Abstract
Cysteine X cysteine (CXC) chemokine receptor 4 (CXCR4) and C-X-C motif chemokine 12 (CXCL12) were originally identified as chemoattractants between immune cells and sites of inflammation. Since studies have validated an increased level of CXCL12 and its receptor in patients with colorectal cancers, CXCL12/CXCR4 axis has been considered as a valuable marker of cancer metastasis. Therefore, identification of CXCR4 inhibitors has great potential to abrogate tumor metastasis. Onbaekwon (OBW) is a complex herbal formula that is derived from the literature of traditional Korean medicine Dongeuibogam. In this study, we demonstrated that OBW suppressed CXCR4 expression in various cancer cell types in a concentration- and time-dependent manner. Both proteasomal and lysosomal inhibitors had no effect to prevent the OBW-induced suppression of CXCR4, suggesting that the inhibitory effect of OBW was not due to proteolytic degradation but occurred at the transcriptional level. Electrophoretic mobility shift assay further confirmed that OBW could block endogenous activation of nuclear factor kappa B, a key transcription factor that regulates the expression of CXCR4 in colon cancer cells. Consistent with the aforementioned molecular basis, OBW abolished cell invasion induced by CXCL12 in colon cancer cells. Together, our results suggest that OBW, as a novel inhibitor of CXCR4, could be a promising therapeutic agent contributing to cancer treatment.
Keywords: onbaekwon, CXCR4, CXCL12, metastasis, invasion, colon cancer
Introduction
Colorectal cancer is one of the most common cancers and the third leading cause of cancer-related death worldwide. Death due to colorectal cancer usually results from uncontrolled metastasis.1
Several recent studies have assessed the role of chemokines and chemokine receptors in tumor progression.2 Chemokine networks might play a role in tumor progression because they can affect the growth, adhesion, and organ-specific metastasis of tumor cells.3 The cysteine X cysteine (CXC) chemokine family and their ligands were originally identified as chemoattractants between immune cells and sites of inflammation.4 Cancer cells also use chemokine networks to modulate the host microenvironment and facilitate cancer progression. Recently, studies have validated an increased level of chemokine C-X-C motif chemokine 12 (CXCL12) and its receptor in patients with colorectal cancer, and the CXCL12/CXCR4 (chemokine receptor 4) axis is considered a useful marker of cancer metastasis.5
Over the past centuries, agents derived from natural sources have long received attention from researchers and clinicians because of their safety, efficacy, and immediate availability. Indeed, at least 70% of all drugs approved by the Food and Drug Administration in the past 30 years originated from natural sources.6 Consequently, discovering less toxic and more effective drugs that can improve survival rates and decrease side effects is needed to establish new therapeutic strategies for colorectal cancer.
Onbaekwon (OBW) is a complex herbal formula, derived from the literature of traditional Korean Medicine, Dongeuibogam. The formula has been used traditionally for treating abdominal mass and comprises Aconitum carmichaelii Debx, Evodia rutaecarpa Bentham,Gleditsia sinensis Lam, among others.
Evodiamine is a phytochemical from a traditional Chinese medicine named Evodia rutaecarpa Benth, which is widely used for the treatment of gastrointestinal disorders and hypertension.7 Aconitum carmichaelii Debx has been used to relieve pain and treat rheumatic arthritis and other inflammatory conditions for over 2000 years. Moreover, Chuanwu (A carmichaelii) has an anti-arthritic effect in complete Freund’s adjuvant (CFA)-induced arthritic rats,8 and methanol extracts of crude Aconitum roots have anti-inflammatory effects in terms of inhibiting acid-induced vascular permeability and carrageen-induced hind paw edema in mice.9 In contrast, saponin isolated from Gleditsia sinensis Lam has been reported to show therapeutic potential against rheumatoid arthritis and tumors, 2 angiogenesis-related diseases.10,11 However, the anticancer mechanism of processed OBW remains unclear.
In this study, we investigated the effect of OBW on CXCR4 expression and its inhibition of colon tumor cell invasion. Our results show that this OBW-induced suppression of CXCR4 expression was evident in various CXCR4-overexpressing tumor cell lines. This downregulation occurred at both the transcriptional and translational levels and led to inhibition of CXCL12-induced invasion by colon tumor cells.
Materials and Methods
Materials and Chemical
RPMI1640, Dulbecco’s modified Eagle’s medium (DMEM), and fetal bovine serum (FBS) were purchased from Hyclone (Logan, UT).Trypsin-EDTA (ethylenediaminetetraacetic acid; 0.25%) and antibiotic-antimycotic were obtained from Gibco BRL (Grand Island, NY). Lactacystin and chloroquine were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antibodies against CXCR4 (ab2074) was obtained from Abcam (Cambridge, MA). β-Actin was used as a loading control (Cell Signaling, Danvers, MA). CXCL12 was purchased from R&D system (Minneapolis, MN).
