Abstract
Bovine tuberculosis (bTB) is a known endemic disease of cattle in Ethiopia; however, there is lack of a comprehensive information on the status and distribution of the disease in the country. The objectives of this systematic review and meta-analysis were to provide a pooled prevalence estimate of bTB at a national level, assess the level of in-between variance among study reports and illustrate the spatial distribution pattern in the country. Articles published on bTB from January 2000 to December, 2016 in English language were included in the review. Pubmed, CAB direct, AJOL and Web of Science were the databases used in electronic search. A total of 127 articles were retrieved from online sources, of which 56 articles were selected for data extraction based on the specified inclusion criteria. From these selected published articles, 114 animal level data were extracted for quantitative analysis. A pooled prevalence estimate of bovine tuberculosis in Ethiopia was found to be 5.8% (95% CI: 4.5, 7.5). In a multivariable meta-regression analysis, breed and production system explained 40.9% of the explainable proportion of the in-between study variance computed. Prevalence of bovine tuberculosis in Holstein-Friesians, 21.6% (95% CI: 14.7–30.7), was higher than in local zebus 4.1 (95% CI: 3.4–4.9). Cattle kept under intensive and semi-intensive production systems had higher prevalence, 16.6% (95% CI: 12.4–21.6) of bTB than those kept in extensive livestock production system, 4.6 (95% CI: 3.4–6.2). Bovine tuberculosis is widely distributed across major livestock producing regions of Ethiopia. However, no valid data could be retrieved from Benishanul-Gumuz, Harari and Dire Dawa. Data obtained on bTB from Somali and Gambella regional states are also few and further studies are suggested in these regions. In conclusion, this review showed that bTB in cattle in Ethiopia is widespread with high prevalence in intensive and semi-intensive management systems that keep exotic breeds and their crosses in urban and peri-urban areas. Thus, it is suggested that the design and implementation of bTB control strategies in Ethiopia should prioritize these hotspots in order to reduce the impact of the disease on the growing dairy sector.
Keywords: bovine tuberculosis, breed, cattle husbandry, geographic distribution, Ethiopia
Introduction
Bovine tuberculosis (bTB) is a chronic granulomatous disease of cattle caused predominantly by Mycobacterium bovis (M. bovis). The disease is transmitted between animals primarily by inhalation although transmission through ingestion is also common in cattle grazing on pasture contaminated with M. bovis. The disease causes significant animal health-induced economic loss, and its impacts include reduction in productivity, movement restrictions, screening costs, culling of affected animals, and trade restrictions (OIE, 2016). On top of its economic impacts, bTB is transmitted to humans, and prior to mandatory pasteurization about one-fourth of TB cases in children was caused by M. bovis in developed countries while 15% of human TB up until the end of 1990’s was believed to be caused by M. bovis in developing countries (Ashford et al., 2001). A more recent study however, reported a much lower figure of 2.8% of human TB to be attributed to M. bovis in Africa (Muller et al., 2013).
Bovine TB has been eliminated or eradication programs are in progress in several developed countries (CFSPH, 2009). Nonetheless, significant pockets of infection remain in wildlife in Canada, the United Kingdom, the United States and New Zealand (OIE, 2016). Globally, the prevalence of the disease is estimated to be 9% based on the results of skin tests (Vordermeier et al., 2016). Although there is still scarcity of data on the prevalence of bTB in developing countries, the disease is widespread in African and Asian countries (OIE, 2016). According to the review made by Cosivi et al. (1998), approximately 85% of the cattle and 82% of the human population of Africa are living in areas where bTB is endemic and partly controlled or not controlled at all. In Asia, 94% of the cattle and 94% of the human population live in countries where bTB is not controlled or where limited control strategy is applied (Cosivi et al., 1998).
Global economic assessment of loss associated with bTB and cost-benefits analysis from its control were multifaceted and figures obtained from different countries provide variable results because of differences in epidemiological situations, livestock systems, natural reservoirs, time horizons, and absence of commonly agreed analytical frameworks (Zinsstag et al., 2006). In Ethiopia, Tschopp et al. (2012) estimated the economic cost of bTB to be in the range of 75.2 million to 385 million US$ in the rural extensive livestock production and from 500,000 −4.9 million US$ in the urban livestock production systems between the years 2005 to 2011. These figures demonstrated losses lower than 1% of the net present value of livestock in the rural and 3.9–6.2% in urban livestock production systems per year. In both cases the costs associated with bTB were within the margin of error (i.e the losses may not be different from zero). In their conclusion, Tschopp et al. (2012), emphasized on the urgent need for control of the disease in the urban production system in Ethiopia for non-economic reasons; including concerns over spread of bTB through dairy cattle trade from high prevalence urban system to low prevalence sedentary rural system and for public health reasons.
Since the report of Hailemariam (1975), several fragmented studies ascertain the presence of bTB in Ethiopia. The estimated animal level prevalence ranges from less than one to 47% at study level, (Ameni et al., 2007a, 2007b, 2010; Berg et al., 2009; Tschopp et al., 2010b; Tsegaye et al., 2010; Gumi et al., 2011; Firdessa et al., 2012) and from no detection of infection (0%) (Tschopp et al., 2015) to 90% (Firdessa et al., 2012) at specific localities level depending on the prevailing production systems and breeds of cattle kept. Most of these studies were carried out based on tuberculin skin testing and postmortem inspection, although few of the studies were supported by isolation and molecular typing of the causative agents. In the former case, bovine purified protein derivative (PPD) alone or together with avian PPD has been used to determine exposure status in live animals (Asseged et al., 2000; Ameni and Regassa, 2001; Fikru et al., 2005; Ameni and Erkihun, 2007).
Regardless of the diagnostic test types used, several reports were available since the early 2000’s, in various parts of the Ethiopia. Many of these reports however, were conducted by graduate and undergraduate students to fulfill their research requirements in the academic programs to which they were enrolled, therefore these studies were noted to have limitations in producing country level picture for one or the other reasons including, the scope of study objectives, methodology used, target population and geographic coverage. Besides, to our knowledge there has never been national level survey or surveillance report that can demonstrate the magnitude of the diseases. There are few compiled qualitative reviews on bTB in animals and humans in Ethiopia (Shitaye et al., 2007; Tschopp and Assefa, 2016), yet, no single review is available based on quantitative analysis of study results conforming to Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA). In a situation where such a gap is noted, producing a review based on data integration from selected articles with objectively defined criteria is worthwhile (Bornstein et al., 2009; Dohoo et al., 2009). Such qualitative analysis would give complimentary picture on the level and scopes of studies made so far from which fellow researchers visualize the existing research gap. Besides, the information will provide policy input for appropriate strategy on the way forward. The current systematic review and meta-analysis is conducted with the aim of providing a pooled prevalence estimate of bTB in Ethiopia. This review also attempts to assess the level of in-between study variance and quantify the true heterogeneity attributed to the hypothesized predictors captured in the final model. The review also illustrated the spatial distribution pattern of bTB in the country based on the available study reports.
Material and methods
Literature Search Strategy
Search for published articles on bTB in Ethiopia was conducted from January 2, 2017 to February 21, 2017. PubMed, CAB direct, African Journals OnLine and Web of Science were the databases used. Key strings were: bovine, cattle, large ruminants, livestock, TB, wasting diseases, mycobacteriosis, zoonotic mycobacteria, M. bovis and Ethiopia. The strings were rearranged to phrases as close as “bovine tuberculosis in Ethiopia” and literature searches were limited to articles published both in English and Amharic (Federal working language) since 2000. Reference lists of reviews and all retrieved articles were also manually searched during the same period to maximize article recovery.
Inclusion and exclusion criteria
A preliminary screening was made based on the title and abstract content of the manuscripts. Those articles thought to have reasonable reflection on the review question were fully scanned and rated using an evaluation form with a rating scales for the following criteria: i) TB in cattle or bovine species (yes/no), ii) clarity of objective/s (estimation of prevalence or otherwise), iii) appropriateness of methodology including the study design and diagnostic details, iv) clarity of result presentation and v) the study period. The study designs considered were cross-sectional and cohort. With regards to the diagnostic techniques, detailed meat inspection (DMI) for abattoir based studies, and single intradermal tuberculin (SIDT) and comparative intradermal tuberculin (CIDT) tests at cut-off values of 4 mm and above for skin test based studies were valid for the review. Sampling strategies reported in the original studies were either random or purposive. In line with predefined criteria, manuscripts that were rated to be of moderate quality and above were included in the review while abstracts, proceedings, review articles, case and outbreak reports, articles published before the year 2000 and those articles rated of low quality due to poorly executed or poorly described methodology or lack of usable data due to vague result presentation were excluded from the review.
