Abstract
Objective
To evaluate (1) systematic assessment of exercise tolerance in adolescents shortly after sport-related concussion (SRC) and (2) the prognostic utility of such assessment.
Design
Prospective randomized controlled trial.
Setting
University and community sports medicine centers.
Participants
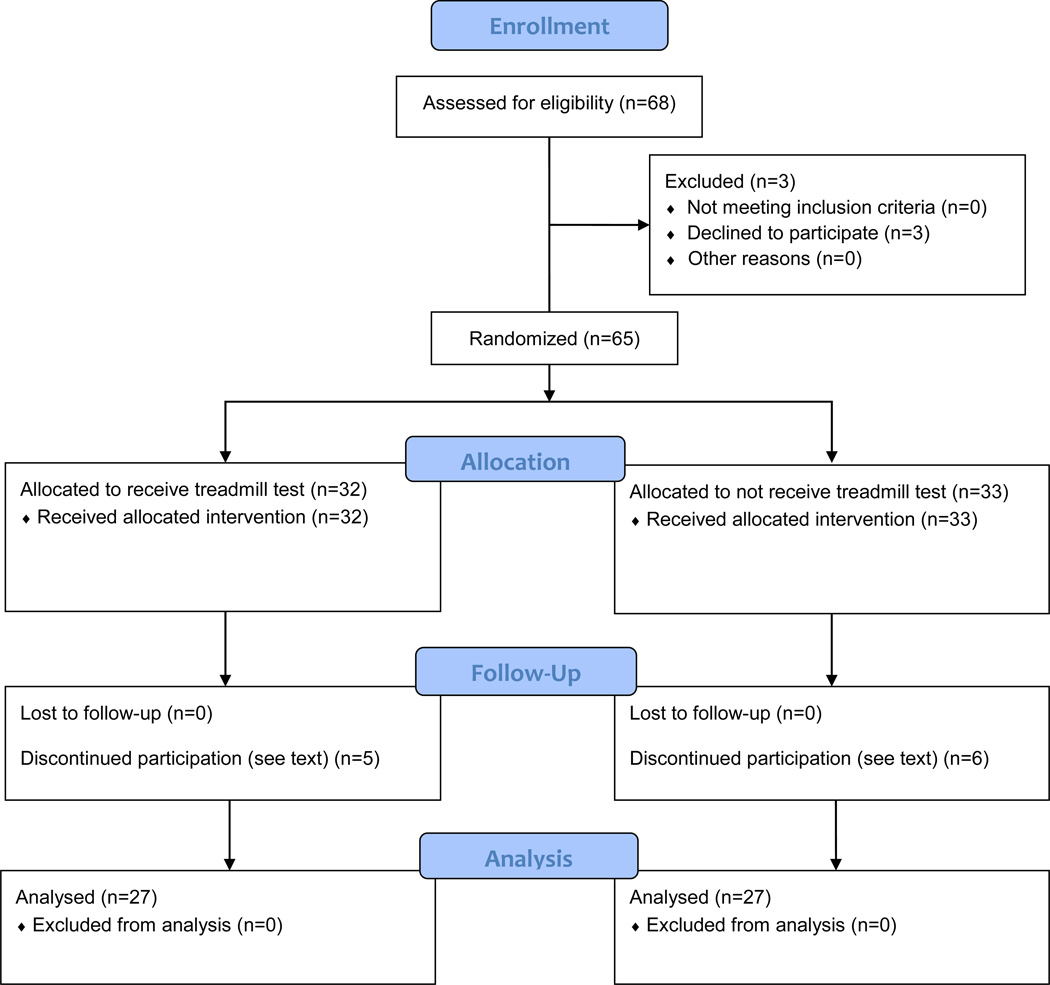
Adolescents with SRC (1–9 days from injury). Sixty-five were randomized and 54 completed the study (mean age 15 y, 4 days post injury).
Interventions
Buffalo Concussion Treadmill Test (BCTT, n=27) or not (controls, n=27) on Visit day #1. Heart rate threshold (HRt) at symptom exacerbation represented level of exercise tolerance. Participants reported symptoms daily for14 days and then had follow up BCTT (n=54). Recovery was defined as returning to normal level of symptoms and exercise tolerance, verified by independent physician examination.
Main Outcome Measures
Days to recovery and typical (≤ 21 days) vs. prolonged recovery (> 21 days). Mixed effects linear models and linear regression techniques examined symptom reports and time to recovery. Linear Regression assessed the association of HRt with recovery time.
Results
Days to recovery (p=0.7060) and typical vs. prolonged recovery (p=0.1195) were not significantly different between groups. Symptom severity scores decreased in both groups over 14 days (p<0.0001), were similar (p=0.2984), and did not significantly increase the day after the BCTT (p=0.1960). Lower HRt on visit day 1 was strongly associated with prolonged recovery time (p=0.0032).
Conclusions
Systematic evaluation of exercise tolerance using the BCTT within one week after SRC did not affect recovery. The degree of early exercise intolerance after SRC was important for prognosis. This has implications for school academic and team preparation.
Keywords: exercise, tolerance, treadmill test, sport, concussion, adolescent
INTRODUCTION
Concussion often resolves along a sequential course within 7–10 days in adults.1 In some cases, however, symptoms persist for weeks to months, which is called post-concussion syndrome (PCS).1 Rest has long been a mainstay of concussion treatment because of fears of exacerbation of symptoms, delayed recovery, and/or brain re-injury with exertion early after concussion.1,2 Experimental human data on post-concussion activity are limited; thus, current expert consensus states that a period of at least 24–48 hours of complete rest should be implemented prior to gradually returning to activity after sport-related concussion (SRC).1 In support of this, a recent prospective randomized controlled study showed that resumption of normal activities after 2 days of recommended rest following concussion improved recovery when compared with a recommendation for 5 days of strict bed rest.3 The question of when it is safe to start structured exercise or training after SRC, however, is not known. There has been no research on whether it is even safe to conduct a provocative exercise test to determine exercise tolerance during the acute phase of concussion recovery.
