Abstract
The aim of this study was to investigate the anticancer effect of a combination of D-fraction polysaccharide from Grifola frondosa (DFP) and vitamin C (VC) on hepatocellular carcinoma in vitro. DFP is a bioactive extract from the maitake mushroom. Anticancer activity was demonstrated using various concentrations of DFP alone or in combination with VC against the human hepatocarcinoma SMMC-7721 cell line. To investigate the anticancer mechanism, studies designed to detect cell apoptosis were conducted. Results from the MTT assay indicated that a combination of DFP (0.2 mg/mL) and VC (0.3 mmol/L) led to a 70% reduction in cell viability. Flow cytometry results indicated that DFP/VC treatment induced apoptosis in approximately 65% SMMC-7721 cells. Cell cycle analysis identified cell cycle arrest at the G2/M phase following DFP/VC treatment for 48 hours. In addition, cellular morphological changes were observed using transmission electron microscopy. Western blot analysis revealed that the upregulation of BAX, downregulation of Bcl-2, activation of poly-(ADP-ribose)-polymerase (PARP), and the release of cytochrome c were observed in cells treated with the combination of DFP/VC, which showed that the mechanism of anticancer activity in the SMMC-7721 hepatocarcinoma cells involved induction of apoptosis.
Keywords: Grifola frondosa, D-fraction, vitamin C, apoptosis, hepatic carcinoma, SMMC-7721
Introduction
The global incidence of hepatocellular carcinoma (HCC) has risen steadily for the past 10 years. In 2012, the top 3 causes of death worldwide were lung cancer, liver cancer, and gastric cancer according to data from the World Health Organization.1 Nearly 28% of patients with liver cancer present with metastatic disease at diagnosis, and 30% to 40% of patients postsurgical resection develop liver cancer recurrence with metastatic disease. The prognosis of metastatic HCC patients is very poor, with limited treatment options, resulting in a median survival of only 6 to 12 months.2,3 At present, standard treatments for HCC are complete resection of the tumor, transcatheter arterial chemoembolization, radiotherapy, and chemotherapy, all of which offer limited control of disease progression. Thus, research has focused on identifying effective drugs for the treatment of HCC. Grifola frondosa, also called maitake mushroom, is a basidiomycete fungus belonging to the Polyporaceae family. A novel polysaccharide-peptide isolated from the cultured mycelia of G frondosa GF9801 has been reported to have antitumor activity, which includes significant inhibition of SGC-7901 cell proliferation.4
Recent research demonstrated that G frondosa polysaccharide exerted its anticancer activity by inducing apoptosis in breast cancer MCF-7 cells.5 The same finding was published by Shomori et al6 where maitake water extract induced gastric cancer cell apoptosis. A recent study showed that a sulfate synthesized from G frondosa polysaccharides induced liver cancer cell HepG2 apoptosis via the notch1-NF-κB signal transduction pathway.7 Furthermore, Soares et al8 reported that a component of the maitake polysaccharide (D-Fraction, DFP) increased BAK-1 gene expression and promoted the release of cytochrome c, thereby initiating MCF-7 cell apoptosis. In addition, in vivo skin experiments showed that DFP significantly enhanced the expression of tumor necrosis factor–α (TNF-α), immunoglobulin G (IgG), T lymphocytes, and caspase-3 mRNA.9 Gene microarray analyses suggested that DFP could regulate the expression of genes involved in apoptosis; inhibit cell proliferation, migration, and metastasis; and block cell cycle in breast cancer cells; it alters the activity of IGFBP-7, ITGA2, ICAM3, among others.10
Vitamin C (VC), also known as ascorbic acid, is a water-soluble enzyme that is found in plants and serves as an enzymatic cofactor in animals. This powerful antioxidant can effectively quench the generation of free radicals in many processes involving biological extracellular and intracellular reactions.11 Recent studies demonstrated that VC reduces the adverse reactions induced by chemotherapy in cancer treatment.12,13 Lee et al14 suggested that VC could inhibit E3 ubiquitin ligase, which induced SIAH1-mediated ubiquitination of the anti-apoptotic protein p34SEI-1, decreasing the levels of expressed protein, leading to tumor cell apoptosis. Moreover, VC appears to induce osteosarcoma cell apoptosis.15 While DFP or VC alone can induce apoptosis in a variety of tumor cell lines, further studies examining the anticancer effects of combination of substances are needed. In this study, the mechanism of antitumor activity of the combination of DFP and VC was investigated in human HCC SMMC-7721 cells.
Materials and Methods
Preparation of the Grifola frondosa Polysaccharide and Cell Culture
The D-fraction polysaccharide of G frondosa (DFP) was prepared from the powdered fruit bodies of G frondosa (Zelang Biotech Co, Nanjing, China), as previously reported.16 Briefly, the hot water–soluble fraction from the powder of G frondosa was concentrated, and alcohol was added to yield a final concentration of 80%, which was subsequently centrifuged (8000 rpm, 4°C, 30 minutes). Acid-soluble matter was derived from the precipitates. Following the subsequent addition of sodium hydroxide, the mixture was again centrifuged (5000 rpm, 4°C, 15 minutes). An alkaline-soluble matter was obtained followed by dialysis, which removed the low-molecular-weight substances. The concentration of polysaccharide was determined by the phenol–sulfuric acid method. VC was purchased from Shanghai Seebio Biotech Co. The human HCC SMMC-7721 cells (a gift from Dr Song in Lanzhou University) were maintained in Dulbecco’s modified Eagle’s medium (Gibco, Grand Island, NY, USA) containing 10% fetal bovine serum, penicillin (100 units/mL), and streptomycin (100 µg/mL). SMMC-7721 cells were seeded at the initial cell density of 6 × 105 cells/mL in 60-mm dishes or T-25 flasks. When cells were treated with varying concentrations of DFP, VC, and combinations of DFP and VC for specified times, the serum concentration of the medium was reduced to 8%.
