Abstract
Lavandula angustifolia is the most widely cultivated Lavandula species. The extraction of its flower and leaves has been used as herbal medicine. In this study, the in vitro antitumor activities were tested on human prostate cancer PC-3 and DU145 cell lines. Flow cytometry technology was applied to study apoptosis induction and cell cycle arrest. The PC-3 cell line was used to establish subcutaneous xenograft tumors in nude mice. Paraffin sections from xenograft tumor specimens were used in the TUNEL (terminal deocynucleotide transferase dUTP nick end labeling) assay and an immunohistochemistry assay to detect cell proliferation markers Ki67 and PCNA. Lavender essential oil, linalool, and linalyl acetate showed stronger inhibitory effect on PC-3 cells than on DU145 cells. The apoptotic cell populations observed in PC-3 cells treated with lavender essential oil, linalool, and linalyl acetate were 74.76%, 67.11%, and 56.14%, respectively. The PC-3 cells were mainly arrested in the G2/M phase. In the xenograft model with PC-3 cell transplantation, essential oil and linalool significantly suppressed tumor growth. The immunosignals of Ki67 and PCNA in the essential oil, linalool, and linalyl acetate treatment groups were significantly lower than that of the control group in xenograft tumor sections. The TUNEL assay indicated that each of the 3 phytochemicals significantly induced apoptosis compared to the control group. This study provides novel insight and evidence on the antiproliferative effect of L angustifolia essential oil and its major constituents on human prostate cancer. The antitumor effect was associated with cell proliferation inhibition and apoptosis induction in xenograft tumors.
Keywords: Lavandula angustifolia, linalool, linalyl acetate, prostate cancer, PC-3
Introduction
Prostate cancer is by far the most commonly diagnosed cancer among American men, and it is the second leading cause of death by cancer.1 Conventional prostate cancer treatments include prostatectomy, radiotherapy, hormonotherapy, chemotherapy, and biological therapy. Side effects such as sexual function disorder, impotence, diabetes, and cardiovascular disease have been observed.2 Recent surveys reveal that complementary medications, such as natural products, can improve the treatment in many cancer patients with minimal side effects.3,4
Androgens are the major growth factors for the normal prostate. Recent studies suggest that the androgen receptor and its signaling pathway significantly contribute to the metastatic prostate cancer progression.5 Although many patients initially respond to androgen depletion therapy, most of them will eventually progress and develop castration-resistant disease.6 Due to the complexity of the androgen receptor–targeted therapy for prostate cancer, newer agents must be discovered.7 Meanwhile, oncogenic fusion proteins have been discovered in prostate cancer tissues. It adds one more layer of complexity to the protein targeting network in prostate cancer cells.8
Transmembrane protease, serine 2 (TMPRSS2) is an androgen-responsive gene. The overexpression of ERG (ETS-related gene) proto-oncogene is one of the distinct characteristics of prostate cancer. The fusion of TMPRSS2 with ERG is a frequent event in prostate cancers, especially in hormone refractory prostate cancer.9,10 TMPRSS2-ERG contributes to androgen-independence in prostate cancer cells by disrupting androgen receptor signaling pathways.11 Overexpression of ERG may contribute to the development of castrate-resistant prostate cancer. In addition, androgen treatment can activate ataxia telangiectasia mutated (ATM) in normal prostate epithelial cells and the knockdown of ATM can induce TMPRSS2-EGR fusion transcription.12 Many studies have shown that microRNA (miRNA) act as tumor suppressors. For personalizing prostate cancer therapy development, miRNA signature identification and validation, particularly in TMPRSS2-ERG-postive prostate cancer cells, has been emerging as a promising trend.13,14
The cytokine tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) has attracted considerable attention as a novel anticancer agent owing to its selectivity toward tumor cells. However, prostate cancer cells such as DU145 and PC-3 are resistant to 100 ng/mL TRAIL-induced apoptosis.15,16 TRAIL sensitizers from natural sources are considered attractive with less toxicity and resistance in normal cells.17,18 TRAIL binds to 2 death receptors, DR4 and DR5, and 3 decoy receptors, DcR1, DcR2, and osteoprotegerin.19 Phytochemicals that increase DR4 and DR5 expression levels can augment the TRAIL-induced apoptosis.20