The adolescent age group is arguably most at risk for the long term consequences of repetitive concussions. There are data to suggest that adolescents take longer to recover than adults and children after concussion.4–6 Adolescent athletes base their perception of recovery primarily on somatic symptoms (e.g., headache, nausea, fatigue, etc.), yet these perceptions may be incongruent with objective testing.7 This supports the need for more objective, valid, and reliable measures for concussion diagnosis and management. Provocative exercise testing of adolescents in the acute phase has not been used despite potential usefulness in classifying the severity of the injury and providing valuable information about prognosis. The thinking appears to be that since adolescents take longer to recover it is not wise to risk anything that might prolong recovery.
Exercise to the maximum capacity of each sport without exacerbation of symptoms after SRC is recommended before an athlete returns to play.1 Exercise is good for the human brain8 but experimental animal data imply that it may be detrimental to human concussion recovery if implemented too soon after injury. For example, premature exercise within the first week after experimental rodent concussion impaired cognitive performance whereas aerobic exercise performed 14 to 21 days after concussion improved performance.9 Forced exercise immediately after rodent concussion elevated the cortisol/ACTH stress response whereas voluntary exercise did not.10 Thus, animal data suggest that uncontrolled or forced activity too soon after concussion is detrimental to recovery, which aligns with retrospective human data in athletes showing that high levels of activity soon after concussion impairs neurocognitive performance when compared with those who report moderate levels of activity.11
In humans who remain symptomatic for weeks to months after concussionit has been shown that symptom-limited exercise testing using the Buffalo Concussion Treadmill Test (BCTT) is reliable and safe.12,13 Provocative exercise testing using the BCTT has been very useful in identifying levels of exercise tolerance and developing exercise prescriptions for PCS patients. The safety and utility of provocative exercise testing, however, during the acute phase of recovery after SRC is not known.3 The benefit of an early assessment of exercise tolerance after concussion is that it may provide a functional biomarker of concussion severity. For example, an adolescent who is able to exercise to near voluntary exhaustion before experiencing exacerbation of symptoms is clearly closer to recovery than an adolescent who experiences symptom exacerbation at a very low level of exercise.13 An exercise test should be used only if it is safe and provides useful information. A necessary first step is to prospectively assess whether a provocative exercise test early after SRC increases symptoms in the short term or delays recovery in the long term. It is also possible that the degree of exercise tolerance soon after injury could be a more objective physiological indicator for prognosis of recovery after SRC than symptoms or neurocognitive performance, both of which are highly variable and are not specific to brain injury.14,15
Since exercise may benefit concussion recovery there is need for research on the safety or utility of assessing exercise tolerance early after concussion. Thus, the purpose of this study was to determine the safety and prognostic utility of systematic evaluation of exercise tolerance using the BCTT in adolescents early after SRC. We hypothesized that exercise up to the symptom-exacerbation threshold on the BCTT using pre-determined stopping criteria would not increase symptoms in the days after testing or delay recovery in adolescents when compared with those who did not exercise in the acute post-injury period. With respect to prognostic utility, we hypothesized that the heart rate threshold for exercise intolerance in the acute phase would bear an important relationship to time and speed of recovery (given that exercise tolerance is an important component of recovery). Our thinking was that if provocative exercise testing did not provide useful information about prognosis then it was not worth attempting in acutely injured patients, even if the procedure appeared to be relatively safe.
METHODS
Study design
We conducted a randomized controlled trial of provocative exercise testing (BCTT) on the day of clinic presentation (Visit #1) in adolescents who sustained SRC within 1–10 days of injury, conducted between March of 2013 and February of 2015 (Figure 1). We conducted a second follow up visit approximately 14 days after the first visit or approximately 21 days post injury (Visit #2).
Figure 1.
Study Design
Subjects
Male and female high school athletes referred to the University at Buffalo Concussion Management Clinics and to the Excelsior Concussion Clinic in Western New York who satisfied the inclusion/exclusion criteria were asked to participate. After randomization on Visit #1, 11 subjects (6 males and 5 females), 6 from the no BCTT (control) group and 5 from the BCTT group, withdrew from the study. Six subjects that withdrew did not return for Visit #2 without explanation; 2 withdrew because of lack of transport to the clinic for follow up appointments; one withdrew because a parent refused to allow their child to receive the exercise test; and 3 did not complete at least 75% of their daily online symptom reports. Subjects who withdrew did not differ on any characteristics or study variables from those who remained in the study. The trial ended because we met our recruitment goal, which was based on a sample size calculation. The study was approved by the University at Buffalo Institutional Review Board and all subjects and their guardians signed informed consent. This RCT was registered with clinicaltrials.gov: 030-387696.
Procedures
Inclusion Criteria
Male or female adolescents (aged 14–19 years) who sustained SRC within 1–10 days of clinic presentation were evaluated by a study physician who diagnosed the concussion. Concussion was defined according to the 4th International Conference on Concussion in Sport as a complex pathophysiological process affecting the brain, induced by biomechanical forces, resulting in a graded set of clinical symptoms that may or may not involve loss of consciousness.1
Exclusion Criteria13
Subjects with evidence of focal neurological deficit; inability to exercise because of orthopedic injury, cervical spine injury, diabetes or known heart disease; increased cardiac risk; current diagnosis of ADHD, learning disorder, depression, or anxiety; history of moderate or severe TBI; or greater than 3 prior concussions (because these factors are associated with delayed recovery16); and inability to understand English.