Isolation of Onbaekwon
Onbaekwon is composed of Aconitum carmichaelii 250 g, Evodia rutaecarpa, Platycodi Radix, Bupleuri Radix, Acori Rhizoma, Asteris Radix, Coptidis Rhizoma, Zingiberis Rhizoma, Cinnamomi Cortex, Tiglii Fructus, Hoelen, Gleditsia sinensis Lam, Machili Cortex, Ginseng Radix 100 g each, Zanthoxyli Fructus 75 g. The herbal materials were purchased from Kyung Hee Pharm (Wonju, Korea) and identified by Prof Seong Woo Yoon (Kyung Hee University Hospital at Gangdong, Seoul, Korea). The voucher specimen was registered and deposited at East-West Medical Research Institute, Kyung Hee University. Dried specimen (1625 g) was ground into powder, and extracted twice with 80% ethanol (1 L × 3) for 2 hours, and filtered through a filter paper. The filtrate was evaporated in vacuo and dried with freezer to produce an ethanol extract of OBW (325 g). OBW was dissolved in dimethyl sulfoxide as a 580 mg/mL stock solution and stored at 4°C. Further dilution was done in cell culture medium.
Cell Line and Cell Culture
The immortalized human colon cancer HCT116, breast cancer MDA-MB-231, and liver cancer HepG2 and Hep3B were cultured in DMEM supplemented with 10% FBS and 1% antibiotics. Breast cancer MCF7 was cultured in RPMI 1640 supplemented with 10% FBS and 1% antibiotics. Cells were maintained at 37°C in an atmosphere of 5% CO2-95% air. All cells were passaged at 80% confluences in 0.25% trypsin-EDTA for 3 to 5 minutes.
Western Blotting
For detection of CXCR4, OBW-treated whole cell extracts were lysed with RIPA buffer (150 mM NaCl, 10 mM Tris [pH 7.2], 0.1% sodium dodecyl sulfate [SDS], 1% triton X-100, 1% deoxycholate, and 5 mM EDTA) enriched with a complete protease inhibitor cocktail tablet (Roche Diagnostics, Mannheim, Germany), and then incubated on ice for 30 minutes with regular vortex before centrifuging at 14 000 rpm at 4°C for 15 minutes. Protein concentration was determined by using bicinchoninic acid protein assay kit (Pierce Biotechnology, Rockford, IL). The protein samples were boiled in SDS sample buffer for 5 minutes and were resolved on a 10% SDS-polyacrylamide gel. After electrophoresis, proteins were transferred onto polyvinyl difluoride (PVDF) membrane, which was blocked with 5% nonfat dry milk in tris-buffered saline with 0.1% tween-20 (TBST) and incubated with primary antibody at the appropriate final concentration followed by hybridization with horseradish peroxidase-conjugated anti-rabbit or anti-mouse secondary antibodies. For each step, the membrane was washed with TBST 3 times for 10 minutes and the transferred proteins were incubated with super-signal pico-chemiluminescent substrate or dura-luminol substrate (Thermo Scientific, Waltham, MA) for 2 minutes according to the manufacturer’s instruction and visualized with imagequant LAS 4000 (Fujifilm Life Science, Roche Diagnostics).
RNA Analysis and Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
Total RNA was extracted using Trizol reagent according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA). And cDNA synthesis was performed using the AccuPower Rocketscript cycle RT premix (Bioneer, Daejeon, Korea) according to the manufacture’s protocol. The relative expression of CXCR4 was analyzed by quantitative RT-PCR with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as an internal control. The following pairs of forward and reverse primer sets were used: CXCR4, sense: 5′-CCG TGG CAA ACT GGT ACT TT-3′, antisense: 5′-TTT CAG CCA ACA GCT TCC TT-3′; GAPDH, sense: 5′-CAG CCT CAA GAT CAT CAG CA-3′, antisense: 5′-GTC TTC TGG GTG GCA GTG AT-3′. The RT-PCR reaction mixture contained 2.5 µL of 10× Taq reaction buffer, 0.5 µL of 10 mM dNTP, 1 µL each of forward and reverse primers, and 2 µL template DNA in a final volume of 25 µL. Amplification products were resolved by 1.5% agarose gel electrophoresis stained with safe dye and photographed by imagequant LAS 4000.
Real-Time Quantitative PCR
Real-time PCR was performed on the cDNA using the selective primers for CXCR4 (sense: 5′-CCG TGG CAA ACT GGT ACT TT-3′, antisense: 5′-TTT CAG CCA ACA GCT TCC TT-3′) and GAPDH (sense: 5′-CAG CCT CAA GAT CAT CAG CA-3′, antisense: 5′-GTC TTC TGG GTG GCA GTG AT-3′). PCR was performed in a Light Cycler 480 (Roche Diagnostics, Indianapolis, IN) using the Light Cycler DNA Master SYBR Green Kit (Roche Diagnostics) according to the manufacturer’s instruction. The PCR thermal profile was 95°C for 10 minutes, and 40 cycles of 95°C for 10 seconds, 55°C for 30 seconds followed by a cooling step at 40°C for 30 seconds. For relative quantification, the crossing point (Cp) value of CXCR4 was normalized by keeping the Cp value of GAPDH as a control.
