Summary
The interleukin (IL)-1 family of cytokines is currently comprised of 11 members that have pleiotropic functions in inflammation and cancer. IL-1α and IL-1β were the first members of the IL-1 family to be described, and both signal via the same receptor, IL-1R. Over the last decade, much progress has been made in our understanding of biogenesis of IL-1β and its functions in human diseases. Studies from our lab and others have highlighted the critical role of Nod-like receptors (NLRs) and multi-protein complexes known as inflammasomes in the regulation of IL-1β maturation. Recent studies have increased our appreciation of the role played by IL-1α in inflammatory diseases and cancer. However, the mechanisms that regulate the production of IL-1α and its bioavailability are relatively understudied. In this review, we summarize the distinctive roles played by IL-1α in inflammatory diseases and cancer. We also discuss our current knowledge about the mechanisms that control IL-1α biogenesis and activity, and the major unanswered questions in its biology.
Keywords: IL-1, cell death, autoinflammatory disorders, cancer
IL-1 cytokine and receptor family
Interleukins were first identified as pyrogenic molecules produced by leukocytes incubated with lipopolysaccharide (LPS) that also enhance the T cell response to lectins (1, 2). Dinarello et al., identified two distinct pyrogens produced by monocytes and neutrophils upon stimulation with heat-killed Staphylococcus (3, 4). With the advent of DNA sequencing strategies, these pyrogenic molecules were later described as IL-1α and IL-1β (5). The IL-1 family is now comprised of 11 members, (IL-1α, IL-1β, IL-18, IL- 33, IL-36α, IL-36β, IL-36γ, IL-1R antagonist (IL-1Ra), IL-36Ra, IL-37, and IL-38) which can have complimentary or distinct biological functions (6). Most of the genes encoding the members of the IL-1 cytokine family are located on human chromosome 2, except for IL-18 and IL-33 encoding genes that are located on chromosomes 11 and 9 respectively. Cytokines of the IL-1 family share a common structure at the C-terminus, which is comprised by of a typical β-trefoil fold consisting of 12-β-strands connected by 11 loops (7).
While IL-1 receptor antagonist (IL-1Ra) possesses a leader secretion sequence that targets it for secretion via the endoplasmic reticulum (ER)-golgi pathway, all other IL-1 cytokines require unconventional pathways of maturation and/or release from the cell. IL-1α, IL-33 and IL-36 possess a nuclear localization signal and localize to the nucleus under homeostatic conditions. However, the function of nuclear localization of these cytokines is unclear. IL-1β and IL-18 are expressed as inactive zymogens that are activated by cleavage in the N-terminal region by caspase-1, which is itself activated by formation of a multi-protein complex known as the inflammasome (8, 9). The release of other IL-1 cytokines in bio-active forms is also thought to require some form of inflammatory cell death (10). Therefore, the activity of IL-1 cytokines is tightly regulated not only at the level of expression, but also maturation by activated caspases and release from the cell by cell death pathways.
Binding of the cognate IL-1 agonist to the specific receptor leads to the recruitment of the co-receptor, followed by activation of intracellular signaling. Receptors of the IL-1 cytokines typically possess three immunoglobulin (Ig) domains and one Toll-like/IL-1R (TIR) domain (11, 12). Signaling downstream of the receptors can be further modulated by membrane-bound and soluble decoy receptors, accessory proteins and antagonists. The IL-1 receptor family is comprised of several members including IL-1R1 (commonly referred to as IL-1R), decoy receptor IL-1R2, IL-1R accessory protein (IL-1RaP or IL- 1R3), IL-1R4 (T1 or ST2), IL-18Rα (IL-1R5), IL-36R (IL-1R6), IL-18R accessory protein (IL-18Rβ or IL-1R7), IL-1R8 (TIR8), IL-1R9 (IL-1RAPL2), and IL-1R10 (TIGIRR) (11, 12). IL-1α and IL-1β are agonists of IL-1R, while IL-18 and IL-36 bind to IL-18Rα and IL-36R respectively to induce activation of downstream signaling in a MyD88-dependent manner. IL-1R1, IL-1R2, IL-1R4, and IL-36R share the co-receptor IL-1RaP, while IL- 18Rα utilizes IL-18Rβ as the accessory receptor (12). The decoy receptors are unable to initiate a signaling cascade as they lack the cytoplasmic TIR domains. IL-1Ra and IL- 36Ra are soluble receptor antagonists that preclude activation of the cognate receptors (11, 12). Furthermore, a secretory form of IL-1R1 (sIL-1R1) also inhibits IL-1α, IL-1β, and IL-1Ra (13). Furthermore, IL-1R2 can be cleaved and solubilized by matrix metalloproteases (MMPs) to act as an IL-1 antagonist (14). Unsurprisingly, a tight control of agonist and antagonist activity is required to mount an appropriate immune response, and mutations associated with dysregulated activity of IL-1 cytokines are frequently associated with autoinflammatory diseases and cancer. While the regulation and function of IL-1β and other IL-1 cytokines has been reviewed elsewhere (7, 15) here we will focus on our current understanding of regulation and function of IL-1α in inflammatory diseases and cancer.
IL-1α expression and sub-cellular localization
Both IL-1α and IL-1β signal via IL-1R. IL-1α is constitutively expressed in epithelial, endothelial and stromal cells and can be upregulated in hematopoietic and non- hematopoietic cells by a number of stimuli, including Toll-like receptor (TLR) agonists (6, 16), inflammatory cytokines (including IL-1α and IL-1β) (17, 18), oxidative stress (19–21), fatty acid induced mitochondrial uncoupling and hormones (18, 22, 23). Il1a promoter lacks canonical TATA and CAAT box regulatory regions, but contains binding sites for the transcription factor Sp1 (24, 25) which mediates expression during homeostasis. Il1a promoter also contains binding sites for AP1 and NF-κB transcription factors (26–28) which up-regulate expression during inflammatory stimulation. In contrast, expression of IL-1β is largely restricted to immune cells, where it is induced by AP1 and NF-κB transcription factors. In myeloid cells, a long noncoding anti-sense Il1a transcript (AS-IL- 1α) is required for Il1a transcription (29). However, the mechanism by which AS-IL-1α promotes IL-1α expression is unknown.
Both IL-1α and IL-1β are translated into 31 kDa pro-forms. However, unlike pro-IL-1β, pro-IL-1α has a functional nuclear localization signal (NLS) in the N-terminal domain (30, 31). In epithelial cells, myeloid cells, and keratinocytes, pro-IL-1α is shuttled to the nucleus upon translation. In contrast, in vascular smooth muscle cells, NLS of pro-IL-1α is masked by interaction with intracellular form of IL-1R2 that leads to its cytosolic retention (32). The mechanisms that regulate sub-cellular targeting of IL-1α in different cell types and under different stimulation conditions are largely unknown. HS-1- associated protein X (HAX)-1 is a protein associated with mitochondrial, endoplasmic reticulum and the nuclear membranes, and can bind to pro-IL-1α and promote its nuclear localization (33, 34). Using over-expression systems, pro-IL-1α has been suggested to interact with histone acetyltransferases p300, P300/CBP-associated factor (PCAF) and GCN5 in the nucleus (35, 36) and modulate gene expression independently of IL-1R (37, 38). Furthermore, pro-IL-1α is post-transnationally modified, including myristoylation or acetylation on Lys82 (39, 40) and phosphorylation at Ser90 (41, 42). The functions of these modifications are also largely unknown (Figure 1).
Figure 1. Post-translational modifications and sub-cellular localization of IL-1α.
A) Pro-IL-1α is translated as a 31-kDa full-length protein and is composed of an N-terminal domain (NTD), nuclear localization signal (NLS) and C-terminal domain (CTD). NLS directs IL-1α translocation to the nucleus, while the CTD is the catalytically active domain capable of signaling via IL-1R. Pro-IL-1α is also modified post-translationally, including myristoylation on Lys82, phosphorylation at Ser90 and glycosylation at D64. The functions of these modifications are largely unknown. B) Upon translation, IL-1α can be targeted to the plasma membrane, nucleus or the cytosol. Myristoylation and glycosylation are associated with the membrane-bound form of IL-1α. Binding to HAX-1 promotes nuclear localization, while binding to IL-1R2 sequesters pro-IL-1α in an inactive form in the cytosol. Pro-IL-1α can also be cleaved by calpain and other inflammatory caspases to yield the inactive NTD and bioactive CTD. The CTD is released from the cell upon loss of membrane integrity, and can signal via IL-1R. Stimuli that control sub-cellular targeting of IL-1α, and their functions are relatively understudied.
Like pro-IL-1β, pro-IL-1α is also cleaved by proteases, including caspase-1, to yield the bioactive 17 kDa form and the 16 kDa N-terminal cleavage product termed as the pro- piece (43). Pro-IL-1α can also be cleaved by calpain, chymase, elastase and granzyme B in the intracellular or extracellular space to yield mature IL-1α (44–47). While cleavage of pro-IL-1β by caspase-1 is required to generate bioactive IL-1β, both pro-IL-1α and mature IL-1α bind to IL-1R with similar kinetics (48) and have similar biological activity on epithelial and hematopoietic cells, as judged by their ability to induce secretion of IL-6 and TNF (49). Therefore, the biological role of proteolytic processing of IL-1α is currently unknown. Caspase-1 activation by inflammasomes does, however, facilitate secretion of IL-1α (and other inflammasome-associated cytokines) by inducing an inflammatory form of cell death termed as pyroptosis (43, 50). In addition to the cytokines (IL-1 and IL-18), active caspase-1 also cleaves the cell death executioner, Gasdermin D (GSDMD) (51- 54). Upon cleavage, the N-terminal fragment of GSDMD oligomerizes and inserts into the cell membrane, leading to loss of osmotic potential, cell swelling and eventual bursting that is accompanied by release of cytoplasmic contents, including IL-1α and IL- 1β, from the cell (51, 52, 54–60). Therefore, inflammasome activation and subsequent pyroptosis is a mechanism of release of IL-1α from the cell, even though the processing of IL-1α by caspase-1 is dispensable for its bioactivity. As they signal via the same receptor, release of IL-1α alongside IL-1β during inflammasome activation may supplement IL-1R activation by IL-1β. In certain cell types, binding of cytosolic IL-1R2 holds pro-IL-1α in an inactive state, as the pro-IL-1α-IL1R2 complex that is released upon necrotic death cannot signal via IL-1R (32). This inhibition of IL-1α activity by IL- 1R2 can be relieved by active caspase-1, as it can cleave IL-1R2, which leads to its dissociation from pro-IL-1α (32). Therefore, inflammasome activation may indirectly promote IL-1α bioavailability by relieving IL-1R2 inhibition. However, proof of such inhibition of IL-1α, or the requirement of inflammasomes for its bioactivity has not been confirmed under in vivo settings.
A membrane-associated form of the full-length pro-IL-1α has also been observed. Exposure of innate immune cells or endothelial cells to inflammatory stimuli such as LPS or heat-killed Mycobacterium tuberculosis leads to up-regulation of IL-1α expression, and pro-IL-1α can be detected in the cytoplasm, the nucleus and the plasma membrane (61–63). This membrane-associated form of pro-IL-1α is also bioactive, as it can signal via IL-1R in a paracrine manner to promote T cell proliferation and chemokine secretion (64–67). This membrane-association property of IL-1α is unique in the IL-1 family, and simultaneous localization in the nucleus, cytoplasm and the plasma membrane is unique amongst all known cytokines. Membrane-bound pro-IL-1α is glycosylated (66) suggesting the involvement of a lectin in its membrane targeting or localization. Plasma- membrane-bound pro-IL-1α can also be cleaved by extracellular proteases to yield the mature form of IL-1α, or cause its inactivation (47, 67, 68). The mechanisms that regulate the cytosolic, cellular membrane, or nuclear trafficking of IL-1α, and their relative functions are major questions in the biology of IL-1α.
IL-1α as an apical instigator of inflammation
IL-1α is expressed under homeostatic conditions by multiple hematopoietic and non- hematopoietic cells, and can be upregulated by a diverse array of inflammatory stimuli. Furthermore, IL-1α does not depend on proteolytic processing for its bioactivity. Therefore, cell death caused by injury due to sterile or infectious insults leads to release of bioactive IL-1α that signals via IL-1R to induce inflammatory responses. IL-1R is expressed constitutively by a broad range of cell types and NF-κB and MAPK activation downstream of IL-1R induces production of pro-inflammatory mediators such as cyclooxygenase type-2 (COX-2), IL-6, tumor necrosis factor (TNF), that further promote production of IL-1α and IL-1β, amplifying the inflammatory stimuli provided by the initial release of IL-1α (16, 69, 70). Physiological manifestations of IL-1 signaling include fever, hypotension, vasodilation and increased sensitivity to pain (71).