Data extraction
Data extraction template was developed on biologically plausible predictors which were consistently reported in most of the selected published articles. The template was further reviewed and enriched by all co-authors and pilot tested before use. Two authors were engaged fully in literature search and data extraction independently based on agreed milestones. Finally, the selected articles and the dataset generated were crosschecked by third author and ambiguities were ruled out by group discussions or through telephone conversations. The dataset constituted authors’ name, year of publication, web-link, manuscript title, journal name and volume in addition to hypothesized biologically meaningful predictors, i.e. breed, production systems, type of study population (study set-up), agro-ecology, study design, sampling technique, diagnostic technique, housing type, herd composition and herd contacts. Sample size, number positive, number negative, prevalence and respective confidence intervals were also generated along each predictor.
Statistical analysis
After coding and editing, the data were imported to Stata 12.1 (STATA, 2012) and the apparent prevalence estimates were log-transformed using the formula: lp = ln [p/ (1-p)], where lp= the logit-event estimate; ln= the natural logarithm; p= study level estimate. The variance of the logit estimate was calculated as, v (lp)= 1/(np) + 1/[n (1-p)], where v= variance and n= sample size. The standard error of logit-prevalence (SE) was also generated using the formula ln_p = Sqrt(1/n × p × (1-p)). Besides, Cochran’s Q statistic (Cochran, 1954) to test for heterogeneity and the Higgin’s statistic (I2) to quantify the proportion of the true variation due to heterogeneity between studies (Higgins et al., 2003) were calculated. The pattern and level of heterogeneity was also further assessed and illustrated in Galbraith plot as per the advice given by Anzures-Cabrera and Higgins (2010) due to large number of studies that limit comfortable presentation using a forest plot. A pooled prevalence estimate (effect size) was calculated in DerSimonian and Laird (1986) random effect model, using the formula, p = 1/(1 + e−lp)*100, where ‘e’ is the base of natural logarithm. Following pooled prevalence estimate in random effect model, univariable meta-regression was run and those predictors with liberal p-values (p<0.25) were checked for multi-collinearity in cross-tabulation (gamma statistics) (Dohoo et al., 2009). Multivariable meta-regression analysis on non-collinear predictors (gamma between-0.6 and +0.6) followed to quantify the explainable proportion of the heterogeneity noted between the study reports (R2). If the computed R2 values fall outside the proportion limit (0, 1), adjustment was made either to “0”, if negative, or to “1”when it was above 1 (Borenstein et al., 2009). Sensitivity analyses were performed to assess the stability of the estimate by performing influence plot analysis, i.e., a single study report in meta-analysis was omitted each time the analysis is run to observe the influence of the omitted individual study in the dataset on the final pooled estimate (Dohoo et al., 2009). Potential publication bias was assessed by visual inspection of a funnel plot asymmetry (Borenestien et al., 2009). The Begg’s rank correlation (Begg and Mazumdar, 1994) and the Egger’s weighted regression methods (Egger et al., 1997) were used to assess the significance level of the bias noted (p<0.05). Proportional dot map showing the spatial distribution of bTB and associated apparent mean prevalence was generated using ArcGIS version 10.4.1 software (ESRI, Redlands, California, USA). To this effect the raw data were organized along report frequency, mean and variability for each district. Finally, the report was organized as per PRISMA guidelines (Liberati et al., 2009; Moher et al., 2009).
Results
Identified study reports
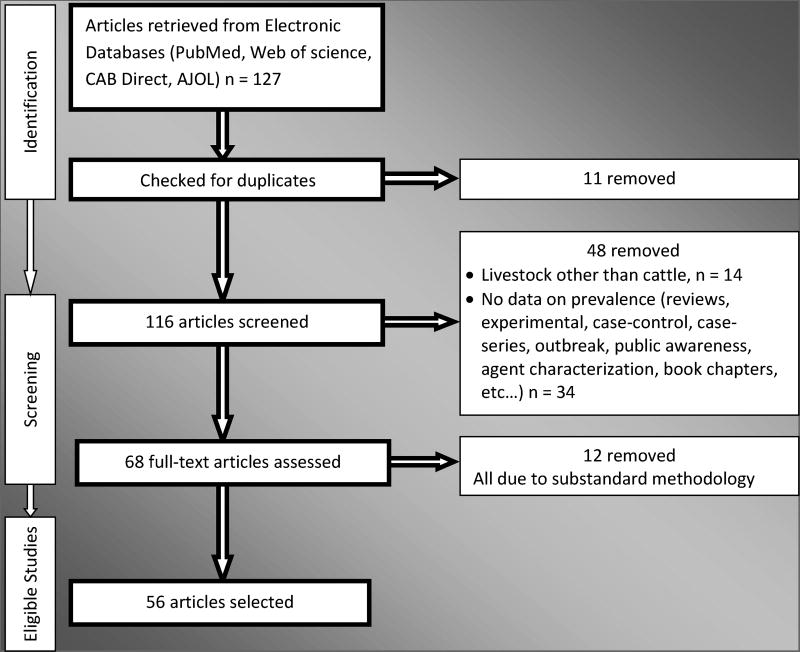
The number of published articles retrieved using the aforementioned databases were 127, of which 11 were rejected for duplication in one or the other form. In the subsequent review of the titles and abstracts of the remaining articles, 48 of them were rejected for one or the other reasons including study design, dealing with species other than cattle, or published prior to 2000 benchmark stated in the inclusion criteria. Full-text assessment of the articles was made on 68 of them and 56 articles were finally selected for data extraction (Figure 1). From the selected published articles, 114 animal level data were extracted for quantitative analyses.
Figure 1. Literature selection flow diagram for systematic review of bovine tuberculosis in Ethiopia.
Characteristics of the reports
Only articles written in English language were retrieved. All the selected studies were conducted using a cross-sectional study design and the cattle breeds considered were zebu, crosses of Holstein-Friesian and zebu and pure Holstein-Friesians. In regard to variation between production systems, the reviewed articles obtained their data from cattle that were kept under the prevailing livestock husbandry systems in the country including: intensive and/or semi-intensive, highland extensive, and pastoral and/or agro-pastoral production systems. However, for the studies conducted in abattoirs, as opposed to field or farm populations, tracing of the agro-ecologies was difficult and as a result, these reports were then categorized as un-specified. From practical point of view, animals presented to slaughter in the abattoirs were dominated by local zebu breeds. Detailed data on the presence of herd contacts, presence of other livestock species in the herd, whether cattle were housed together with their owners or kept separately in their own shelters were captured. The sampling techniques used were also assessed and registered as either random, purposive or haphazard depending on the description in the materials and methods sections of the articles. Authors reported to have used different diagnostic techniques including DMI, SIDT test both at ≥2 mm and ≥4 mm cut-off points, CIDT test also at > 2mm and >4 mm cut-off points, and gamma-interferon assay. In this review however, prevalence report made based on SIDT, CIDT tests (both made at ≥4 mm cut-off points) and DMI technique were considered (Corner, 1994; OIE, 2016). To visualize spatial distribution patterns of bTB in Ethiopia, reports were clustered under administrative states for regression analysis based on the study locations. Gambella and Somali regional states were omitted from analysis due to a single study report from each. For spatial display, district names were used to reveal the study locations and the corresponding apparent prevalence. The study period was also treated in three separate levels, i.e. 2000–2005, 2006–2010 and period after 2010. In spite of the list of predictors accounted for, sufficient comparable categories could not be retrieved for study design, herd composition and patterns of housing. In some of the predictor categories where explicit information on the attribute was not available, including breed and production systems ‘unidentified’ category was designated separately. Therefore, group level quantitative analysis of those predictors could not be made. A pooled estimate given in meta-analysis below was made based on the entire study reports conducted on 92,482 animals of which 7,529 were positive using one of the three diagnostic test considered for the current quantitative review (Table 1).
Table 1.