Cell Viability Assay
Cell viability was determined by the MTT assay following the vendor’s protocol (Amresco, Cleveland, OH, USA). The SMMC-7721 cells were seeded at the initial cell density of 5 × 103 cells/well in 96-well plates and cultured with varying concentrations of DFP or VC for specified times. At harvest time, the MTT reagent (0.5 mg/mL) was added to all wells in the 96-well plate, followed by incubation for 4 hours. Following the incubation, the supernatant was removed from the wells and dimethyl sulfoxide was added to each well to dissolve the formazan crystals. The absorbance was then measured using a spectrophotometer microplate reader (Bio-RAD, Hercules, CA, USA) at a wavelength of 492 nm. The cell inhibitory rate was expressed as a percentage (%) of 1 minus the ratio of viable cell number after drug treatment relative to the control cell number. The IC50 (50% inhibition concentration, defined as the drug concentration resulting in 50% inhibition vs the untreated culture) of DFP and VC was calculated using the data generated from the MTT experiment. This experiment was repeated 3 times, and the results were statistically analyzed using the unpaired Student’s t test.
Isobologram Method
To determine the synergistic or antagonistic interactions of DFP and VC, we used the isobologram method.17 The SMMC-7721 cells were exposed to various concentrations of VC (0.05, 0.1, 0.15, 0.20, 0.25, 0.30 mmol/L) with specific concentrations of DFP, including 1/8, 1/4, 1/2, 3/4 of the IC50. According to the comparison of the IC50s of DFP or VC with the new IC50 drug combination, the interaction of DFP and VC were determined. The fractional inhibitory concentration (FIC) for each combination of VC and DFP was calculated as follows: FICDFP = (concentration of DFP in the combination at the end point)/(concentration of DFP alone required to achieve the end point) and FICVC = (concentration of VC in the combination at the end point)/(concentration of VC alone required to achieve the end point). The combinations resulting in an additive anticancer effect (FICDFP + FICVC = 1) are represented by a straight line (unity line) on the isobologram. When the combination results in synergy, that is, stronger antitumor effects than the sum of the individual effects (FICDFP + FICVC < 1), the FIC value falls below the unity line. When the combination results in an antagonistic antitumor effect (FICDFP + FICVC > 1), the FIC value rises above the unity line.
Detection of Apoptotic Cells and Cell Cycle by Flow Cytometry
Apoptotic and necrotic cells were measured using a double staining method with the Annexin V-FITC/propidium iodide (PI) apoptosis detection kit (KeyGen Biotech Co, Nanjing, China). The 1 × 105 SMMC-7721 cells were processed according to the manufacturer’s instructions after they were treated with DFP and VC for 48 hours. Five hundred microliters of binding buffer containing 5 µL Annexin V-FITC and 5 µL PI was added to each cell suspension, and the resultant mixture was incubated for 20 min at room temperature in the dark. Detection of the stained cells was completed using a NovoCyte flow cytometer (ACEA Biosciences Inc, San Diego, CA, USA). For the cell cycle analysis, approximately 1 × 106 cells were collected and fixed overnight in 70% ethanol at 4°C. Cells were then resuspended in 400 µL PI solution (containing 0.2 mg/mL RNase) followed by incubation at room temperature for 1 hour. Twenty thousand nuclei were analyzed from each sample, and flow cytometry (FCM) software was used to quantify cell cycle compartments and estimate the cell cycle phase fractions. This experiment was repeated 3 times, and the results were statistically analyzed.
Detection of Apoptosis by Transmission Electron Microscopy
After DFP and VC treatment for 24 hours, cells were fixed with 2% glutaraldehyde in 0.1 mol/L PBS (pH 7.4), followed by 1% osmic acid. After dehydration, thin sections were stained with uranyl acetate and lead citrate for observation using JEM-1230 transmission electron microscopy (JEOL, Tokyo, Japan).
Western Blot Analysis of Apoptosis-Related Proteins
After incubation with control treatment, DFP, VC, and DFP and VC for 48 hours, approximately 2 × 106 cells were harvested and lysed in 200 µL of RIPA (radioimmunoprecipitation assay) lysis buffer by “freeze-thaw” at −80°C to obtain total protein extracts. The protein concentration was determined using the BCA protein assay (Erwan Biotechnology Co, Shanghai, China). Equal amounts of cell lysate (20 µg) were first subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE; Sigma Co, St Louis, MO, USA) and then transferred to a nitrocellulose membrane. The blot membrane was incubated for 90 minutes with the primary antibodies against Bcl-2, BAX, or poly(ADP-ribose) polymerase (PARP) (Immunoway Biotechnology Co, Plano, Texas, USA), followed by a 30-minute incubation with the secondary antibody conjugates. The specific immunoreactive protein bands were then detected by chemiluminescence using the manufacturer’s protocol (Sigma Co, St. Louis, MO, USA).