Potential subjects were identified after a standard clinical evaluation that consisted of a thorough history and physical examination by one of several physicians with extensive experience in concussion management. The specific exclusion criteria were listed on a form used by the physicians to exclude participants. The physical examination was standardized among the study physicians via a 2-hour training session and included instruction on assessment of cervical, oculomotor and vestibular function.17 Once diagnosis of concussion was confirmed by the physician, a research associate explained the purpose of the study and subjects signed informed consent/assent and parental consent was obtained for minors. Subjects were then randomly assigned to receive the BCTT or standard care (no treadmill test, the control group) on Visit #1. A computer-generated process of random assignment was used, in groups of 3, stratified by sex and clinic location. Sequentially numbered envelopes containing group assignment information were located in each clinic. Research assistants and subjects were not blinded to treadmill allocation but treating physicians were blinded to subject allocation. Recovery definition among the evaluating physicians was standardized to include: normal symptom severity score on the PCSS (<5 for boys and < 9 for girls),18 demonstrated exercise tolerance on the BCTT,19 a normal physical examination (normal oculomotor, vestibular, cervical and neurological examinations), and normal cognitive performance on ImPACT.20 All 54 subjects performed ImPACT on Visit #1. Subjects randomized to the BCTT (n = 27) performed ImPACT prior to treadmill testing. Subjects reported symptoms daily between Visit #1 and 14 days later (Visit #2) via a password-protected online data form. Subjects received an email and text message reminder to access the online data form to record their symptoms on the Post-Concussion Symptom Scale (PCSS), a validated instrument with normative data for males and females.18 Subjects were asked to record symptoms at approximately the same time each day, preferably in the evening.
Physicians provided standard care blinded to group assignment and made recovery and return to school and play determinations according to international concussion consensus guidelines.1 In addition, physicians were provided the recent ImPACT results and consulted a neuropsychologist regarding unusual ImPACT scores. The only difference between the two groups was that half the subjects performed the BCTT on Visit #1. At Visit #2, all 54 subjects performed ImPACT and then had the BCTT, including the subjects that had not been tested on the treadmill Visit #1. Subjects who were not recovered at Visit #2 (14 days after Visit #1) were followed with regular bi-weekly visits with the concussion management team until their symptoms and their physical examinations normalized and they were exercise tolerant.
The Buffalo Concussion Treadmill Test (BCTT)
The starting speed was 3.2–3.6 mph (depending on subject height) at 0% incline. After one minute the incline was increased by 1 degree and then by 1 degree each minute thereafter while maintaining the same speed until the subject could not continue, whether because of symptom exacerbation or fatigue. Heart rate (HR, by Polar HR monitor, Model #FIT N2965, Kempele, Finland), rating of perceived exertion (RPE), and symptoms were assessed every minute and the test was stopped at exacerbation of symptoms or at voluntary exhaustion (RPE ≥17). Symptom exacerbation was defined as ≥3 point change from that day’s pre-treadmill resting overall symptom score on a 1–10 point visual analog scale (VAS) where a point was given for each increase in a symptom, e.g., headache, and/or the appearance of a new symptom, e.g., dizziness.13 For safety reasons, the test was performed under supervision of 2 CPR-trained research personnel.
Statistical Analysis
The objectives in this study were twofold: 1) to examine the impact of the BCTT on recovery and 2) to examine the prognostic utility of the heart rate threshold (HRt: average heart rate over the final minute) of the BCTT in patients at Visit #1 in predicting time to recovery. Time to recovery was calculated as the number days from the time of injury to the first day in which the daily symptom severity score fell below 5 on the PCSS, confirmed by a physical examination that revealed no signs of concussion, and demonstrated exercise tolerance.
In assessing Objective 1, the impact of the BCTT, we were interested in how symptom scores and recovery times were associated with performing the BCTT. To this end, we examined several associations: 1) the association between the days to recovery with BCTT/control status; 2) the association of binary days to recovery (≤ 21 days, > 21 days) with BCTT/control status; 3) the association of symptom scores at Visit # 1 and the very next day after testing with BCTT/control status; and 4) the association of symptom scores from Visit # 1 to Visit # 2 (14 days after first visit) with BCTT/control status.
For the analysis of Objective 1, we used linear regression for days to recovery, logistic regression for binary days to recovery, and mixed effects linear models to assess whether the symptom severity scores changed between Visit #1 and the day after administration of the BCTT, compared with controls after their first visit, and whether this change was significantly different in the BCTT group versus the control group. Also, mixed effects linear models were used to analyze the first 14 days of daily symptom severity data within each group (BCTT/control).
For Objective 2, we were interested in the association between the average heart rate in beats per minute (bpm) over the final minute of the BCTT in the 27 subjects randomized to the BCTT at Visit # 1 and their time to recovery. The average heart rate in bpm was measured by Polar HR (see BCTT in Procedures) and is denoted by heart rate threshold (HRt). To assess its prognostic utility, we examined several associations: 1) the association between HRt and days to recovery; 2) the association between HRt and binary days to recovery (≤21 days, > 21 days); 3) the association between a binary HRt (<135 BPM, ≥ 135 BPM) and days to recovery; and 4) the association between a binary HRt and binary days to recovery (≤21 days, >21 days). The binary HRt variable was based on a cut point of 135 bpm that was determined via k-means (k=2). As an exploratory aim within this objective, we examined the additional impact (if any) of including HRt or binary HRt to a prognostic model with total symptom scores at Visit #1 for explaining days to recovery or binary days to recovery (≤21 days, > 21days). In this way, we could explore the prognostic value of HRt relative to other possible prognostic markers such as total symptom score at the initial visit.
For the analysis of Objective 2, we used linear regression for the association between HRt and binary HRt and days to recovery and we used logistic regression for HRt/ binary HRt and binary days to recovery. The exploratory aim in this objective also employed the above techniques to create a full model including symptom score at Visit #1 and HRt (or binary HRt) and a reduced model including only symptom score at Visit #1. The full and reduced models were then compared via ANOVA for the linear regression models (F-test) and Analysis of Deviance for the logistic regression models (Chi-squared test). All data analyses were performed using the R programming language.21
RESULTS
As can be seen in Table 1, consistent with expectations with random assignment, there were no differences in age, sex, number of prior concussions, days since injury, or symptom scores between the two groups at Visit #1. There was no evidence that allocation concealment was not maintained.
