Abstract
The accurate diagnosis of interstitial lung disease (ILD) is essential for optimal prognostication and management. While connective tissue disease (CTD) is among the most common causes of ILD, some patients have features suggestive of autoimmunity without meeting criteria for a specific CTD. To help define and study this disease entity more uniformly, a 2015 research statement proposed consensus-based criteria and coined the term “interstitial pneumonia with autoimmune features” (IPAF). In this review, we summarize and compare previously proposed criteria to characterize these patients, provide an overview of the IPAF criteria and highlight recent investigations aimed at characterizing IPAF cohorts. We then call attention to questions that have arisen with the application of the IPAF criteria and discuss future areas of study.
Keywords: interstitial lung disease (ILD), interstitial pneumonia with autoimmune features (IPAF), connective tissue disease (CTD), idiopathic pulmonary fibrosis (IPF)
Introduction
Interstitial lung disease (ILD) is composed of a heterogeneous group of diffuse parenchymal lung processes and accounts for about 15% of conditions seen in general pulmonary practice.1 Among the most common ILDs are connective tissue disease-associated ILD (CTD-ILD) and idiopathic pulmonary fibrosis (IPF). Because CTD-ILD generally follows a favorable clinical course compared to IPF, and because therapies differ substantially between the two processes, an accurate diagnosis is critical.2,3 All patients undergoing ILD evaluation should undergo high-resolution computed tomography (HRCT), surgical lung biopsy when indicated and multidisciplinary discussion between clinicians, a radiologist and pathologist (when needed) with ILD expertise.4 Consultation with a rheumatologist should also be considered for patients with suspected CTD-ILD.
Up to 30% of newly diagnosed ILD will be due to CTD.5,6 While most patients will manifest a radiographic and/or histopathologic pattern of non-specific interstitial pneumonia (NSIP)7,8, a substantial minority will manifest usual interstitial pneumonia (UIP), which is diagnostic of IPF in the absence of other known causes of ILD.9 Despite a similar pattern as those with IPF, those with CTD-associated UIP generally experience better outcomes, 10–12 underscoring the importance of assessing all patients with ILD, including those with UIP, for occult CTD. While some patients will develop ILD several months to years before other CTD features, others will have signs or symptoms suggestive of a CTD without meeting specific CTD criteria. Because these patients may represent a unique phenotype, several classification systems have been proposed in recent years.13–18 The need for a standardized classification system and improved understanding of this patient population led to the formation of the European Respiratory Society (ERS)/American Thoracic Society (ATS) Task Force on Undifferentiated Forms of Connective Tissue Disease-associated Interstitial Lung Disease. This group produced an official ERS/ATS research statement proposing criteria for patients with “interstitial pneumonia with autoimmune features” (IPAF).19 In this article, we provide historical context for the emergence of IPAF criteria, outline specific features comprising the IPAF criteria, review several recently characterized IPAF cohorts, highlight unanswered questions that have arisen with the application of these criteria and discuss areas needing further study.
Pre-IPAF Classification
In the fifteen years prior to the release of the IPAF criteria, several unique, yet overlapping classification systems were proposed to help characterize patients with features of autoimmunity who failed to meet overt CTD definitions. Among the first investigations to call attention to this disease entity were those that focused on non-specific interstitial pneumonia (NSIP), a radiographic and pathologic pattern commonly encountered in CTD-ILD.6 The strong link between NSIP and CTD, along with high frequency of circulating autoantibodies in patients with NSIP, led investigators to hypothesize that idiopathic NSIP represented an “autoimmune interstitial pneumonia”13 or “undifferentiated connective tissue disease” (UCTD).14
Kinder and colleagues proposed formal criteria for UCTD, which required at least one symptom and one circulating autoantibody suggestive of CTD.14 Those meeting proposed UCTD criteria were more likely to have surgical lung biopsy (SLB)-proven NSIP, supporting the hypothesis that NSIP was an autoimmune phenomenon and that UCTD-ILD was a pulmonary manifestation of CTD. Because UCTD, as defined by rheumatologists, describes patients with a milder disease course that is not frequently complicated by ILD20, Fischer and colleagues suggested that “lung-dominant” CTD (LD-CTD) was more appropriate nomenclature.17 Criteria for LD-CTD included the combination of ILD, failure to meet definitive criteria for a CTD, no alternative etiology for ILD and one autoantibody or two histopathologic features suggestive of CTD.
Corte and colleagues refined UCTD criteria proposed by Kinder and colleagues by developing an algorithm to predict the presence of NSIP on SLB using clinical and serologic features suggestive of CTD.15 Compared to the broader UCTD criteria proposed by Kinder and colleagues, those proposed by Corte had a higher specificity for predicting biopsy-proven NSIP. A similar classification system was proposed by Vij and colleagues, who suggested the term “autoimmune-featured interstitial lung disease” (AIF-ILD) to describe this patient population.16 As opposed to the UCTD cohort with NSIP characterized by Corte and colleagues, UIP predominated in the AIF-ILD cohort characterized by Vij and colleagues. Despite these differences, survival was similar between patients meeting UCTD/AIF-ILD criteria and those with IPF at each center.