List of study reports on bovine tuberculosis in Ethiopia selected for meta-analysis
| Authors | Study setup | Dx. Test | Sample size | APP |
|---|---|---|---|---|
| Asseged et al., 2000 | Farm/field | SIDT | 1241 | 10.5 |
| Ameni and Regessa, 2001 | Farm/field | CIDT | 416 | 14.2 |
| Ameni and Wudie, 2003 | Abattoir | DMI | 1125 | 5.2 |
| Ameni et al., 2003a | Farm/field | CIDT | 763 | 7.8 |
| Ameni et al., 2003b | Farm/field | CIDT | 1168 | 46.9 |
| Ameni et al., 2003c | Farm/field | CIDT | 735 | 4.2 |
| Asseged et al., 2004 | Abattoir | DMI | 1350 | 1.5 |
| Teklu et al., 2004 | Abattoir | DMI | 751 | 4.5 |
| Laval and Ameni, 2004 | Farm/field | SIDT | 460 | 4.1 |
| Laval and Ameni, 2004 | Farm/field | CIDT | 320 | 1.6 |
| Fikru et al., 2005 | Farm/field | SIDT | 353 | 3.4 |
| Shitaye et al., 2006 | Farm/field | CIDT | 2098 | 18.7 |
| Ameni and Erkihun, 2007 | Farm/field | CIDT | 524 | 11.1 |
| Ameni et al., 2007a | Farm/field | CIDT | 5424 | 13.5 |
| Elias et al., 2008 | Farm/field | CIDT | 1869 | 23.7 |
| Yacob et al., 2008 | Abattoir | DMI | 638 | 0.9 |
| Fetene and Kebede, 2009 | Farm/field | CIDT | 1207 | 9.7 |
| Demelash et al., 2009 | Abattoir | DMI | 3322 | 10.1 |
| Berg et al., 2009 | Abattoir | DMI | 32779 | 4.6 |
| Tsegaye et al., 2010 | Farm/field | CIDT | 1132 | 34.1 |
| Regassa et al., 2010 | Abattoir | DMI | 1023 | 1.1 |
| Tschopp et al., 2010a | Farm/field | CIDT | 499 | 0.8 |
| Amenu et al., 2010 | Farm/field | CIDT | 425 | 6.4 |
| Ameni et al., 2010 | Abattoir | DMI | 1138 | 5 |
| Tschopp et al., 2010b | Farm/field | CIDT | 5377 | 1 |
| Tschopp et al., 2011 | Farm/field | CIDT | 1214 | 1.6 |
| Bekele and Belay, 2011 | Abattoir | DMI | 780 | 2.7 |
| Gumi et al., 2011 | Farm/field | CIDT | 473 | 5.5 |
| Dinka and Duressa, 2011 | Farm/field | CIDT | 625 | 12.2 |
| Tigre et al., 2012 | Farm/field | CIDT | 384 | 21.4 |
| Tigre et al., 2012 | Abattoir | DMI | 1102 | 5.4 |
| Ewnetu et al., 2012 | Abattoir | DMI | 720 | 5.8 |
| Gumi et al., 2012 | Farm/field | CIDT | 411 | 2.4 |
| Firdessa et al., 2012 | Farm/field | CIDT | 2926 | 32.2 |
| Mekbib et al., 2013 | Abattoir | DMI | 500 | 5 |
| Tschopp et al., 2013 | Farm/field | CIDT | 584 | 0.3 |
| Mamo et al., 2013 | Farm/field | CIDT | 1087 | 11 |
| Zeru et al., 2013 | Abattoir | DMI | 768 | 6.4 |
| Aylate et al., 2013 | Abattoir | DMI | 1029 | 6.1 |
| Ayana et al., 2013 | Farm/field | CIDT | 371 | 1.3 |
| Ayana et al., 2013 | Abattoir | DMI | 487 | 9.3 |
| Romha et al., 2013 | Farm/field | CIDT | 484 | 6.6 |
| Romha et al., 2013 | Abattoir | DMI | 582 | 5.8 |
| Zeweld et al., 2013 | Farm/field | CIDT | 423 | 7.3 |
| Terefe, 2014 | Abattoir | DMI | 486 | 6.8 |
| Woyessa et al., 2014 | Abattoir | DMI | 1183 | 5.7 |
| Gebrezgabiher et al., 2014 | Abattoir | DMI | 768 | 2.6 |
| Zeweld, 2014 | Farm/field | CIDT | 524 | 2.7 |
| Zeru et al., 2014 | Farm/field | CIDT | 480 | 11.3 |
| Bussa, 2014 | Farm/field | CIDT | 508 | 1.8 |
| Biru et al., 2014 | Farm/field | CIDT | 835 | 11.4 |
| Romha et al., 2014 | Farm/field | CIDT | 440 | 4.3 |
| Tschopp et al., 2015 | Farm/field | CIDT | 220 | 0 |
| Nuru et al., 2015 | Farm/field | CIDT | 788 | 1.3 |
| Alemu et al., 2016a | Abattoir | DMI | 500 | 13.2 |
| Dejene et al., 2016 | Farm/field | CIDT | 2550 | 5.5 |
| Duguma et al., 2016 | Farm/field | CIDT | 554 | 3.8 |
| Alemu et al., 2016b | Abattoir | DMI | 500 | 10.6 |
| Bekele et al., 2016 | Farm/field | CIDT | 558 | 17 |
| Woldemariam et al., 2016 | Farm/field | CIDT | 501 | 4.4 |
Dx. Technique: Diagnostic technique, App: Apparent prevalence, CIDT: Comparative Intradermal Tuberculin test, SIDT: Single Intra-dermal Tuberculin test, DMI: Detailed Meat Inspection,
Meta-analysis
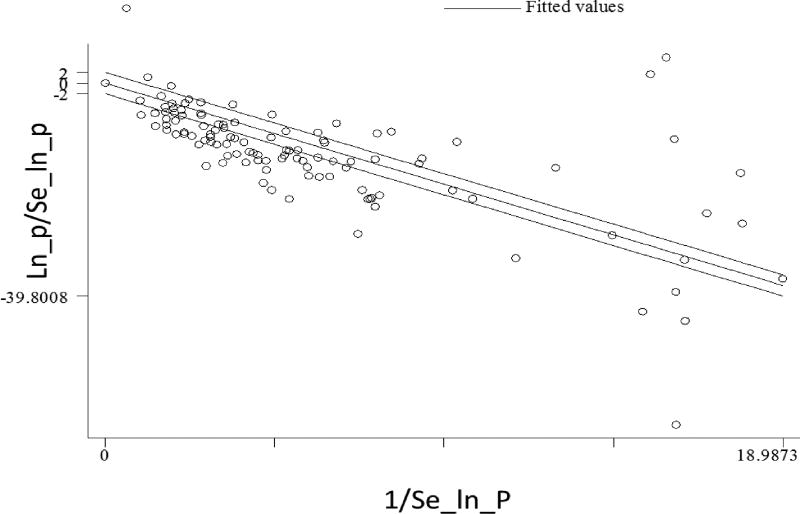
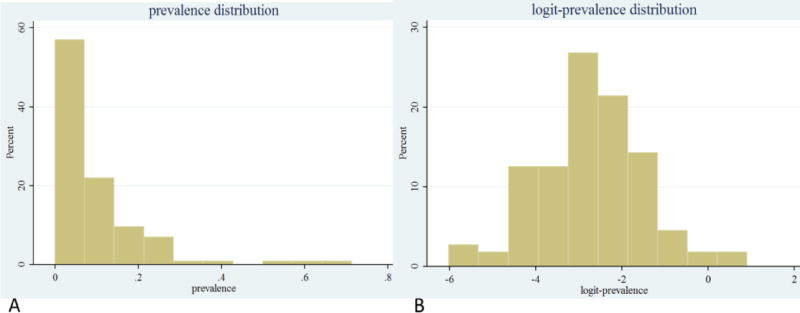
The estimated pooled prevalence of bovine tuberculosis in Ethiopia was 5.8% (95% CI: 4.5, 7.5). This analysis was computed in a random effect model due to calculated Cochran value (Q) 7114.9851 (df = 113 and p < 0.001) and Higgins statistics (I2= 98.4). The graphical assessment in Galbraith plot (Figure 2) revealed that over 70% of the study reports are outside the +2 unit lines and provided clear evidence on the heterogeneity of study reports. The raw and logit-transformed effect size distribution patterns are also demonstrated on Figure (3).
Figure 2. Galbraith plot for assessing bovine tuberculosis study reports heterogeneity in Ethiopia.
The plot is a scatter plot of standardized effect estimates against inverse standard error. In such graphical assessment points outside the confidence bounds (positioned 2 units above and below the regression line) are study reports which have major contribution to heterogeneity noted. In the absence of heterogeneity we could expect all points to lie within the confidence limits. This is one of the evidence for choosing random effect model as opposed to fixed effect model for pooled estimate made in meta-analysis.
Figure 3. Apparent (A) and logit-prevalence (B) estimate distributions for the animal-level studies of bovine tuberculosis prevalence in Ethiopia.
The right skewed histogram (figure 3.A), illustrates distribution pattern of apparent prevalence reports, while figure 3.B is a normalized one, following logit-transformation of each prevalence estimate up on which all the analyses were made.
Meta-regression
Univariable meta-regression
The univariable meta-regression was conducted with and without controlling the effect of sample size. The predictors considered were: year of study report, sample size, type of sampling, breed, production system, agro-ecology, types of study population (abattoir Vs field), herd contact, diagnostic technique and administrative states (Table 2).
Table 2.
Proportion of individual predictor’s effect on heterogeneity and associated statistical significance adjusted for sample size.
| Model | Without sample size | With sample size | ||
|---|---|---|---|---|
|
| ||||
| proportion | p value | proportion | p value | |
| Sample size | - | - | 0.52 | 0.249 |
| Year | 1.42 | 0.119 | 2.46 | 0.009 |
| Type of sampling | 0.0 | 0.489 | 0.0 | 0.541 |
| Type of study population | 0.0 | 0.564 | 0.0 | 0.780 |
| Breed | 20.44 | <0.001 | 25.44 | <0.001 |
| Production systems | 9.0 | 0.001 | 8.9 | 0.002 |
| Herd contact | 0.0 | 0.329 | 0.0 | 0.362 |
| Agro-ecology | 0.32 | 0.287 | 0.16 | 0.438 |
| Diagnostic technique | 0.0 | 0.789 | 0.0 | 0.943 |
| Administrative states | 1.07 | 0.160 | 1.83 | 0.142 |
As it is depicted in the model (Table 2) the proportion of effect attributed to each predictor ranged from 0.0 to 25.44%. The highest proportion was noted for breed while herd contact, diagnostic test used, type of study population and type of sampling were noted to have no observable effect for heterogeneity among the study reports.