Lavandula angustifolia Mill. (Lamiaceae) belongs to the mint family, which is native to the western Mediterranean.21,22 In the form of oil extraction, the flower and leaves of L angustifolia are used as herbal medicine.23 Over the past decade, substantial scientific evidences have been accumulated to suggest that the extracts of L angustifolia have antibacterial, antioxidant, and anticancer activities.23,24 It has been used in the treatment of gastrointestinal disorders, irritability, and rheumatism. Studies have showed that the distillation extract of L angustifolia had cytostatic and apoptotic effects on human lung cancer (A-549), breast cancer (MCF-7), cervical cancer (HeLa), and prostate cancer (PC-3).25
The main components of L angustifolia distillation products are linalool (26% to 49%) and linalyl acetate (17.6% to 53%), and they are responsible for the characteristic flavor and the therapeutic properties.22,26 Linalool and linalyl acetate are naturally occurring phytochemicals showing anti-inflammatory activity in carrageenan-induced edema rats.27 Cell line–based studies suggested that linalool had antiproliferative activity against SW 620, Hep G2, A-549, and T-47D cells via inducing apoptosis and activating antitumor immunity.28 An in vivo sarcoma-180 solid tumor model study stated that the antitumor potential of linalool is achieved by oxidative stress modulation.29 Linalool also exhibited very high antimicrobial activity against Staphylococcus epidermidis, Pseudomonas aeruginosa, and Candida albicans.30 Linalyl acetate is the acetate form of linalool. It has a typical prodrug behavior.27 Linalyl acetate can alter the melanogenesis of melanoma cells through oxidative stress and the JNK and ERK signaling pathways.31
In this study, the antiproliferative effects of the L angustifolia essential oil and its main components, linalool and linalyl acetate, on human prostate cancer cells were investigated. Efficacies of the compounds were evaluated and compared in both in vitro cell culture assays and xenograft tumor model.
Materials and Methods
Materials
The plant Lavandula angustifolia was harvested from Malong, Yunnan, China (N25°23′, E103°37′). The extract (essential oil) was kindly provided by Yunnan Nuoye Biotech, Inc. Human prostate cancer cell lines, PC-3 and DU145, were obtained from the Chinese Academy of Sciences Cell Bank. Linalool, linalyl acetate, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT), and the Annexin V-FITC Apoptosis Detection Kit were obtained from Sigma Aldrich (St Louis, MO). The DNA content quantitation assay kit was purchased from KeyGEN BioTECH (Nanjing, Jiangsu, China).
Liquid Chromatography/Mass Spectrometry (LC/MS)
The presence of linalool and linalyl acetate in the essential oil was assessed by LC (Ekspert Ultra LC 100, Eksigent part of AB SCIEX)/MS (3200 Q TRAP LC/MS/MS system, AB SCIEX) with a reverse-phase column (Thermo Accucore C18). The chromatography conditions were a mobile phase A of ddH2O with 0.1% formic acid and B of acetonitrile with 0.1% formic acid. The gradient program was (a) 10% solvent B for 0.5 minutes; (b) a linear gradient from 10% to 90% solvent B over 4 minutes; (c) 0.5 minutes with 90% solvent B; and (d) 3 minutes with 10% solvent B. EMS+ was used as the scan type.
Cytotoxicity Assay
Human prostate cancer cell lines, PC-3 and DU145, were maintained in F-12 and Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Gibico, Life Technologies). Cells were plated in a clear 96-well flat-bottomed plate at the density of 2500 cells/well in 90 µL of growth medium. After 12 hours, L angustifolia essential oil, linalool, or linalyl acetate solution was added at different concentrations. One percent dimethyl sulfoxide (DMSO) in phosphate-buffered saline (PBS) and 10% trichloroacetic acid solution was used as negative and positive controls, respectively. After the treatment for 48 hours, MTT was added and incubated at 37°C for 4 hours. After the incubation, the medium was carefully removed and 50 µL DMSO was added to each well. After 10-minute incubation at 37°C, the plate was then read at 540 nm on a plate reader.