Given the multiple proposed classification schemes for relatively similar cohorts, Assayag and colleagues compared each set of criteria (Kinder, Corte, Fischer, Vij) to better characterize these overlapping groups.18 These investigators found that all four sets of criteria described a similar group of patients, but that more patients met the Fischer and Kinder criteria as compared to the Vij and Corte. This analysis also suggested that meeting each set of criteria was associated with better survival compared to patients with chronic fibrosing interstitial pneumonia who did not meet criteria, though only the Corte criteria remained an independent predictor of survival after adjusting for the gender-age-physiology (GAP) score.21
IPAF Research Statement: The Criteria
The need for consensus when studying this patient population resulted in the 2015 publication of the joint ERS/ATS research statement, coining the term IPAF.19 IPAF criteria (Table 1) required 1) the presence of ILD by HRCT or SLB 2) exclusion of an alternate etiology for the ILD 3) failure to meet criteria for a defined CTD and 4) at least one feature from at least two clinical, serologic and morphologic domains. While there existed extensive overlap between IPAF and UCTD/LD-CTD/AIF-ILD criteria with regard to the clinical and serologic domains, the inclusion of the morphologic domain in the IPAF criteria represented an acknowledgment that incorporation of HRCT, SLB and other multi-compartment features could help identify additional patients with features of autoimmunity.
Table 1.
Classification criteria for interstitial pneumonia with autoimmune features (IPAF), adapted from Fischer et al. After ruling out other causes of ILD, patients who do not meet established criteria for CTD must satisfy one criterion from two separate domains to meet IPAF criteria.
| Clinical Domain | Serologic Domain | Morphologic domain |
|---|---|---|
| Mechanic’s hands (distal digital fissuring) | ANA≥1:320 titer (diffuse, speckled, homogeneous) OR | HRCT patterns: |
| Distal digital tip ulceration | ANA nucleolar pattern (any titer) | NSIP |
| Inflammatory arthritis/joint stiffness≥60 minutes | ANA centromere pattern (any titer) | OP |
| Palmar telangiectasia | RF≥2× upper limit of normal | NSIP with OP overlap |
| Raynaud's phenomenon | Anti-CCP | LIP |
| Unexplained digital edema | Anti-dsDNA | Histopathology patterns: |
| Gottron's sign (fixed rash on digital extensor surfaces) | Anti-Ro (SS-A) | NSIP |
| Anti-La (SS-B) | OP | |
| Anti-ribonucleoprotein | NSIP with OP overlap | |
| Anti-Smith | LIP | |
| Anti-topoisomerase (Scl-70) | Interstitial lymphoid aggregates with germinal centers | |
| Anti-tRNA synthetase | Diffuse lymphoplasmacytic infiltration | |
| Anti-PM-Scl | Multi-compartment involvement: | |
| Anti-MDA-5 | Unexplained pleural effusion/thickening | |
| Unexplained pericardial effusion/thickening | ||
| Unexplained intrinsic airway disease | ||
| Unexplained pulmonary vasculopathy |
Abbreviations: ANA-antinuclear antibody, HRCT-high resolution computed tomography, NSIP-nonspecific interstitial pneumonia, OP-organizing pneumonia, LIP-lymphocytic interstitial pneumonia.
Clinical domain
Clinical features most strongly associated with specific CTDs based on the task force consensus opinion are included in this domain. These include distal digital fissuring (mechanic’s hands), digital tip ulceration, inflammatory arthritis or polyarticular morning stiffness lasting over 60 minutes, palmar telangiectasia, Raynaud’s phenomenon, unexplained digital edema and unexplained fixed rash on the digital extensor surfaces (Gottron’s sign). The task force aimed to include signs and symptoms specific for autoimmune etiologies but whose absence does not rule out the presence of a CTD. Clinical findings included in previously proposed criteria, including alopecia, dysphagia, photosensitivity, oral ulcers, weight loss, sicca symptoms, myalgia, arthralgia and proximal muscle weakness were not included, as they were felt to be insufficiently specific for CTD.
Serologic domain
The task force again emphasized specificity in this domain by selecting autoantibodies with strong CTD association and by requiring moderately elevated titers for less specific autoantibodies, such as antinuclear antibody (ANA) and rheumatoid factor (RF). To satisfy the IPAF serologic domain with an ANA or RF, patients must have ANA ≥ 1:320 (or nucleolar or centromere pattern at any titer) or RF at ≥2 times the upper limit of normal. Anti-neutrophil cytoplasmic antibody is not included in the IPAF criteria, as this is associated with vasculitis-associated ILD rather than ILD due to CTD.