Multivariable meta-regression
Among the predictors used in univariable model above, five non collinear predictors, namely, year category, breed, production systems, administrative states and sample size were subjected to multivariable meta-regression analysis. The final model accounted only for two predictors, namely, breeds and production systems which together explained 40.9% (R2) of the explainable proportion of heterogeneity between study reports. The collinearity between production systems and breed was rechecked (gamma= 0.56). The prevalence of bTB in Holstein-Friesian breed was noted to be higher by 79.4% compared to the prevalence in zebu; while Friesian zebu crosses had a prevalence that exceeded the prevalence in reference breed by 64.3%. Concerning production systems, the highest prevalence (16.6%) reported in intensive and semi-intensive production system exceeded the reference group by 66.4% (Table 3).
Table 3.
The association of prevalence of bovine tuberculosis in Ethiopia along with selected predictors in meta-regression model (R2 = 40.9%, n = 114).
| Variables | Category/Range | Prev.(95%CI) | #Reports | Coeff | *p value | p value |
|---|---|---|---|---|---|---|
| Sample size | 7–14314 | 114 | −0.00007 | 0.249 | 0.343 | |
| Year | 2011–2016 | 6.2 (4.6,8.2) | 62 | reference | ||
| 2006–2010 | 6.0 (4.3, 8.3) | 34 | −0.05 | 0.852 | 0.984 | |
| 2000–2005 | 7.8 (4.1,14.6) | 18 | 0.27 | 0.401 | 0.383 | |
| Production systems | Highland extensive | 4.6 (3.4,6.2) | 56 | reference | ||
| Pastoral/agro-pastoral | 4.5 (3.6,5.7) | 12 | −0.03 | 0.724 | 0.936 | |
| Intensive/semi-intensive | 16.6 (12.4,21.6) | 26 | 0.68 | <0.001 | 0.034 | |
| Breed | Zebu | 4.1 (3.4,4.9) | 58 | reference | ||
| Cross | 9.9 (7.4,13.3) | 24 | 0.59 | <0.001 | 0.030 | |
| Holstein Friesian | 21.6 (14.7,30.7) | 17 | 1.35 | <0.001 | <0.001 | |
| Administrative State | Oromia | 6.6 (5.5,8.1) | 46 | reference | ||
| SNNP | 4.0 (2.9,5.6) | 19 | −0.51 | 0.105 | 0.062 | |
| Addis Ababa | 10.6 (6.7,16.4) | 17 | −0.08 | 0.069 | 0.785 | |
| Amhara | 3.6 (2.2,5.8) | 13 | −0.53 | 0.076 | 0.086 | |
| Tigray | 8.8 (5.8,13.3) | 12 | 0.11 | 0.256 | 0.717 | |
| Afar | 6.7 (3.4,12.7) | 3 | 0.38 | 0.872 | 0.566 |
Distribution
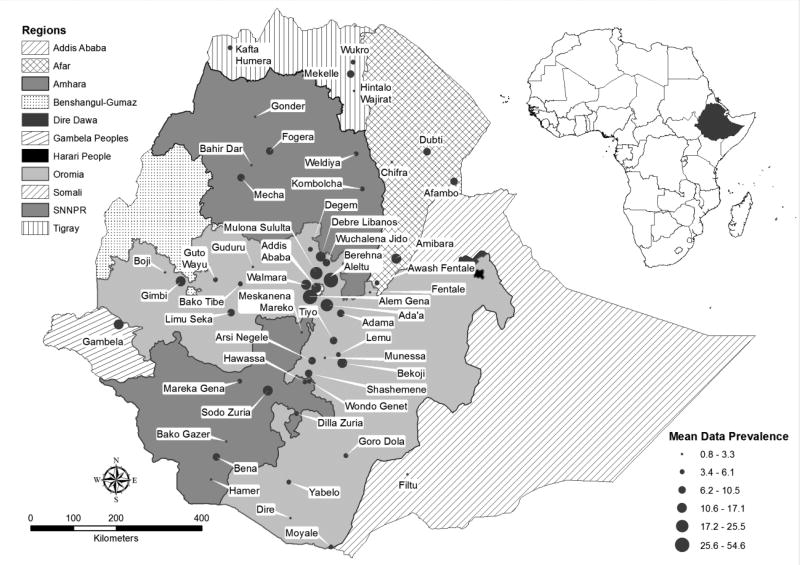
Valid reports on bTB in cattle were available from all regions of the country except Benishangul-Gumuz, Harari and Dire Dawa, yet the intensity of the studies was highly variable from region to region. Many of the studies were conducted in and around urban areas in the central highlands of Ethiopia. As it is clearly depicted on the map (Figure 4), in some of the regions like Somali and Gambella regional states, sufficient number of reports were lacking.
Figure 4. Distribution and mean prevalence of bovine tuberculosis in districts of Ethiopia.
Most of the studies were conducted in Addis Ababa, Amhara, Oromia and Southern Nations and Nationalities Peoples regions while no valid published study was obtained from Benishangul-Gumuz, Harari and Dire Dawa regions. On the other hand, few studies were undertaken in Afar, Gambella, Somali and Tigray regions. Variable animal level prevalence of bTB were recorded in the districts of the regions ranging from 0.8% to 54.6%; the highest prevalence being reported in intensive farms in and around cities while the lowest prevalence being recorded in grazing animals in rural areas.
Literature bias
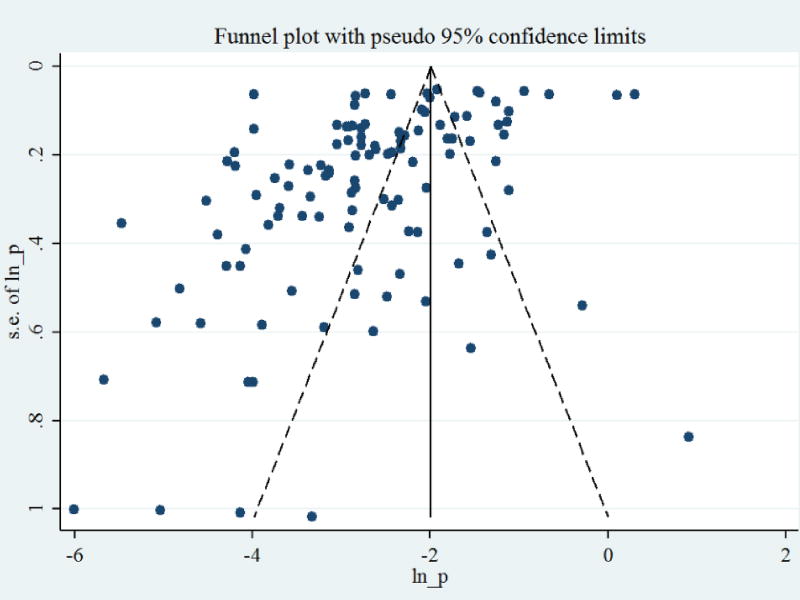
The visual interpretation of Funnel plot (Figure 5) and Egger’s statistics (weighted regression) revealed the presence of bias (p <0.001) whereas, Begg’s statistics (rank correlation method) did not (p>0.05). In the assessment of estimate stability, no individual study was found to have a profound influence on pooled estimate computed (figure not shown).
Figure 5. Funnel plot of logit-prevalence estimate of bovine tuberculosis in Ethiopia.
The figure displays each study’s estimated effect plotted against its SE and evaluates the relationship between study results and their precision. The lack of symmetry around the inverted funnel plot illustrates the presence of potential bias due to increasing number of study reports with low prevalence and study reports based on smaller sample size.
Discussion
In this review, a total of 56 cross-sectional studies were considered and meta-analysis was made based on the results of these studies. A pooled prevalence estimate of bTB in Ethiopia was 5.8% with 95% CI of 4.5 % to 7.5%. This pooled estimate (also called effect size) is computed by integration of several individual study estimates that range from 0.0% (Tschopp et al., 2015) to 46.9% (Ameni et al., 2003b). A summary of such primary studies using meta-analysis is the preferred approach (Borenstein et al., 2009) as it improves the precision and increases the statistical power of the estimate made on the status of bTB at national level for the specified time period. Analytical procedures for pooling prevalence estimate of different studies consider the existing in-between individual study variance (I2). In the current review I2 was 98.4% indicating high heterogeneity. Academicians, policy and research fellows are advised to understand the calculated effect size in this context.