Consistent with the hypothesis of IL-1α as an apical driver of inflammation during sterile injury, infiltration of neutrophils into the peritoneal cavity upon administration of necrotic cells was found to be dependent on the release of IL-1α from necrotic cells, and signaling via IL-1R in radio-resistant cells (68, 72, 73). These and subsequent studies have shown that release of IL-1α from dying cells is a major driver of many inflammatory processes (6, 73–75). IL-1α is therefore referred to as an “alarmin” and a critical danger- associated molecular pattern (DAMP) during sterile insults. Mechanisms are also in place to prevent IL-1α release and initiation of downstream inflammatory responses during regulated non-inflammatory cell death. During apoptosis, release of cytosolic pro- IL-1α is prevented by its sequestration into the nucleus, followed by clearance of the nuclei-containing apoptotic bodies by scavenging phagocytes (76, 77). Binding of cytosolic IL-1α by IL-1R2 may also serve to sequester IL-1α during inflammatory signaling (32).
IL-1α and auto-inflammatory disorders
Auto-inflammatory disorders refer to the inflammatory diseases that are caused by in- born errors in the innate immune system and develop independently of involvement of T cells or B cells. Dysregulation of IL-1α or IL-1β production is associated with numerous auto-inflammatory disorders, and as they signal via the same receptor, they were initially thought to be functionally redundant. Studies in the last decade utilizing mouse models of auto-inflammatory diseases have helped determine specific roles of these cytokines, and the molecular mechanisms that control their production, bio-availability and downstream signaling events. Here, we will briefly discuss auto-inflammation induced by IL-1β (reviewed in (78)) and focus on complimentary and contrasting mechanisms by which IL-1α provokes autoinflammatory diseases.
Proline-serine-threonine phosphatase-interacting protein 2 (PSTPIP2) is a member of the Fes/CIP4 homology-bin/amphiphysin/rvs (F-BAR) family of proteins and interacts with protein tyrosine phosphatases of the PEST (a domain rich in proline, glutamic acid, serine, and threonine) family (79–81). Mice carrying a homozygous L98P missense mutation in Pstpip2, referred to as Pstpip2cmo mice, develop a severe autoinflammatory disease affecting the bones that mimics chronic recurrent multifocal osteomyelitis (CRMO) in humans (82–84). Our group and others have shown that IL-1β, but not IL-1α, is required for disease development in Pstpip2cmo mice (85, 86). Furthermore, caspase-1 functions redundantly with caspase-8 to induce IL-1β maturation in Pstpip2cmo neutrophils, while elastase and neutrophil proteinase 3 are dispensable (85, 87, 88). Bone marrow chimera studies further reveal that IL-1β produced by hematopoietic cells signals via IL-1R on non-hematopoietic cells to mediate auto-inflammation (87).
Auto-inflammatory diseases familial cold autoinflammatory syndrome (FCAS), Muckle- Wells syndrome (MWS) and neonatal-onset multisystem inflammatory disease (NOMID) are associated with mutations in NLRP3 (previously named as cryopyrin) and are collectively referred to as cryopyrin-associated periodic syndromes (CAPS) (89–94). Whole body or myeloid-cell specific knock-in (KI) mice expressing NLRP3 mutations associated with CAPS develop auto-inflammation that is associated with increased levels of circulating IL-1β (95, 96). Myeloid cells from human patients or mouse models of CAPS secrete IL-1β after TLR priming alone, and auto-inflammation in these mice is rescued by genetic deletion of ASC (97) or caspase-1 (98). However, IL-1β, IL-18 and IL-1R have a partial role, suggesting that other inflammasome-associated functions such as cell death can also contribute towards CAPS-pathology (95, 96, 98, 99). In contrast, in a mouse model of Familial Mediterranean Fever (FMF) wherein the auto-inflammation is caused by a gain-of-function mutation in the inflammasome sensor Pyrin (100–102), IL-1β, ASC and caspase-1 have a non-redundant role in mediating auto-inflammation, while IL-1α and caspase-8 are dispensable (103).
Inflammasomes and IL-1 cytokines can also promote auto-inflammation induced by other cytokines. Sharpincpdm mice are deficient in Shank-associated RH domain interacting protein (SHARPIN), a member of linear ubiquitin chain assembly complex (LUBAC), and develop severe dermatitis (104–108). Auto-inflammatory dermatitis in Sharpincpdm mice is completely rescued by genetic deletion of TNF (108), TNFR1 or downstream signaling molecules (109–111). However, deletion of IL-1β, IL-1R, NLRP3 or caspase-1 significantly delays dermatitis in Sharpincpdm mice (112, 113). In line with these findings, IL-1R signaling also upregulates the production of TNF (114). These studies show that concerted production of IL-1β and TNF can form an inflammatory loop, which is capable of causing destructive auto-inflammation, unless curtailed by negative regulators like IL-10 (115, 116).
In recent years, IL-1α has also emerged as an apical driver of cutaneous inflammation (117–119), colon inflammation and cancer (120), cardiovascular disease (121) and neural inflammation (122). We have shown that IL-1α has a non-redundant role in driving auto-inflammatory disease in a mouse model of neutrophilic dermatoses (119). Neutrophilic dermatoses are a heterogeneous group of autoinflammatory skin disorders that are associated with cutaneous inflammatory lesions and include Sweet’s syndrome, pyoderma gangrenosum, subcorneal pustular dermatosis and hidradenitis suppurativa (123–125). Increased neutrophil infiltration at the affected sites is a defining clinical feature of neutrophilic dermatoses. A homozygous Tyr208Asn mutation in the Ptpn6 gene (that encodes for the phosphatase SHP1) results in a severe form of autoinflammatory skin disease in mice (referred to as Ptpn6spin mice) that mimics neutrophilic dermatoses in humans (119, 126–128). Furthermore, splice variants or mutations in PTPN6 gene are associated with neutrophilic dermatoses such as Sweet’s syndrome and pyoderma gangrenosum in humans (129). SHP-1 has a negative regulatory role in inflammatory signaling in immune cells, and modulation of SHP-1 expression is associated with skin inflammation, multiple sclerosis, psoriatic arthritis and cancer in humans (130–134). While SHP1-deficient mice have severe inflammation associated with early mortality, Ptpn6spin mice retain approximately 30% of the SHP-1 phosphatase activity and present a milder phenotype with no disease-associated mortality (119). Ptpn6spin mice develop footpad swelling at 8–16 weeks of age that is associated with marked neutrophilia, intraepidermal pustules and cutaneous tissue damage (119). The neutrophilic dermatoses in Ptpn6spin mice is rescued by MyD88 or IL- 1R deficiency demonstrating a role for IL-1 signaling in provoking disease in these mice (135).
Our studies show that IL-1α has a non-redundant role in driving SHP1-mediated neutrophilic dermatoses as genetic ablation of IL-1α, but not IL-1β protects the Ptpn6spin mice from disease development (119). Transgenic mice that overexpress IL-1α in epidermal cells also develop an inflammatory skin disease, underscoring the pathogenic role of excess IL-1α production in skin inflammation (136). Bone marrow chimera studies further reveal that while Ptpn6spin mutation in hematopoietic cells is required for disease development in these mice, the non-hematopoietic cells are the primary source of pathogenic IL-1α (137). Microabrasion injury in the footpads also accelerates disease progression and enhances severity in pre-diseased Ptpn6spin mice, suggesting that passive release of IL-1α from epidermal cells during necrotic cell death is an instigator of disease (119). Microabrasion in the footpads of WT mice also leads to enhanced TNF-α, G-CSF and KC production in an IL-1α-dependent manner (137). However, production of IL-1α, as well as G-CSF and KC is unaffected in the microabrated footpads of TNF-α- deficient mice (137). Furthermore, genetic deletion of TNF-α or TNFR adaptor tumor necrosis factor receptor type 1-associated death domain protein (TRADD) in Ptpn6spin mice only partially protects mice from the disease (137). Taken together, these data show that IL-1α is the apical cytokine that induces production of other pro- inflammatory cytokines and ultimately recruits neutrophils to drive the autoinflammatory disease in Ptpn6spin mice.
Unlike CAPS and FMF, the IL-1 driven cutaneous inflammation in Ptpn6spin mice is independent of inflammasomes as genetic deletion of caspase-1, ASC or NLRP3 does not affect disease development (119). Instead, disease development depends on receptor-interacting protein kinase 1 (RIPK1) – a central hub in NF-κB signaling, apoptosis and necroptosis (138–140). Necroptosis is a regulated form of inflammatory cell death that depends on the kinase activity of RIPK1, which along with RIPK3 activates the cell death executioner mixed lineage kinase domain–like (MLKL) (138–140). MLKL is a pseudokinase that forms pores in the plasma membrane, leading to loss of osmolarity and eventual death of the cell (141). Ptpn6spin mice that are also genetically deficient in RIPK3, MLKL or RIPK1 kinase activity develop the disease with the same severity as the Ptpn6spin mice (137). While RIPK1-deficient mice die perinatally, fetal liver chimeras with Ptpn6spin and RIPK1-deficient bone marrow donor cells are protected from disease development (119). Recent reports show that perinatal death of RIPK1-deficient mice can be rescued by simultaneous deletion of both RIPK3 and caspase-8 (142–144). In line with a critical role for RIPK1 in inducing disease in these mice, simultaneous germline deletion of caspase-8, RIPK3 and RIPK1, but not RIPK3 and/or caspase-8, protects Ptpn6spin mice from disease (137). These studies demonstrate that IL-1α production from non-hematopoietic cells and scaffolding activity of RIPK1 is required for disease development in Ptpn6spin mice, while RIPK3 and MLKL mediated necroptosis is dispensable. Ubiquitination of RIPK1 allows it to act as a scaffold to support transforming growth factor-β activated kinase 1 (TAK1) recruitment to the RIPK1 complex, which is central to signaling events downstream of both IL-1R and TLRs (145, 146). Our recent study shows that scaffolding activity of RIPK1 coordinates with TAK1 to provoke disease (137). We also show that SHP-1 controls activation of spleen tyrosine kinase (SYK), which further regulates MyD88 phosphorylation in myeloid cells (147). Consequently, Ptpn6spin mice that are also deficient in TAK1 or SYK in myeloid cells are also protected from disease development. Overall, studies from the Ptpn6spin mice demonstrate that IL- 1α production from non-hematopoietic cells acts on myeloid cells in a SYK, MyD88, RIPK1 and TAK1 dependent manner, and this pathway is negatively regulated by SHP-1 to restrain excessive IL-1R signaling and associated auto-inflammation (Figure 2) (142, 148–150).
Figure 2. SHP-1 negatively regulates autoinflammation induced by IL-1α.
IL-1α from non-hematopoietic cells signals via IL-1R on myeloid cells in a MyD88, receptor- interacting protein kinase 1 (RIPK1) and transforming growth factor-β activated kinase 1 (TAK1)-dependent manner to induce expression of pro-inflammatory cytokines. Src homology region 2 domain-containing phosphatase 1 (SHP1) controls phosphorylation of spleen tyrosine kinase (SYK), which further regulates MyD88 phosphorylation in myeloid cells, leading to restrained IL-1R signaling. Mice that express a mutant form of SHP1 (Ptpn6spin mice) that has reduced SHP1 activity develop an auto-inflammatory disease that are is dependent on IL-1α production from non-hematopoietic cells, and SYK, MyD88, RIPK1 and TAK1 mediated signaling in myeloid cells.
Ptpn6spin mouse model has therefore proved extremely useful in delineating cellular and molecular checkpoints that regulate the production of IL-1α, and its downstream signaling events. This model could also be useful in exploring mechanisms that control the bioavailability of IL-1α, and perhaps the function of IL-1α maturation and post- translational modifications. Interestingly, modulation of the gut microbiota by diet or antibiotic usage can affect the expression of IL-1α and IL-1β in the extra-intestinal sites, and development of the associated auto-inflammatory disorders (88, 119, 151). Therefore, manipulation of gut microbiota, and specific blockade of IL-1α or IL-1β could be a potential therapeutic strategy for treatment of autoinflammatory disorders.
