoveries. First, readily accessible BOECs and hard to obtain human PA endothelial cells have similar metabolic reprogramming, supporting the use of BOECs in PAH research. Second, loss of BMPR2 drives proliferation by promoting Warburg metabolism. Thus, metabolic therapies may be an ideal way to treat PAH. Third, they address a controversy in the PAH field: What is the predominant change in endothelial cell fate in PAH: increased proliferation versus increased apoptosis? These theories are opposing, although this paradox may be related to temporal aspects of pathogenesis (i.e. the increased apoptosis is postulated to be an early injury response whereas the proliferative response is thought to occur later in the disease). In their study Caruso provides clear evidence that the net effect of the Warburg metabolic shift in PAH is accelerated endothelial cell proliferation.
Two observations emerge from Caruso’s data that were not discussed. First, they state that endothelial cells don’t use glucose oxidation and interpret the benefits of inhibiting PKM2 solely as reflecting decreased glycolysis. However, in PAH BOECs there was suppression of glucose oxidation and this was reversed by both miR-124 mimic and siPTBP1 (Caruso Figure 7H)7. Thus, endothelial cells may be quantitatively less reliant on glucose oxidation than PASMC, but if a cell has mitochondria, and is not “Warburgian”, it will oxidize glucose. Second, while the study focuses on the miR-124/PTBP1/PKM pathway in endothelial cells, examination of the PASMC shows identical upregulation of PTBP1 (Caruso Figures 3 and 8)7.
Zhang shows that this miR-124-PTBP1-PKM2-Warburg pathway creates a similar proliferative phenotype in fibroblasts derived from patients with PAH or calves with hypoxic pulmonary hypertension (PH)8. Zhang et al also show that mitochondrial pyruvate uptake is diminished due to MPT downregulation, as occurs in cancer11. They conclude that miR-124-mediated PTBP1upregulation underlies the increase in PKM2/PKM1 in PAH fibroblasts. Unlike Caruso et al, they found no increase in absolute expression of PKM2; rather the ratio shift was largely driven by PKM1 downregulation8. This PKM isoform shift both increased glycolysis and suppressed glucose oxidation. Inhibiting PKM2 in fibroblasts enhanced mitochondrial respiratory activity and rescued complex I activity. Both Warburg metabolism and increased fibroblast proliferation were reversed by normalizing the PKM2/PKM1 ratio, achieved by miR-124 overexpression or PTBP1 knockdown.
This Warburg shift also accounted for inflammatory signaling in the fibroblast. Inhibiting the miR-124/PTBP1/PKM pathway reduces macrophage IL-1β expression, consistent with a dynamic glycolytic-mitochondrial interaction between fibroblasts and inflammatory cells in PAH. This is consistent with findings in colorectal cancer, where PKM2 is an inflammatory mediator, acting through STAT3 signaling12.
The Zhang paper also hints at this pathway’s therapeutic relevance8. The PKM2 tetramer has greater activity than the dimeric form13. Zhang et al stabilized the enzyme in its tetrameric form using TEPP-46, a small molecule, and shikonin, a Chinese herbal remedy. These interventions promote coupled glycolysis and attenuate the proliferative phenotype.
One limitation shared by both these elegant studies is the lack of in vivo evaluation of the therapeutic impact in a preclinical PAH model. The lack of this intervention is surprising since this pathway is deranged in the SU5416 rat model. It is feasible to deliver modified miRs and siRNA to the lung vasculature by nebulization with therapeutic benefit3, 4. Therapeutic interventions in other epigenetic Warburg pathways in PAH have been tested. For example, the DNMT-SOD2-HIF-1α-PDK-PDH pathway can be inhibited using decitabine, dichloroacetate or anti-miRs. These interventions reduce cell proliferation, increase apoptosis and regress experimental PAH.
Future Directions
The articles leave several important areas to address in future studies.
First, while both papers focus on miR-124 and PTBP1 as regulators of the metabolic switch, PKM2 is also directly regulated by DNA methylation, transcription factors, and PKM2-specific miRs, including miR-122, -133a -133b and -326 (Figure)9. It is unknown whether these mechanisms are important to PKM2 dysregulation in PAH.
Second, while the authors acknowledge that some of the observed Warburg effect likely reflects the pseudohypoxic state (related to HIF-1α) originally described in PAH PASMC2, they do not study the interactions between these pathways. The PKM2 isoform is a transcriptional coactivator of HIF-1α and increases HIF-1α activity by enhancing its binding to hypoxia response elements14. It would be profitable to see how the miR-124/PTBP1/PKM and DNMT-SOD2-HIF-PDK-PDH pathways interact in PAH.