Table 1.
Characteristics of patients in each group (BCTT: n=27 and Control: n=27).
| Characteristic | BCTT (n=27) | Control (n=27) | P-value |
|---|---|---|---|
| Age (mean in years ± standard deviation (SD) |
15.19±1.45 | 15.63±1.36 | 0.2498 |
| Gender (count) | |||
| Male | 18 | 19 | |
| Female | 9 | 8 | >0.9999 |
| # Prior Concussions (count) |
|||
| 0 | 18 | 21 | |
| 1 | 9 | 4 | |
| 2 or more | 0 | 2 | 0.1253 |
| Days Since Injury prior to Visit #1 (median and IQR) |
Median = 4 IQR = 3 |
Median = 5 IQR =3 |
0.4626 |
| Initial PCSS at Visit #1 (median and IQR) |
Median = 31 IQR = 33 |
Median = 17 IQR = 27 |
0.1001 |
| PCSS at day after Visit #1 (median and IQR) |
Median = 28 IQR = 27 |
Median = 16 IQR = 21.5 |
0.3268 |
| PCSS at Day 3 (median and IQR) |
Median=21.0 IQR=38.25 |
Median=15.5 IQR=17.5 |
0.2065 |
| PCSS at Day 4 (median and IQR) |
Median=24.0 IQR=31.50 |
Median=13.0 IQR=22.0 |
0.2002 |
| PCSS at Day 5 (median and IQR) |
Median=22.0 IQR=28.0 |
Median=7.0 IQR=23.0 |
0.1414 |
| PCSS at Day 6 (median and IQR) |
Median=15.0 IQR=20.50 |
Median=11.0 IQR=20.0 |
0.4517 |
| PCSS at Day 7 (median and IQR) |
Median=7.5 IQR=24.5 |
Median=7.0 IQR=19.0 |
0.3783 |
| Time to Recovery (Days, mean ± SD) |
27.5±36.6 | 23.5±39.4 | 0.7060 |
BCTT (Buffalo Concussion Treadmill Test); PCSS (Post Concussion Symptom Scale severity score); P-value for age, time to recovery via two sample t test, for gender and # prior concussions via Chi-squared, while Days since injury, PCSS Visit # 1, and PCSS Day after Visit #1 and Days 3–7 p-values are from Wilcoxon Rank Sum test. IQR denotes interquartile range.
Results for Objective 1
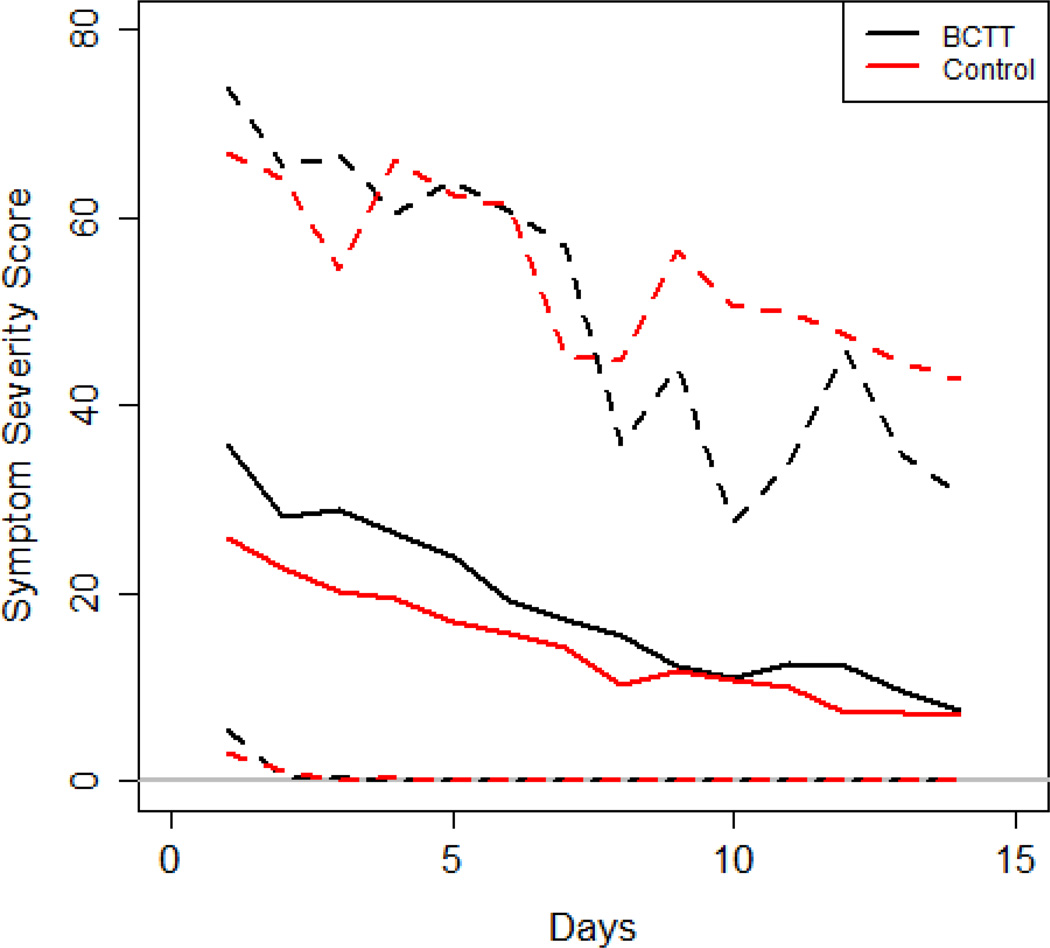
Using a simple linear regression, days to recovery was not significantly associated with BCTT status (R2 =0.0028, p=0.7060). Using a simple logistic regression, the binary days to recovery variable (≤21 days, >21 days) was not significantly associated with BCTT status (McFadden’s R2=0.0433, p=0.1195). Regarding symptom scores, Figure 2 is a plot of the daily PCSS reports of all 54 subjects from clinic Visit #1 through clinic Visit #2 (14 days after Visit #1). Figure 2 shows that the rate of symptom recovery of those that received the BCTT during the acute phase after concussion was quite similar to the rate of recovery of those that did not receive the BCTT. We examined symptom scores for the first two time points in Figure 2 (Visit #1 and the day after Visit #1) via mixed effects linear models. The group variable (BCTT/control) was not significantly associated with the symptom score (Table 1. F-value=1.7157, p=0.1960). This suggests there is no short term (within 24 hours) acute increase in symptom scores due to use of the BCTT.