Invasion Assay
In vitro invasion assay was done using Bio-Coat Matrigel invasion assay system (BD Biosciences, Lexington, KY) according to the manufacturer’s instructions. Cancer cells (5 × 104/mL) were suspended in medium and seeded into the Matrigel-precoated transwell chambers with polycarbonate membranes of 8 µm pore size. After preincubation with or without pomolic acid (25 µM), transwell chambers were then placed into 24-well plates, in which was added the basal medium only or basal medium containing 100 ng/mL CXCL12. After incubation (24 hours for MCF7 and MDA-MB-231), the upper surface of transwell chamber was wiped off with a cotton swab and invading cells were fixed and stained with a Diff-Quick stain. The invading cell numbers were counted in 5 randomly selected microscope fields (×100).
Electrophoretic Mobility Shift Assay
A DIG Gel Shift kit (Roche, Mannheim, Germany) was used for electrophoretic mobility shift assay. The NF-κB oligonucleotide probe (NF-κB: 5′-CTT GAA GGG ATT TCC CTG GCT TGA AGG GAT TTC CCT GG-3′—only sense strands are shown; consensus sequences for NF-κB are underlined) containing the NF-κB binding motif was end-labeled with DIG-ddUTP. For the binding reaction, 10 µg of the sample protein was incubated at room temperature for 30 minutes with a DIG-labeled probe.The DNA-protein complexes were separated by electrophoresis in 6% nondenatured polyacrylamide gels using 0.5× TBE as a running buffer. After electrophoresis, the gels were transferred to nylon membranes and detected chemiluminescently. Signal intensity was quantified by an image analyzer (Las4000).
Statistical Analysis
The results obtained from each experiment are expressed as mean ± standard deviation (SD) from at least 3 independent experiments. The significance level was set at P < .05 for each analysis using Student’s t test.
Results
OBW Suppresses CXCR4 Protein Levels in HCT116 Cells
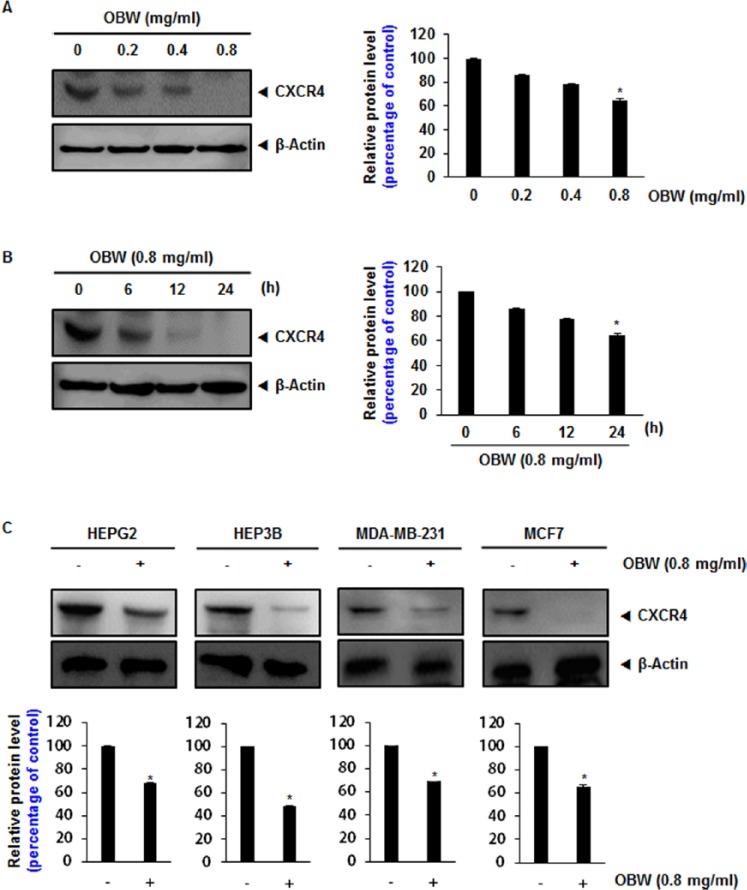
The chemokine receptor CXCR4 has been directly implicated in colorectal cancer metastasis.12 Therefore, the effect of OBW on CXCR4 expression in HCT116 cells was investigated. Following incubation of HCT116 cells with various concentrations of OBW for 24 hours or with 0.8 mg/mL OBW for various times, CXCR4 expression was suppressed in a dose- and time-dependent manner (Figure 1A and B). Exposure of cells to 0.8 mg/mL OBW for 24 hours significantly inhibited CXCR4 expression.
Figure 1.
OBW suppresses CXCR4 protein levels in HCT116 cells.