Morphologic domain
The morphologic domain is separated into three sub-domains: radiographic, pathologic and multi-compartment. The radiographic and pathologic sub-domains focus on HRCT and SLB features associated with CTD, and were largely in line with those proposed by Fischer and colleagues as part of LD-CTD criteria. HRCT features include NSIP, organizing pneumonia (OP), NSIP with OP overlap and lymphocytic interstitial pneumonia (LIP). While NSIP is the most common HRCT pattern observed in CTD-ILD, OP is also common and LIP is strongly correlated with Sjogren’s disease.22 Although UIP pattern can be seen in the setting of CTD, particularly rheumatoid arthritis, it is not included in IPAF criteria given lack of specificity.
Similar to the radiographic sub-domain, the pathologic sub-domain includes NSIP, OP, NSIP with OP and LIP (Figure 1). Like HRCT, NSIP is also the most commonly encountered SLB pattern observed in CTD-ILD.7,8 Interstitial lymphoid aggregates with germinal centers and diffuse lymphoplasmacytic infiltration were also included in the pathologic sub-domain, given their strong association with CTD.10 While pathologic UIP is commonly observed in patients with rheumatoid arthritis (RA) and systemic sclerosis (SSc)23,24, this finding was not included in the pathologic sub-domain due to lack of specificity.
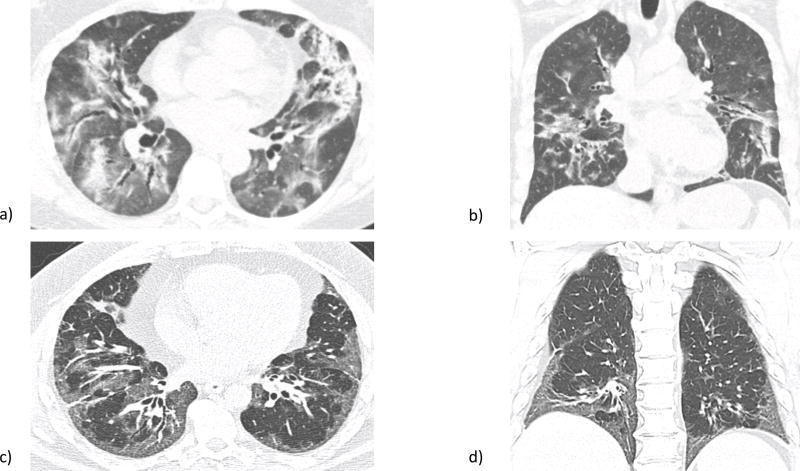
Figure 1.
a) Axial and b) sagittal high resolution computed tomography (HRCT) views of organizing pneumonia (OP), part of the radiographic subdomain of interstitial pneumonia with autoimmune features (IPAF). Notable is airspace consolidation mainly in the periphery. c) Axial and d) sagittal HRCT views of nonspecific interstitial pneumonia (NSIP), which also qualifies under the IPAF radiographic subdomain. Ground glass opacities predominate, particularly in the basilar regions in this patient. Images courtesy of Dr. Jonathan Chung, associate professor of radiology, University of Chicago, Chicago, IL.
Recognition that CTD often results in extra-parenchymal thoracic manifestations also led to inclusion of a multi-compartment sub-domain. Such manifestations include unexplained pleural or pericardial effusion or thickening, intrinsic airways disease (defined as airflow obstruction, bronchiolitis, or non-traction bronchiectasis) and pulmonary vasculopathy. Unexplained pleural or pericardial effusion or thickening on HRCT has been shown to occur more frequently with CTD-ILD than idiopathic interstitial pneumonia.25 Concomitant airway disease is often present in patients with connective tissue diseases, particularly rheumatoid arthritis26, and features such as a disproportionately reduced FEV1 or elevated residual volume on PFTs, mosaic attenuation or bronchiectasis on HRCT or follicular or constrictive bronchiolitis on SLB could be used to make this determination.19 Pre-capillary pulmonary hypertension is a prevalent clinical manifestation in CTDs, particularly scleroderma27, and is defined as a mean pulmonary artery pressure of over 25 mm Hg in the setting of a pulmonary arterial wedge pressure of less than 15 mm.28 While right heart catheterization is necessary before initiating therapy in these patients, echocardiography may serve as an effective screening tool.29
Application of IPAF Criteria to ILD Cohorts
Several groups have applied IPAF criteria to ILD cohorts in recent years. These studies, which include centers from North America and Europe, demonstrated substantial heterogeneity between cohorts with regard to phenotype and outcomes.
Prevalence and Distribution of Features
The true prevalence of IPAF is difficult to ascertain given variability in how criteria are applied and the patient populations in which they are measured. Our group retrospectively applied IPAF criteria to all patients with IIP and UCTD-ILD (by Corte criteria) followed at our center (n=422) and identified 144 patients (34%) who met inclusion criteria.30 These patients were predominantly white, had a mean age of 63 years, had a slight (52%) female predominance, and over half (55%) endorsed a history of smoking. Similar to prior findings by Ferri and colleagues31, the most common clinical feature in our cohort was Raynaud’s phenomenon (28%) and the most common serologic feature was ANA seropositivity (76%). When assessing morphologic features, NSIP was the most common pattern observed on HRCT (32%) and SLB (23%). Despite these morphologic features, the majority of patients in this cohort had UIP on either HRCT or SLB. Most patients (51%) met IPAF criteria through a combination of serologic and morphologic domains, and only 26% met all three domains.