Apart from very few farm based control efforts (Ameni et al., 2007b; Firdessa et al., 2012), there has never been bTB control intervention in the country; however, the computed estimate is moderate, perhaps it is linked to numerical dominance of local cattle breeds in extensive farming system and abattoir population that have much lower prevalence as evidenced in this review. In this connection, production systems and breed of cattle were the key risk factors identified in the final model to affect the prevalence of bTB in the study population. A pooled prevalence estimate of bTB in intensive and/or semi-intensive farms (16.6%) based on 26 studies is much greater than the pooled prevalence (4.6%) of either on 56 studies conducted in highland extensive production or the pooled prevalence (4.5%) of 12 studies conducted in pastoral production systems. Perhaps, this reflects the effect of the production systems on prevalence of bTB in the country and warrant the need for prioritization of control towards intensive or semi-intensive dairy production systems. Similar observations were made in Tanzania and Uganda (Shirima et al., 2003; Inangolet et al., 2008). Previously, it was reported that intensive farming facilitates transmission of bTB among cattle as individual animals are kept in close proximity to one another (Constable et al., 2017). In addition, overcrowding itself was claimed to stress cattle and compromise their ability to thrive with the disease. Lesions in intensively managed cattle were predominantly found in the lung and associated lymphnodes, while mesenteric lymphnodes are the primary foci in grazing cattle suggesting the mode of transmission of the disease in the respective production systems (Kaneene and Pfeiffer, 2006).
The current quantitative review revealed breed differences to be one of the factors that significantly influence pooled prevalence of bTB in cattle in Ethiopia. As indicated previously in the result section, a pooled prevalence (21.6%) of 17 studies conducted on Holstein-Friesian and a pooled prevalence (9.9%) of 24 studies conducted on crosses were by far greater than a pooled prevalence (4.1%) of 58 studies conducted on zebu cattle. This agrees with the suggestion that cattle genetics affects susceptibility/resistance of cattle to bTB (Brotherstone et al., 2010; Finaly et al., 2012). Studies conducted in Ethiopia in this regard demonstrated that both prevalence and severity of TB lesion were found to be higher in Holstein-Friesian cattle as compared to zebu cattle and their crosses kept together on communal pasture in the central highlands of the country (Ameni et al., 2006; Ameni et al., 2007a). Likewise, an experimental study comparing susceptibility of Holstein-Friesian calves to Boran (zebu) calves exposed to low doses of M. bovis in South Africa showed that half of Holstein-Friesian calves developed typical lesions while none of the Boran calves developed bTB suggestive lesion (Vordermeier et al., 2012). The finding indicated that zebu breeds are more likely to be resistant to bTB infection as compared to Holstein-Friesian breed. Benkirane (1998) also suggested that zebus are more resistant to bTB than high producing European breeds of cattle. The lack of pronounced collinearity between production systems (herd level factor) and breed (individual level) in this review might be an evidence for possible impact of each risk factor operating at different levels.
Prevalence of bTB generally showed a decreasing trend as one moves from central areas of the country to the peripheries in all geographic directions; and from accessible regional towns to more remote locations where traditional extensive livestock husbandry dominates. The generalization is not absolute as differences could be observed over short distances or even in the same area as reported by different investigators at different times. Prevalence of bTB appears to be the highest in central areas of the country with the highest intensity in Addis Ababa and the surrounding sub-urban areas. This could be due to relatively long history of intensive dairying with exotic European breeds in central Ethiopia following their introduction during the last imperial regime with the objectives of supplying dairy products to the burgeoning human population in Addis Ababa and its surroundings (Ahmed et al., 2004). These areas still remain areas of major dairy cattle concentration and serve as source of improved dairy cattle to the rest of the country (Firdessa et al., 2012), as well as the sources of bTB for the newly emerging dairy areas.
There is no significant difference with regard to prevalence of bTB between different administrative regions of the country (p>0.05). This is because epidemiology of bTB is not affected by animal health activities in any of the administrative states, as none of them have adopted any viable control intervention. Results of this review suggest that intensification of the dairy sector in any of the regions possibly enhance the prevalence as there is no working regulatory imposition on biosecurity that limits animal movement. This affects the dairy sector adversely, since trade in dairy cows was observed to be unidirectional in Ethiopia and was usually from the central areas of the country where bTB is more prevalent to the regional cities and zonal towns (Firdessa et al., 2012). The real impact of such trade on bTB prevalence and spread from region to region needs to be supported by molecular fingerprinting of isolates from all regions of the country. Even then, exact tracing of animals to the herds of their origin would be a challenge as no animal identification and traceability system is currently in place. Absence of valid reports from Benishangul-Gumuz, Dire Dawa and Harari regions, and only one study each from Somali and Gambella regions calls for further studies in these regions.
The final multivariable logistic regression model built in the current review explained about 41% of the explainable proportion of heterogeneity among the studies. This indicates that there are other risk factors of the disease that are not captured in the analysis for various reasons. Important among these factors were age of animals, herd size, population density, hygienic status of farms and sources of replacement animals. Data obtained from studies conducted in Ethiopia with regards to these factors were so heterogeneous and overlapping, which suggests that there were no meaningful categories to be developed for analysis. We believe that some of the heterogeneity not captured in the current analyses might have been explained by the missing predictors. Apart from the lack of consistency among the studies described previously, many of the studies suffer from shortage of clear description of the study area with regards to geo-referencing, animal husbandry practices, presence or absence of other co-existing animal species, co-habitation with humans, presence or absence of informal culling practices and farm hygiene. Some of the studies failed to describe how individual animals included in the studies were selected. Others put too much emphasis on the technicalities of the diagnostic tests used and failed to include epidemiological determinants that might have been altered to control bTB. It is suggested that future studies on bTB in Ethiopia need to be designed by incorporating these weaknesses and the findings of the current quantitative review should be viewed in the light of these limitations.
In the sensitivity analysis of the pooled estimate, the observed disparity between Egger’s and Begg’s test statistics over statistical significance is explainable with a greater statistical power of the regression test over the rank correlation, respectively (STB, 1998). The bias noted could be linked to increasing number of studies with smaller sample sizes and lower prevalence that have larger variance for their point estimate and possibly reduced precision. Absence of an influential study in this review validates the robustness of a pooled estimate given.
Bovine TB control based on test and slaughter of reactor animals is considered to be the best policy in developed countries. The strategy was effective and resulted in bTB official free status in many countries, and lowered individual and herd level prevalence in others (EFSA and ECDPC, 2014; Constable et al., 2017). This strategy is practically impossible in developing countries like Ethiopia due to costs related to compensation (Zinsstag et al., 2006). Alternatively, skin testing and segregation of animals into positive and negative herds in one government bull-dam farm at Holeta decreased the prevalence of the disease from 48% to 14% in a year and to 1% in three consecutive years (Ameni et al., 2007b). Although such intervention could be considered as an alternative to the test and slaughter policy, long term sustainability of the alternative is questionable given the critical shortage of land faced by dairy farmers to establish such physically separated herds (Land O’Lakes, 2010; Gizaw et al., 2016) and the eventual fate of bTB positive animals in the absence of any regulation and/or policy in place. The future assignment should be identification of control strategies that could be implemented in Ethiopia. The strategies should be supported by legal framework for their effective implementation. On top of the main control strategies, subordinate/supportive control methods such as registration and identification of dairy cows, control of the trade/movement of dairy cows, strengthening the biosecurity of dairy farms and pasteurization of milk before distribution to the consumers will play greater role in minimizing the spread of bTB and its impact on public health.
Conclusions
The quantitative review showed that a pooled prevalence estimate of bTB in Ethiopia is 5.8%. The pooled prevalence was affected both by breed and management systems. The estimate computed for intensive and or semi-intensive production systems was higher than that of extensive production systems, reflecting the effect of the intensification on the prevalence of bTB. Holstein-Friesians and their local crosses had higher pooled prevalence compared to local zebu breed. Perhaps these could be strong epidemiological evidences on the importance of genetics and management on prevalence of bTB in Ethiopia. Our result suggest that efforts towards control of bTB in the country need to target intensive and semi-intensive dairy farms in urban and peri-urban areas that keep genetically improved cattle.
Highlights.
Results of 56 eligible studies were combined for quantitative review.
The estimated pooled prevalence of bovine tuberculosis in Ethiopia is 5.8% however; the overall prevalence is dominated by the overwhelming number of zebu cattle included in the studies.
Holstein Friesian and their crosses with zebu cattle were more significantly affected than zebu.
Intensive cattle husbandry was associated with increased prevalence
Acknowledgments
This review was financially supported by the fund obtained from the National Institute of Health (NIH) for a Research Project entitled “Systems Biology for Molecular Analysis of Tuberculosis in Ethiopia” with a reference number U01-HG007472. Authors would like to acknowledge all researchers who devoted their time and resources to produce such relevant information on tuberculosis in cattle in Ethiopia. The work of Mr Emmor Nile is appreciated for his intellectual input in the development of bTB distribution map in Ethiopia.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
None
Author’s contribution
BS took part in design, literature selection, data extraction and preparation of the draft manuscript; KA crafted the design, did data analysis and interpretation; KD participated in literature selection, data extraction and write-up; GAy participated in write-up; GM participated in write-up and GAm participated in design, write-up and critically reviewed and enriched the manuscript.