Figure 2.
The sample mean (solid lines), 5th and 95th sample percentiles (dashed lines) for each day's symptom severity scores for the BCTT group (black) and the control group (red).
Using mixed effects linear models to analyze the entire 14-day daily symptom severity score data with each group, we found that the symptom scores significantly decreased over time (F-value = 53.00, p<0.0001, see Figure 2) and that the symptom score was not significantly associated with BCTT/control group (F=1.1035, p=0.2984) or the interaction term for group and time (F=1.0385, p=0.4121). Hence, we conclude that symptom scores are decreasing over time as the subjects are recovering; however, there is no evidence that the symptom recovery trend changed significantly in the BCTT group versus the control group. We examined the symptom severity scores for each day in the first week using a Wilcoxon-Rank sum test (Table 1). The p-values at each day are all larger than 0.05, suggesting that there are no significant differences in symptom scores between the groups on any day during the 7 day period. For each subject we computed the difference in symptom score between Day 1 and Day 2 and tested for significance with BCTT/Control status via a two sample t test. The mean decrease in symptom score from Day 1 to Day 2 was 8.64 for the BCTT group and 4.00 for the Control group (p=0.07). This suggests that there is no immediate increase in symptom score from Day 1 to Day 2, and albeit not statistically significant, the BCTT group had a larger reduction in symptoms immediately after the treadmill test when compared with the control group. For Objective 1, our results suggest that the BCTT does not significantly affect symptom reporting in the short-term or delay recovery or increase symptoms in the long term.
Results for Objective 2
Using simple linear regression, HRt is significantly associated with days to recovery (R2=0.2982, p=0.0032). The estimate for HRt is −0.82 with a standard error of 0.2517 (t-score=−3.26, p=0.0032) indicating that, on average, every 1 beat per minute increase in heart rate threshold resulted in a .82 day shorter recovery period. Using logistic regression, HRt is significantly associated with binary days to recovery (McFadden’s R2=0.2543, p=0.0149). Using k-means clustering (k=2), we dichotomize the HRt scores into a low HRt (≤135 bpm) and a high HRt (>135 bpm). The binary HRt is significantly associated with days to recovery (R2=0.3641, p=0.0009) and binary days to recovery (McFadden’s R2= 0.4525, p=0.0017). Table 2 shows the contingency table for the binary HRt score and binary days to recovery (Fisher’s test p=0.0003, odds ratio = 44.75), indicating that subjects with a low HR threshold (<135 bpm) are approximately 45 times more likely to have a prolonged recovery. Both measures of HRt (bpm, binary bpm) are significantly associated with time to recovery (days, binary based on 21 days cut point) with the binary HRt score being more significant. As for direction of association throughout these models, the coefficients suggest that a lower HRt is significantly associated with longer recovery times.
Table 2.
Contingency table showing number of participants who recovered in a normal time period versus those with prolonged recovery based on heart rate threshold for the BCTT group on first visit (n=27).
| Low HR Threshold (≤135 BPM) | High HR Threshold (>135 BPM) | |
|---|---|---|
| Normal Recovery (≤ 21 days) | 1 | 17 |
| Prolonged Recovery (> 21 days) | 7 | 2 |
As the exploratory aim in this objective, we were interested in examining other prognostic variables and we focused on total symptom score at Visit #1. We found that symptom score is not significantly associated with days to recovery (R2=0.0825, p=0.1463). Nevertheless, we explored the association of HRt with days to recovery in a model that also includes total symptom score. As expected, HRt is significantly associated (F=8.9254, p=0.0063) with time to recovery in a model that includes total symptom score (same for binary HRt, F=11.184, p=0.0027). Similarly, HRt is significantly associated (Chi-sq=7.2314, p=0.0072) with binary time to recovery in a model that includes total symptom score (same for binary HRt, Chi-sq=13.20, p=0.0003). Thus, HRt added significant prognostic value to a model that also includes total symptom score at Visit #1.
There were no adverse medical events as a result of the treadmill test. No athletes reported an increase in symptoms over time independent of group assignment and no athlete reported an increased symptom score at Visit #2 when compared to Visit #1. Before returning for Visit #2, no subject had completed a formal return to play protocol. The only medications recommended were as-needed acetaminophen (for headache) or melatonin (for sleep). No subjects were kept out of school for more than 2 days and no subjects were participating in physical/vestibular therapy during the 14 days between study visits. As of Visit #2, 81% (22/27) randomized to the BCTT and 78% (21/27) randomized to no BCTT were recovered, which is consistent with the rate of recovery after SRC reported in the literature.1
DISCUSSION
We used a randomized trial design to evaluate whether administration of a provocative exercise test to adolescents during the acute phase after SRC would cause a short-term increase in symptoms or possibly delay recovery. We also wanted to know if the measure of exercise tolerance (i.e. the HRt at exercise intolerance on the treadmill) provided valid information about time to recovery and therefore was worth any other possible risk of assessing the acutely injured athlete. Using the pre-determined stopping criterion of symptom exacerbation on the BCTT, we found that systematic evaluation of exercise tolerance a median of 4 days after injury did not cause a short-term increase in symptoms (i.e., within 24 hours) and that the rate of symptom recovery during 14 days of follow up was not significantly different when compared with those who did not perform the treadmill test. If there was going to be an adverse effect of exercise testing on symptoms, we would have seen an increase in symptom score for a BCTT subject from visit one (when assessment occurred) to the day after.. We did not. Thus, the BCTT appears to do no harm when administered within a week of SRC. Secondly, the degree of exercise intolerance within the first week after concussion, as indicated by the HRt at symptom exacerbation on the BCTT, strongly predicted recovery by 2–3 weeks after concussion injury in adolescents. The lower the HRt at symptom exacerbation, that is, the greater the level of early exercise intolerance, the longer adolescents required to recover from SRC. Thus, the BCTT may be useful for prognosis when administered within a week of injury to identify adolescents likely to experience delayed recovery after SRC. This is very useful information for school academic and athletic team planning. We understand that further validation of HRt as a prognostic marker including estimates of sensitivity, specificity, and positive predictive value will need to be obtained from planned future independent studies.