Chartrand and colleagues characterized a cohort of 56 patients meeting IPAF criteria with substantially lower UIP prevalence.32 These patients were younger (mean age 55) and were predominantly white women. Raynaud’s phenomenon (39%) and ANA seropositivity (48%) were again the most commonly observed clinical and serologic features, respectively. Morphologically, a majority of patients had NSIP on HRCT (52%) or SLB (33%), and only 9% of patients had UIP. As compared to our cohort, over half of patients manifested a feature in all three domains.
A European IPAF cohort was characterized by Ahmad and colleagues33, who screened 778 patients and identified 57 (7.3%) meeting criteria. Similar to our cohort, these patients had a mean age of 64 and relatively even gender distribution. Raynaud’s phenomenon was again the most common clinical feature (74%), ANA seropositivity the most common serologic feature (82%) and NSIP the most common feature on HRCT (53%). Few patients in this cohort underwent SLB.
Outcomes
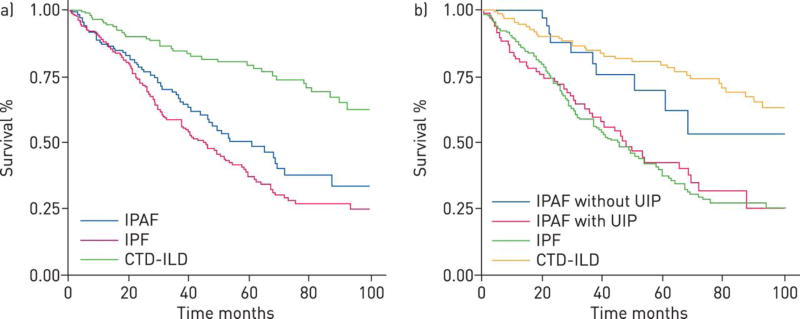
Outcome data for presently characterized IPAF cohorts also demonstrate variability. Our group described significantly worse survival in those meeting IPAF criteria when compared to a CTD-ILD cohort and only marginally better survival compared to an IPF cohort (Figure 2a).30 After stratifying the cohort based on the presence of UIP on HRCT and/or SLB, IPAF patients without UIP had similar survival as a CTD-ILD cohort, while those with UIP had similar survival as an IPF cohort (Figure 2b). In exploratory analysis, satisfying the clinical domain was associated with improved survival, while satisfying the serologic and morphologic domains were not. Within the morphologic domain, satisfying HRCT criteria was associated with improved survival, while satisfying the multi-compartment domain was associated with worse survival. This discordance likely arose from the large number of patients with IPF who met IPAF criteria through fulfillment of the serologic domain and multi-compartment sub-domain.
Figure 2.
Kaplan–Meier survival curves of interstitial pneumonia with autoimmune features (IPAF), idiopathic pulmonary fibrosis (IPF) and connective tissue disease (CTD)-interstitial lung disease (ILD) cohorts. Overall a) IPAF cohort survival was significantly worse than the CTD-ILD cohort (p<0.001) and marginally better than the IPF cohort (p=0.07). After stratification of the IPAF cohort by the presence of a usual interstitial pneumonia pattern on high-resolution computed tomography and/or surgical lung biopsy b) IPAF patients without usual interstitial pneumonia (UIP) demonstrated survival similar to those with CTD-ILD (p=0.45), while those with UIP demonstrate survival similar to those with IPF (p=0.51). Reproduced with permission from the ©ERS 2015.
Chartrand and colleagues did not observe any deaths during the 5-year follow-up period of their study.32 This discordance in survival may stem from the vastly different prevalence of UIP between studies. However, despite a higher prevalence of NSIP in their study, Ahmad and colleagues demonstrated similar survival patterns between their IPAF and IPF cohorts.33 Another possible explanation for the differences in outcome between IPAF cohorts lies in the high percentage of patients with a positive tRNA synthetase antibody in the Chartrand study, which may correlate with a more inflammatory-predominant ILD responsive to immunosuppression (discussed further below).
When assessing changes in longitudinal pulmonary function, Chartrand and colleagues showed that most patients meeting IPAF criteria experienced stability in forced vital capacity over time. These authors noted that all but one patient was treated with immunosuppression, which included systemic corticosteroids and often a steroid-sparing agent such mycophenolate mofetil or azathioprine.32 Collins and colleagues also showed that patients meeting IPAF criteria demonstrated stability in pulmonary function over a 1-year follow-up period, but similar trends were observed in CTD-ILD and lone IPF cohorts during this time.34
Additional predictors of mortality in those meeting IPAF criteria have been described. Ahmad and colleagues showed history of smoking cigarettes to be an independent predictor of mortality in their cohort.33 Chung and colleagues recently showed the presence of mosaic attenuation on HRCT to be associated with worse survival in IPAF.35 These investigators also showed that increased PA diameter, which may predict the presence of pulmonary hypertension36, was also associated with worse survival.