IL-1α is also suggested to promote inflammation in the skin in humans. Expression of IL- 1α, IL-6, platelet-derived growth factor (PDGF)-α, collagen and IL-1R is increased in fibroblasts isolated from skin lesions of systemic sclerosis (SSc) patients (152–154). This IL-1α was localized primarily in the nucleus, but also detected in the cytoplasm and the cell membrane. Underscoring the importance of IL-1α as an apical driver of SSc, knock- down of IL-1α also reduced the proliferation, and levels of secreted IL-6, PDGF-α and pro-collagen in SSc-associated fibroblasts. On the other hand, levels of IL-6, PDGF-α and collagen are increased in normal fibroblasts when pro-IL-1α is over-expressed (152, 153). IL-1α has also been suggested to be a sensor of genotoxic stress (40). Upon induction of DNA-damage by exposure to UV or genotoxic agents, IL-1α co-localizes with gH2AX and HDAC-1 at the sites of DNA-damage. While the function of IL-1α nuclear localization is not known, acetylation of IL-1α within the NLS is required for this nuclear localization. Furthermore, secretion of IL-1α from the skin lesions, via unknown mechanisms, drives neutrophil-infiltration in the affected tissue (40).
IL-1α is also associated with driving inflammation at mucosal barrier surfaces. IL-1α, but not IL-1β-deficient mice are protected from acute colitis induced by oral feeding of cytotoxic agent dextran sodium sulfate (DSS) (155, 156). Furthermore, epithelial cells are the primary source of this pathogenic IL-1α (155). We also demonstrated that another member of the IL-1 cytokine family, IL-33, promotes IgA production in the intestine, which in turn supports symbiotic gut microbiota (156). Consequently, IL-33- deficient mice have a dysbiotic microbiota characterized by increased levels of mucolytic and colitogenic bacteria. Upon cytotoxic stress induced by DSS administration, this dysbiotic microbial landscape promotes the release of IL-1α in the colon tissue, which acts as a critical driver of colitis. The increased susceptibility of IL-33-deficient mice to acute and chronic colitis can be ameliorated by genetic or biochemical ablation of IL-1α, underscoring the importance of IL-1α in driving colitis induced by exacerbated epithelial injury (156).
IL-1α also plays a significant role in the development of atherosclerotic lesions (157, 158). Fatty acids induce the expression of IL-1α in macrophages that accumulate in the atherosclerotic plaques. This macrophage-associated IL-1α drives vasculitis in an IL-1R- dependent manner (157, 158). IL-1α is also a critical driver of ischemia during myocardial infarction and brain injury (158, 159). It is unknown if a secreted or cell membrane-associated form mediates this function of IL-1α, or if proteolytic processing or post-translational modifications of IL-1α are required.
Overall, these studies indicate that IL-1α is an apical regulator of inflammation. Upon cellular injury, release of the pre-formed pool of IL-1α, or its shuttling to the plasma membrane can induce signaling via ubiquitously expressed IL-1R in an autocrine and paracrine manner. IL-1R signaling further leads to expression of cytokines and chemokines, including IL-1α and IL-1β that leads to recruitment of inflammatory cells to the site of damage. Therefore, the initial round of IL-1α–IL-1R1 signaling can lead to self-perpetuating inflammation until the anti-inflammatory mediators like IL-10 and IL- 1R2 suppress it.
IL-1α and microbial diseases
Consistent with its role as an alarmin, IL-1α also plays a non-redundant role in instigating host defense against multiple infectious agents. Exposure to Mycobacterium tuberculosis leads to upregulation of IL-1α and IL-1β expression in macrophages and epithelial cells (160). IL-1 signaling cooperates with TNF signaling to promote granuloma formation, which help sequester the bacteria. The absence of IL-1α, IL-1R or TNFR in the host leads to failure of granuloma formation, and increased mortality in the host (63, 160). The synergy between IL-1α and TNF signaling is also protective from gut-derived sepsis induced by Pseudomonas aeruginosa colonization and cyclophosphamide induced immune-suppression (161). IL-1α-induced chemokines IL-8, CXCL1, CXCL2 and G-CSF can further promote IL-1α expression, forming an inflammatory feedback loop that can exacerbate neutrophil infiltration following Staphylococcus aureus or Chlamydia trachomatis infection (162, 163). Therefore, IL-1α is also an apical instigator of inflammatory responses during bacterial infections and similar to cooperation between IL-1α and TNF in mediating auto-inflammation in Ptpn6spin mice, cooperation between IL- 1α and TNF mediates host immune responses during infections. In contrast, inflammasomes and IL-1β play a major part in mediating host-defense to bacteria such as Streptococcus pneumonia, Citrobacter rodentium and Francisella novicida (164–169).
IL-1α is also the initiator of neutrophil influx in the lungs following infection with an intracellular bacterial pathogen Legionella pneumophila, the cause of severe pneumonia called Legionnaires’ disease (170). IL-1α production in hematopoietic cells is necessary for neutrophil infiltration following L. pneumophila infection, while IL-1β has a relatively minor role. Furthermore, while inflammasome activation is necessary for IL-1β secretion, it is dispensable for IL-1α release following L. pneumophila infection (170). IL-1 also exacerbates neutrophilia in response to Haemophilus influenzae infection and cigarette smoke exposure, in a CXCR2-dependent manner (171). Specific IL-1α blockade also restricts intestinal inflammation induced by Yersinia enterocolitica (172) or cytotoxic agent DSS (156). In contrast, exogenous administration of IL-1α ameliorates intestinal ischemia and reperfusion injury by reducing intestinal permeability and bacterial translocation during thermal injury and endotoxemia (173). The mechanisms that control the bio-availability of IL-1α following these infections or treatments are not known. It is also unknown if specific sub-cellular localization, post-translational modification or proteolytic processing of IL-1α is required for these functions.
IL-1α also plays a role in neutrophil recruitment and host-defense following viral, fungal and protozoan infections. Interaction of adenovirus with β3 integrin on marginal zone macrophages leads to upregulation of IL-1α, which integrates with complement signaling to induce recruitment of neutrophils to the marginal zone of the spleen (174, 175). This IL-1α-dependent neutrophil recruitment is necessary for elimination of virus-containing cells (174, 175). IL-1α production from keratinocytes following Candida albicans infection also leads to G-CSF production from endothelial cells at the site of infection, which promotes neutrophil recruitment (176). IL-1α also boosts granulopoiesis in the bone marrow, increasing the number of circulating neutrophils (176). Therefore, IL-1α signaling in both hematopoietic and non-hematopoietic cells is required for neutrophil generation and recruitment to the site of infection. These findings are similar to the concerted action of IL-1α in both hematopoietic and non-hematopoietic compartments for induction of auto-inflammatory disease in the Ptpn6spin mice (137).
IL-1α expression also precedes IL-1β expression in the brain after systemic infection with the fungal pathogen Cryptococcus neoformans (177) and supports neutrophil recruitment in the lung following Apsergillus fumigatus infection (178). IL-1α induced neutrophil recruitment can also be a driving factor for tissue damage during infections. In the genetically susceptible BALB/c mice, protozoan parasite Leishmania major induces pathology in an IL-1α-dependent manner (179). However, transient local administration of IL-1α during cutaneous leishmaniasis promotes IFN-γ production from T cells, which is protective for the host (180). IL-1α also drives neutrophil infiltration in lungs during RSV infection, in an IL-1R-dependent manner (181). Therefore, IL-1α can drive immunopathology or protective immune responses during infections, and IL-1α activity must be tightly controlled to protect from destructive inflammation while also boosting anti-microbial immunity.
IL-1α and cancer
Malignant cells, tumor-infiltrating immune cells and stromal cells can express IL-1α, IL- 1β and IL-1R. IL-1 signaling in the tumor tissue and its microenvironment can an affect tumor progression in different ways. IL-1R signaling leads to expression of inflammatory mediators, including IL-6 and COX2, which promote survival and proliferation of malignant cells. IL-1 derived from the tumor microenvironment can act on cancer stem cells to upregulate the expression of “stemness” associated genes, like Bmi1 and Nestin, promote proliferation and epithelial-mesenchymal transition (182–184). IL-1 cytokines can also induce production of pro-inflammatory cytokines, chemokines, prostaglandins and growth factors, which can, in turn, activate β-catenin signaling in proliferating cells (185–189). IL-1 signaling also promotes expression of chemokines and adhesion molecules that lead to recruitment of immune cells into the tumor tissue, and metalloproteases that promote tumor invasion and metastatic escape (190). IL-1 signaling also drives myelopoiesis in the bone marrow (191) and differentiation of monocytes into suppressor cells (referred to as myeloid-derived suppressor cells, MDSCs) that further promote production of pro-survival factors and suppress anti-tumor immune responses (184, 191–193). On the other hand, IL-1 signaling can also boost anti-tumor immunity. IL-1 signaling contributes to recruitment and activation of antigen presenting cells to the lymph nodes where they can induce activation, expansion and development of immunological memory in CD4+ and CD8+ T cells specific to tumor- associated antigens (194–197). Therefore, IL-1 signaling can both promote and regress cancer growth, probably depending on the characteristics of the tumor and its microenvironment (Figure 3).
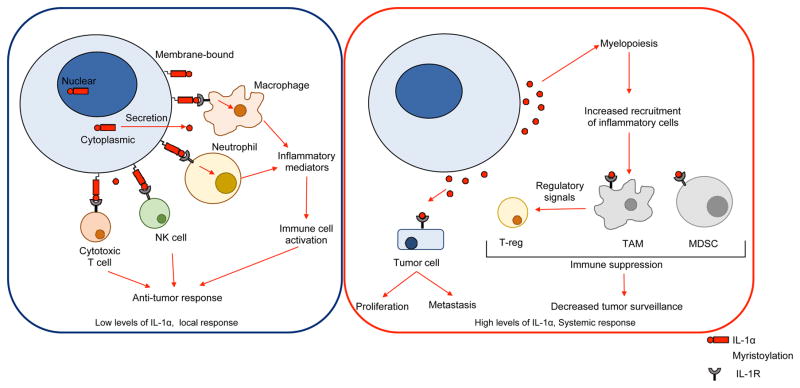
Figure 3. Functions of IL-1α in cancer.
In the tumor microenvironment, low levels of cell-surface or secreted form of IL-1α are associated with anti-tumor responses. Activation of macrophages and neutrophils through IL-1R signaling promotes inflammatory responses and T cell activation through antigen presentation. Furthermore, IL-1R signaling promotes tumor killing through activation of cytotoxic T cells and natural killer (NK) cells. On the other hand, increased release of IL-1α is associated with pro- tumorigenic responses. IL-1α signaling increases myelopoiesis and accumulation of tumor associated macrophages (TAMs) and myeloid derived suppressor cells (MDSCs) within the tumor microenvironment, which in turn promotes differentiation of regulatory T cells (Tregs). Together, these cell types suppress immune cell activation and reduce tumor surveillance. Furthermore, IL-1α can signal via IL-1R on tumor cells to promote proliferation and invasiveness.
IL-1α and IL-1β can have complimentary or contrasting functions in different types of cancers. Expression of both IL-1α and IL-1β is increased during genotoxic stress, and their secretion by necrosis or pyroptosis promotes the expression of vascular endothelial growth factor (VEGF) in the tumor tissue (198–200). 3-Methylcholanthrene (MCA) induced fibrosarcoma development requires IL-1β, but not IL-1α expression in the host (201). However, IL-1α expression in the 3-MCA-induced fibrosarcoma cell line is required for tumor development upon implantation in a naïve host (202). 3-MCA-induced tumor cell lines from IL-1α-deficient mice have increased expression of MHCI, co-stimulatory and adhesion molecules, and this increased immunogenicity of IL-1α-deficient tumor cells is associated with increased activation of CD8+ T cells and NK cells, which control tumor growth (202). Like IL-1α, IL-1β also promotes tumor growth and invasiveness. Fibrosarcoma cells that are genetically modified to express the mature form of IL-1β ligated to a signal peptide (ssIL-1β) constitutively secrete mature IL-1β via the canonical ER-golgi secretion pathway (192, 203). Upon implantation into a naïve host, these tumor cells demonstrate increased growth, angiogenesis and invasiveness (192, 203–206). Furthermore, both IL-1β and VEGF synergize to promote angiogenesis in the tumors (207). Increased IL-1α production is associated with distant metastases, and poor survival rate in head and neck squamous cell carcinoma and gastric carcinoma (208, 209). IL-1α is also a major driver of colon cancer that is associated with chronic inflammation in the colon (156). A single nucleotide polymorphism (SNP) in the IL1a gene that leads to A114S substitution is associated with increased risk of ovarian cancer (210). The A114S mutant form of pro-IL-1α form is more readily cleaved by inflammatory proteases, underscoring the idea of increased IL-1 secretion leading to increased tumorigenicity and metastasis (211).