We have shown previously that the BCTT is very useful as a final screen for return to practice in asymptomatic athletes after SRC.19 The rationale for using the BCTT while patients are still symptomatic is to use the data to prescribe individualized, low-level, sub-symptom threshold aerobic exercise as a treatment in the first week after concussion to see if early but controlled activity speeds recovery or reduces symptom burden. The beneficial effects of controlled exercise upon autonomic function, cerebral blood flow and neuroplasticity should be evaluated for efficacy in those recently concussed. Before attempting this, however, it was important to ensure that the test for systematically assessing exercise tolerance itself was safe in the acute recovery period. Our data show that it is safe and suggest that a low HRt early after concussion identifies those adolescents who are slow to recover, which is consistent with animal data showing that early uncontrolled or forced exercise is detrimental to recovery.10 If we want to assess whether controlled exercise in the acute recovery phase may help adolescents recover from SRC and prevent some of them from suffering delayed recovery, it was important to ensure that the test of exercise tolerance itself did no harm.
Consistent with reports in the literature,1 15% of our subjects did not recover within 2–3 weeks of their initial assessment (i.e., within 21 days after injury). There was, however, no statistically significant difference in the number of subjects who had prolonged recovery from their concussion whether they performed early treadmill testing or not. Treadmill exercise testing to develop an individualized sub-threshold exercise prescription is a clinically reliable test.12 It has been shown to be safe in patients with PCS who are outside of the acute recovery phase,22 theoretically because PCS patients are beyond the period of acute metabolic crisis of increased vulnerability where excessive post-injury activity may delay recovery.23 The present study confirms that it is also safe to systematically assess exercise capacity early after SRC in adolescents provided the patient is not allowed to exceed the individual symptom-limited threshold.
Efforts to improve prognosis of concussion recovery have traditionally focused on demographic and medical history data (sex, number of prior concussions), symptom type and burden, and computerized neuropsychological test performance.16 These variables have not consistently been successful at predicting time to recovery from SRC, perhaps because there have been varying definitions of concussion recovery (e.g., symptom resolution, normal cognitive test performance, return of balance, return to sport, etc.). The Zurich guidelines provide the most widely accepted definition of recovery, i.e., normalization of symptoms and the ability to exercise to the maximum requirements of the sport without exacerbation of symptoms.1 Exercise intolerance can thus be considered to be an objective sign of concussion suggesting that return of normal exercise tolerance signals physiological recovery.24,25 It is therefore not surprising that a systematic assessment of exercise tolerance during acute SRC would best predict time to recovery (of exercise tolerance). However, it is notable that in this study, in addition to being exercise tolerant, recovery was defined as fully recovered by assessment of a physician blinded to the exercise test results (normal level of symptoms, normal physical examination, and normal cognitive function on ImPACT).
A persistent problem for treating clinicians is that there is no specific imaging or serum biomarker for concussion; therefore, the diagnosis is a clinical one, which is based upon a traumatic mechanism of injury that results in the rapid onset of (typically) short-lived impairment of neurological function characterized by a graded set of symptoms.1 It is being increasingly recognized that there is a differential diagnosis for the subjective symptoms after head injury because they do not always emanate from the brain (e.g., they may be from a cervical injury)15 and so the lack of a biomarker challenges clinicians when the diagnosis of concussion is in question. The results of this study suggest that exercise intolerance may be emerging as one of the best systemic physiological biomarkers of concussion and that return of normal exercise tolerance may be one physiological biomarker of concussion recovery. Exercise intolerance has been shown to relate to abnormal control of cerebral blood flow (CBF) during exercise25 and a small controlled study showed that recovery of exercise tolerance was associated with return to normal brain function on functional MRI during a cognitive task.26 Since most clinicians are not present at the time of injury, the diagnosis of concussion depends upon the degree of clinical certainty after the history and physical examination. If the clinician has a question of whether concussion is responsible for the patient’s symptoms after head injury, demonstration of early exercise intolerance could increase clinician confidence that a brain injury is the source. In a similar vein, confirmation of return of exercise tolerance could increase confidence that the athlete is physiologically recovered and ready to begin the graduated return to play process. The results of this study demonstrate that the degree of exercise intolerance shortly after SRC strongly predicts time to recovery. The provocative exercise test (BCTT) can thus be conceptualized as a clinical representation of abnormal concussion cerebrovascular physiology. This may account for exercise intolerance being more predictive of recovery than non-physiological variables.19
The usual care for concussion, especially for children and adolescents, has been to prescribe rest until the patient becomes asymptomatic.1 This ‘rest is best’ model was based on animal research that suggested aerobic activity delayed recovery after simulated concussion in rodents.9,27 Sub-threshold aerobic exercise22 and multimodal physiotherapy28 have been shown to be of benefit for those who are slow to recover but the optimal amount of rest, when to start exercise, and how to progress safely in the acute phase of concussion recovery have not yet been established. The potential value of systematic evaluation of exercise tolerance early after injury is that it may serve as a first step in developing a safe, sub-threshold exercise program, potentially to use exercise as “medicine”29 to speed recovery from SRC in adolescents and perhaps reduce the risk of delayed recovery due to prolonged autonomic dysfunction in some of these patients.30 Since concussion is a uniquely individual injury, we believe that determination of each individual’s level of exercise tolerance after concussion is best done systematically using a physiological test like the BCTT. Thus, it was important to ensure that the test itself does not do harm.