Detection of Cytochrome c Release by Western Blot Analysis
The whole cell total protein and cytoplasm protein were extracted after 48 hours of drug incubation. The extraction method of the whole cell total protein is the same method described above for the BAX protein. The extraction method of cytoplasmic protein was as follows: approximately 2 × 106 cells were harvested and solubilized in 200 µL RIPA lysis buffer. The cytoplasmic protein was then extracted using a nuclear and cytoplasmic protein extraction kit (Beyotime Biotechnology Co, Shanghai, China). After addition of the cytoplasmic protein lysis solution to the cellular precipitate and centrifugation at 12 000 rpm for 20 minutes at 4°C, the cytoplasmic protein was partitioned into the supernatant from the cellular precipitate. Subsequently, the targeted protein was separated by SDS-PAGE under reducing conditions using a 12% gel and electrotransferred to a polyvinylidene difluoride membrane (Millipore Co, Bedford, MA, USA). After blocking with 5% skim milk in PBS(−) containing 0.01% Tween-20 (T-PBS), the membrane was blotted with a mouse anti-human cytochrome c monoclonal antibody (Immunoway Biotechnology Co, Plano, TX, USA) overnight at 4°C. The membrane was washed with T-PBS once for 30 minutes and 5 times for 3 minutes each, and then incubated with peroxidase-labeled antimouse IgG (1:10000) for 40 minutes at room temperature. After washing in T-PBS as described above, signals were observed with an enhanced chemiluminescence system (LI-COR, Lincoln, NE, USA).
Assays of the Activity of Caspase-3, Caspase-8, and Caspase-9
Cells were seeded at a density of 8 × 105 cells/mL in 10-mm diameter culture dishes. After cells were incubated with 0.2 mg/mL DFP, 0.3 mM VC, and combinations of both for 24 and 48 hours, the activities of caspase-3, caspase-8, and caspase-9 in SMMC-7721 cells were examined. In brief, cells were treated with cell lysis buffer and then centrifuged at 4°C for 10 minutes. The resultant supernatants were then used for further evaluation. The total protein concentration was determined using the BCA protein assay kit. The activity of caspase enzymes was measured using the caspase activity assay kits according to the manufacturer’s instructions (Beyotime Biotechnology Co, Shanghai, China). The assay was based on the spectrophotometric detection of the chromophore p-nitroanilide (pNA), which is cleaved from each caspase-specific substrate by activated caspases (DEVD-pNA for activated caspase-3, IETD-pNA for activated caspase-8, and LEHD-pNA for activated caspase-9). This series of experiments was repeated 3 times, and the data were analyzed statistically.
Statistical Analysis
Statistical analysis was performed with Statistical Product and Service Solutions (IBM SPSS, Somers, NY, USA) version 18.0 software. All data were presented as mean ± standard deviation (). Statistical differences between groups were assessed with either one-way analysis of variance or the unpaired Student’s t test. Values of P < .05 were considered statistically significant.
Results
Effects of DFP and VC on SMMC-7721 Cell Proliferation
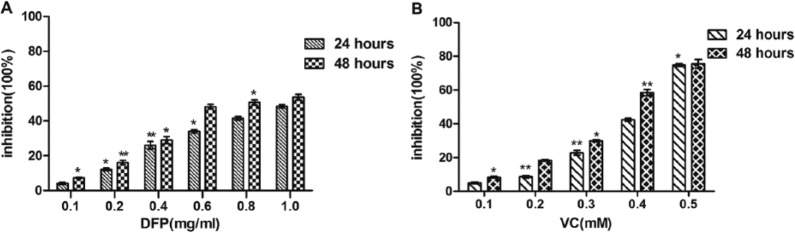
SMMC-7721 cells were cultured separately with various concentrations of DFP or VC. After 24 and 48 hours of drug exposure, the MTT assay showed that both DFP and VC alone had time- and dose-dependent antitumor effects. DFP produced a 4.23% to 49.43% reduction in cell viability in the dose range of 0.1 to 1.0 mg/mL at 24 hours and 7.34% to 54.95% at 48 hours (Figure 1A). In contrast, VC produced a 4.93% to 74.87% reduction in cellular viability over the dose range of 0.1 to 0.5 mM at 24 hours and 8.37% to 77.19% at 48 hours (Figure 1B). Differences in cell viability rates between the DFP and VC drug treatment groups compared with the control group were statistically significant (DFP: *P < .05; VC: **P < .01). The IC50 values of DFP and VC at 48 hours were 0.80 ± 0.08 mg/mL and 0.368 ± 0.07 mM, respectively.
Figure 1.
Effects of DFP or VC on SMMC-7721 cell viability. Cells were treated with different concentrations of DFP (0.2-1.0 mg/mL) (A) or VC (0.1-0.5mmol/L) (B) for 24 and 48 hours. Cell inhibition (absorbance at 492 nm) was determined by an MTT assay and compared with control. Data are expressed as mean ± SD of 3 separate experiments (*P < .5; **P < .1 with control). DFP, D-fraction of Grifola frondosa polysaccharide; VC, vitamin C.