In contrast with these observations, overexpression of cytosolic or membrane-bound forms of IL-1α lead to regression of fibrosarcoma (212, 213). The protection conferred by overexpression of IL-1α is associated with enhanced immune-surveillance mechanisms that depend on CD8+ T cells, NK cells and M1-differentiated macrophages (214, 215). IL-1α-mediated anti-tumor immune response also leads to development of immunological memory that is protective from re-challenge with parental tumor cells (214, 215). Similarly, IL-1β (and IL-18) maturation by the NLRP3 inflammasome activated by chemotherapeutic drugs contributes towards activation of anti-tumor CD8+ T cells (197). However, it was suggested that chemotherapy-enhanced production of pro- inflammatory mediators potentiate tumor invasiveness and metastasis (216, 217). Taken together, these studies suggest that while IL-1 signaling is inherently pro-tumorigenic, focused, local stimulation provided by membrane-bound or low levels of secreted of IL-1 can be beneficial by boosting anti-tumor immunity.
Clinical trials with an IL-1α-blocking monoclonal antibody has shown promising outcomes in patients with end-stage cancers (147, 218). MABp1 (XBiotech Inc.) is a naturally occurring human interleukin-1α neutralizing antibody. Monotherapy with MABp1 improved survival rate, increased lean body weight, normalized paraneoplastic thrombocytosis, decreased levels of serum IL-6 and associated systemic inflammation and cachexia without significant side-effects in patients with advanced non-small cell lung cancer, ovarian cancer and other refractory cancers (147, 219–221). MABp1 treatment also improved clinical parameters in patients with unresectable or metastatic colon cancer (222). Therefore, it is clear that IL-1α is a clinically significant driver of cancer and associated complications. Further clinical trials with these and other types of cancers, and in earlier stages of the disease, are warranted.
Senescence-associated secretory phenotype (SASP) is a phenomenon characterized by spontaneous production of low-levels IL-6, IL-8 and other inflammatory mediators by aged cells undergoing senescence (223). Sustained production of inflammatory mediators at low levels leads to chronic systemic inflammation. This low-level chronic inflammation has been associated with age-related pathologies and cancer as IL-6 and other inflammatory mediators promote angiogenesis and proliferation of malignant cells (223). IL-1α expression after exposure to ionizing radiation enhances SASP, and inhibition of mTOR by rapamycin treatment represses IL-1α translation, resulting in suppression of SASP (223, 224). Initial IL-1α production results in amplification of production of pro-inflammatory and pro-tumorigenic factors, and consequently SASP in fibroblasts and epithelial cells is associated with worsening prognosis for cancer (194). Therefore, similar to auto-inflammatory disorders and infections, IL-1α can also be an apical driver of SASP.
Conclusions and future perspectives
New studies propose a paradigm of IL-1α-dependent pro-inflammatory priming as the initiator of several major human diseases. Certain unique properties of IL-1α support its critical function as an apical instigator of inflammation. First, IL-1α is ubiquitously expressed by both hematopoietic and non-hematopoietic cell in vivo, and its expression is rapidly upregulated by a wide variety of danger- and pathogen-associated molecular patterns. Second, IL-1α is very promiscuous in its sub-cellular localization, and can function as an IL-1R agonist upon secretion from the cell or as a cell membrane bound molecule. Third, IL-1α can translocate to the nucleus to modulate gene expression independently of IL-1R or as a sequestration mechanism during non-inflammatory cell death. Fourth, while the other IL-1R ligand, IL-1β, requires inflammasome activation for its maturation and pyroptosis for its secretion, IL-1α is bioavailable in a much wider set of cellular scenarios. IL-1α is bioactive in both the pro-form and mature form, and therefore both pyroptotic and necrotic cell death yields bioactive IL-1α. Despite increasing appreciation of the importance of IL-1α in the pathogenesis of human diseases, the factors that control its bioavailability remain poorly understood. The major unanswered questions in IL-1 biology include the mechanisms that control sub-cellular targeting of IL-1α, and their relative biological functions. Mechanisms that regulate IL-1α secretion from the cell, and the function of its cleavage by inflammatory proteases are also unknown. Due to its ubiquitous expression, potent activity and bioavailability, IL- 1α has emerged as a major player and drug target in many diseases. Further understanding of the regulation IL-1α will undoubtedly help develop novel therapeutics for the vast array of ailments associated with this cytokine.
Acknowledgments
Funding Statement: This work was supported by grants from the US National Institutes of Health (AI101935, AI124346, AR056296, and CA163507) and the ALSAC to T.-D.K.
We would like to thank Deepika Sharma and Subbarao Malireddi for helpful editing of the manuscript.
Footnotes
Conflict of Interest Statement: Authors have no conflict of interest
References
- 1.Beeson P. Temperature-elevating effect of a substance obtained from polymorphonuclear leucocytes. The Journal of clinical investigation. 1948;27:524. [PubMed] [Google Scholar]
- 2.Gery I, Gershon RK, Waksman BH. Potentiation of the T-lymphocyte response to mitogens. Journal of Experimental Medicine. 1972;136:128–142. doi: 10.1084/jem.136.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinarello CA, Goldin NP, Wolff SM. Demonstration and characterization of two distinct human leukocytic pyrogens. Journal of Experimental Medicine. 1974;139:1369–1381. doi: 10.1084/jem.139.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello CA, Renfer L, Wolff SM. Human leukocytic pyrogen: purification and development of a radioimmunoassay. Proceedings of the National Academy of Sciences. 1977;74:4624–4627. doi: 10.1073/pnas.74.10.4624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.March CJ, Mosley B, Larsen A, et al. Cloning, sequence and expression of two distinct human interleukin-1 complementary DNAs. Nature. 1985;315:641–647. doi: 10.1038/315641a0. [DOI] [PubMed] [Google Scholar]
- 6.Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39:1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Palomo J, Dietrich D, Martin P, Palmer G, Gabay C. The interleukin (IL)-1 cytokine family–Balance between agonists and antagonists in inflammatory diseases. Cytokine. 2015;76:25–37. doi: 10.1016/j.cyto.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 8.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14:1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 9.Sharma D, Kanneganti T-D. The cell biology of inflammasomes: Mechanisms of inflammasome activation and regulation. The Journal of cell biology. 2016;213:617–629. doi: 10.1083/jcb.201602089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lukens JR, Gross JM, Kanneganti TD. IL-1 family cytokines trigger sterile inflammatory disease. Front Immunol. 2012;3:315. doi: 10.3389/fimmu.2012.00315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thomas C, Bazan JF, Garcia KC. Structure of the activating IL-1 receptor signaling complex. Nature structural & molecular biology. 2012;19:455–457. doi: 10.1038/nsmb.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Boraschi D, Tagliabue A, editors. The interleukin-1 receptor family. Elsevier; 2013. [DOI] [PubMed] [Google Scholar]
- 13.Burger D, Chicheportiche R, Giri J, Dayer J. The inhibitory activity of human interleukin-1 receptor antagonist is enhanced by type II interleukin-1 soluble receptor and hindered by type I interleukin-1 soluble receptor. Journal of Clinical Investigation. 1995;96:38. doi: 10.1172/JCI118045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Symons JA, Young PR, Duff GW. Soluble type II interleukin 1 (IL-1) receptor binds and blocks processing of IL-1 beta precursor and loses affinity for IL-1 receptor antagonist. Proceedings of the National Academy of Sciences. 1995;92:1714–1718. doi: 10.1073/pnas.92.5.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, Joosten LA. Inflammasome-independent regulation of IL-1-family cytokines. Annual review of immunology. 2015;33:49–77. doi: 10.1146/annurev-immunol-032414-112306. [DOI] [PubMed] [Google Scholar]
- 16.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3:cm1–cm1. doi: 10.1126/scisignal.3105cm1. [DOI] [PubMed] [Google Scholar]
- 17.Kimura H, Inukai Y, Takii T, et al. MOLECULAR ANALYSIS OF CONSTITUTIVE IL-1α GENE EXPRESSION IN HUMAN MELANOMA CELLS: AUTHOCRINE STIMULATION THROUGH NF-κB ACTIVATION BY ENDOGENOUS IL-1α. Cytokine. 1998;10:872–879. doi: 10.1006/cyto.1998.0369. [DOI] [PubMed] [Google Scholar]
- 18.Itoh Y, Hayashi H, Miyazawa K, Kojima S, Akahoshi T, Onozaki K. 17β-Estradiol induces IL-1α gene expression in rheumatoid fibroblast-like synovial cells through estrogen receptor α (ERα) and augmentation of transcriptional activity of Sp1 by dissociating histone deacetylase 2 from ERα. The Journal of Immunology. 2007;178:3059–3066. doi: 10.4049/jimmunol.178.5.3059. [DOI] [PubMed] [Google Scholar]
- 19.Rider P, Kaplanov I, Romzova M, et al. The transcription of the alarmin cytokine interleukin-1 alpha is controlled by hypoxia inducible factors 1 and 2 alpha in hypoxic cells. Frontiers in immunology. 2012:3. doi: 10.3389/fimmu.2012.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCarthy DA, Ranganathan A, Subbaram S, et al. Redox-control of the alarmin, interleukin-1α. Redox biology. 2013;1:218–225. doi: 10.1016/j.redox.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy DA, Clark RR, Bartling TR, Trebak M, Melendez JA. Redox control of the senescence regulator interleukin-1α and the secretory phenotype. Journal of Biological Chemistry. 2013;288:32149–32159. doi: 10.1074/jbc.M113.493841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tynan GA, Hearnden CH, Oleszycka E, et al. Endogenous oils derived from human adipocytes are potent adjuvants that promote IL-1α–dependent inflammation. Diabetes. 2014;63:2037–2050. doi: 10.2337/db13-1476. [DOI] [PubMed] [Google Scholar]
- 23.Freigang S, Ampenberger F, Weiss A, et al. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1[alpha] and sterile vascular inflammation in atherosclerosis. Nat Immunol. 2013;14:1045–1053. doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- 24.McDowell TL, Symons JA, Duff GW. Human interleukin-1α gene expression is regulated by Sp1 and a transcriptional repressor. Cytokine. 2005;30:141–153. doi: 10.1016/j.cyto.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 25.Wierstra I. Sp1: emerging roles—beyond constitutive activation of TATA-less housekeeping genes. Biochemical and biophysical research communications. 2008;372:1–13. doi: 10.1016/j.bbrc.2008.03.074. [DOI] [PubMed] [Google Scholar]
- 26.Alheim K, McDowell TL, Symons JA, Duff GW, Bartfai T. An AP-1 site is involved in the NGF induction of IL-1α in PC12 cells. Neurochemistry international. 1996;29:487–496. doi: 10.1016/0197-0186(96)00017-4. [DOI] [PubMed] [Google Scholar]
- 27.Bailly S, Fay M, Israel N, Gougerot-Pocidalo M. The transcription factor AP-1 binds to the human interleukin 1 alpha promoter. European cytokine network. 1995;7:125–128. [PubMed] [Google Scholar]
- 28.Mori N, Prager D. Transactivation of the interleukin-1alpha promoter by human T-cell leukemia virus type I and type II Tax proteins. Blood. 1996;87:3410–3417. [PubMed] [Google Scholar]
- 29.Chan J, Atianand M, Jiang Z, et al. Cutting edge: a natural antisense transcript, AS-IL1α, controls inducible transcription of the proinflammatory cytokine IL-1α. The Journal of Immunology. 2015;195:1359–1363. doi: 10.4049/jimmunol.1500264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wessendorf J, Garfinkel S, Zhan X, Brown S, Maciag T. Identification of a nuclear localization sequence within the structure of the human interleukin-1 alpha precursor. Journal of Biological Chemistry. 1993;268:22100–22104. [PubMed] [Google Scholar]