(A) HCT116 cells (1 ×106) were treated with the indicated concentration of OBW for 24 hours. Whole cell extracts were prepared and 20 µg of protein was resolved via SDS-PAGE, electrotransferred onto PVDF membranes, and probed for CXCR4. (B) HCT116 cells (1 × 106) were treated with 0.8 mg/mL OBW for the indicated time. Whole cell extracts were prepared and analyzed by western blot analysis with antibodies against CXCR4. (C) Liver and breast cancer cells were incubated with 0.8 mg/mL for 24 hours. Whole cell extracts were prepared and analyzed by Western blot analysis with antibodies against CXCR4. The same blots were stripped and reprobed with a β-actin antibody to show equal protein loading. Representative results of 3 independent experiments are shown. *P< .05 versus control. Data are expressed as mean ± SD.
OBW Suppresses CXCR4 Expression in Various Cell Types
CXCR4 is overexpressed in a wide variety of tumor cells. CXCR4 has been implicated in metastasis of breast and liver cancer.13,14 Thus, we investigated the effect of OBW on CXCR4 expression in liver (HEPG2, HEP3B) and breast (MDA-MB-231, MCF7) cancer cell lines. Cells were treated with 0.8 mg/mL OBW for 24 hours and their CXCR4 expression levels were examined. OBW inhibited CXCR4 expression in all cell lines tested (Figure 1C). Thus, CXCR4 suppression by OBW is not cell-type specific.
Downregulation of CXCR4 by OBW Is Not Due to Its Degradation
Because CXCR4 undergoes ubiquitination at its lysine residue followed by degradation,15,16 we next explored the possibility that OBW enhances the rate of degradation by activating proteasomes. To determine this, we examined the effect of lactacystin, a proteasome inhibitor, on OBW-induced degradation of CXCR4 in HCT116 cells.
Cells were pretreated with lactacystin for 1 hour before being exposed to OBW. Lactacystin had no effect on OBW-induced degradation of CXCR4 (Figure 2A), suggesting that the effects of OBW on CXCR4 expression are not mediated by enhanced degradation.
Figure 2.
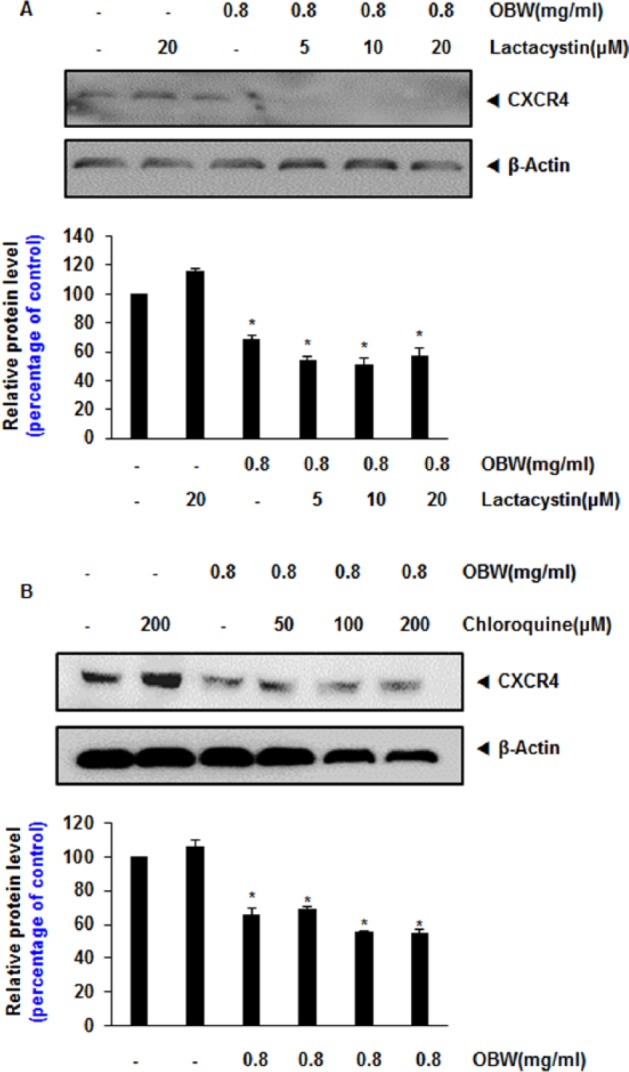
Downregulation of CXCR4 by OBW is not due to its degradation.
(A) HCT116 cells (1 × 106) were treated with the indicated concentration of lactacystin for 1 hour at 37°C followed by treatment with 0.8 mg/mL OBW for 24 hours. Whole cell extracts were prepared and analyzed by western blot analysis with antibodies against CXCR4. (B) HCT116 cells were treated with the indicated concentration of chloroquine for 1 hour at 37°C followed by treatment with 0.8 mg/mL OBW for 24 hours. Whole cell extracts were prepared and analyzed by western blot analysis with antibodies against CXCR4. The same blots were stripped and reprobed with a β-actin antibody to show equal protein loading. *P< .05 versus control. Data are expressed as mean ± SD.