Synergistic Cytotoxic Effect of DFP and VC
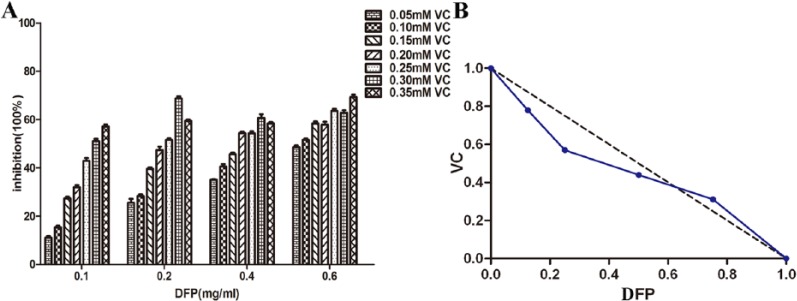
Vitamin C has previously been proposed as a modulator of bioactivity of β-glucan in DFP.18 In this study, various combinations and concentrations of DFP with VC were tested in SMMC-7721 cells. The results of the MTT assay showed that a synergistic effect was observed when cells were treated with both DFP and VC for 48 hours. As shown in Figure 2, using 0.1 mg/mL DFP (1/8 IC50) in combination with 0.289 mM VC (4/5 IC50), or 0.2 mg/mL DFP (1/4 IC50) with 0.210 mM VC (1/2 IC50), or 0.4 mg/mL DFP (1/2 IC50) with 0.161 mM VC (2/5 IC50) inhibited the proliferation of SMMC-7721 cells by more than 50%. However, the combination of 0.6 mg/mL DFP (3/4 IC50) with 0.092 mM VC (1/4 IC50) produced an antagonistic effect where cells were inhibited by less than 50%. The analysis using the isobologram method revealed that the specific combination of 0.2 mg/mL DFP and 0.3 mM VC had the most synergistic effect (FICDFP + FICVC = 0.82, synergism defined as <1). Additionally, the 0.2 mg/mL DFP and 0.3 mM VC combination produced ~70% inhibition while both 0.2 mg/ml DFP or 3 mM VC alone had little inhibitory effect (DFP: 16.03% ± 1.80 and VC: 29.85% ± 1.02). DFP at >0.6 mg/mL decreased cell viability. Based on this finding, 0.2 mg/mL DFP and 0.3 mM VC doses were chosen as the final concentration for further evaluation.
Figure 2.
Isobologram analysis: synergy effect of the combinations of DFP and VC to inhibit cell proliferation in SMMC-7721cells. FICDFP + FICVC = 0.91, 0.82, 0.94 (all <1) when used DFP 0.1, 0.2, 0.4 mg/mL, respectively; antagonistic effect of the combinations of DFP and VC, FICDFP + FICVC = 1.27(>1) when used DFP 0.6 mg/mL. FIC, fractional inhibitory concentration; DFP, D-fraction of Grifola frondosa polysaccharide; VC, vitamin C.
DFP and VC Promote Apoptosis and Cell Cycle Arrest of SMMC-7721 Cells
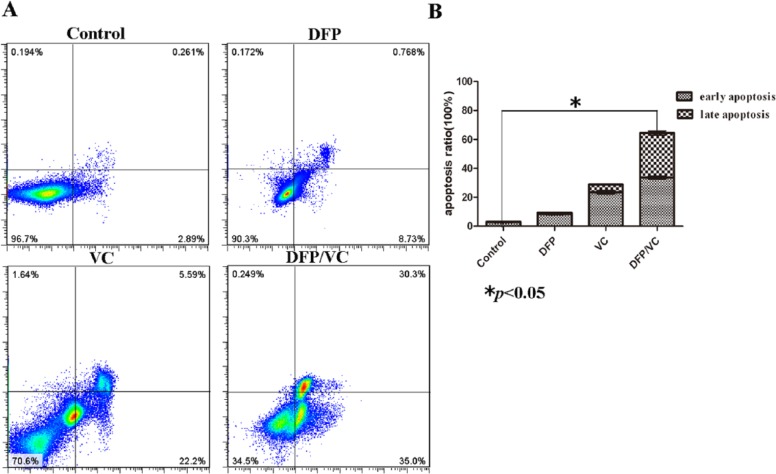
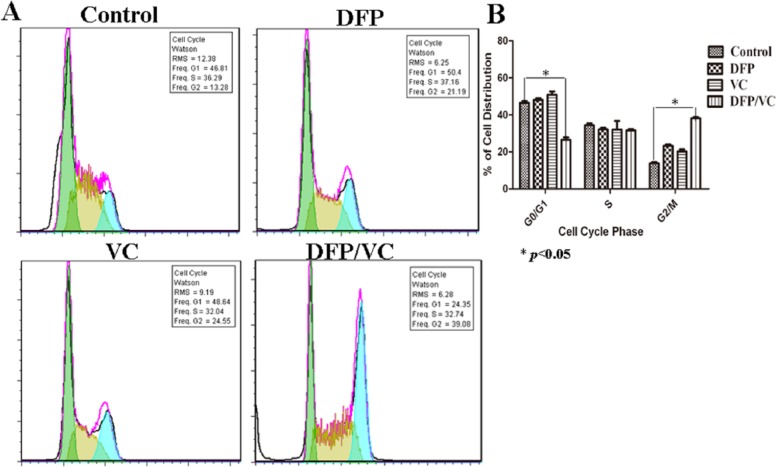
Flow cytometry results revealed that marked (65.30%) cell apoptosis was achieved using the combination of DFP and VC, while 9.50% and 27.79% cell apoptosis were detected with DFP and VC alone, respectively (Figure 3A). The difference in apoptosis rates between the control group and the DFP/VC groups was statistically significant (*P < .05) (Figure 3B). In addition, the percentage of cells in different phases of the cell cycle was determined by FCM. As shown in Figure 4A, the DFP and VC treatment reduced the cell population in the G0/G1 phase and increased the percentage of cells in the G2/M-phase. Compared with the control group, the percentage of cells in the G2/M phase increased from 13.28% to 39.08% following treatment with DFP and VC for 48 hours (Figure 4B).