- 31.Luheshi NM, Rothwell NJ, Brough D. The Dynamics and Mechanisms of Interleukin-1α and β Nuclear Import. Traffic. 2009;10:16–25. doi: 10.1111/j.1600-0854.2008.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zheng Y, Humphry M, Maguire JJ, Bennett MR, Clarke MC. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1α, controlling necrosis-induced sterile inflammation. Immunity. 2013;38:285–295. doi: 10.1016/j.immuni.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Suzuki Y, Demoliere C, Kitamura D, Takeshita H, Deuschle U, Watanabe T. HAX-1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family tyrosine kinases. The Journal of Immunology. 1997;158:2736–2744. [PubMed] [Google Scholar]
- 34.Kawaguchi Y, Nishimagi E, Tochimoto A, et al. Intracellular IL-1α-binding proteins contribute to biological functions of endogenous IL-1α in systemic sclerosis fibroblasts. Proceedings of the National Academy of Sciences. 2006;103:14501–14506. doi: 10.1073/pnas.0603545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buryskova M, Pospisek M, Grothey A, Simmet T, Burysek L. Intracellular interleukin-1α functionally interacts with histone acetyltransferase complexes. Journal of Biological Chemistry. 2004;279:4017–4026. doi: 10.1074/jbc.M306342200. [DOI] [PubMed] [Google Scholar]
- 36.Zamostna B, Novak J, Vopalensky V, Masek T, Burysek L, Pospisek M. N- terminal domain of nuclear IL-1α shows structural similarity to the C-terminal domain of Snf1 and binds to the HAT/Core module of the SAGA complex. PloS one. 2012;7:e41801. doi: 10.1371/journal.pone.0041801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Werman A, Werman-Venkert R, White R, et al. The precursor form of IL-1α is an intracrine proinflammatory activator of transcription. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:2434–2439. doi: 10.1073/pnas.0308705101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lamacchia C, Rodriguez E, Palmer G, Gabay C. Endogenous IL-1α is a chromatin-associated protein in mouse macrophages. Cytokine. 2013;63:135–144. doi: 10.1016/j.cyto.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Stevenson FT, Bursten SL, Fanton C, Locksley RM, Lovett DH. The 31-kDa precursor of interleukin 1 alpha is myristoylated on specific lysines within the 16-kDa N-terminal propiece. Proceedings of the National Academy of Sciences. 1993;90:7245–7249. doi: 10.1073/pnas.90.15.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Idan C, Peleg R, Elena V, et al. IL-1α is a DNA damage sensor linking genotoxic stress signaling to sterile inflammation and innate immunity. Scientific reports. 2015:5. doi: 10.1038/srep14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beuscher H, Nickells M, Colten H. The precursor of interleukin-1 alpha is phosphorylated at residue serine 90. Journal of Biological Chemistry. 1988;263:4023–4028. [PubMed] [Google Scholar]
- 42.Kobayashi Y, Appella E, Yamada M, Copeland T, Oppenheim J, Matsushima K. Phosphorylation of intracellular precursors of human IL-1. The Journal of Immunology. 1988;140:2279–2287. [PubMed] [Google Scholar]
- 43.Groß O, Yazdi AS, Thomas CJ, et al. Inflammasome activators induce interleukin-1α secretion via distinct pathways with differential requirement for the protease function of caspase-1. Immunity. 2012;36:388–400. doi: 10.1016/j.immuni.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 44.Kobayashi Y, Yamamoto K, Saido T, Kawasaki H, Oppenheim JJ, Matsushima K. Identification of calcium-activated neutral protease as a processing enzyme of human interleukin 1 alpha. Proceedings of the National Academy of Sciences. 1990;87:5548–5552. doi: 10.1073/pnas.87.14.5548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carruth LM, Demczuk S, Mizel SB. Involvement of a calpain-like protease in the processing of the murine interleukin 1 alpha precursor. Journal of Biological Chemistry. 1991;266:12162–12167. [PubMed] [Google Scholar]
- 46.Kavita U, Mizel SB. Differential sensitivity of interleukin-1α and-β precursor proteins to cleavage by calpain, a calcium-dependent protease. Journal of Biological Chemistry. 1995;270:27758–27765. doi: 10.1074/jbc.270.46.27758. [DOI] [PubMed] [Google Scholar]
- 47.Afonina IS, Tynan GA, Logue SE, et al. Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1α. Molecular cell. 2011;44:265–278. doi: 10.1016/j.molcel.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mosley B, Urdal D, Prickett K, et al. The interleukin-1 receptor binds the human interleukin-1 alpha precursor but not the interleukin-1 beta precursor. Journal of Biological Chemistry. 1987;262:2941–2944. [PubMed] [Google Scholar]
- 49.Kim B, Lee Y, Kim E, et al. The interleukin-1α precursor is biologically active and is likely a key alarmin in the IL-1 family of cytokines. Frontiers in immunology. 2013:4. doi: 10.3389/fimmu.2013.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanneganti T-D, Özören N, Body-Malapel M, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature. 2006;440:233. doi: 10.1038/nature04517. [DOI] [PubMed] [Google Scholar]
- 51.Liu X, Zhang Z, Ruan J, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–158. doi: 10.1038/nature18629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sborgi L, Ruhl S, Mulvihill E, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. The EMBO journal. 2016;35:1766–1778. doi: 10.15252/embj.201694696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kayagaki N, Stowe IB, Lee BL, et al. Caspase-11 cleaves gasdermin D for non- canonical inflammasome signalling. Nature. 2015;526:666–671. doi: 10.1038/nature15541. [DOI] [PubMed] [Google Scholar]
- 54.Shi J, Zhao Y, Wang K, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–665. doi: 10.1038/nature15514. [DOI] [PubMed] [Google Scholar]
- 55.Fettelschoss A, Kistowska M, LeibundGut-Landmann S, et al. Inflammasome activation and IL-1β target IL-1α for secretion as opposed to surface expression. Proceedings of the National Academy of Sciences. 2011;108:18055–18060. doi: 10.1073/pnas.1109176108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perregaux DG, Gabel CA. Post-translational processing of murine IL-1: evidence that ATP-induced release of IL-1α and IL-1β occurs via a similar mechanism. The Journal of Immunology. 1998;160:2469–2477. [PubMed] [Google Scholar]
- 57.Yazdi AS, Drexler SK. Regulation of interleukin 1α secretion by inflammasomes. Annals of the rheumatic diseases. 2012 doi: 10.1136/annrheumdis-2012-202252. annrheumdis-2012-202252. [DOI] [PubMed] [Google Scholar]
- 58.Ding J, Wang K, Liu W, et al. Pore-forming activity and structural autoinhibition of the gasdermin family. Nature. 2016;535:111–116. doi: 10.1038/nature18590. [DOI] [PubMed] [Google Scholar]
- 59.Fink SL, Cookson BT. Caspase-1-dependent pore formation during pyroptosis leads to osmotic lysis of infected host macrophages. Cell Microbiol. 2006;8:1812–1825. doi: 10.1111/j.1462-5822.2006.00751.x. [DOI] [PubMed] [Google Scholar]
- 60.Fink SL, Bergsbaken T, Cookson BT. Anthrax lethal toxin and Salmonella elicit the common cell death pathway of caspase-1-dependent pyroptosis via distinct mechanisms. Proceedings of the National Academy of Sciences. 2008;105:4312–4317. doi: 10.1073/pnas.0707370105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kurt-Jones EA, Beller DI, Mizel SB, Unanue ER. Identification of a membrane- associated interleukin 1 in macrophages. Proceedings of the National Academy of Sciences. 1985;82:1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kurt-Jones E, Kiely J, Unanue E. Conditions required for expression of membrane IL 1 on B cells. The Journal of Immunology. 1985;135:1548–1550. [PubMed] [Google Scholar]
- 63.Di Paolo NC, Shafiani S, Day T, et al. Interdependence between interleukin-1 and tumor necrosis factor regulates TNF-dependent control of Mycobacterium tuberculosis infection. Immunity. 2015;43:1125–1136. doi: 10.1016/j.immuni.2015.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kurt-Jones EA, Fiers W, Pober JS. Membrane interleukin 1 induction on human endothelial cells and dermal fibroblasts. The Journal of Immunology. 1987;139:2317–2324. [PubMed] [Google Scholar]
- 65.Kurt-Jones E, Virgin H, Unanue E. In vivo and in vitro expression of macrophage membrane interleukin 1 in response to soluble and particulate stimuli. The Journal of Immunology. 1986;137:10–14. [PubMed] [Google Scholar]
- 66.Brody D, Durum S. Membrane IL-1: IL-1 alpha precursor binds to the plasma membrane via a lectin-like interaction. The Journal of Immunology. 1989;143:1183–1187. [PubMed] [Google Scholar]
- 67.Giri J, Lomedico P, Mizel S. Studies on the synthesis and secretion of interleukin 1. I. A 33,000 molecular weight precursor for interleukin 1. The Journal of Immunology. 1985;134:343–349. [PubMed] [Google Scholar]
- 68.Bakouche O, Brown D, Lachman L. Subcellular localization of human monocyte interleukin 1: evidence for an inactive precursor molecule and a possible mechanism for IL 1 release. The Journal of Immunology. 1987;138:4249–4255. [PubMed] [Google Scholar]
- 69.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 70.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annual review of immunology. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 71.Lee J-K, Kim S-H, Lewis EC, Azam T, Reznikov LL, Dinarello CA. Differences in signaling pathways by IL-1β and IL-18. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8815–8820. doi: 10.1073/pnas.0402800101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eigenbrod T, Park J-H, Harder J, Iwakura Y, Núñez G. Cutting edge: critical role for mesothelial cells in necrosis-induced inflammation through the recognition of IL-1α released from dying cells. The Journal of Immunology. 2008;181:8194–8198. doi: 10.4049/jimmunol.181.12.8194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kono H, Karmarkar D, Iwakura Y, Rock KL. Identification of the cellular sensor that stimulates the inflammatory response to sterile cell death. The Journal of Immunology. 2010;184:4470–4478. doi: 10.4049/jimmunol.0902485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Rider P, Carmi Y, Voronov E, Apte RN, editors. Interleukin-1α. Elsevier; 2013. [DOI] [PubMed] [Google Scholar]
- 75.Rock KL, Latz E, Ontiveros F, Kono H. The sterile inflammatory response. Annual review of immunology. 2009;28:321–342. doi: 10.1146/annurev-immunol-030409-101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cohen I, Rider P, Carmi Y, et al. Differential release of chromatin-bound IL-1α discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proceedings of the National Academy of Sciences. 2010;107:2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Berda-Haddad Y, Robert S, Salers P, et al. Sterile inflammation of endothelial cell-derived apoptotic bodies is mediated by interleukin-1α. Proceedings of the National Academy of Sciences. 2011;108:20684–20689. doi: 10.1073/pnas.1116848108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gurung P, Kanneganti T-D. Autoinflammatory skin disorders: the inflammasome in focus. Trends in molecular medicine. 2016;22:545–564. doi: 10.1016/j.molmed.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chitu V, Nacu V, Charles JF, et al. PSTPIP2 deficiency in mice causes osteopenia and increased differentiation of multipotent myeloid precursors into osteoclasts. Blood. 2012;120:3126–3135. doi: 10.1182/blood-2012-04-425595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsujita K, Suetsugu S, Sasaki N, Furutani M, Oikawa T, Takenawa T. Coordination between the actin cytoskeleton and membrane deformation by a novel membrane tubulation domain of PCH proteins is involved in endocytosis. J Cell Biol. 2006;172:269–279. doi: 10.1083/jcb.200508091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu Y, Dowbenko D, Lasky LA. PSTPIP 2, a second tyrosine phosphorylated, cytoskeletal-associated protein that binds a PEST-type protein-tyrosine phosphatase. Journal of Biological Chemistry. 1998;273:30487–30496. doi: 10.1074/jbc.273.46.30487. [DOI] [PubMed] [Google Scholar]