As CXCR4 also undergoes ligand-dependent lysosomal degradation,17 we next investigated the ability of chloroquine, a lysosomal inhibitor, to block OBW-induced degradation of CXCR4. Cells were pretreated with chloroquine for 1 hour before being exposed to OBW. Chloroquine at 200 µmol/L reduced CXCR4 degradation only slightly (Figure 2B), indicating that this was not the primary pathway of suppression of CXCR4 expression.
Downregulation of CXCR4 by OBW Occurs at the Transcriptional Level
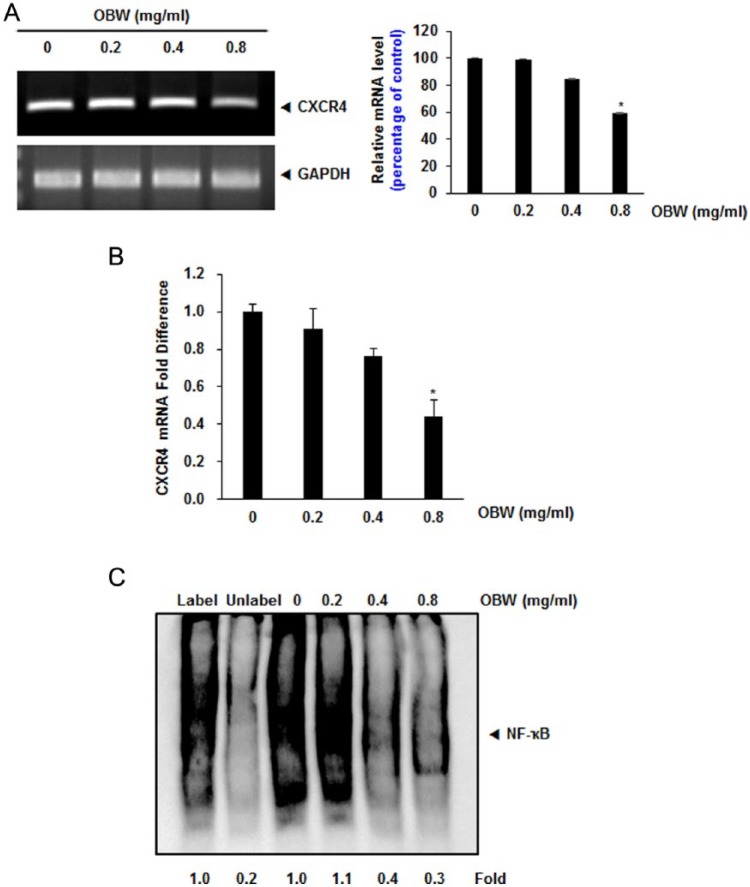
Because OBW did not downregulate CXCR4 expression by enhancing its degradation, we investigated whether suppression occurred at the transcriptional level. HCT116 cells were treated with various concentrations of OBW and then mRNAs were extracted for analysis by RT-PCR and real-time PCR.OBW was found to downregulate CXCR4 mRNA levels (Figure 3A and B).
Figure 3.
Downregulation of CXCR4 by OBW occurs at the transcriptional level.
(A) HCT116 cells (1 × 106) were treated with OBW at the indicated concentrations. Total RNA was isolated and analyzed by RT-PCR assays. GAPDH was used to show equal loading of total RNA. *P< .05 versus control. Data are expressed as mean ± SD. (B) HCT116 cells (1 × 106) were treated with OBW at the indicated concentrations. Total RNA was isolated and analyzed by real-time PCR assays. The crossing point (Cp) value of CXCR4 was normalized by keeping the Cp value of GAPDH as a control; bars show the standard error (*P< .05). (C) OBW suppresses constitutive activation of NF-κB in HCT116 cells.Cells were incubated with the indicated concentrations of OBW for 24 hours, and then the nuclear extracts were assayed for NF-κB activation in HCT116cells. *P< .05 versus control. Data are expressed as mean ± SD.
OBW Suppresses Constitutive Activation of NF-κB in HCT116 Cells
Earlier studies reported that NF-κB, a transcription factor related to expression of various genes, binds to several regions of the CXCR4 promoter. Thus, OBW likely influences CXCR4 expression by suppressing NF-κB activation. We performed a DNA-binding assay to explore the effect of OBW on constitutive NF-κB activation in HCT116 cells, and we found that treatment with 0.8 mg/mL OBW for 24 hours significantly inhibited NF-κB activation (Figure 3C).
OBW Suppresses CXCL12-Induced Colon Cancer Cell Invasion
Several lines of evidence implicate that CXCR4 in colon cancer metastasis, motility, and migration was induced by exposure to CXCL12.18 Therefore, colon cancer metastasis may be inhibited by silencing of CXCR4. The correlation between downregulation of CXCR4 by OBW and colon cancer cell migration was examined using an in vitro invasion assay. CXCL12 induced invasion of colon cancer cells, which was abrogated by OBW treatment (Figure 4A and B). Furthermore, OBW reduced the expression of the tumor metastasis and progression marker CXCR4.