Figure 3.
Effect of DFP with or without VC on SMMC-7721cell apoptosis. (A) Flow cytometry analysis: cell apoptosis was induced by DFP/VC using Annexin V-FITC/propidium iodide staining after 48 hours. The cells are characterized as early apoptosis (bottom right quadrant), late apoptosis (top right quadrant), necrotic (top left quadrant), and healthy cells (bottom left quadrant) based on the flow cytometry results, and the total apoptosis rate is summarized in a bar chart on the right. (B) Histogram of corresponding apoptotic ratios. *P < .05; **P < .01 compared with control. DFP, D-fraction of Grifola frondosa polysaccharide; VC, vitamin C.
Figure 4.
Effect of DFP or/and VC on cell cycle in SMMC-7721 cells. (A) Diagram of cell cycle analyzed by flow cytometry. (B) Histogram of cell cycle. *P < .05; **P < .01 compared with control. DFP, D-fraction of Grifola frondosa polysaccharide; VC, vitamin C.
Morphological Changes
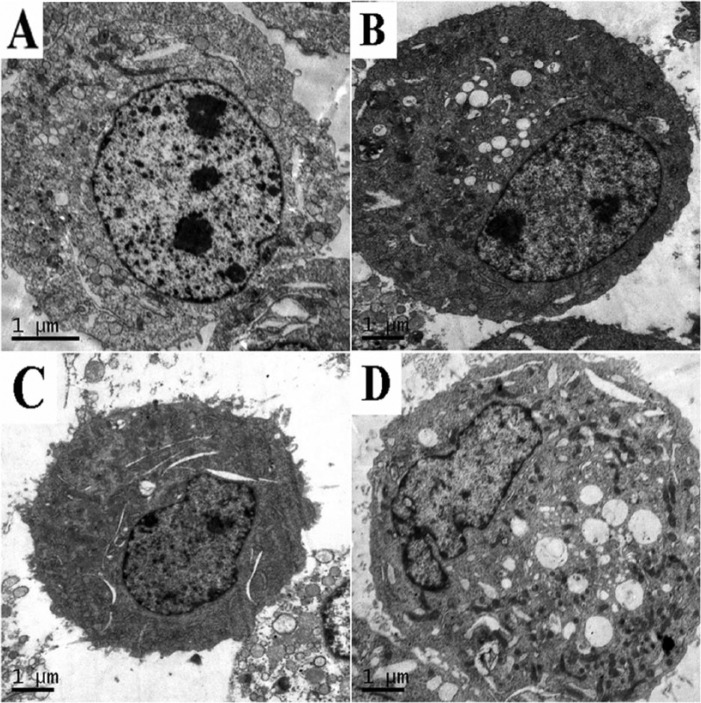
DFP and VC treatment for 24 hours altered the morphology of SMMC-7721 cells compared with the control group, as observed by transmission electron microscopy. Visible cells showed typical characteristics of early apoptosis such as loose cytoplasm, changes in cell nucleus, and chromatin edge set (Figure 5D), whereas cells treated only with DFP or VC did not show these changes (Figure 5B and C).
Figure 5.
Detection of apoptosis (original magnification, 10 000×). (A) Control; (B) 0.2 mg/mL DFP; (C) 0.3 mmol/L VC; (D) DFP/VC-treated cell 24 hours. DFP, D-fraction of Grifola frondosa polysaccharide; VC, vitamin C.
Effects of DFP and VC Combination on Apoptosis Regulators
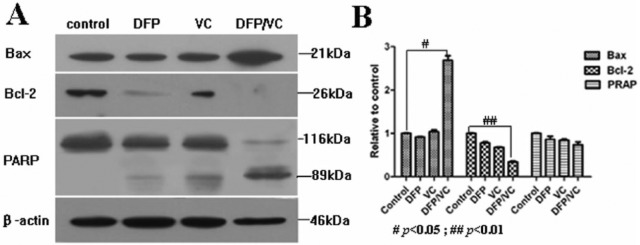
Based on the observed synergistic effect of the combination of DFP and VC in inducing SMMC-7721 cell death, the possible molecular mechanisms of apoptosis were investigated. The expression of 3 key apoptosis-related proteins was analyzed by Western blot after cells were treated for 48 hours with DFP, VC, and DFP and VC. Compared with DFP or VC alone, the combination of DFP and VC upregulated the expression of the pro-apoptotic protein, BAX, and activation of the pro-apoptotic PARP (poly-(ADP-ribose)-polymerase). Conversely, the expression level of the Bcl-2 protein decreased in SMMC-7721 cells (Figure 6). Because the upregulation and increased activation of BAX and PARP combined with the downregulation of Bcl-2 is indicative of apoptosis,19,20 it is plausible that DFP and VC combination induces cell death through apoptosis, possibly via the mitochondrial signaling transduction pathways.