Limitations of the study include that it was performed on young healthy adolescent athletes who sustained SRC and therefore the results should not be generalized to younger children, to adults with risk factors for heart disease, or to concussions incurred by other mechanisms of injury (car accidents, work injuries, etc.). Subjects were not blinded to treadmill allocation; therefore, it is possible that the information/instructions provided as part of the informed consent proceedings influenced their self-report of symptoms and performance on other measures of the clinical assessment. We think this is unlikely. The use of early exercise evaluation in athletes with ADHD, a learning disorder, psychological disorders, and a history of 3 or more concussions requires further study. Another potential limitation is that the concussion centers involved in this trial are accustomed to evaluating exercise tolerance in patients with concussion. It will require extra training on the part of others to gain experience and become comfortable with the nuances of exercise testing in concussed patients.
In summary, clinicians can safely assess exercise tolerance using the pre-determined stopping criterion of symptom exacerbation on the BCTT in adolescents within the first week after SRC. The degree of early exercise intolerance appears to have prognostic utility and may serve as a physiological biomarker for the severity of concussion. Moreover, return of normal exercise tolerance could serve as a physiological biomarker of concussion recovery. While not every adolescent needs a treadmill test after concussion, the information from the test could help clinicians prescribe evidence-based, specific instructions to patients (i.e., at what HR level to limit exercise intensity) for safe resumption of activity following several days of complete rest. This information could help team physicians establish prognosis for readiness to return to play shortly after injury that would be very valuable to athletic team planning. Early identification of students who are at risk for prolonged recovery will help schools direct their limited academic resources to these patients. Systematic evaluation of exercise tolerance does not require baseline testing or specialist consultation, works equally well in males and females, and clinicians can partner with a physical therapist or athletic trainer, practitioners with equipment to evaluate exercise tolerance after concussion. The principles of a gradual increase in exercise intensity, the use of pre-determined stopping criteria,13 and the use of a heart rate monitor to establish the threshold can be performed on a variety of testing devices, such as a treadmill or stationary bike.31 The results of this study should be confirmed in other groups and extended to randomized controlled studies of the efficacy of early individualized sub-threshold aerobic exercise treatment on improving recovery in the acute phase after SRC.
Acknowledgments
We gratefully acknowledge the assistance of Christopher Stawitz, ATC and Anthony Surace, ATC, for their tremendous efforts in diligently collecting much of the clinical data.
We gratefully appreciate the support of The Ralph C. Wilson Foundation, Program for Understanding Childhood Concussion and Stroke, The Robert Rich Family Foundation, The Buffalo Sabres Foundation, and the National Football League Charities for their financial support.
Research reported in this publication was supported by the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number 1R01NS094444. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
The authors report no conflicts of interest.
REFERENCES
- 1.McCrory P, Meeuwisse W, Aubry M, et al. Consensus statement on concussion in sport--the 4th International Conference on Concussion in Sport held in Zurich, November 2012. Clin J Sport Med. 2013;23(2):89–117. doi: 10.1097/JSM.0b013e31828b67cf. [DOI] [PubMed] [Google Scholar]
- 2.Schnadower D, Vazquez H, Lee J, Dayan P, Roskind CG. Controversies in the evaluation and management of minor blunt head trauma in children. Current opinion in pediatrics. 2007;19(3):258–264. doi: 10.1097/MOP.0b013e3281084e85. [DOI] [PubMed] [Google Scholar]
- 3.Thomas DG, Apps JN, Hoffmann RG, McCrea M, Hammeke T. Benefits of Strict Rest After Acute Concussion: A Randomized Controlled Trial. Pediatrics. 2015;135(2):213–223. doi: 10.1542/peds.2014-0966. [DOI] [PubMed] [Google Scholar]
- 4.Carson JD, Lawrence DW, Kraft SA, et al. Premature return to play and return to learn after a sport-related concussion: physician's chart review. Canadian family physician Medecin de famille canadien. 2014;60(6):e310, e312–e315. [PMC free article] [PubMed] [Google Scholar]
- 5.Kostyun RO, Hafeez I. Protracted recovery from a concussion: a focus on gender and treatment interventions in an adolescent population. Sports health. 2015;7(1):52–57. doi: 10.1177/1941738114555075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Field M, Collins MW, Lovell MR, Maroon J. Does age play a role in recovery from sports-related concussion? A comparison of high school and collegiate athletes. J Pediatr. 2003;142(5):546–553. doi: 10.1067/mpd.2003.190. [DOI] [PubMed] [Google Scholar]
- 7.Sandel NK, Lovell MR, Kegel NE, Collins MW, Kontos AP. The relationship of symptoms and neurocognitive performance to perceived recovery from sports-related concussion among adolescent athletes. Applied neuropsychology. Child. 2013;2(1):64–69. doi: 10.1080/21622965.2012.670580. [DOI] [PubMed] [Google Scholar]
- 8.Guiney H, Lucas SJ, Cotter JD, Machado L. Evidence cerebral blood-flow regulation mediates exercise-cognition links in healthy young adults. Neuropsychology. 2015;29(1):1–9. doi: 10.1037/neu0000124. [DOI] [PubMed] [Google Scholar]