Figure 4.
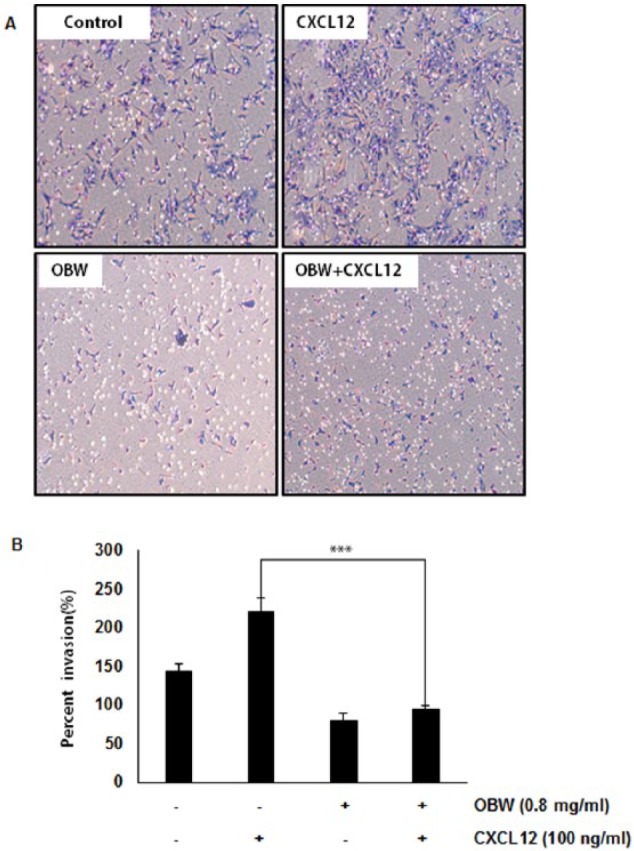
OBW suppresses CXCL12-induced colon cancer cell invasion.
(A and B) HCT116 cells (5 × 104) were seeded on the top chamber of matrigel. After incubation with or without 0.8 mg/mL OBW for 24 hours, transwell chambers were then placed in 24-well plates, to which we added the basal medium only or basal medium containing 100 ng/mL CXCL12. After incubation, cell invasion ability was observed. (B) Columns give the mean numbers of invaded cells; bars give the standard error (***P< .001).
Discussion
Chemokine receptors, which belong to the G-protein-coupled receptor family, are involved in regulation of the immune response, inflammation, leukocyte trafficking, and cytoskeletal rearrangement.19 A number of chemokine receptors are expressed in tumor cells, and CXCR4 has been shown to play an important role in cancer metastasis.20-22 CXCR4 expression is higher in embryonic or dedifferentiated cells than in normal cells,23 and CXCR4 expression was an independent predictor of poor survival in cancer patients.24 Interaction of CXCL12 with CXCR4 triggers molecular events that facilitate cell migration, invasion, and metastasis. Thus, disruption of the CXCR4/CXCL12 axis is likely to reduce metastasis, making this factor a promising target for cancer treatment.21 The goal of the present study was to determine the effect of OBW on CXCR4 expression.Our results showed for the first time that OBW suppresses the expression of CXCR4, irrespective of cell type. Moreover, downregulation of CXCR4 was not due to proteolytic degradation of the receptor but rather through suppression of transcription. Furthermore, suppression of CXCR4 led to downregulation of invasion induced by the CXCL12 ligand in HCT116 colon cancer cells.
CXCR4 has been reported to be overexpressed in a variety of tumors and promotes metastasis.25-27 Our data also showed that OBW suppressed CXCR4 expression in various tumor cell lines, including liver and breast cancer cells, thereby indicating that the effect of OBW on CXCR4 is not limited to a single cell type.
The mechanism underlying overexpression of this receptor in tumor cells is unclear. However, recent studies have documented the ligand-dependent downregulation of the CXCR4 expression by lysosomal degradation,16 which involves atrophin-interacting protein 4-mediated ubiquitination and degradation.17 However, our data indicate that OBW does not downregulate CXCR4 through this mechanism. As such, as downregulation of CXCR4 by OBW did not occur at the posttranslational level, we postulated that the effect would be at the transcriptional level.
Inflammatory cytokines produced by tumor cells28,29 or inflammatory cells in the tumor microenvironment can promote tumor progression by inducing the expression of genes dependent on the NF-κB signaling pathway.30 NF-κB was also shown to induce the expression of CXCR4.26,31,32 Because NF-κB has been shown to upregulate the expression of several pro-metastatic and pro-angiogenic genes including interleukin 6 (IL-6), IL-8, and vascular endothelial growth factor (VEGF),33-35 the NF-κB complex is normally confined to the cytosol through its interaction with the IκB protein. On stimulation, IκB is degraded and NF-κB is activated. Transient transfection of an NF-κB expression plasmid upregulated CXCR4 expression in human prostate cancer cells.35 In this study, we presented evidence that OBW suppresses CXCR4 expression through NF-κB inhibition in colon cancer cells.