Third, while the authors propose PKM2’s proliferative effects are mediated via altered metabolism, there are 5 other mechanisms by which PK may regulate cell accumulation: 1) Although not assessed in these articles, it is likely that PKM2 reduces mitochondrial-mediated apoptosis, as it does in cancer15. 2) In addition, PKM2 is the cytosolic receptor for thyroid hormone16, relevant because PAH is associated with a very high prevalence of thyroid disease. 3) PKM2 is a protein kinase that activates extracellular signal related kinase 1 and 2 (ERK1/2), thereby stimulating mitosis17. 4) In response to stimuli that abound in PAH and cancer, such as activation of platelet-derived growth factor receptors (PDGF-R), PKM2 translocates to the nucleus18, 19. Nuclear PKM2 activates β-catenin and induces c-Myc expression. cMyc reinforces glycolysis by upregulation of the glucose transporter, GLUT1 and lactate dehydrogenase A (LDHA). Nuclear PKM2 also phosphorylates histones and regulates cyclin D1 expression, accelerating the G1-S phase transition9. There is precedent for metabolic enzymes acting within the nucleus, notably the elegant demonstration that nuclear PDH generates the acetyl-coA required for histone regulation20. 5) PKM2 also increases glutaminolysis. This represents an energetic compensation for the loss of mitochondrial ATP and, in cancer, promotes cell survival and cell growth. In the pressure-overloaded RV, induction of glutaminolysis promotes hypertrophy and hypokinesis and suppresses glucose oxidation21.
Conclusion
The miR-124/PTBP1/PKM2 pathway joins the DNMT-SOD2-HIF-PDK-PDH pathway, and several others epigenetic pathways as the means by which abnormal pulmonary vascular cells achieve a Warburg metabolic state that promotes cell proliferation. Warburg metabolism and its epigenetic regulators are high-value therapeutic targets in PAH.
Acknowledgments
I gratefully acknowledge the editorial assistance of Dr. Kimberley Dunham-Snary and Dr. Asish Das Gupta for proof reading this editorial.
Funding Sources: SLA and this research are supported by a CIHR Foundation Grant, NIH-RO1-HL071115, 1RC1HL099462, a Tier 1 Canada Research Chair in Mitochondrial Dynamics, the William J Henderson Foundation and the CIHR Vascular Network and the Canadian Vascular Network.
Footnotes
Disclosures: No relevant disclosures
References
- 1.Archer SL. Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. The New England journal of medicine. 2013;369:2236–51. doi: 10.1056/NEJMra1215233. [DOI] [PubMed] [Google Scholar]
- 2.Archer SL, Marsboom G, Kim GH, Zhang HJ, Toth PT, Svensson EC, Dyck JR, Gomberg-Maitland M, Thebaud B, Husain AN, Cipriani N, Rehman J. Epigenetic attenuation of mitochondrial superoxide dismutase 2 in pulmonary arterial hypertension: a basis for excessive cell proliferation and a new therapeutic target. Circulation. 2010;121:2661–71. doi: 10.1161/CIRCULATIONAHA.109.916098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hong Z, Chen KH, DasGupta A, Potus F, Dunham-Snary K, Bonnet S, Tian L, Fu J, Breuils-Bonnet S, Provencher S, Wu D, Mewburn J, Ormiston ML, Archer SL. MicroRNA-138 and MicroRNA-25 Down-regulate Mitochondrial Calcium Uniporter, Causing the Pulmonary Arterial Hypertension Cancer Phenotype. Am J Respir Crit Care Med. 2017;195:515–529. doi: 10.1164/rccm.201604-0814OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meloche J, Potus F, Vaillancourt M, Bourgeois A, Johnson I, Deschamps L, Chabot S, Ruffenach G, Henry S, Breuils-Bonnet S, Tremblay E, Nadeau V, Lambert C, Paradis R, Provencher S, Bonnet S. Bromodomain-Containing Protein 4: The Epigenetic Origin of Pulmonary Arterial Hypertension. Circ Res. 2015;117:525–35. doi: 10.1161/CIRCRESAHA.115.307004. [DOI] [PubMed] [Google Scholar]