Unanswered Questions
Since publication of the consensus statement, several important questions have arisen with regard to each of the IPAF domains. Features composing the clinical domain were chosen with specificity in mind and were fewer than previously proposed clinical criteria for UCTD, AIF-ILD and LD-CTD. Two features excluded from the IPAF clinical domain were dysphagia and myopathy. Esophageal dysfunction complicates many CTDs, and is suggested by symptomatic gastroesophageal reflux (GER), dysphagia, eating-associated aspiration, esophageal dilation on HRCT, or abnormal esophageal motility on functional testing. This feature is especially prevalent in scleroderma-spectrum CTD.37 While GER is non-specific and commonly encountered in patients with IPF38, some have suggested that esophageal dysmotility may be more specific for CTD and should be reconsidered in subsequent IPAF criteria iterations.39,40 Similarly, because proximal muscle weakness and myalgia are among the most common clinical manifestations in patients with polymyositis and dermatomyositis as a whole41, some have advocated for inclusion of these features in the IPAF clinical domain.39
A question surrounding the serologic domain has stemmed from whether patients meeting IPAF criteria by the presence of a circulating tRNA synthetase antibody, and additional feature within the clinical or morphologic domain, should instead be characterized as having the anti-synthetase syndrome.42 While Connors and colleagues suggested that the presence of a circulating tRNA synthetase antibody in the setting of ILD is sufficient to diagnose the anti-synthetase syndrome43, Solomon and colleagues proposed that inflammatory arthritis, Raynaud’s or mechanics hands (at least two of three) be present in such patients without overt polymyositis/dermatomyositis to meet criteria for anti-synthetase syndrome.44 Mejia and colleagues recently demonstrated that all patients with ILD and a circulating tRNA synthetase antibody who failed to meet criteria for dermatomyositis subsequently met IPAF criteria and that survival was similar between the two groups.45 Over one third of patients included in the IPAF cohort characterized by Chartrand and colleagues had a positive tRNA synthetase antibody, which given the proclivity of this group to respond to immunosuppression46–48, may explain the low mortality in this group compared to other IPAF cohorts.
Multiple questions have arisen in the application of the morphologic domain. While UIP is not a feature that satisfies the HRCT or SLB sub-domains, it remains unclear how patients with IPAF SLB features should be characterized in the setting of background UIP. A recent investigation by Adegunsoye and colleagues found that a large minority of cases with UIP have another pathologic feature that satisfies the IPAF pathologic sub-domain.49 Because survival is typically better among patients with UIP due to CTD compared to IPF11,12, it may be reasonable for patients with UIP and concurrent NSIP, organizing pneumonia, lymphoid aggregates with germinal centers or diffuse lymphoplasmacytic infiltrate to satisfy the IPAF SLB sub-domain.
Another issue with the morphologic domain stems from the requirement that a feature within the multi-compartment sub-domain be “not otherwise explained.” Because a history of smoking cigarettes can lead to intrinsic airways disease vis-à-vis emphysema-mediated obstruction, such history may explain these findings when present. Additionally, because World Health Organization group III pulmonary hypertension can complicate chronic lung disease, it remains unclear when to consider the presence of pulmonary vasculopathy to be not otherwise explained. Some have also pointed out that there exists considerable uncertainty at which point, and by which measure, intrinsic airways disease and pulmonary vasculopathy are considered sufficiently present to justify a multi-compartment designation.50
Future Directions
As an increasing number of IPAF cohorts are characterized, it will be important to begin validating individual domains, and features within domains, as they relate to clinically relevant endpoints. Because IPAF seeks to identify patients with occult CTD, one relevant endpoint will be the percentage of patients meeting IPAF criteria that go on to develop overt CTD. Two single-center studies suggested that 10% of patients with UIP and 17% of patients with NSIP on SLB ultimately developed a connective tissue disease.51,52 This contrasts the findings by Chartrand and colleagues, who showed that no patients in their IPAF cohort developed CTD during a five year follow-up period.32 Validating individual IPAF criteria against outcomes will also be important, as the identification of specific features predictive of differential survival will assist in risk stratification.
The response to therapy by patients meeting IPAF criteria will be another critical area of study. Substantial variability existed between the recently characterized IPAF cohorts with respect to immunosuppressive use. Whether this influenced survival differences between the IPAF cohorts, or whether it merely reflected phenotypic differences in these cohorts, remains unclear. Additionally, because data suggest that the IPAF criteria may characterize a highly heterogeneous phenotype, some patients may be more appropriately treated with anti-fibrotic therapy, specifically those who would otherwise be diagnosed with IPF.