References
- Ahmed MAM, Ehui S, Assefa Y. Dairy Development in Ethiopia. EPTD Discussion Paper No. 123. International Food Policy Research Institute 2033 K Street, NW Washington; DC 20006 U.S.A: 2004. p. 73. [Google Scholar]
- Alemu B, Nazir S, Tintagu T, Teklu A. Diagnostic efficacy of abattoir meat inspection for detecting bovine tuberculosis at Adama municipal abattoir, Ethiopia. Comp. Clin. Pathol. 2016b;25(4):885–893. [Google Scholar]
- Alemu J, Mamo G, Ameni G, Pal M. Molecular Epidemiology of Bovine Tuberculosis in Cattle and its Public Health Implications in Gambella Region, Ethiopia. Molecular Microbiology Research. 2016a;6(1):1–15. [Google Scholar]
- Ameni G, Regasa A. Survey on bovine tuberculosis in cattle and its public health implications to cattle raising families in WolaitaSoddo, southern Ethiopia. Ethiopian J Anim. Prod. 2001;1:55–62. [Google Scholar]
- Ameni G, Erkihun A. Bovine tuberculosis on small-scale dairy farms in Adama town, central Ethiopia, and farmer awareness of the disease. Rev. Sci. Tech. 2007;26(3):711–719. [PubMed] [Google Scholar]
- Ameni G, Wudie A. Preliminary study on bovine tuberculosis in Nazreth municipality abattoir of central Ethiopia. Bull. Anim. Hlth. Prod. Afr. 2003;51:125–132. [Google Scholar]
- Ameni G, Amenu K, Tibbo M. Bovine Tuberculosis: Prevalence and risk factor assessment in cattle and cattle owners in Wuchale-Jida District, central Ethiopia. Int. J. Appl. Res. Vet. Med. 2003a;1(1):17–26. [Google Scholar]
- Ameni G, Aseffa A, Engers H, Young D, Gordon S, Hewinson G, Vordermeier M. High Prevalence and Increased Severity of Pathology of Bovine Tuberculosis in Holsteins Compared to Zebu Breeds under Field Cattle Husbandry in Central Ethiopia. Clin. Vaccine Immunol. 2007a;14(10):1356–1361. doi: 10.1128/CVI.00205-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameni G, Aseffa A, Engers H, Young D, Hewinson G, Vordermeier M. Cattle Husbandry in Ethiopia Is a Predominant Factor Affecting the Pathology of Bovine Tuberculosis and Gamma Interferon Responses to Mycobacterial Antigens. Clin. Vaccine Immunol. 2006;13(9):1030–1036. doi: 10.1128/CVI.00134-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameni G, Aseffa A, Sirak A, Engers H, Young DB, Hewinson GR, Vordermeie MH, Gordon SV. Effect of skin testing and segregation on the incidence of bovine tuberculosis, and molecular typing of Mycobacterium bovis in Ethiopia. Vet Rec. 2007b;161(23):782–786. [PMC free article] [PubMed] [Google Scholar]
- Ameni G, Bekele S, Tolosa T. Preliminary Study on the Impact of Bovine Tuberculosis on the Reproductive Efficiency and Productivity of Holstein Dairy Cows in Central Ethiopia. Bull. Anim. Hlth. Prod. Afr. 2010;58:222–226. [Google Scholar]
- Ameni G, Bonnet P, Tibbo M. A cross-sectional study of bovine tuberculosis in selected dairy farms in Ethiopia. Int. J. Appl. Res. Vet. Med. 2003b;1(4):253–258. [Google Scholar]
- Ameni G, Desta F, Firdessa R. Molecular typing of Mycobacterium bovis isolated from tuberculosis lesions of cattle in north eastern Ethiopia. Vet Rec. 2010;167(4):138–41. doi: 10.1136/vr.b4881. [DOI] [PubMed] [Google Scholar]
- Ameni G, Belay M, Medhin G. A Preliminary Study on Bovine Tuberculosis in Stallholder Farms and its Zoonotic Implication to Owners in Fitche Town, Central Ethiopia. The Sudan J. Vet. Res. 2003c;18:37–46. [Google Scholar]
- Amenu K, Thys E, Regassa A, Marcotty T. Brucellosis and Tuberculosis in Arsi-Negele District, Ethiopia: Prevalence in Ruminants and People’s Behaviour towards Zoonoses. Tropicultura. 2010;28(4):205–210. [Google Scholar]
- Anzures-Cabrera J, Higgins JPT. Graphical displays for meta-analysis: An overview with suggestions for practice. Res. Syn. Meth. 2010;1:66–80. doi: 10.1002/jrsm.6. www.interscience.wiley.com. [DOI] [PubMed] [Google Scholar]
- Ashford DA, Whintey E, Raghunathan P, Cosivi P. Epidemiology of selected mycobacteria that infect humans and other animals. Rev. Sci. Tech. 2001;20:325–337. doi: 10.20506/rst.20.1.1266. [DOI] [PubMed] [Google Scholar]
- Asseged B, Lübke-Becker A, Lemma E, Taddele K, Bayleyegn M, Britton S. Bovine Tuberculosis: Cross-sectional and Epidemiological Study in and around Addis Ababa. Bull. Anim. Hlth. Prod. Afr. 2000;48(2):71–80. [Google Scholar]
- Asseged B, Woldesenbet Z, Yimer E, Lemma E. Evaluation of Abattoir Inspection for the diagnosis of Mycobacterium bovis Infection in Cattle at Addis Ababa Abattoir. Trop. Anim. Health Prod. 2004;36:537–546. doi: 10.1023/b:trop.0000040934.32330.44. [DOI] [PubMed] [Google Scholar]
- Ayana T, Tafess K, Mamo G, Sisay TT, Ameni G. Isolation and molecular characterization of non-tuberculosis mycobacteria from skin positive reactors and pathological lesions of cattle at Bako, Western Ethiopia. Afr. J. Micro. Res. 2013;7(20):2190–2197. [Google Scholar]
- Aylate A, Shah SN, Aleme H, Gizaw TT. Bovine tuberculosis: prevalence and diagnostic efficacy of routine meat inspection procedure in Woldiya municipality abattoir north Wollo zone, Ethiopia. Trop. Anim. Health Prod. 2013;45:855–864. doi: 10.1007/s11250-012-0298-7. [DOI] [PubMed] [Google Scholar]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- Bekele M, Belay I. Evaluation of Routine Meat Inspection Procedure to Detect Bovine Tuberculosis Suggestive Lesions in Jimma Municipal Abattoir, South West Ethiopia. Glob. Vet. 2011;6(2):172–179. [Google Scholar]
- Bekele M, Mamo G, Mulat S, Ameni G, Beyene G, Tekeba E. Epidemiology of Bovine Tuberculosis and Its Public Health Significance in Debre- Zeit Intensive Dairy Farms, Ethiopia. Biomedicine and Nursing. 2016;2(2):8–18. [Google Scholar]
- Benkirane A. Bovine tuberculosis in Africa. World Animal Review. [Accessed on March 16, 2017];1998 :90–1998/1. Available online at: http://www.fao.org/docrep/W8600T/w8600t09.htm#TopOfPage.