Figure 6.
Effects of DFP or/and VC on apoptosis regulators after SMMC-7721 cells were addressed with DFP, VC, and DFP/VC for 48 hours. (A) Expressions of Bcl-2, BAX, and PARP were analyzed with Western blotting. Autoradiographs of those regulators (on the X-ray film) in control, DFP, VC, and DFP/VC-treated cells are shown. In addition, β-actin is shown as a protein loading control. (B) Histogram of Western blotting results, #P < .05; ## P < .01 compared with control. DFP, D-fraction of Grifola frondosa polysaccharide; PARP, (poly-(ADP-ribose)-polymerase); VC, vitamin C.
Cytochrome c Release Into the Cytoplasm of SMMC-7721 Cells
To investigate whether DFP and VC combination induces the release of cytochrome c from mitochondria into the cytoplasm, the expression of cytochrome c protein in whole cell and cytosolic fractions of SMMC-7721 cells were simultaneously determined through comparison of the protein levels within each fraction. Western blot analysis results indicated that the expression of cytochrome c in the cytosol increased after 48 hours of treatment with DFP, VC, and DFP and VC, although the expression level of cytochrome c in the whole cell fraction remained unchanged between the control and 3 active treatment groups (Figure 7).
Figure 7.
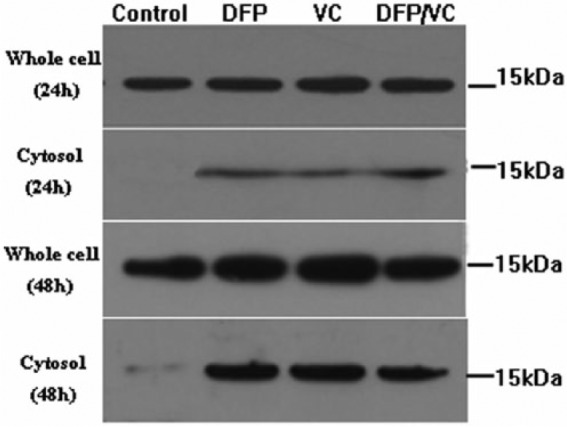
Detection of cytochrome c release by Western blotting. Cytochrome c is released after treatment of SMMC-7721 cells with DFP, VC, and DFP/VC for 48 hours. The total protein level of cytochrome c at the cellular remains unchanged (whole cell), but the levels in cytosol were distinct among the 4 groups (cytosol). DFP, D-fraction of Grifola frondosa polysaccharide; VC, vitamin C.
Activity Assay of Caspase-3, Caspase-8, and Caspase-9
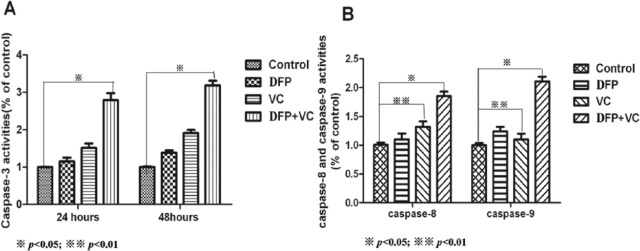
Caspase-3 activity in SMMC-7721 cells that were treated for 24 hours with DFP, VC, and DFP and VC increased by 1.2-, 1.8-, and 3.5-fold, respectively, compared with controls, and these differences were statistically significant (Figure 8A). The activity of caspase-8 and caspase-9 in SMMC-7721 cells was different following exposure to the different treatments. There was a significant difference in caspase-8 activity (P < .05), but not of caspase-9 activity (P > .05) between VC-treated and control cells. A different activity profile was revealed following treatment with DFP, where caspase-9 activity was significantly increased in cells compared with controls (P < .05), whereas the activity of caspase-8 remained unchanged between the groups. Moreover, the activities of caspase-8 and caspase-9 in cells treated with the combination of DFP and VC significantly increased compared with the control cells after 24 hours of treatment (approximately 2.1- and 2.6-fold for caspase-8 and caspase-9, respectively) (Figure 8, *P < .05; **P < .01).
Figure 8.
Activation of caspase-3, caspase-8, and caspase-9 in SMMC-7721 cells treated with DFP, VC, DFP/VC. (A) Caspase-3 activity significantly increased in cells treated with DFP/VC after 24 and 48 hours (*P < .05). (B) Caspase-8 activity significantly increased in cells treated with VC and DFP/VC while caspase-9 activity significantly increased in cells treated with DFP and DFP/VC after 24 hours (*P < .05, **P < .01 compared with control). DFP, D-fraction of Grifola frondosa polysaccharide; VC, vitamin C.