- 82.Chitu V, Ferguson PJ, De Bruijn R, et al. Primed innate immunity leads to autoinflammatory disease in PSTPIP2-deficient cmo mice. Blood. 2009;114:2497–2505. doi: 10.1182/blood-2009-02-204925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ferguson PJ, Bing X, Vasef MA, et al. A missense mutation in pstpip2 is associated with the murine autoinflammatory disorder chronic multifocal osteomyelitis. Bone. 2006;38:41–47. doi: 10.1016/j.bone.2005.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grosse J, Chitu V, Marquardt A, et al. Mutation of mouse Mayp/Pstpip2 causes a macrophage autoinflammatory disease. Blood. 2006;107:3350–3358. doi: 10.1182/blood-2005-09-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lukens JR, Gross JM, Calabrese C, et al. Critical role for inflammasome- independent IL-1β production in osteomyelitis. Proceedings of the National Academy of Sciences. 2014;111:1066–1071. doi: 10.1073/pnas.1318688111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cassel SL, Janczy JR, Bing X, et al. Inflammasome-independent IL-1beta mediates autoinflammatory disease in Pstpip2-deficient mice. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:1072–1077. doi: 10.1073/pnas.1318685111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gurung P, Burton A, Kanneganti T-D. NLRP3 inflammasome plays a redundant role with caspase 8 to promote IL-1β–mediated osteomyelitis. Proceedings of the National Academy of Sciences. 2016;113:4452–4457. doi: 10.1073/pnas.1601636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lukens JR, Gurung P, Vogel P, et al. Dietary modulation of the microbiome affects autoinflammatory disease. Nature. 2014 doi: 10.1038/nature13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kuemmerle-Deschner JB, editor. CAPS—pathogenesis, presentation and treatment of an autoinflammatory disease. Springer; 2015. [DOI] [PubMed] [Google Scholar]
- 90.Hoffman HM, Wanderer AA, Broide DH. Familial cold autoinflammatory syndrome: phenotype and genotype of an autosomal dominant periodic fever. Journal of Allergy and Clinical Immunology. 2001;108:615–620. doi: 10.1067/mai.2001.118790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hoffman HM, Mueller JL, Broide DH, Wanderer AA, Kolodner RD. Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle–Wells syndrome. Nature genetics. 2001;29:301. doi: 10.1038/ng756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feldmann J, Prieur A-M, Quartier P, et al. Chronic infantile neurological cutaneous and articular syndrome is caused by mutations in CIAS1, a gene highly expressed in polymorphonuclear cells and chondrocytes. The American Journal of Human Genetics. 2002;71:198–203. doi: 10.1086/341357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Aksentijevich I, Nowak M, Mallah M, et al. De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): A new member of the expanding family of pyrin-associated autoinflammatory diseases. Arthritis & Rheumatology. 2002;46:3340–3348. doi: 10.1002/art.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Neven B, Callebaut I, Prieur AM, et al. Molecular basis of the spectral expression of CIAS1 mutations associated with phagocytic cell-mediated autoinflammatory disorders CINCA/NOMID, MWS, and FCU. Blood. 2004;103:2809–2815. doi: 10.1182/blood-2003-07-2531. [DOI] [PubMed] [Google Scholar]
- 95.Meng G, Zhang F, Fuss I, Kitani A, Strober W. A mutation in the Nlrp3 gene causing inflammasome hyperactivation potentiates Th17 cell-dominant immune responses. Immunity. 2009;30:860–874. doi: 10.1016/j.immuni.2009.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brydges SD, Mueller JL, McGeough MD, et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity. 2009;30:875–887. doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brydges SD, Mueller JL, McGeough MD, et al. Inflammasome-mediated disease animal models reveal roles for innate but not adaptive immunity. Immunity. 2009;30:875–887. doi: 10.1016/j.immuni.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Brydges SD, Broderick L, McGeough MD, Pena CA, Mueller JL, Hoffman HM. Divergence of IL-1, IL-18, and cell death in NLRP3 inflammasomopathies. The Journal of clinical investigation. 2013;123:4695–4705. doi: 10.1172/JCI71543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Agostini L, Martinon F, Burns K, McDermott MF, Hawkins PN, Tschopp J. NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle- Wells autoinflammatory disorder. Immunity. 2004;20:319–325. doi: 10.1016/s1074-7613(04)00046-9. [DOI] [PubMed] [Google Scholar]
- 100.Bernot A, Da Silva C, Petit J-L, et al. Non-founder mutations in the MEFV gene establish this gene as the cause of familial Mediterranean fever (FMF) Human molecular genetics. 1998;7:1317–1325. doi: 10.1093/hmg/7.8.1317. [DOI] [PubMed] [Google Scholar]
- 101.Chae JJ, Cho YH, Lee GS, et al. Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity. 2011;34:755–768. doi: 10.1016/j.immuni.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ancient missense mutations in a new member of the RoRet gene family are likely to cause familial Mediterranean fever. The International FMF Consortium. Cell. 1997;90:797–807. doi: 10.1016/s0092-8674(00)80539-5. [DOI] [PubMed] [Google Scholar]
- 103.Sharma D, Sharma BR, Vogel P, Kanneganti T-D. IL-1β and Caspase-1 Drive Autoinflammatory Disease Independently of IL-1α or Caspase-8 in a Mouse Model of Familial Mediterranean Fever. The American Journal of Pathology. 2017;187:236–244. doi: 10.1016/j.ajpath.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.HogenEsch H, Gijbels M, Offerman E, Van Hooft J, Van Bekkum DW, Zurcher C. A spontaneous mutation characterized by chronic proliferative dermatitis in C57BL mice. The American journal of pathology. 1993;143:972. [PMC free article] [PubMed] [Google Scholar]
- 105.Seymour R, Hasham M, Cox G, et al. Spontaneous mutations in the mouse Sharpin gene result in multiorgan inflammation, immune system dysregulation and dermatitis. Genes and immunity. 2007;8:416. doi: 10.1038/sj.gene.6364403. [DOI] [PubMed] [Google Scholar]
- 106.Tokunaga F, Nakagawa T, Nakahara M, et al. SHARPIN is a component of the NF-[kgr]B-activating linear ubiquitin chain assembly complex. Nature. 2011;471:633–636. doi: 10.1038/nature09815. [DOI] [PubMed] [Google Scholar]
- 107.Ikeda F, Deribe YL, Skånland SS, et al. SHARPIN forms a linear ubiquitin ligase complex regulating NF-κB activity and apoptosis. Nature. 2011;471:637. doi: 10.1038/nature09814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gerlach B, Cordier SM, Schmukle AC, et al. Linear ubiquitination prevents inflammation and regulates immune signalling. Nature. 2011;471:591–596. doi: 10.1038/nature09816. [DOI] [PubMed] [Google Scholar]
- 109.Rickard JA, Anderton H, Etemadi N, et al. TNFR1-dependent cell death drives inflammation in Sharpin-deficient mice. Elife. 2014;3:e03464. doi: 10.7554/eLife.03464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Douglas T, Champagne C, Morizot A, Lapointe J-M, Saleh M. The inflammatory caspases-1 and-11 mediate the pathogenesis of dermatitis in Sharpin-deficient mice. The Journal of Immunology. 2015;195:2365–2373. doi: 10.4049/jimmunol.1500542. [DOI] [PubMed] [Google Scholar]
- 111.Kumari S, Redouane Y, Lopez-Mosqueda J, et al. Sharpin prevents skin inflammation by inhibiting TNFR1-induced keratinocyte apoptosis. Elife. 2014;3:e03422. doi: 10.7554/eLife.03422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Douglas T, Champagne C, Morizot A, Lapointe JM, Saleh M. The Inflammatory Caspases-1 and -11 Mediate the Pathogenesis of Dermatitis in Sharpin-Deficient Mice. Journal of immunology (Baltimore, Md : 1950) 2015;195:2365–2373. doi: 10.4049/jimmunol.1500542. [DOI] [PubMed] [Google Scholar]
- 113.Gurung P, Sharma BR, Kanneganti TD. Distinct role of IL-1beta in instigating disease in Sharpincpdm mice. Scientific reports. 2016;6:36634. doi: 10.1038/srep36634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ikejima T, Okusawa S, Ghezzi P, Van Der Meer JW, Dinarello CA. Interleukin-l induces tumor necrosis factor (TNF) in human peripheral blood mononuclear cells in vitro and a circulating TNF-Iike activity in rabbits. Journal of Infectious Diseases. 1990;162:215–223. doi: 10.1093/infdis/162.1.215. [DOI] [PubMed] [Google Scholar]
- 115.Gurung P, Lamkanfi M, Kanneganti T-D. Cutting Edge: SHARPIN Is Required for Optimal NLRP3 Inflammasome Activation. The Journal of Immunology. 2015;194:2064–2067. doi: 10.4049/jimmunol.1402951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gurung P, Li B, Malireddi RS, Lamkanfi M, Geiger TL, Kanneganti T-D. Chronic TLR stimulation controls NLRP3 inflammasome activation through IL-10 mediated regulation of NLRP3 expression and caspase-8 activation. Scientific reports. 2015:5. doi: 10.1038/srep14488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Milora KA, Miller SL, Sanmiguel JC, Jensen LE. Interleukin-1alpha released from HSV-1-infected keratinocytes acts as a functional alarmin in the skin. Nat Commun. 2014;5:5230. doi: 10.1038/ncomms6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cohen I, Rider P, Vornov E, et al. IL-1alpha is a DNA damage sensor linking genotoxic stress signaling to sterile inflammation and innate immunity. Sci Rep. 2015;5:14756. doi: 10.1038/srep14756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lukens JR, Vogel P, Johnson GR, et al. RIP1-driven autoinflammation targets IL-1alpha independently of inflammasomes and RIP3. Nature. 2013;498:224–227. doi: 10.1038/nature12174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bersudsky M, Luski L, Fishman D, et al. Non-redundant properties of IL- 1alpha and IL-1beta during acute colon inflammation in mice. Gut. 2014;63:598–609. doi: 10.1136/gutjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- 121.Freigang S, Ampenberger F, Weiss A, et al. Fatty acid-induced mitochondrial uncoupling elicits inflammasome-independent IL-1alpha and sterile vascular inflammation in atherosclerosis. Nature immunology. 2013;14:1045–1053. doi: 10.1038/ni.2704. [DOI] [PubMed] [Google Scholar]
- 122.Brough D, Denes A. Interleukin-1alpha and brain inflammation. IUBMB Life. 2015;67:323–330. doi: 10.1002/iub.1377. [DOI] [PubMed] [Google Scholar]
- 123.Angelone DF, Wessels MR, Coughlin M, et al. Innate Immunity of the Human Newborn Is Polarized Toward a High Ratio of IL-6/TNF-{alpha} Production In Vitro and In Vivo. Pediatr Res. 2006 doi: 10.1203/01.pdr.0000228319.10481.ea. [DOI] [PubMed] [Google Scholar]
- 124.Berk DR, Bayliss SJ. Neutrophilic dermatoses in children. Pediatric dermatology. 2008;25:509–519. doi: 10.1111/j.1525-1470.2008.00765.x. [DOI] [PubMed] [Google Scholar]
- 125.Cohen PR. Neutrophilic dermatoses: a review of current treatment options. American journal of clinical dermatology. 2009;10:301–312. doi: 10.2165/11310730-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 126.Shultz LD, Schweitzer PA, Rajan TV, et al. Mutations at the murine motheaten locus are within the hematopoietic cell protein-tyrosine phosphatase (Hcph) gene. Cell. 1993;73:1445–1454. doi: 10.1016/0092-8674(93)90369-2. [DOI] [PubMed] [Google Scholar]
- 127.Nesterovitch AB, Szanto S, Gonda A, et al. Spontaneous insertion of a b2 element in the ptpn6 gene drives a systemic autoinflammatory disease in mice resembling neutrophilic dermatosis in humans. The American journal of pathology. 2011;178:1701–1714. doi: 10.1016/j.ajpath.2010.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Tsui HW, Siminovitch KA, de Souza L, Tsui FW. Motheaten and viable motheaten mice have mutations in the haematopoietic cell phosphatase gene. Nature genetics. 1993;4:124–129. doi: 10.1038/ng0693-124. [DOI] [PubMed] [Google Scholar]
- 129.Nesterovitch AB, Gyorfy Z, Hoffman MD, et al. Alteration in the gene encoding protein tyrosine phosphatase nonreceptor type 6 (PTPN6/SHP1) may contribute to neutrophilic dermatoses. The American journal of pathology. 2011;178:1434–1441. doi: 10.1016/j.ajpath.2010.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cao H, Hegele RA. Identification of polymorphisms in the human SHP1 gene. Journal of human genetics. 2002;47:445–447. doi: 10.1007/s100380200062. [DOI] [PubMed] [Google Scholar]