We further investigated the effect of OBW on CXCL12-induced invasion of colon cancer cells. The role of CXCL12 in promotion of invasive growth is well documented, and the intracellular signals triggered by CXCR4 activation have been investigated extensively.36 This argues in favor of a critical role for the CXCR4 receptor in tumor invasiveness and progression and the potential of OBW to downregulate CXCL12-induced invasion of colon cancer cells. In summary, we found that OBW downregulated the expression of CXCR4, a key receptor in the cross-talk between tumor cells and their microenvironment, and thus, that the antimetastasis effects of OBW may be mediated through CXCR4 regulation. Further in vivo studies are needed to demonstrate the relevance of these observations to cancer treatment.
Conclusion
OBW exerts potent antimetastasis effects by inhibiting expression of CXCR4 in colon cancer cells. These inhibitory effects are mediated by the suppression of NF-κB activation. These findings support the traditional use of OBW in various anticancer treatments and also suggest that OBW might be useful in the development of new therapeutic agents for metastasis diseases.
Footnotes
Declaration of Conflicting Interest: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (NRF-2013R1A1A1062064) and this research was also supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2016R1A6A1A03011325).
References
- 1. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [DOI] [PubMed] [Google Scholar]
- 2. Balkwill FR. The chemokine system and cancer. J Pathol. 2012;226:148-157. [DOI] [PubMed] [Google Scholar]
- 3. Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761-1767. [DOI] [PubMed] [Google Scholar]
- 4. Oppenheim JJ, Zachariae CO, Mukaida N, Matsushima K. Properties of the novel proinflammatory supergene “intercrine” cytokine family. Annu Rev Immunol. 1991;9:617-648. [DOI] [PubMed] [Google Scholar]
- 5. Salvucci O, Bouchard A, Baccarelli A, et al. The role of CXCR4 receptor expression in breast cancer: a large tissue microarray study. Breast Cancer Res Treat. 2006;97:275-283. [DOI] [PubMed] [Google Scholar]
- 6. Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bak EJ, Park HG, Kim JM, Kim JM, Yoo YJ, Cha JH. Inhibitory effect of evodiamine alone and in combination with rosiglitazone on in vitro adipocyte differentiation and in vivo obesity related to diabetes. Int J Obes (Lond). 2010;34:250-260. [DOI] [PubMed] [Google Scholar]
- 8. Xue L, Zhang HY, Qin L, Wang XC, Wang L. Effect of chuanwu and baishao used separately or in combination on adjuvant arthritis in rats [in Chinese]. Zhongguo Zhong Yao Za Zhi. 2000;25:175-178. [PubMed] [Google Scholar]
- 9. Hikino H, Konno C, Takata H, et al. Antiinflammatory principles of Aconitum roots. J Pharmacobiodyn. 1980;3:514-525. [DOI] [PubMed] [Google Scholar]
- 10. Pak KC, Lam KY, Law S, Tang JC. The inhibitory effect of Gleditsia sinensis on cyclooxygenase-2 expression in human esophageal squamous cell carcinoma. Int J Mol Med. 2009;23:121-129. [PubMed] [Google Scholar]
- 11. Tang WK, Chui CH, Fatima S, et al. Inhibitory effects of Gleditsia sinensis fruit extract on telomerase activity and oncogenic expression in human esophageal squamous cell carcinoma. Int J Mol Med. 2007;19:953-960. [PubMed] [Google Scholar]
- 12. Murakami T, Kawada K, Iwamoto M, et al. The role of CXCR3 and CXCR4 in colorectal cancer metastasis. Int J Cancer. 2013;132:276-287. [DOI] [PubMed] [Google Scholar]
- 13. Zhao H, Guo L, Zhao H, Zhao J, Weng H, Zhao B. CXCR4 over-expression and survival in cancer: a system review and meta-analysis. Oncotarget. 2015;6:5022-5040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sun Y, Mao X, Fan C, et al. CXCL12-CXCR4 axis promotes the natural selection of breast cancer cell metastasis. Tumour Biol. 2014;35:7765-7773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernandis AZ, Cherla RP, Chernock RD, Ganju RK. CXCR4/CCR5 down-modulation and chemotaxis are regulated by the proteasome pathway. J Biol Chem. 2002;277:18111-18117. [DOI] [PubMed] [Google Scholar]
- 16. Marchese A, Benovic JL. Agonist-promoted ubiquitination of the G protein-coupled receptor CXCR4 mediates lysosomal sorting. J Biol Chem. 2001;276:45509-45512. [DOI] [PubMed] [Google Scholar]