Discussion
The potential antitumor effects of the combination of the DFP and VC on the growth of human HCC SMMC-7721 cells were investigated in our study. DFP or VC, when applied separately, decreased cell viability in a dose- and time-dependent manner. The IC50 values of DFP and VC were 0.798 mg/mL and 0.3682 mM, respectively. In addition, the analytical results of the isobologram indicate that DFP (0.2 mg/mL) and VC (0.3 mM) at their respective optimal concentrations have synergistic cytotoxic effects in SMMC-7721 cells (70%). However, an antagonistic effect was observed when the concentration of DFP >0.6 mg/mL. This finding may be due to the possibility that VC is the dominant contributor to the observed antitumor effect when the 2 drugs are administered in combination. Mechanistically, VC has direct antitumor effect while DFP regulates the intracellular conditions that facilitate the antitumor activity of VC. DFP at low concentrations has no direct inhibitory effect on SMMC-7721 cells, but may influence the level of cell metabolism and oxidative stress, thereby sensitizing the cells to VC. However, DFP at high concentrations can promote cell growth, which may overcome the inhibitory effect of VC when used in combination.
In addition, the FCM results revealed that more cell apoptosis (65.30%) was induced by the combination than that by the individual agents and that the number of cells in the G2/M phase of the cell cycle increased (from 13.28% to 39.08%) compared with controls. The morphological changes associated with apoptosis in cells were observed by transmission electron microscopy.
Many signal transduction pathways are involved in apoptosis.21 The Bcl-2 protein is mainly located on the outer membrane of mitochondria and prevents the release of cytochrome c and the resultant activation of caspase. It is also known that BAX can promote cell apoptosis. Therefore, the changes in the expression levels of various apoptosis-related proteins, namely upregulation of BAX and downregulation of Bcl-2, and the mitochondrial release of cytochrome c, were investigated to confirm the induction of apoptosis through the intracellular mitochondrial signal transduction pathways. Furthermore, activation of caspase-3, which is essential for the induction of apoptosis and cleavage of PARP, an endogenous substrate of caspase-3, provides additional evidence of apoptosis at the molecular level.22,23
Western blot analysis of cells treated with the combination of DFP and VC showed that BAX was clearly upregulated compared with controls. Meanwhile, Bcl-2 expression was downregulated. Although the total expression level of cytochrome c remained constant independent of DFP and/or VC treatment, the cytochrome c level in cytoplasm was increased by the DFP and VC combination treatment compared with controls. These results suggest that cytochrome c was released from mitochondria into the cytoplasm. In addition, the expression of PARP was enhanced in our study. These overall results suggest that the combination of DFP and VC induces SMMC-7721 cell apoptosis through interruption of the mitochondrial signal transduction pathway.
We also evaluated the activity of caspase-3, a key mediator in the apoptosis pathway, which is responsible for initiation of degradation of cellular proteins through the caspase cascade. The results of our colorimetric evaluation revealed that DFP and/or VC induced a significant increase in activation levels of caspase-3 in a time-dependent manner.
Caspase-8 activates downstream caspases in the apoptotic process through direct or indirect cleavage of Bid and induces cytochrome c release from mitochondria.24 Kim et al25 reported that caspase-9 is the critical upstream molecule involved in the apoptotic protease cascade and becomes activated by cytochrome c and dATP. In addition, caspase-3 is known to be activated by caspase-8 and/or caspase-9. To determine whether a caspase-8-dependent or caspase-9-dependent pathway is operating in the present apoptotic cell system, the activation of caspase-8 and caspase-9 was analyzed. As shown in Figure 8, treatment of SMMC-7721cells for 24 hours with DFP resulted in an increase in caspase-8 but not in caspase-9 activity. Interestingly, a contrary result was observed in cells following VC treatment, while caspase-8 and caspase-9 were both activated by the combination treatment of DFP and VC. This result demonstrates that DFP may activate caspase-8 while VC may activate caspase-9; the apoptosis of SMMC-7721 cells is induced ultimately by the activation of caspase-3 through the activation of either caspase-8 or caspase-9.
Conclusion
The present study demonstrated that the combined use of DFP and VC can produce synergistic cytotoxic effects on SMMC-7721 cells, resulting in cell apoptosis. This apoptosis is mainly attributed to interruption of the mitochondrial signal transduction pathways. Therefore, the combined application of DFP and VC may have clinical implications in researching a new, improved, and safer therapeutic modality for treating advanced HCC. Nevertheless, additional studies of the efficacy of the combination of DFP and VC in actual HCC cases are needed, such as those in animals bearing HCC (in vivo).
Footnotes
Authors’ Note: Fei Zhao and Yong-Feng Wang contributed equally to this study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Fundamental Research Funds for the Central Universities from Northwest University for Nationalities, China (No. 1920140070) and Natural Science Foundation of Gansu Province of China (No. 145RJZA221).