- 131.Christophi GP, Hudson CA, Gruber RC, et al. SHP-1 deficiency and increased inflammatory gene expression in PBMCs of multiple sclerosis patients. Laboratory investigation; a journal of technical methods and pathology. 2008;88:243–255. doi: 10.1038/labinvest.3700720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Eriksen KW, Woetmann A, Skov L, et al. Deficient SOCS3 and SHP-1 expression in psoriatic T cells. J Invest Dermatol. 2010;130:1590–1597. doi: 10.1038/jid.2010.6. [DOI] [PubMed] [Google Scholar]
- 133.Tibaldi E, Brunati AM, Zonta F, et al. Lyn-mediated SHP-1 recruitment to CD5 contributes to resistance to apoptosis of B-cell chronic lymphocytic leukemia cells. Leukemia. 2011;25:1768–1781. doi: 10.1038/leu.2011.152. [DOI] [PubMed] [Google Scholar]
- 134.Wu C, Sun M, Liu L, Zhou GW. The function of the protein tyrosine phosphatase SHP-1 in cancer. Gene. 2003;306:1–12. doi: 10.1016/s0378-1119(03)00400-1. [DOI] [PubMed] [Google Scholar]
- 135.Croker BA, Lawson BR, Rutschmann S, et al. Inflammation and autoimmunity caused by a SHP1 mutation depend on IL-1, MyD88, and a microbial trigger. Proceedings of the National Academy of Sciences. 2008;105:15028–15033. doi: 10.1073/pnas.0806619105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Groves RW, Mizutani H, Kieffer JD, Kupper TS. Inflammatory skin disease in transgenic mice that express high levels of interleukin 1 alpha in basal epidermis. Proceedings of the National Academy of Sciences. 1995;92:11874–11878. doi: 10.1073/pnas.92.25.11874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gurung P, Fan G, Lukens JR, Vogel P, Tonks NK, Kanneganti T-D. Tyrosine Kinase SYK Licenses MyD88 Adaptor Protein to Instigate IL-1α-Mediated Inflammatory Disease. Immunity. 2017;46:635–648. doi: 10.1016/j.immuni.2017.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Weinlich R, Green DR. The two faces of receptor interacting protein kinase-1. Molecular cell. 2014;56:469–480. doi: 10.1016/j.molcel.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Berger SB, Kasparcova V, Hoffman S, et al. Cutting Edge: RIP1 kinase activity is dispensable for normal development but is a key regulator of inflammation in SHARPIN-deficient mice. Journal of immunology. 2014;192:5476–5480. doi: 10.4049/jimmunol.1400499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Polykratis A, Hermance N, Zelic M, et al. Cutting edge: RIPK1 Kinase inactive mice are viable and protected from TNF-induced necroptosis in vivo. Journal of immunology. 2014;193:1539–1543. doi: 10.4049/jimmunol.1400590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dondelinger Y, Declercq W, Montessuit S, et al. MLKL compromises plasma membrane integrity by binding to phosphatidylinositol phosphates. Cell reports. 2014;7:971–981. doi: 10.1016/j.celrep.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 142.Dillon CP, Weinlich R, Rodriguez DA, et al. RIPK1 blocks early postnatal lethality mediated by caspase-8 and RIPK3. Cell. 2014;157:1189–1202. doi: 10.1016/j.cell.2014.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kaiser WJ, Daley-Bauer LP, Thapa RJ, et al. RIP1 suppresses innate immune necrotic as well as apoptotic cell death during mammalian parturition. Proc Natl Acad Sci U S A. 2014;111:7753–7758. doi: 10.1073/pnas.1401857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rickard JA, O’Donnell JA, Evans JM, et al. RIPK1 regulates RIPK3-MLKL- driven systemic inflammation and emergency hematopoiesis. Cell. 2014;157:1175–1188. doi: 10.1016/j.cell.2014.04.019. [DOI] [PubMed] [Google Scholar]
- 145.Lee TH, Shank J, Cusson N, Kelliher MA. The kinase activity of Rip1 is not required for tumor necrosis factor-alpha-induced IkappaB kinase or p38 MAP kinase activation or for the ubiquitination of Rip1 by Traf2. J Biol Chem. 2004;279:33185–33191. doi: 10.1074/jbc.M404206200. [DOI] [PubMed] [Google Scholar]
- 146.Ajibade AA, Wang HY, Wang RF. Cell type-specific function of TAK1 in innate immune signaling. Trends in immunology. 2013;34:307–316. doi: 10.1016/j.it.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 147.Coleman KM, Gudjonsson JE, Stecher M. Open-label trial of MABp1, a true human monoclonal antibody targeting interleukin 1α, for the treatment of psoriasis. JAMA dermatology. 2015;151:555–556. doi: 10.1001/jamadermatol.2014.5391. [DOI] [PubMed] [Google Scholar]
- 148.Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 149.Wu XN, Yang ZH, Wang XK, et al. Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell Death Differ. 2014;21:1709–1720. doi: 10.1038/cdd.2014.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Thapa RJ, Nogusa S, Chen P, et al. Interferon-induced RIP1/RIP3-mediated necrosis requires PKR and is licensed by FADD and caspases. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:E3109–3118. doi: 10.1073/pnas.1301218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nakamura Y, Franchi L, Kambe N, Meng G, Strober W, Núñez G. Critical role for mast cells in interleukin-1β-driven skin inflammation associated with an activating mutation in the nlrp3 protein. Immunity. 2012;37:85–95. doi: 10.1016/j.immuni.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kawaguchi Y, Hara M, Wright TM. Endogenous IL-1α from systemic sclerosis fibroblasts induces IL-6 and PDGF-A. Journal of Clinical Investigation. 1999;103:1253. doi: 10.1172/JCI4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Kawaguchi Y, McCarthy SA, Watkins SC, Wright TM. Autocrine activation by interleukin 1alpha induces the fibrogenic phenotype of systemic sclerosis fibroblasts. The Journal of rheumatology. 2004;31:1946–1954. [PubMed] [Google Scholar]
- 154.Kawaguchi Y, Harigai M, Hara M, et al. Increased interleukin 1 receptor, type I, at messenger RNA and protein level in skin fibroblasts from patients with systemic sclerosis. Biochemical and biophysical research communications. 1992;184:1504–1510. doi: 10.1016/s0006-291x(05)80053-1. [DOI] [PubMed] [Google Scholar]
- 155.Bersudsky M, Luski L, Fishman D, et al. Non-redundant properties of IL-1α and IL-1β during acute colon inflammation in mice. Gut. 2013 doi: 10.1136/gutjnl-2012-303329. gutjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- 156.Malik A, Sharma D, Zhu Q, et al. IL-33 regulates the IgA-microbiota axis to restrain IL-1α–dependent colitis and tumorigenesis. The Journal of Clinical Investigation. 2016;126:4469–4481. doi: 10.1172/JCI88625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chamberlain J, Francis S, Brookes Z, et al. Interleukin-1 regulates multiple atherogenic mechanisms in response to fat feeding. PloS one. 2009;4:e5073. doi: 10.1371/journal.pone.0005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lugrin J, Parapanov R, Rosenblatt-Velin N, et al. Cutting edge: IL-1α is a crucial danger signal triggering acute myocardial inflammation during myocardial infarction. The Journal of Immunology. 2015;194:499–503. doi: 10.4049/jimmunol.1401948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Luheshi NM, Kovács KJ, Lopez-Castejon G, Brough D, Denes A. Interleukin-1α expression precedes IL-1β after ischemic brain injury and is localised to areas of focal neuronal loss and penumbral tissues. Journal of neuroinflammation. 2011;8:186. doi: 10.1186/1742-2094-8-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Guler R, Parihar SP, Spohn G, Johansen P, Brombacher F, Bachmann MF. Blocking IL-1α but not IL-1β increases susceptibility to chronic Mycobacterium tuberculosis infection in mice. Vaccine. 2011;29:1339–1346. doi: 10.1016/j.vaccine.2010.10.045. [DOI] [PubMed] [Google Scholar]
- 161.Matsumoto T, Tateda K, Miyazaki S, et al. Paradoxical synergistic effects of tumour necrosis factor and interleukin 1 in murine gut-derived sepsis with Pseudomonas aeruginosa. Cytokine. 1999;11:366–372. doi: 10.1006/cyto.1998.0434. [DOI] [PubMed] [Google Scholar]
- 162.Olaru F, Jensen LE. Staphylococcus aureus stimulates neutrophil targeting chemokine expression in keratinocytes through an autocrine IL-1α signaling loop. Journal of Investigative Dermatology. 2010;130:1866–1876. doi: 10.1038/jid.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cheng W, Shivshankar P, Zhong Y, Chen D, Li Z, Zhong G. Intracellular interleukin-1α mediates interleukin-8 production induced by Chlamydia trachomatis infection via a mechanism independent of type I interleukin-1 receptor. Infection and immunity. 2008;76:942–951. doi: 10.1128/IAI.01313-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kafka D, Ling E, Feldman G, et al. Contribution of IL-1 to resistance to Streptococcus pneumoniae infection. International immunology. 2008;20:1139–1146. doi: 10.1093/intimm/dxn071. [DOI] [PubMed] [Google Scholar]
- 165.Lupfer CR, Anand PK, Liu Z, et al. Reactive oxygen species regulate caspase- 11 expression and activation of the non-canonical NLRP3 inflammasome during enteric pathogen infection. 2014 doi: 10.1371/journal.ppat.1004410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Liu Z, Zaki MH, Vogel P, et al. Role of inflammasomes in host defense against Citrobacter rodentium infection. Journal of Biological Chemistry. 2012;287:16955–16964. doi: 10.1074/jbc.M112.358705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Man SM, Karki R, Malireddi RS, et al. The transcription factor IRF1 and guanylate-binding proteins target activation of the AIM2 inflammasome by Francisella infection. Nature immunology. 2015;16:467–475. doi: 10.1038/ni.3118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Man SM, Karki R, Sasai M, et al. IRGB10 Liberates Bacterial Ligands for Sensing by the AIM2 and Caspase-11-NLRP3 Inflammasomes. Cell. 2016;167:382–396. e317. doi: 10.1016/j.cell.2016.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Man SM, Karki R, Kanneganti TD. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunological Reviews. 2017;277:61–75. doi: 10.1111/imr.12534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Barry KC, Fontana MF, Portman JL, Dugan AS, Vance RE. IL-1α signaling initiates the inflammatory response to virulent Legionella pneumophila in vivo. The Journal of Immunology. 2013;190:6329–6339. doi: 10.4049/jimmunol.1300100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Nikota JK, Shen P, Morissette MC, et al. Cigarette Smoke Primes the Pulmonary Environment to IL-1α/CXCR-2–Dependent Nontypeable Haemophilus influenzae–Exacerbated Neutrophilia in Mice. The Journal of Immunology. 2014;193:3134–3145. doi: 10.4049/jimmunol.1302412. [DOI] [PubMed] [Google Scholar]
- 172.Dube PH, Revell PA, Chaplin DD, Lorenz RG, Miller VL. A role for IL-1α in inducing pathologic inflammation during bacterial infection. Proceedings of the National Academy of Sciences. 2001;98:10880–10885. doi: 10.1073/pnas.191214498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tadros T, Traber DL, Heggers JP, Herndon DN. Effects of interleukin-1α administration on intestinal ischemia and reperfusion injury, mucosal permeability, and bacterial translocation in burn and sepsis. Annals of surgery. 2003;237:101. doi: 10.1097/00000658-200301000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Di Paolo NC, Miao EA, Iwakura Y, et al. Virus binding to a plasma membrane receptor triggers interleukin-1α-mediated proinflammatory macrophage response in vivo. Immunity. 2009;31:110–121. doi: 10.1016/j.immuni.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Di Paolo NC, Baldwin LK, Irons EE, Papayannopoulou T, Tomlinson S, Shayakhmetov DM. IL-1α and complement cooperate in triggering local neutrophilic inflammation in response to adenovirus and eliminating virus-containing cells. PLoS pathogens. 2014;10:e1004035. doi: 10.1371/journal.ppat.1004035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Altmeier S, Toska A, Sparber F, Teijeira A, Halin C, LeibundGut-Landmann S. IL-1 coordinates the neutrophil response to C. albicans in the oral mucosa. PLoS pathogens. 2016;12:e1005882. doi: 10.1371/journal.ppat.1005882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Maffei CM, Mirels LF, Sobel RA, Clemons KV, Stevens DA. Cytokine and inducible nitric oxide synthase mRNA expression during experimental murine cryptococcal meningoencephalitis. Infection and immunity. 2004;72:2338–2349. doi: 10.1128/IAI.72.4.2338-2349.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Caffrey AK, Lehmann MM, Zickovich JM, et al. IL-1α signaling is critical for leukocyte recruitment after pulmonary Aspergillus fumigatus challenge. PLoS pathogens. 2015;11:e1004625. doi: 10.1371/journal.ppat.1004625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Voronov E, Dotan S, Gayvoronsky L, et al. IL-1-induced inflammation promotes development of leishmaniasis in susceptible BALB/c mice. International immunology. 2010;22:245–257. doi: 10.1093/intimm/dxq006. [DOI] [PubMed] [Google Scholar]