- 9.Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125(1):129–139. doi: 10.1016/j.neuroscience.2004.01.030. [DOI] [PubMed] [Google Scholar]
- 10.Griesbach GS, Tio DL, Vincelli J, McArthur DL, Taylor AN. Differential effects of voluntary and forced exercise on stress responses after traumatic brain injury. J Neurotrauma. 2012;29(7):1426–1433. doi: 10.1089/neu.2011.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majerske CW, Mihalik JP, Ren D, et al. Concussion in sports: postconcussive activity levels, symptoms, and neurocognitive performance. J Athl Train. 2008;43(3):265–274. doi: 10.4085/1062-6050-43.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leddy JJ, Baker JG, Kozlowski K, Bisson L, Willer B. Reliability of a graded exercise test for assessing recovery from concussion. Clin J Sport Med. 2011;21(2):89–94. doi: 10.1097/JSM.0b013e3181fdc721. [DOI] [PubMed] [Google Scholar]
- 13.Leddy JJ, Willer B. Use of Graded Exercise Testing in Concussion and Return-to-Activity Management. Curr Sports Med Rep. 2013;12(6):370–376. doi: 10.1249/JSR.0000000000000008. [DOI] [PubMed] [Google Scholar]
- 14.Boake C, McCauley SR, Levin HS, et al. Diagnostic criteria for postconcussional syndrome after mild to moderate traumatic brain injury. J Neuropsychiatry Clin Neurosci. 2005;17(3):350–356. doi: 10.1176/jnp.17.3.350. [DOI] [PubMed] [Google Scholar]
- 15.Leddy JJ, Baker JG, Merchant A, et al. Brain or strain? Symptoms alone do not distinguish physiologic concussion from cervical/vestibular injury. Clin J Sport Med. 2015;25(3):237–242. doi: 10.1097/JSM.0000000000000128. [DOI] [PubMed] [Google Scholar]
- 16.Leddy JJ, Sandhu H, Sodhi V, Baker JG, Willer B. Rehabilitation of Concussion and Post-concussion Syndrome. Sports health. 2012;4(2):147–154. doi: 10.1177/1941738111433673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matuszak JM, McVige J, McPherson J, Willer B, Leddy J. A Practical Concussion Physical Examination Toolbox: Evidence-Based Physical Examination for Concussion. Sports health. 2016;8(3):260–269. doi: 10.1177/1941738116641394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lovell MR, Iverson GL, Collins MW, et al. Measurement of symptoms following sports-related concussion: reliability and normative data for the post-concussion scale. Appl Neuropsychol. 2006;13(3):166–174. doi: 10.1207/s15324826an1303_4. [DOI] [PubMed] [Google Scholar]
- 19.Darling SR, Leddy JJ, Baker JG, et al. Evaluation of the Zurich Guidelines and Exercise Testing for Return to Play in Adolescents Following Concussion. Clin J Sport Med. 2013 doi: 10.1097/JSM.0000000000000026. [DOI] [PubMed] [Google Scholar]
- 20.Collins MW, Field M, Lovell MR, et al. Relationship between postconcussion headache and neuropsychological test performance in high school athletes. The American journal of sports medicine. 2003;31(2):168–173. doi: 10.1177/03635465030310020301. [DOI] [PubMed] [Google Scholar]
- 21.R: A language and environment for statistical computing [computer program] Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 22.Leddy JJ, Kozlowski K, Donnelly JP, Pendergast DR, Epstein LH, Willer B. A preliminary study of subsymptom threshold exercise training for refractory post-concussion syndrome. Clinical Journal of Sport Medicine. 2010;20(1):21–27. doi: 10.1097/JSM.0b013e3181c6c22c. [DOI] [PubMed] [Google Scholar]
- 23.Giza CC, Difiori JP. Pathophysiology of sports-related concussion: an update on basic science and translational research. Sports health. 2011;3(1):46–51. doi: 10.1177/1941738110391732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozlowski KF, Graham J, Leddy JJ, Devinney-Boymel L, Willer BS. Exercise intolerance in individuals with postconcussion syndrome. J Athl Train. 2013;48(5):627–635. doi: 10.4085/1062-6050-48.5.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clausen M, Pendergast DR, Willer B, Leddy J. Cerebral Blood Flow During Treadmill Exercise Is a Marker of Physiological Postconcussion Syndrome in Female Athletes. J Head Trauma Rehabil. 2016;31(3):215–224. doi: 10.1097/HTR.0000000000000145. [DOI] [PubMed] [Google Scholar]
- 26.Leddy JJ, Cox JL, Baker JG, et al. Exercise treatment for postconcussion syndrome: a pilot study of changes in functional magnetic resonance imaging activation, physiology, and symptoms. J Head Trauma Rehabil. 2013;28(4):241–249. doi: 10.1097/HTR.0b013e31826da964. [DOI] [PubMed] [Google Scholar]
- 27.Griesbach GS, Gomez-Pinilla F, Hovda DA. The upregulation of plasticity-related proteins following TBI is disrupted with acute voluntary exercise. Brain Res. 2004;1016(2):154–162. doi: 10.1016/j.brainres.2004.04.079. [DOI] [PubMed] [Google Scholar]
- 28.Schneider KJ, Iverson GL, Emery CA, McCrory P, Herring SA, Meeuwisse WH. The effects of rest and treatment following sport-related concussion: a systematic review of the literature. Br J Sports Med. 2013;47(5):304–307. doi: 10.1136/bjsports-2013-092190. [DOI] [PubMed] [Google Scholar]
- 29.Sallis R. Exercise is medicine: a call to action for physicians to assess and prescribe exercise. Phys Sportsmed. 2015;43(1):22–26. doi: 10.1080/00913847.2015.1001938. [DOI] [PubMed] [Google Scholar]
- 30.Leddy JJ, Kozlowski K, Fung M, Pendergast DR, Willer B. Regulatory and autoregulatory physiological dysfunction as a primary characteristic of post concussion syndrome: implications for treatment. NeuroRehabilitation. 2007;22(3):199–205. [PubMed] [Google Scholar]
- 31.Dematteo C, Volterman KA, Breithaupt PG, Claridge EA, Adamich J, Timmons BW. Exertion Testing in Youth with Mild Traumatic Brain Injury/Concussion. Med Sci Sports Exerc. 2015;47(11):2283–2290. doi: 10.1249/MSS.0000000000000682. [DOI] [PubMed] [Google Scholar]