- 17. Bhandari D, Trejo J, Benovic JL, Marchese A. Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor CXCR4. J Biol Chem. 2007;282:36971-36979. [DOI] [PubMed] [Google Scholar]
- 18. Matsusue R, Kubo H, Hisamori S, et al. Hepatic stellate cells promote liver metastasis of colon cancer cells by the action of SDF-1/CXCR4 axis. Ann Surg Oncol. 2009;16:2645-2653. [DOI] [PubMed] [Google Scholar]
- 19. Moyer RA, Wendt MK, Johanesen PA, Turner JR, Dwinell MB. Rho activation regulates CXCL12 chemokine stimulated actin rearrangement and restitution in model intestinal epithelia. Lab Invest. 2007;87:807-817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balkwill F. The significance of cancer cell expression of the chemokine receptor CXCR4. Semin Cancer Biol. 2004;14:171-179. [DOI] [PubMed] [Google Scholar]
- 21. Ramsey DM, McAlpine SR. Halting metastasis through CXCR4 inhibition. Bioorg Med Chem Lett. 2013;23:20-25. [DOI] [PubMed] [Google Scholar]
- 22. Furusato B, Mohamed A, Uhlen M, Rhim JS. CXCR4 and cancer. Pathol Int. 2010;60:497-505. [DOI] [PubMed] [Google Scholar]
- 23. Salvucci O, Yao L, Villalba S, Sajewicz A, Pittaluga S, Tosato G. Regulation of endothelial cell branching morphogenesis by endogenous chemokine stromal-derived factor-1. Blood. 2002;99:2703-2711. [DOI] [PubMed] [Google Scholar]
- 24. Ahn JY, Seo K, Weinberg OK, Arber DA. The prognostic value of CXCR4 in acute myeloid leukemia. Appl Immunohistochem Mol Morphol. 2013;21:79-84. [DOI] [PubMed] [Google Scholar]
- 25. Porcile C, Bajetto A, Barbero S, Pirani P, Schettini G. CXCR4 activation induces epidermal growth factor receptor transactivation in an ovarian cancer cell line. Ann N Y Acad Sci. 2004;1030:162-169. [DOI] [PubMed] [Google Scholar]
- 26. Proudfoot AE. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol. 2002;2:106-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gschwind A, Prenzel N, Ullrich A. Lysophosphatidic acid-induced squamous cell carcinoma cell proliferation and motility involves epidermal growth factor receptor signal transactivation. Cancer Res. 2002;62:6329-6336. [PubMed] [Google Scholar]
- 28. Kulbe H, Hagemann T, Szlosarek PW, Balkwill FR, Wilson JL. The inflammatory cytokine tumor necrosis factor-alpha regulates chemokine receptor expression on ovarian cancer cells. Cancer Res. 2005;65:10355-10362. [DOI] [PubMed] [Google Scholar]
- 29. Bachelder RE, Wendt MA, Mercurio AM. Vascular endothelial growth factor promotes breast carcinoma invasion in an autocrine manner by regulating the chemokine receptor CXCR4. Cancer Res. 2002;62:7203-7206. [PubMed] [Google Scholar]
- 30. Bohonowych JE, Hance MW, Nolan KD, Defee M, Parsons CH, Isaacs JS. Extracellular Hsp90 mediates an NF-kappaB dependent inflammatory stromal program: implications for the prostate tumor microenvironment. Prostate. 2014;74:395-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Helbig G, Christopherson KW, 2nd, Bhat-Nakshatri P, et al. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278:21631-21638. [DOI] [PubMed] [Google Scholar]
- 32. Zhi Y, Duan Y, Zhou X, et al. NF-kappaB signaling pathway confers neuroblastoma cells migration and invasion ability via the regulation of CXCR4. Med Sci Monit. 2014;20:2746-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cho JS, Han IH, Lee HR, Lee HM. Prostaglandin E2 induces IL-6 and IL-8 production by the EP receptors/Akt/NF-kappaB pathways in nasal polyp-derived fibroblasts. Allergy Asthma Immunol Res. 2014;6:449-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shibata A, Nagaya T, Imai T, Funahashi H, Nakao A, Seo H. Inhibition of NF-kappaB activity decreases the VEGF mRNA expression in MDA-MB-231 breast cancer cells. Breast Cancer Res Treat. 2002;73:237-243. [DOI] [PubMed] [Google Scholar]
- 35. Kukreja P, Abdel-Mageed AB, Mondal D, Liu K, Agrawal KC. Up-regulation of CXCR4 expression in PC-3 cells by stromal-derived factor-1alpha (CXCL12) increases endothelial adhesion and transendothelial migration: role of MEK/ERK signaling pathway-dependent NF-kappaB activation. Cancer Res. 2005;65:9891-9898. [DOI] [PubMed] [Google Scholar]
- 36. Song ZY, Gao ZH, Chu JH, Han XZ, Qu XJ. Downregulation of the CXCR4/CXCL12 axis blocks the activation of the Wnt/beta-catenin pathway in human colon cancer cells. Biomed Pharmacother. 2015;71:46-52. [DOI] [PubMed] [Google Scholar]