Wound Healing Assay
Human prostate cancer cells, PC-3 and DU145, were cultured to >90% confluence in 6-well plates. The cells were rinsed with PBS and starved in low serum medium (0.5% FBS in F-12 or DMEM) overnight. On the bottom of the dish, a line was drawn with a marker. A sterile 200 µL pipet tip was used to scratch through the cells to create wounds perpendicular to the marker line. The cells were gently washed with PBS and incubated with 1.5 mL medium or medium with added drug. Pictures were taken by Nikon Eclipse 80i microscope system using phase-contrast objective (10×). All the images were analyzed by the TScratch program (www.cse-lab.ethz.ch).
Apoptosis
The induction of apoptosis was assessed using a Sigma Annexin V-FITC Apoptosis Detection Kit. According to the manufacturer’s instructions, 1 × 106 cells/mL human prostate cancer cells, PC-3 and DU145, were treated with lavender essential oil (0.05%, v/v), linalool (2.5 µM), or linalyl acetate (2.5 µM) for 24 hours. Both adherent and floating cells were collected and suspended in 1× binding buffer. Cells were stained with Annexin V-FITC conjugate and propidium iodide (PI). After 10-minute incubation at ambient temperature in dark, the samples were quantified by flow cytometry (PARTEC CyFlow Space). The experiments were repeated thrice.
Cell Cycle Arrest
The cell cycle analysis was performed using a KeyGEN BioTECH DNA content quantitation assay kit. Human prostate cancer cells, PC-3 or DU145, were treated with lavender essential oil (0.05%, v/v), linalool (2.5 µM), or linalyl acetate (2.5 µM) for 24 hours. After the treatment, cells were collected and fixed with 70% ethanol at 4°C for 2 hours. DNA was stained with PI in the presence of 1% DNase-free RNase A at 37°C for 30 minutes before flow cytometry analysis (PARTEC CyFlow Space). The distribution of cells in distinct cell cycle phases was determined using FloMax Ver. 2.82 (QA GmbH, Munster, Germany) cell cycle analysis software. The experiments were repeated 3 times.
Western Blot
DU145 and PC-3 cells were seeded in 6-well plates. Cells were treated with L angustifolia essential oil (0.05%, v/v), linalool (2.5 µM), or linalyl acetate (2.5 µM) for 24 hours. After the treatment, cells were washed with PBS and collected. Cells were lysed with RIPA buffer on ice for 30 minutes. The protein concentration was determined by BCA kit (Beyotime Biotechnology, Shanghai, China). The proteins were separated on 10% SDS-PAGE and electrotransferred onto polyvinylidene fluoride membrane. The membrane was blocked with 5% bovine serum albumin (BSA) and immunoblotted with antibodies against DR4, DR5, and β-actin (Abcam Plc, Cambridge, MA).
Maximum Tolerable Dose
Male Balb/c nude mice (SPF, 6 weeks) were purchased from HFK Bioscience Co, Ltd (Certificate No. 11401300024642; Beijing, China). The animals were fed with standard commercial diet purchased from Keaoxieli Feed Co, Ltd (Certificate No. 11002900015471; Beijing, China). Nude mice were housed in SPF animal experiment center that is specific pathogen-free with a 12-hour light-dark cycle. All animal usage and operation procedures were approved by Kunming Medical University Experimental Animal Ethics Committee (Approval No. KMMU 2015008).
Five groups of nude mice (n = 5) received subcutaneous injection of lavender essential oil, linalool, or linalyl acetate at 5 different doses (10, 25, 50, 100, and 200 mg/kg body weight). One additional group of animals received the drug vehicle in PBS as control. Animals were monitored for 7 days for any sign of toxicity. For necropsy, major organs, such as heart, liver, spleen, kidney, and intestine, were collected. Tissue and organ damage, bleeding, or necrosis was evaluated by hematoxylin and eosin (HE) stain. Briefly, tissue samples were fixed in 10% neutral formalin and embedded in paraffin. Alum hematocylin stain solution was used to stain the nuclei after the sections were deparaffinized and rehydrated. The samples were differentiated in 1% HCl-MeOH solution and washed separately with 70%, 85%, 95%, and 100% methanol for 5 minutes. Then, the samples were stained with eosin for 2 minutes. Sections were washed, dehydrated, cleared, and mounted for microscopic observation.