- Berg S, Firdessa R, Habtamu M, Gadisa E, Mengistu A, Yamuah L, Ameni G, Vordermeier M, Robertson BD, Smith NH, Engers H, Young D, Hewinson RG, Aseffa A, Gordon SV. The Burden of Mycobacterial Disease in Ethiopian Cattle: Implications for Public Health. PLoS ONE. 2009;4(4):e5068. doi: 10.1371/journal.pone.0005068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biru A, Ameni G, Sori T, Disassa F, Teklu A, Tafess K. Epidemiology and public health significance of bovine tuberculosis in and around Sululta District, Central Ethiopia. Afr. J. Micro. Res. 2014;8(24):2352–2358. [Google Scholar]
- Borenstein M, Hedges LV, Higgins JPT, Rothstein H. Introduction to Meta-analyses. Wiley; Chichester, UK: 2009. [Google Scholar]
- Brotherstone S, White IMS, Coffey M, Downs SH, Mitchell AP, Clifton-Hadley RS, More SJ, Good AM, Woolliams JA. Evidence of genetic resistance of cattle to infection with Mycobacterium bovis. J. Dairy Sci. 2010;93:1234–1242. doi: 10.3168/jds.2009-2609. [DOI] [PubMed] [Google Scholar]
- Bussa MW. Survey of Bovine Tuberculosis in Guduru Cattle Breeding and Research Center, Western Ethiopia. Survey of Bovine Tuberculosis in Guduru Cattle Breeding and Research Center, Western Ethiopia. Journal of Biology, Agriculture and Healthcare. 2014;4(20):215–219. [Google Scholar]
- CFSPH. Bovine tuberculosis. Center for Food Security and Public Health, Iowa State University, College of Veterinary Medicine; Ames, Iowa 50011: 2009. www.cfsph.iastate.edu. [Google Scholar]
- Cochran WG. The combination of estimates from different experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- Constable PD, Hinchcliff KW, Done SH, Grünberg W. Bovine Tuberculosis. Veterinary Medicine: a Textbook of the Diseases of Cattle, Horses, Sheep, Pigs, and Goats. 11. Elsevier; St. Louis, Missouri: 2017. pp. 2015–2023. [Google Scholar]
- Corner LA. Post mortem diagnosis of Mycobacterium bovis infection in cattle. Vet Microbiol. 1994;40(1–2):53–63. doi: 10.1016/0378-1135(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Cosivi O, Grange JM, Daborn CJ, Ravilione MC, Fujikura T, Cousins D, Robinson RA, Huchzermeyer HF, Kantor AK, De Kantor I, Meslin FX. Zoonotic tuberculosis due to Mycobacterium bovis in developing countries. Emerg. Infect. Dis. 1998;4:59–70. doi: 10.3201/eid0401.980108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejene SW, Heitkönig IM, Prins HH, Lemma FA, Mekonnen DA, Alemu ZE, Kelkay TZ, de Boer WF. Risk Factors for Bovine Tuberculosis (bTB) in Cattle in Ethiopia. PLoS ONE. 2016;11(7):e0159083. doi: 10.1371/journal.pone.0159083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demelash B, Inangolet I, Oloya J, Asseged B, Badaso M, Yilkal A, Skjerve E. Prevalence of Bovine tuberculosis in Ethiopian slaughter cattle based on post-mortem examination. Trop. Anim. Health Prod. 2009;41:755–765. doi: 10.1007/s11250-008-9248-9. [DOI] [PubMed] [Google Scholar]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control. Clin. Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- Dinka H, Duressa A. Prevalence of bovine tuberculosis in Arsi Zones of Oromia, Ethiopia. Afr. J. Agric. Res. 2011;6(16):3853–3858. [Google Scholar]
- Dohoo I, Martin W, Stryhn H. Veterinary Epidemiologic Research. 2. AVC Inc; Charlottetown, Prince Edward Island: 2009. pp. 739–766. [Google Scholar]
- Duguma A, Abera S, Zewdie W, Belina D, Haro G. Status of bovine tuberculosis and its zoonotic implications in Borana zone, Southern Ethiopia. Trop. Anim. Health Prod. 2016;49(3):445–450. doi: 10.1007/s11250-016-1213-4. [DOI] [PubMed] [Google Scholar]
- EFSA and ECDPC. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-borne Outbreaks in 2012. European Food Safety Authority, European Centre for Disease Prevention and Control. EFSA Journal. 2014;12(2):3547. [Google Scholar]
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias K, Hussein D, Asseged B, Wondwossen T, Gebeyehu M. Status of bovine tuberculosis in Addis Ababa dairy farms. Rev. Sci. Tech. 2008;27(3):915–923. doi: 10.20506/rst.27.3.1850. [DOI] [PubMed] [Google Scholar]
- Ewnetu L, Melaku A, Birhanu A. Bovine Tuberculosis Prevalence in Slaughtered Cattle at Akaki Municipal Abattoir, Based on Meat Inspection Methods. Glob. Vet. 2012;9(5):541–545. [Google Scholar]
- Fetene T, Kebede N. Bovine tuberculosis of cattle in three districts of northwestern Ethiopia. Trop. Anim. Health Prod. 2009;41:273–277. doi: 10.1007/s11250-008-9186-6. [DOI] [PubMed] [Google Scholar]
- Fikru R, Bonnet P, Moges W. Prevalence of bovine tuberculosis in indigenous Zebu cattle under extensive farming system in Western Ethiopia. Bull. Anim. Hlth. Prod. Afr. 2005;53(1):85–88. [Google Scholar]
- Finlay EK, Berry DP, Wickham B, Gormley EP, Bradley DG. A Genome Wide Association Scan of Bovine Tuberculosis Susceptibility in Holstein-Friesian Dairy Cattle. PLoS ONE. 2012;7(2):e30545. doi: 10.1371/journal.pone.0030545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firdessa R, Tschopp R, Wubete A, Sombo M, Hailu E, Erenso G, Kiros T, Yamuah L, Vordermeier M, Hewinson RG, Young D, Gordon SV, Sahile M, Aseffa A, Berg S. High Prevalence of Bovine Tuberculosis in Dairy Cattle in Central Ethiopia: Implications for the Dairy Industry and Public Health. PLoS ONE. 2012;7(12):e52851. doi: 10.1371/journal.pone.0052851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebrezgabiher G, Romha G, Ameni G. Prevalence Study of Bovine Tuberculosis and Genus Typing of its Causative Agents in Cattle Slaughtered at Dilla Municipal Abattoir, Southern Ethiopia. African J. Basic and Appl. Sci. 2014;6(4):103–109. [Google Scholar]
- Gizaw S, Megersa A, Muluye M, Hoekstra D, Gebremedhin B, Tegegne A. LIVES Working Paper 23. Nairobi, Kenya: International Livestock Research Institute (ILRI); 2016. [Accessed on 3 March, 2017]. Smallholder dairy farming systems in the highlands of Ethiopia: System-specific constraints and intervention options. Available online at: https://cgspace.cgiar.org/bitstream/handle/10568/27914/ipmsWP31.pdf. [Google Scholar]
- Gumi B, Schelling E, Firdessa R, Aseffa A, Tschopp R, Yamuah L, Young D, Zinsstag J. Prevalence of bovine tuberculosis in pastoral cattle herds in the Oromia region, southern Ethiopia. Trop. Anim. Health Prod. 2011;43:1081–1087. doi: 10.1007/s11250-010-9777-x. [DOI] [PubMed] [Google Scholar]
- Gumi B, Schelling E, Firdessa R, Erenso G, Biffa D, Aseffa A, Tschopp R, Yamuah L, Young D, Zinsstag J. Low prevalence of bovine tuberculosis in Somali pastoral livestock, southeast Ethiopia. Trop. Anim. Health Prod. 2012;44:1445–1450. doi: 10.1007/s11250-012-0085-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hailemariam S. A brief analysis of the activities of the meat inspection and quarantine division. Ministry of Agriculture; Addis Ababa, Ethiopia: 1975. [Google Scholar]
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta- analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inangolet FO, Demelash B, Olya J, Opuda-Asibo J, Skjerve E. Cross-sectional study of bovine tuberculosis in the transhumant and agro-pastoral cattle herdsin the border areas of Katakwi and Morota districts, Uganda. Trop. Anim. Health Prod. 2008;40:501–508. doi: 10.1007/s11250-007-9126-x. [DOI] [PubMed] [Google Scholar]
- Kaneene JB, Pfeiffer D. Epidemiology of Mycobacterium bovis. In: Thoen CO, Steele JH, Gilsdorf MJ, editors. Mycobacterium bovis Infection in Animals and Humans. 2. Blackwell Publishing; Ames, Iowa: 2006. pp. 34–48. [Google Scholar]
- Land O’Lakes Inc. End Markets and Food Security Cooperative Agreement 663-A-00-05-00431-00. Land O’Lakes Inc; Addis Ababa, Ethiopia: 2010. [Accessed on 3 March, 2017]. The next stage in dairy development for Ethiopia Dairy Value Chains. Available online at: https://www.usaid.gov/sites/default/files/documents/1860/Dairy%20Industry%20Development%20Assessment_0.pdf. [Google Scholar]
- Laval G, Ameni G. Prevalence of bovine tuberculosis in zebu cattle under traditional animal husbandry in Boji district of western Ethiopia. Rev. Méd.Vét., 155. 2004;10:494–499. [Google Scholar]
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamo G, Abebe F, Worku Y, Hussein N, Legesse M, Tilahun G, Medhin G, Bjune G, Ameni G. Bovine tuberculosis and its associated risk factors in pastoral and agro-pastoral cattle herds of Afar Region, Northeast Ethiopia. J. Vet. Med. Anim. Health. 2013;5(6):171–179. [Google Scholar]