Conclusion
The IPAF criteria represent an important step forward in the classification of patients with ILD and features of autoimmunity who fail to meet overt CTD criteria. It must be stressed that this guideline is a research statement based on expert consensus, rather than a validated classification system. Substantial work lies ahead to begin validating these criteria, determining how they inform clinical decision-making, and identifying IPAF subgroups most appropriate for ILD-specific therapies.
Acknowledgments
Source of Funding: Dr. Oldham has received a grant from the National Center for Advancing Translational Sciences, award number KL2 TR001859.
Footnotes
Conflicts of Interest: None declared for Dr. Lee. Dr. Oldham has received speaking fees and sat on advisory boards for Genentech and Boehringer Ingelheim.
References
- 1.Gulati M. Diagnostic Assessment of Patients with Interstitial Lung Disease. Primary Care Respiratory Journal. 2011;20:120–7. doi: 10.4104/pcrj.2010.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cottin V. Significance of connective tissue diseases features in pulmonary fibrosis. European respiratory review : an official journal of the European Respiratory Society. 2013;22:273–80. doi: 10.1183/09059180.00003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lauretis A, Veeraraghavan S, Renzoni E. Review series: Aspects of interstitial lung disease: connective tissue disease-associated interstitial lung disease: how does it differ from IPF? How should the clinical approach differ? Chronic respiratory disease. 2011;8:53–82. doi: 10.1177/1479972310393758. [DOI] [PubMed] [Google Scholar]
- 4.Travis WD, Costabel U, Hansell DM, et al. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. American journal of respiratory and critical care medicine. 2013;188:733–48. doi: 10.1164/rccm.201308-1483ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mittoo S, Gelber AC, Christopher-Stine L, Horton MR, Lechtzin N, Danoff SK. Ascertainment of collagen vascular disease in patients presenting with interstitial lung disease. Respiratory medicine. 2009;103:1152–8. doi: 10.1016/j.rmed.2009.02.009. [DOI] [PubMed] [Google Scholar]
- 6.Kligerman SJ, Groshong S, Brown KK, Lynch DA. Nonspecific interstitial pneumonia: radiologic, clinical, and pathologic considerations. Radiographics. 2009;29:73–87. doi: 10.1148/rg.291085096. [DOI] [PubMed] [Google Scholar]
- 7.Kim DS, Yoo B, Lee JS, et al. The major histopathologic pattern of pulmonary fibrosis in scleroderma is nonspecific interstitial pneumonia. Sarcoidosis, vasculitis, and diffuse lung diseases : official journal of WASOG. 2002;19:121–7. [PubMed] [Google Scholar]
- 8.Yamadori I, Fujita J, Bandoh S, et al. Nonspecific interstitial pneumonia as pulmonary involvement of primary Sjogren's syndrome. Rheumatology international. 2002;22:89–92. doi: 10.1007/s00296-002-0204-0. [DOI] [PubMed] [Google Scholar]
- 9.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. American journal of respiratory and critical care medicine. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song JW, Do K-H, Kim M-Y, Jang SJ, Colby TV, Kim DS. Pathologic and Radiologic Differences Between Idiopathic and Collagen Vascular Disease-Related Usual Interstitial Pneumonia. Chest. 2009;136:23–30. doi: 10.1378/chest.08-2572. [DOI] [PubMed] [Google Scholar]
- 11.Moua T, Zamora Martinez AC, Baqir M, Vassallo R, Limper AH, Ryu JH. Predictors of diagnosis and survival in idiopathic pulmonary fibrosis and connective tissue disease-related usual interstitial pneumonia. Respir Res. 2014;15:154. doi: 10.1186/s12931-014-0154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JH, Kim DS, Park IN, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med. 2007;175:705–11. doi: 10.1164/rccm.200607-912OC. [DOI] [PubMed] [Google Scholar]
- 13.Fujita J, Ohtsuki Y, Yoshinouchi T, et al. Idiopathic non-specific interstitial pneumonia: as an "autoimmune interstitial pneumonia". Respiratory medicine. 2005;99:234–40. doi: 10.1016/j.rmed.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Kinder BW, Collard HR, Koth L, et al. Idiopathic nonspecific interstitial pneumonia: lung manifestation of undifferentiated connective tissue disease? American journal of respiratory and critical care medicine. 2007;176:691–7. doi: 10.1164/rccm.200702-220OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Corte TJ, Copley SJ, Desai SR, et al. Significance of connective tissue disease features in idiopathic interstitial pneumonia. The European respiratory journal. 2012;39:661–8. doi: 10.1183/09031936.00174910. [DOI] [PubMed] [Google Scholar]