- 180.von Stebut E, Ehrchen JM, Belkaid Y, et al. Interleukin 1α promotes Th1 differentiation and inhibits disease progression in Leishmania major–susceptible BALB/c mice. Journal of Experimental Medicine. 2003;198:191–199. doi: 10.1084/jem.20030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Schmitz N, Kurrer M, Bachmann MF, Kopf M. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. Journal of virology. 2005;79:6441–6448. doi: 10.1128/JVI.79.10.6441-6448.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Li Y, Wang L, Pappan L, Galliher-Beckley A, Shi J. IL-1β promotes stemness and invasiveness of colon cancer cells through Zeb1 activation. Molecular cancer. 2012;11:87. doi: 10.1186/1476-4598-11-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li H-J, Reinhardt F, Herschman HR, Weinberg RA. Cancer-stimulated mesenchymal stem cells create a carcinoma stem cell niche via prostaglandin E 2 signaling. Cancer discovery. 2012;2:840–855. doi: 10.1158/2159-8290.CD-12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Soria G, Ofri-Shahak M, Haas I, et al. Inflammatory mediators in breast cancer: coordinated expression of TNFα & IL-1β with CCL2 & CCL5 and effects on epithelial-to-mesenchymal transition. BMC cancer. 2011;11:130. doi: 10.1186/1471-2407-11-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Niu J, Li Z, Peng B, Chiao PJ. Identification of an autoregulatory feedback pathway involving interleukin-1α in induction of constitutive NF-κB activation in pancreatic cancer cells. Journal of Biological Chemistry. 2004;279:16452–16462. doi: 10.1074/jbc.M309789200. [DOI] [PubMed] [Google Scholar]
- 186.Murakami Y, Watari K, Shibata T, et al. N-myc downstream-regulated gene 1 promotes tumor inflammatory angiogenesis through JNK activation and autocrine loop of interleukin-1α by human gastric cancer cells. Journal of Biological Chemistry. 2013;288:25025–25037. doi: 10.1074/jbc.M113.472068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sakurai T, He G, Matsuzawa A, et al. Hepatocyte necrosis induced by oxidative stress and IL-1α release mediate carcinogen-induced compensatory proliferation and liver tumorigenesis. Cancer cell. 2008;14:156–165. doi: 10.1016/j.ccr.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Tjomsland V, Bojmar L, Sandström P, et al. IL-1α expression in pancreatic ductal adenocarcinoma affects the tumor cell migration and is regulated by the p38MAPK signaling pathway. PLoS One. 2013;8:e70874. doi: 10.1371/journal.pone.0070874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Löffek S, Zigrino P, Angel P, Anwald B, Krieg T, Mauch C. High invasive melanoma cells induce matrix metalloproteinase-1 synthesis in fibroblasts by interleukin-1α and basic fibroblast growth factor-mediated mechanisms. Journal of investigative dermatology. 2005;124:638–643. doi: 10.1111/j.0022-202X.2005.23629.x. [DOI] [PubMed] [Google Scholar]
- 190.Xu D, Matsuo Y, Ma J, et al. Cancer cell-derived IL-1α promotes HGF secretion by stromal cells and enhances metastatic potential in pancreatic cancer cells. Journal of surgical oncology. 2010;102:469–477. doi: 10.1002/jso.21530. [DOI] [PubMed] [Google Scholar]
- 191.Pietras EM, Mirantes-Barbeito C, Fong S, et al. Chronic interleukin-1 drives haematopoietic stem cells towards precocious myeloid differentiation at the expense of self-renewal. Nature cell biology. 2016;18:607. doi: 10.1038/ncb3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Song X, Krelin Y, Dvorkin T, et al. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1β-secreting cells. The Journal of Immunology. 2005;175:8200–8208. doi: 10.4049/jimmunol.175.12.8200. [DOI] [PubMed] [Google Scholar]
- 193.Keskinov AA, Shurin MR. Myeloid regulatory cells in tumor spreading and metastasis. Immunobiology. 2015;220:236–242. doi: 10.1016/j.imbio.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 194.Eisenbarth SC, Colegio OR, O’Connor W, Jr, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453:1122. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ben-Sasson SZ, Hogg A, Hu-Li J, et al. IL-1 enhances expansion, effector function, tissue localization, and memory response of antigen-specific CD8 T cells. Journal of Experimental Medicine. 2013;210:491–502. doi: 10.1084/jem.20122006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ben-Sasson SZ, Hu-Li J, Quiel J, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proceedings of the National Academy of Sciences. 2009;106:7119–7124. doi: 10.1073/pnas.0902745106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Ghiringhelli F, Apetoh L, Tesniere A, et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β–dependent adaptive immunity against tumors. Nature medicine. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 198.Feldmeyer L, Keller M, Niklaus G, Hohl D, Werner S, Beer H-D. The inflammasome mediates UVB-induced activation and secretion of interleukin-1β by keratinocytes. Current Biology. 2007;17:1140–1145. doi: 10.1016/j.cub.2007.05.074. [DOI] [PubMed] [Google Scholar]
- 199.Feldmeyer L, Werner S, French LE, Beer H-D. Interleukin-1, inflammasomes and the skin. European journal of cell biology. 2010;89:638–644. doi: 10.1016/j.ejcb.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 200.Beer H-D, Contassot E, French LE. The inflammasomes in autoinflammatory diseases with skin involvement. Journal of Investigative Dermatology. 2014;134:1805–1810. doi: 10.1038/jid.2014.76. [DOI] [PubMed] [Google Scholar]
- 201.Krelin Y, Voronov E, Dotan S, et al. Interleukin-1β–Driven inflammation promotes the development and invasiveness of chemical carcinogen–Induced tumors. Cancer research. 2007;67:1062–1071. doi: 10.1158/0008-5472.CAN-06-2956. [DOI] [PubMed] [Google Scholar]
- 202.Elkabets M, Krelin Y, Dotan S, et al. Host-derived interleukin-1α is important in determining the immunogenicity of 3-methylcholantrene tumor cells. The Journal of Immunology. 2009;182:4874–4881. doi: 10.4049/jimmunol.0803916. [DOI] [PubMed] [Google Scholar]
- 203.Song X, Voronov E, Dvorkin T, et al. Differential effects of IL-1α and IL-1β on tumorigenicity patterns and invasiveness. The Journal of Immunology. 2003;171:6448–6456. doi: 10.4049/jimmunol.171.12.6448. [DOI] [PubMed] [Google Scholar]
- 204.Bunt SK, Sinha P, Clements VK, Leips J, Ostrand-Rosenberg S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. The Journal of Immunology. 2006;176:284–290. doi: 10.4049/jimmunol.176.1.284. [DOI] [PubMed] [Google Scholar]
- 205.Nakao S, Kuwano T, Tsutsumi-Miyahara C, et al. Infiltration of COX-2– expressing macrophages is a prerequisite for IL-1β–induced neovascularization and tumor growth. Journal of Clinical Investigation. 2005;115:2979. doi: 10.1172/JCI23298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Saijo Y, Tanaka M, Miki M, et al. Proinflammatory cytokine IL-1β promotes tumor growth of Lewis lung carcinoma by induction of angiogenic factors: in vivo analysis of tumor-stromal interaction. The Journal of Immunology. 2002;169:469–475. doi: 10.4049/jimmunol.169.1.469. [DOI] [PubMed] [Google Scholar]
- 207.Carmi Y, Dotan S, Rider P, et al. The role of IL-1β in the early tumor Cell– Induced angiogenic response. The Journal of Immunology. 2013;190:3500–3509. doi: 10.4049/jimmunol.1202769. [DOI] [PubMed] [Google Scholar]
- 208.León X, Bothe C, García J, et al. Expression of IL-1α correlates with distant metastasis in patients with head and neck squamous cell carcinoma. Oncotarget. 2015;6:37398. doi: 10.18632/oncotarget.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Tomimatsu S, Ichikura T, Mochizuki H. Significant correlation between expression of interleukin-1α and liver metastasis in gastric carcinoma. Cancer. 2001;91:1272–1276. doi: 10.1002/1097-0142(20010401)91:7<1272::aid-cncr1128>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 210.Charbonneau B, Block MS, Bamlet WR, et al. Risk of Ovarian Cancer and the NF-κB Pathway: Genetic association with IL1A and TNFSF10. Cancer research. 2013 doi: 10.1158/0008-5472.CAN-13-1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kawaguchi Y, Tochimoto A, Hara M, et al. Contribution of single nucleotide polymorphisms of the IL1A gene to the cleavage of precursor IL-1α and its transcription activity. Immunogenetics. 2007;59:441–448. doi: 10.1007/s00251-007-0213-y. [DOI] [PubMed] [Google Scholar]
- 212.Douvdevani A, Huleihel M, Segal S, Apte R. Aberrations in interleukin-1 expression in oncogene-transformed fibrosarcoma lines: constitutive interleukin-1 alpha transcription and manifestation of biological activity. European cytokine network. 1991;2:257–264. [PubMed] [Google Scholar]
- 213.Douvdevani A, Huleihel M, Zöller M, Segal S, Apte RN. Reduced tumorigenicity of fibrosarcomas which constitutively generate il-1α either spontaneously or following il-1α gene transfer. International journal of cancer. 1992;51:822–830. doi: 10.1002/ijc.2910510526. [DOI] [PubMed] [Google Scholar]
- 214.Dvorkin T, Song X, Argov S, et al. Immune phenomena involved in the in vivo regression of fibrosarcoma cells expressing cell-associated IL-1α. Journal of leukocyte biology. 2006;80:96–106. doi: 10.1189/jlb.0905509. [DOI] [PubMed] [Google Scholar]
- 215.Voronov E, Weinstein Y, Benharroch D, et al. Antitumor and immunotherapeutic effects of activated invasive T lymphoma cells that display short-term interleukin 1α expression. Cancer research. 1999;59:1029–1035. [PubMed] [Google Scholar]
- 216.Shaked Y. Balancing efficacy of and host immune responses to cancer therapy: the yin and yang effects. Nature Reviews Clinical Oncology. 2016;13:611–626. doi: 10.1038/nrclinonc.2016.57. [DOI] [PubMed] [Google Scholar]
- 217.Timaner M, Bril R, Kaidar-Person O, et al. Dequalinium blocks macrophage- induced metastasis following local radiation. Oncotarget. 2015;6:27537. doi: 10.18632/oncotarget.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Dinarello CA. Interleukin-1 [alpha] neutralisation in patients with cancer. Lancet Oncology. 2014;15:552. doi: 10.1016/S1470-2045(14)70164-0. [DOI] [PubMed] [Google Scholar]
- 219.Hong DS, Hui D, Bruera E, et al. MABp1, a first-in-class true human antibody targeting interleukin-1α in refractory cancers: an open-label, phase 1 dose- escalation and expansion study. The Lancet Oncology. 2014;15:656–666. doi: 10.1016/S1470-2045(14)70155-X. [DOI] [PubMed] [Google Scholar]
- 220.Yeh K-Y, Li Y-Y, Hsieh L-L, et al. Analysis of the effect of serum interleukin-6 (IL-6) and soluble IL-6 receptor levels on survival of patients with colorectal cancer. Japanese journal of clinical oncology. 2010;40:580–587. doi: 10.1093/jjco/hyq010. [DOI] [PubMed] [Google Scholar]
- 221.Stone RL, Nick AM, McNeish IA, et al. Paraneoplastic thrombocytosis in ovarian cancer. New England Journal of Medicine. 2012;366:610–618. doi: 10.1056/NEJMoa1110352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Hickish T, Andre T, Wyrwicz L, et al. MABp1 as a novel antibody treatment for advanced colorectal cancer: a randomised, double-blind, placebo-controlled, phase 3 study. The Lancet Oncology. 2017;18:192–201. doi: 10.1016/S1470-2045(17)30006-2. [DOI] [PubMed] [Google Scholar]
- 223.Kuilman T, Michaloglou C, Vredeveld LC, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 224.Laberge R-M, Sun Y, Orjalo AV, et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nature cell biology. 2015;17:1049. doi: 10.1038/ncb3195. [DOI] [PMC free article] [PubMed] [Google Scholar]