- 5.Weir EK, Lopez-Barneo J, Buckler KJ, Archer SL. Acute oxygen-sensing mechanisms. The New England journal of medicine. 2005;353:2042–55. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archer SL, Gomberg-Maitland M, Maitland ML, Rich S, Garcia JG, Weir EK. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1alpha-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol Heart Circ Physiol. 2008;294:H570–8. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- 7.Caruso P, Dunmore BJ, Schlosser K, Schoors S, Dos Santos CC, Perez-Iratxeta C, Lavoie JR, Zhang H, Long L, Flockton AR, Frid MG, Upton PD, D’Alessandro A, Hadinnapola C, Kiskin FN, Taha M, Hurst LA, Ormiston ML, Hata A, Stenmark KR, Carmeliet P, Stewart DJ, Morrell NW. Identification of miR-124 as a Major Regulator of Enhanced Endothelial Cell Glycolysis in Pulmonary Arterial Hypertension via PTBP1 and PKM2. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.117.028034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H, Wang D, Li M, Plecita-Hlavata L, D’Alessandro A, Tauber J, Riddle S, Kumar S, Flockton AR, McKeon BA, Frid MG, Reisz JA, Caruso P, El Kasmi KC, Jezek P, Morrell NW, Hu CJ, Stenmark KR. The Metabolic and Proliferative State of Vascular Adventitial Fibroblasts in Pulmonary Hypertension is Regulated through a MiR-124/PTBP1/PKM Axis. Circulation. 2017 doi: 10.1161/CIRCULATIONAHA.117.028069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang W, Lu Z. Pyruvate kinase M2 at a glance. J Cell Sci. 2015;128:1655–60. doi: 10.1242/jcs.166629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai S, Ding M, Wang B, Lu Z, Zhao Q, Shaw K, Yung WK, Weinstein JN, Tan M, Yao J. Tissue-specific isoform switch and DNA hypomethylation of the pyruvate kinase PKM gene in human cancers. Oncotarget. 2014;5:8202–10. doi: 10.18632/oncotarget.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schell JC, Olson KA, Jiang L, Hawkins AJ, Van Vranken JG, Xie J, Egnatchik RA, Earl EG, DeBerardinis RJ, Rutter J. A role for the mitochondrial pyruvate carrier as a repressor of the Warburg effect and colon cancer cell growth. Mol Cell. 2014;56:400–13. doi: 10.1016/j.molcel.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang P, Li Z, Li H, Lu Y, Wu H, Li Z. Pyruvate kinase M2 accelerates pro-inflammatory cytokine secretion and cell proliferation induced by lipopolysaccharide in colorectal cancer. Cell Signal. 2015;27:1525–32. doi: 10.1016/j.cellsig.2015.02.032. [DOI] [PubMed] [Google Scholar]
- 13.Dombrauckas JD, Santarsiero BD, Mesecar AD. Structural basis for tumor pyruvate kinase M2 allosteric regulation and catalysis. Biochemistry. 2005;44:9417–29. doi: 10.1021/bi0474923. [DOI] [PubMed] [Google Scholar]
- 14.Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–44. doi: 10.1016/j.cell.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morfouace M, Lalier L, Oliver L, Cheray M, Pecqueur C, Cartron PF, Vallette FM. Control of glioma cell death and differentiation by PKM2-Oct4 interaction. Cell Death Dis. 2014;5:e1036. doi: 10.1038/cddis.2013.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato H, Fukuda T, Parkison C, McPhie P, Cheng SY. Cytosolic thyroid hormone-binding protein is a monomer of pyruvate kinase. Proc Natl Acad Sci U S A. 1989;86:7861–5. doi: 10.1073/pnas.86.20.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keller KE, Doctor ZM, Dwyer ZW, Lee YS. SAICAR induces protein kinase activity of PKM2 that is necessary for sustained proliferative signaling of cancer cells. Mol Cell. 2014;53:700–9. doi: 10.1016/j.molcel.2014.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang W, Zheng Y, Xia Y, Ji H, Chen X, Guo F, Lyssiotis CA, Aldape K, Cantley LC, Lu Z. ERK1/2-dependent phosphorylation and nuclear translocation of PKM2 promotes the Warburg effect. Nat Cell Biol. 2012;14:1295–304. doi: 10.1038/ncb2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lv L, Xu YP, Zhao D, Li FL, Wang W, Sasaki N, Jiang Y, Zhou X, Li TT, Guan KL, Lei QY, Xiong Y. Mitogenic and oncogenic stimulation of K433 acetylation promotes PKM2 protein kinase activity and nuclear localization. Mol Cell. 2013;52:340–52. doi: 10.1016/j.molcel.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutendra G, Kinnaird A, Dromparis P, Paulin R, Stenson TH, Haromy A, Hashimoto K, Zhang N, Flaim E, Michelakis ED. A nuclear pyruvate dehydrogenase complex is important for the generation of acetyl-CoA and histone acetylation. Cell. 2014;158:84–97. doi: 10.1016/j.cell.2014.04.046. [DOI] [PubMed] [Google Scholar]
- 21.Piao L, Sidhu VK, Fang YH, Ryan JJ, Parikh KS, Hong Z, Toth PT, Morrow E, Kutty S, Lopaschuk GD, Archer SL. FOXO1-mediated upregulation of pyruvate dehydrogenase kinase-4 (PDK4) decreases glucose oxidation and impairs right ventricular function in pulmonary hypertension: therapeutic benefits of dichloroacetate. J Mol Med (Berl) 2013;91:333–46. doi: 10.1007/s00109-012-0982-0. [DOI] [PMC free article] [PubMed] [Google Scholar]