- 16.Vij R, Noth I, Strek ME. Autoimmune-featured interstitial lung disease: a distinct entity. Chest. 2011;140:1292–9. doi: 10.1378/chest.10-2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer A, West SG, Swigris JJ, Brown KK, du Bois RM. Connective tissue disease-associated interstitial lung disease: a call for clarification. Chest. 2010;138:251–6. doi: 10.1378/chest.10-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assayag D, Kim EJ, Elicker BM, et al. Survival in interstitial pneumonia with features of autoimmune disease: a comparison of proposed criteria. Respir Med. 2015;109:1326–31. doi: 10.1016/j.rmed.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Fischer A, Antoniou KM, Brown KK, et al. An official European Respiratory Society/American Thoracic Society research statement: interstitial pneumonia with autoimmune features. Eur Respir J. 2015;46:976–87. doi: 10.1183/13993003.00150-2015. [DOI] [PubMed] [Google Scholar]
- 20.Vaz CC, Couto M, Medeiros D, et al. Undifferentiated connective tissue disease: a seven-center cross-sectional study of 184 patients. Clinical rheumatology. 2009;28:915–21. doi: 10.1007/s10067-009-1175-2. [DOI] [PubMed] [Google Scholar]
- 21.Ley B, Ryerson CJ, Vittinghoff E, et al. A multidimensional index and staging system for idiopathic pulmonary fibrosis. Annals of internal medicine. 2012;156:684–91. doi: 10.7326/0003-4819-156-10-201205150-00004. [DOI] [PubMed] [Google Scholar]
- 22.Ramos-Casals M, Brito-Zeron P, Seror R, et al. Characterization of systemic disease in primary Sjogren's syndrome: EULAR-SS Task Force recommendations for articular, cutaneous, pulmonary and renal involvements. Rheumatology (Oxford) 2015;54:2230–8. doi: 10.1093/rheumatology/kev200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee HK, Kim DS, Yoo B, et al. Histopathologic pattern and clinical features of rheumatoid arthritis-associated interstitial lung disease. Chest. 2005;127:2019–27. doi: 10.1378/chest.127.6.2019. [DOI] [PubMed] [Google Scholar]
- 24.Fischer A, Swigris JJ, Groshong SD, et al. Clinically significant interstitial lung disease in limited scleroderma: histopathology, clinical features, and survival. Chest. 2008;134:601–5. doi: 10.1378/chest.08-0053. [DOI] [PubMed] [Google Scholar]
- 25.Hwang J-H, Misumi S, Sahin H, et al. Computed Tomographic Features of Idiopathic Fibrosing Interstitial Pneumonia: Comparison With Pulmonary Fibrosis Related to Collagen Vascular Disease. Journal of Computer Assisted Tomography May. 2009;33:410–5. doi: 10.1097/RCT.0b013e318181d551. [DOI] [PubMed] [Google Scholar]
- 26.Mori S, Koga Y, Sugimoto M. Small airway obstruction in patients with rheumatoid arthritis. Modern rheumatology. 2011;21:164–73. doi: 10.1007/s10165-010-0376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mukerjee D, St George D, Coleiro B, et al. Prevalence and outcome in systemic sclerosis associated pulmonary arterial hypertension: application of a registry approach. Annals of the rheumatic diseases. 2003;62:1088–93. doi: 10.1136/ard.62.11.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the Diagnosis and Treatment of Pulmonary Hypertension. Revista espanola de cardiologia (English ed) 2016;69:177. doi: 10.1016/j.rec.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Nathan SD, Shlobin OA, Barnett SD, et al. Right ventricular systolic pressure by echocardiography as a predictor of pulmonary hypertension in idiopathic pulmonary fibrosis. Respiratory medicine. 2008;102:1305–10. doi: 10.1016/j.rmed.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oldham JM, Adegunsoye A, Valenzi E, et al. Characterisation of patients with interstitial pneumonia with autoimmune features. The European respiratory journal. 2016;47:1767–75. doi: 10.1183/13993003.01565-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ferri C, Manfredi A, Sebastiani M, et al. Interstitial pneumonia with autoimmune features and undifferentiated connective tissue disease: Our interdisciplinary rheumatology-pneumology experience, and review of the literature. Autoimmun Rev. 2016;15:61–70. doi: 10.1016/j.autrev.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 32.Chartrand S, Swigris JJ, Stanchev L, Lee JS, Brown KK, Fischer A. Clinical features and natural history of interstitial pneumonia with autoimmune features: A single center experience. Respir Med. 2016;119:150–4. doi: 10.1016/j.rmed.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Ahmad K, Barba T, Gamondes D, et al. Interstitial pneumonia with autoimmune features: Clinical, radiologic, and histological characteristics and outcome in a series of 57 patients. Respir Med. 2017;123:56–62. doi: 10.1016/j.rmed.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 34.Collins BF, Spiekerman CF, Shaw MA, et al. Idiopathic Interstitial Pneumonia associated with autoantibodies: A large case series followed over one year. Chest. 2017 doi: 10.1016/j.chest.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung JH, Montner SM, Adegunsoye A, et al. CT Findings, Radiologic-Pathologic Correlation, and Imaging Predictors of Survival for Patients With Interstitial Pneumonia With Autoimmune Features. AJR American journal of roentgenology. 2017:1–8. doi: 10.2214/AJR.16.17121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alhamad EH, Al-Boukai AA, Al-Kassimi FA, et al. Prediction of pulmonary hypertension in patients with or without interstitial lung disease: reliability of CT findings. Radiology. 2011;260:875–83. doi: 10.1148/radiol.11103532. [DOI] [PubMed] [Google Scholar]