Efficacy Study in Nude Mice
Human prostate cancer cell line PC-3 was used to establish the synergetic subcutaneous tumor model. Cell suspension (2 × 106 cells in 0.1 mL PBS) was inoculated subcutaneously into the rear flanks of the male nude mice. Once xenograft tumors were palpable (~30 mm3), animals were randomly divided into 4 groups and were treated with solvent control, lavender essential oil, linalool, or linalyl acetate. Mice received drugs at the dose of 200 mg/kg body weight twice a week for 4 weeks via subcutaneous injection. Tumor growth was measured twice a week with a caliper. Tumor volume was calculated by the following formula: L × W × H × 0.5236. A tumor growth curve was then generated by plotting the relative fold value of tumor volume at each time point compared to its initial volume by the following formula: (Vt−V0)/V0× 100%.
Immunohistochemistry
All the mice were euthanized after the last dose of the treatments on the 28th day. Tumor tissues were collected, washed with PBS, fixed in 10% neutral formalin, and embedded in paraffin. Immunohistochemistry for PCNA and Ki67 was performed with antigen retrieval. Briefly, paraffin-embedded tumor sections were deparaffinized and rehydrated. Antigen recovery was achieved by boiling the tissues in citrate acid buffer (10 mM, pH 6.0). Nonspecific binding sites were blocked using 1% BSA before incubation with antibodies at 4°C overnight. After the treatment of primary and secondary antibodies, the sections were stained with DAB solution. The samples were then counterstained with HE, as described above. The sections were visualized using a DAKO LSAB Detection System (Catalog #K0679, DAKO USA). The immunosignal index was calculated by the following formula: (positive cells number/total cell number) × 100%.
Apoptosis in Xenograft Tumor
The terminal deocynucleotide transferase dUTP nick end labeling (TUNEL) assay was performed to detect apoptotic cell death in xenograft tumors using the in situ TUNEL Cell Apoptosis Detection Kit-Biotin-POD (KGA702). The slide was prepared according to the manufacturer’s instructions. Briefly, paraffin-embedded tumor sections were washed in PBS 3 times after being deparaffinized and rehydrated. Proteinase K (100 µL) was added to the sections and incubated at 37°C for 30 minutes. After the treatment, the sections were washed 3 times in PBS and incubated in 3% H2O2 for 10 minutes at ambient temperature. DNase I (100 µL) was added to the samples and incubated at 37°C for 10 minutes. Samples were washed and dried, 50 µL TdT enzyme mix was added, and reacted at 37°C for 60 minutes. Then, 50 µL streptavidin-HRP working solution was added, and the samples were incubated at 37°C for 30 minutes in dark. After the reaction, 50 µL DAB was added and reacted at ambient temperature for 1 minute. The samples were counterstained with HE, as described above. The sections were visualized and the immunosignal index was calculated.
Statistical Analysis
GraphPad Prism 5 software was used for statistical analysis. A t test was used for a statistical comparison of the 2 means. One-way ANOVA was used to compare differences between groups. In all comparisons, statistical significance was set at P ≤ .05.
Results
Linalool and Linalyl Acetate in the L angustifolia Essential Oil
LC/MS/MS was used to examine if linalool and linalyl acetate are present in the essential oil. The retention times of extractions of 177-178 (linalool + Na+) and 219-220 (linalyl acetate + Na+) were 3.09 minutes and 3.71 minutes, respectively. The content of linalool and linalyl acetate found in the essential oil was 30.5 ± 0.5% and 15.8 ± 0.5%, respectively.
In Vitro Cytotoxicity
Viability assay was performed to evaluate the inhibitory effects of L angustifolia essential oil, linalool, and linalyl acetate on human prostate cancer cells. The IC50 of the essential oil treatment determined in DU145 and PC-3 cells was 0.199 ± 0.026% and 0.037 ± 0.011% (v/v), respectively. The IC50 of linalool and linalyl acetate was 7.22 ± 0.28 µM, 11.74 ± 0.62 µM, in DU145, and 3.06 ± 0.22 µM and 4.98 ± 0.31 µM, respectively, in PC-3. These results indicate that L angustifolia essential oil and its main components, linalool and linalyl acetate, displayed potent cytotoxicity against both PC-3 and DU145 cell lines.