References
- 1. World Health Organization. GLOBOCAN 2012: Estimated Cancer Incidence, Mortality and Prevalence Worldwide in 2012. Geneva, Switzerland: World Health Organization; http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed March 29, 2016. [Google Scholar]
- 2. Wang R, Chen XZ, Zhang MG, Tang L, Wu H. Incidence and mortality of liver cancer in mainland China: changes in first decade of 21st century. Hepatogastroenterology. 2015;62:118-121. [PubMed] [Google Scholar]
- 3. Chen X, Liu HP, Li M, Qiao L. Advances in non-surgical management of primary liver cancer. World J Gastroenterol. 2014;20:16630-16638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cui FJ, Li Y, Xu YY, et al. Induction of apoptosis in SGC-7901 cells by polysaccharide-peptide DFPS1b from the cultured mycelia of Grifola frondosa GF9801. Toxicol In Vitro. 2007;21:417-427. [DOI] [PubMed] [Google Scholar]
- 5. Martin KR, Brophy SK. Commonly consumed and specialty dietary mushrooms reduce cellular proliferation in MCF-7 human breast cancer cells. Exp Biol Med (Maywood). 2010;235:1306-1314. [DOI] [PubMed] [Google Scholar]
- 6. Shomori K, Yamamoto M, Arifuku I, Teramachi K, Ito H. Antitumor effects of a water-soluble extract from Maitake (Grifola frondosa) on human gastric cancer cell lines. Oncol Rep. 2009;22:615-260. [DOI] [PubMed] [Google Scholar]
- 7. Wang CL, Meng M, Liu SB, Hou LH, Cao XH. A chemically sulfated polysaccharide from Grifola frondosa induces HepG2 cell apoptosis by notch1-NF-κB pathway. Carbohydr Polym. 2013;95:282-287. [DOI] [PubMed] [Google Scholar]
- 8. Soares R, Meireles M, Rocha A, et al. Maitake (D-fraction) mushroom extract induces apoptosis in breast cancer cells by BAK-1 gene activation. J Med Food. 2011;14:563-572. [DOI] [PubMed] [Google Scholar]
- 9. Xu H, Liu JH, Shen ZY, Fei Y, Chen XD. Analysis of chemical composition, structure of Grifola frondosa polysaccharides and its effect on skin TNF-α levels, IgG content, T lymphocytes rate and caspase-3 mRNA. Carbohydr Polym. 2010;82:687-691. [Google Scholar]
- 10. Alons EN, Orozco M, Eloy Nieto A, Balogh GA. Genes related to suppression of malignant phenotype induce by Maitake D-fraction in breast cancer cells. J Med Food. 2013;16:602-617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sorice A, Guerriero E, Capone F, Colonna G, Castello G, Costantini S. Ascorbic acid: its role in immune system and chronic inflammation diseases. Mini Rev Med Chem. 2014;14:444-452. [DOI] [PubMed] [Google Scholar]
- 12. Mamede AC, Tavares SD, Abrantes AM, Trindade J, Maia JM, Botelho MF. The role of vitamins in cancer: a review. Nutr Cancer. 2011;63:479-494. [DOI] [PubMed] [Google Scholar]
- 13. Guerriero E, Sorice A, Capone F, et al. Vitamin C effect on mitoxantrone-induced cytotoxicity in human breast cancer cell lines. PLoS One. 2014;9:e115287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee S, Kim J, Jung S, et al. SIAH1-induced p34SEI-1 polyubiquitination/degradation mediates p53 preferential vitamin C cytotoxicity. Int J Oncol. 2015;46:1377-1384. [DOI] [PubMed] [Google Scholar]
- 15. Leon IE, Di Virgilio AL, Porro V, et al. Antitumor properties of a vanadyl (IV) complex with the flavonoid chrysin [VO(chrysin)2EtOH]2 in a human osteosarcoma model: the role of oxidative stress and apoptosis. Dalton Trans. 2013;42:11868-11880. [DOI] [PubMed] [Google Scholar]
- 16. Gu CQ, Li JW, Chao FH. Inhibition of hepatitis B virus by D-fraction from Grifola frondosa: synergistic effect of combination with interferon in HepG2 2.2.15. Antiviral Res. 2006;72:162-165. [DOI] [PubMed] [Google Scholar]
- 17. Briolant S, Garin D, Scaramozzino N, Jouan A, Crance JM. In vitro inhibition of Chikungunya and Semliki Forest viruses replication by antiviral compounds: synergistic effect of interferon-alpha and ribavirin combination. Antiviral Res. 2004;61:111-117. [DOI] [PubMed] [Google Scholar]
- 18. Konno S. Synergistic potentiation of D-fraction with vitamin C as possible alternative approach for cancer therapy. Int J Gen Med. 2009;30:91-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398-6406. [DOI] [PubMed] [Google Scholar]
- 20. Soldani C, Scovassi AI. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7:321-328. [DOI] [PubMed] [Google Scholar]
- 21. Budihardjo I, Oliver H, Lutter M, Luo X, Wang X. Biochemical pathway of caspase activation during apoptosis. Annu Rev Cell Dev Biol. 1999;15:269-290. [DOI] [PubMed] [Google Scholar]
- 22. Porter AG, Janicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99-104. [DOI] [PubMed] [Google Scholar]
- 23. Li P, Nijhawan D, Budihardjo I, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479-489. [DOI] [PubMed] [Google Scholar]
- 24. Miyake K, Bekisz J, Zhao T, Clark CR, Zoon KC. Apoptosis-inducing factor (AIF) is targeted in IFN-α2a-induced Bid-mediated apoptosis through Bak activation in ovarian cancer cells. Biochim Biophys Acta. 2012;1823:1378-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim HE, Jiang X, Du F, Wang X. PHAPI, CAS, and Hsp70 promote apoptosome formation by preventing Apaf-1 aggregation and enhancing nucleotide exchange on Apaf-1. Mol Cell. 2008;30:239-247. [DOI] [PubMed] [Google Scholar]