- Mekibeb A, Fulasa TT, Firdessa R, Hailu E. Prevalence study on bovine tuberculosis and molecular characterization of its causative agents in cattle slaughtered at Addis Ababa municipal abattoir, Central Ethiopia. Trop. Anim. Health Prod. 2013;45:763–769. doi: 10.1007/s11250-012-0287-x. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group: preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. http://dx.doi.org/10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller B, Durr S, Alonso S, Hattendorf J, Laisse CJ, Parsons SD, van Helden PD, Zinsstag J. Zoonotic Mycobacterium bovis-induced tuberculosis in humans. Emerg. Infect. Dis. 2013;19(6):899–908. doi: 10.3201/eid1906.120543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuru A, Mamo G, Teshome L, Zewdie A, Medhin G, Pieper R, Ameni G. Bovine tuberculosis and its risk factors among dairy cattle herds in and around Bahir Dar City, Northwest Ethiopia. Ethiop. Vet. J. 2015;19(2):27–40. [Google Scholar]
- OIE. Manual of Diagnostic tests and vaccines for terrestrial animals. Office Internationale des Epizooties (OIE); Paris, France: 2016. [Google Scholar]
- Regassa A, Tassew A, Amenu K, Megersa B, Abunna F, Mekibib B, Marcotty T, Ameni G. A cross-sectional study on bovine tuberculosis in Hawassa town and its surroundings, Southern Ethiopia. Trop. Anim. Health Prod. 2010;42:915–920. doi: 10.1007/s11250-009-9507-4. [DOI] [PubMed] [Google Scholar]
- Romha G, Ameni G, Berhe G, Mamo G. Epidemiology of mycobacterial infections in cattle in two districts of Western Tigray Zone, northern Ethiopia. Afr. J. Micro. Res. 2013;7(31):4031–4038. [Google Scholar]
- Romha G, Gebreegziabher G, Ameni G. Assessment of bovine tuberculosis and its risk factors in cattle and humans, at and around Dilla town, southern Ethiopia. Animal and Veterinary Sciences. 2014;2(4):94–100. [Google Scholar]
- Shirima GM, Kazwala RR, Kambarage DM. Prevalence of bovine tuberculosis in cattle in different farming systems in the eastern zone of Tanzania. Prev. Vet. Med. 2003;57:167–172. doi: 10.1016/s0167-5877(02)00214-3. [DOI] [PubMed] [Google Scholar]
- Shitaye JE, Tsegaye W, Pavlik I. Bovine tuberculosis infection in animal and human populations in Ethiopia: a review. Vet. Med. (Praha.) 2007;52(8):317–332. [Google Scholar]
- Shitaye JE, Getahun B, Alemayehu T, Skoric M, Treml F, Fictum P, Vrbas V, Pavlik I. A prevalence study of bovine tuberculosis by using abattoir meat inspection and tuberculin skin testing data, histopathological and IS6110 PCR examination of tissues with tuberculous lesions in cattle in Ethiopia. Vet. Med. (Praha.) 2006;51(11):512–522. [Google Scholar]
- STATA. Stata Statistical Software, Stata Corporation, Release 12.1 STATA. College Station; Texas: 2012. [Google Scholar]
- STB (Stata Technical Bulletin) A publication to promote communication among Stata users. STB. 1998;41:12–15. [Google Scholar]
- Teklu A, Asseged B, Yimer E, Gebeyehu M, Woldesenbet Z. Tuberculous lesions not detected by routine abattoir inspection: the experience of the Hossana municipal abattoir, southern Ethiopia. Rev. Sci. Tech. 2004;23(3):957–964. doi: 10.20506/rst.23.3.1534. [DOI] [PubMed] [Google Scholar]
- Terefe D. Gross pathological lesions of bovine tuberculosis and efficiency of meat inspection procedure to detect-infected cattle in Adama municipal abattoir. J. Vet. Med. Anim. Health. 2014;6(2):48–55. [Google Scholar]
- Tigre W, Alemayehu G, Abetu T, Ameni G. Preliminary study on the epidemiology of bovine tuberculosis in Jimma and its surroundings, Southwestern Ethiopia. Afr. J. Micro. Res. 2012;6(11):2591–2597. [Google Scholar]
- Tschopp R, Aseffa A. Bovine Tuberculosis and Other Mycobacteria in Animals in Ethiopia: A Systematic Review. J. J. Epidemiol. Prevent. 2016;2(2):026. [Google Scholar]
- Tschopp R, Abera B, Sourou SY, Guerne-Bleich E, Aseffa A, Wubete A, Zinsstag J, Young D. Bovine tuberculosis and brucellosis prevalence in cattle from selected milk cooperatives in Arsi zone, Oromia region, Ethiopia. BMC Vet. Res. 2013;9:163. doi: 10.1186/1746-6148-9-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp R, Aseffa A, Schelling E, Berg S, Hailu E, Gadisa E, Habtamu M, Argaw K, Zinsstag J. Bovine Tuberculosis at the Wildlife-Livestock-Human Interface in HamerWoreda, South Omo, Southern Ethiopia. PLoS ONE. 2010a;5(8):e12205. doi: 10.1371/journal.pone.0012205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp R, Bekele S, Moti T, Young D, Aseffa A. Brucellosis and bovine tuberculosis prevalence in livestock from pastoralist communities adjacent to Awash National Park, Ethiopia. Prev. Vet. Med. 2015;120:187–194. doi: 10.1016/j.prevetmed.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Tschopp R, Bobosha K, Aseffa A, Schelling E, Habtamu M, Iwnetu R, Hailu E, Firdessa R, Hussein J, Young D, Zinsstag J. Bovine tuberculosis at a cattle-small ruminant-human interface in Meskan, Gurage region, Central Ethiopia. BMC Infect. Dis. 2011;11:318. doi: 10.1186/1471-2334-11-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp R, Hattendorf J, Roth F, Choudhury A, Shaw A, Aseffa A, Zinsstag J. Cost estimate of bovine tuberculosis to Ethiopia. Curr. Top. Microbiol. Immunol. 2012;365:249–68. doi: 10.1007/82_2012_245. [DOI] [PubMed] [Google Scholar]
- Tschopp R, Schelling E, Hattendorf J, Young D, Aseffa A, Zinsstag J. Repeated cross-sectional skin testing for bovine tuberculosis in cattle kept in a traditional husbandry system in Ethiopia. Vet. Rec. 2010b;167:250–256. doi: 10.1136/vr.c3381. [DOI] [PubMed] [Google Scholar]
- Tsegaye W, Aseffa A, Mache A, Mengistu Y, Berg S, Ameni G. Conventional and Molecular Epidemiology of Bovine Tuberculosis in Dairy Farms in Addis Ababa City, the Capital of Ethiopia. Int. J. Appl. Res. Vet. Med. 2010;8(2):143–151. [Google Scholar]
- Vordermeier HM, Jones GJ, Buddle BM, Hewinson RG, Villarreal-Ramos B. Bovine Tuberculosis in Cattle: Vaccines, DIVA Tests, and Host Biomarker Discovery. Annu. Rev. Anim. Biosci. 2016;4:87–109. doi: 10.1146/annurev-animal-021815-111311. [DOI] [PubMed] [Google Scholar]
- Vordermeier M, Ameni G, Berg S, Bishop R, Robertson BD, Aseffa A, Hewinson RG, Young DB. The influence of cattle breed on susceptibility to bovine tuberculosis in Ethiopia. Comp. Immunol. Microbiol. Infect. Dis. 2012;35:227–232. doi: 10.1016/j.cimid.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldemariam T, Mamo G, Mohammed T, Ameni G. Prevalence of bovine tuberculosis in beef feedlot of Borena cattle by using comparative intradermal skin test, Adama, Ethiopia. Ethiop. Vet. J. 2016;20(2):17–29. [Google Scholar]
- Woyessa M, Jibril Y, Ameni G, Duguma R. Molecular Epidemiology of Mycobacterium Tuberculosis Complex at Nekemte Municipality Abattoir, Western Ethiopia. Sci. Technol. Arts Res. J. 2014;3(2):167–173. [Google Scholar]
- Yacob HT, Basu AK, Guesh N. Bovine pulmonary tuberclosis at Bahirdar municipality abattoir, Ethiopia. Bull. Anim. Hlth. Prod. Afr. 2008;56(3):223–229. [Google Scholar]
- Zeru F, Romha G, Ameni G. Gross and Molecular Characterization of Mycobacterium tuberculosis Complex in Mekelle Town Municipal Abattoir, Northern Ethiopia. Glob. Vet. 2013;11(5):541–546. [Google Scholar]
- Zeru F, Romha G, Berhe G, Mamo G, Sisay T, Ameni G. Prevalence of bovine tuberculosis and assessment of Cattle owners’ awareness on its public health implication in and around Mekelle, Northern Ethiopia. J. Vet. Med. Anim. Health. 2014;6(6):159–167. [Google Scholar]
- Zeweld SW. Cultural and molecular detection of zoonotic tuberculosis and its public health impacts in selected districts of Tigray region, Ethiopia. Sokoto Journal of Veterinary Sciences. 2014;12(1):1–12. [Google Scholar]
- Zeweld SW, Reta DH, Abay AG, Zelelew YB. Detection of Human and Bovine Tuberculosis Using an Existing Diagnostic Practice in Residential Districts of Tigray Region, Northern Ethiopia. J. Environ. Occup. Sci. 2013;2(2):77–88. [Google Scholar]
- Zinsstag J, Schelling E, Roth F, Kazwala R. Economics of Bovine Tuberculosis. In: Thoen CO, Steele JH, Gilsdorf MJ, editors. Mycobacterium bovis Infection in Animals and Humans. 2. Blackwell Publishing; Oxford, UK: 2006. pp. 68–83. [Google Scholar]