- 37.Lahcene M, Oumnia N, Matougui N, Boudjella M, Tebaibia A, Touchene B. Esophageal involvement in scleroderma: clinical, endoscopic, and manometric features. ISRN rheumatology. 2011;2011:325826. doi: 10.5402/2011/325826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Raghu G, Freudenberger TD, Yang S, et al. High prevalence of abnormal acid gastro-oesophageal reflux in idiopathic pulmonary fibrosis. The European respiratory journal. 2006;27:136–42. doi: 10.1183/09031936.06.00037005. [DOI] [PubMed] [Google Scholar]
- 39.Collins B, Raghu G. Interstitial pneumonia with autoimmune features: the new consensus-based definition for this cohort of patients should be broadened. The European respiratory journal. 2016;47:1293–5. doi: 10.1183/13993003.02084-2015. [DOI] [PubMed] [Google Scholar]
- 40.Strek ME, Costabel U. Interstitial pneumonia with autoimmune features: a critical appraisal of the new definition. Current opinion in pulmonary medicine. 2016;22:442–9. doi: 10.1097/MCP.0000000000000298. [DOI] [PubMed] [Google Scholar]
- 41.Bohan A, Peter JB, Bowman RL, Pearson CM. Computer-assisted analysis of 153 patients with polymyositis and dermatomyositis. Medicine. 1977;56:255–86. doi: 10.1097/00005792-197707000-00001. [DOI] [PubMed] [Google Scholar]
- 42.Jee AS, Bleasel JF, Adelstein S, Keir GJ, Corte TJ. A call for uniformity in implementing the IPAF (interstitial pneumonia with autoimmune features) criteria. The European respiratory journal. 2016;48:1811–3. doi: 10.1183/13993003.01259-2016. [DOI] [PubMed] [Google Scholar]
- 43.Connors GR, Christopher-Stine L, Oddis CV, Danoff SK. Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest. 2010;138:1464–74. doi: 10.1378/chest.10-0180. [DOI] [PubMed] [Google Scholar]
- 44.Solomon J, Swigris JJ, Brown KK. Myositis-related interstitial lung disease and antisynthetase syndrome. J Bras Pneumol. 2011;37:100–9. doi: 10.1590/s1806-37132011000100015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mejia M, Herrera-Bringas D, Perez-Roman DI, et al. Interstitial lung disease and myositis-specific and associated autoantibodies: Clinical manifestations, survival and the performance of the new ATS/ERS criteria for interstitial pneumonia with autoimmune features (IPAF) Respiratory medicine. 2017;123:79–86. doi: 10.1016/j.rmed.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 46.Labirua-Iturburu A, Selva-O'Callaghan A, Martinez-Gomez X, Trallero-Araguas E, Labrador-Horrillo M, Vilardell-Tarres M. Calcineurin inhibitors in a cohort of patients with antisynthetase-associated interstitial lung disease. Clinical and experimental rheumatology. 2013;31:436–9. [PubMed] [Google Scholar]
- 47.Fischer A, Brown KK, Du Bois RM, et al. Mycophenolate mofetil improves lung function in connective tissue disease-associated interstitial lung disease. J Rheumatol. 2013;40:640–6. doi: 10.3899/jrheum.121043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Witt LJ, Demchuck C, Curran JJ, Strek ME. Benefit of adjunctive tacrolimus in connective tissue disease-interstitial lung disease. Pulm Pharmacol Ther. 2016 doi: 10.1016/j.pupt.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Adegunsoye A, Oldham JM, Valenzi E, et al. Interstitial Pneumonia With Autoimmune Features: Value of Histopathology. Arch Pathol Lab Med. 2017 doi: 10.5858/arpa.2016-0427-OA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Luppi F, Wells AU. Interstitial pneumonitis with autoimmune features (IPAF): a work in progress. The European respiratory journal. 2016;47:1622–4. doi: 10.1183/13993003.00690-2016. [DOI] [PubMed] [Google Scholar]
- 51.Kono M, Nakamura Y, Enomoto N, et al. Usual interstitial pneumonia preceding collagen vascular disease: a retrospective case control study of patients initially diagnosed with idiopathic pulmonary fibrosis. PloS one. 2014;9:e94775. doi: 10.1371/journal.pone.0094775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kono M, Nakamura Y, Yoshimura K, et al. Nonspecific interstitial pneumonia preceding diagnosis of collagen vascular disease. Respiratory medicine. 2016;117:40–7. doi: 10.1016/j.rmed.2016.05.030. [DOI] [PubMed] [Google Scholar]