Wound Healing Assay
The in vitro inhibition effect of the drugs on cell migration was studied by scratch assay. The open image areas were quantified (Figure 1). L angustifolia essential oil, linalool, and linalyl acetate significantly inhibited migration of DU145 and PC-3 cells (P < .05) in both dose- and time-dependent manners. As shown in Figure 1, after the treatment of 0.005% (v/v) of lavender essential oil for 24 hours, the open image areas of PC-3 and DU145 cell monolayer were 10 and 4.25 times larger than that of the control group, respectively.
Figure 1.
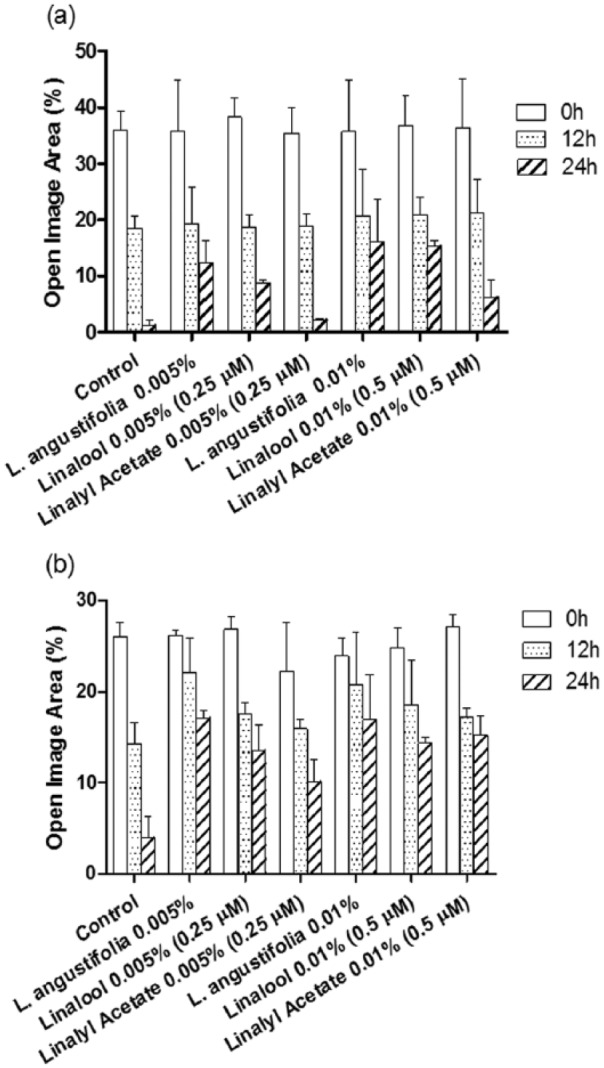
The quantified open image area in wound healing assay of DU145 (a) and PC-3 (b) cells treated by L angustifolia, linalool, or linalyl acetate at different time points (mean ± SD, n = 3).
Apoptosis and Cell Cycle Arrest
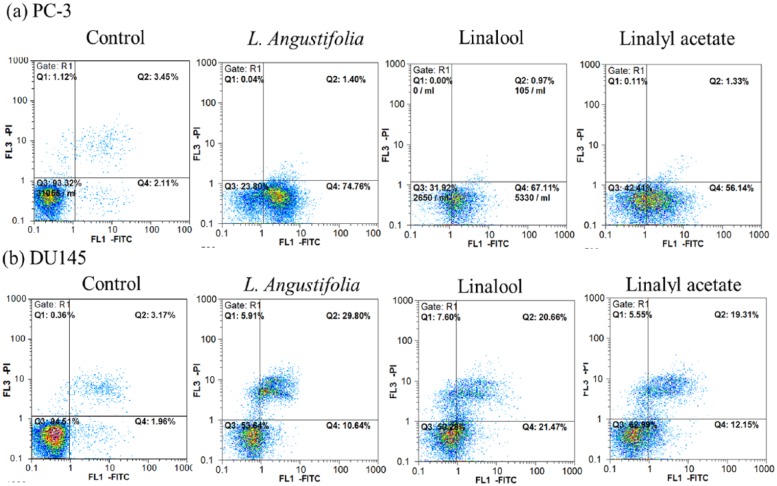
Annexin-V/PI staining was used to examine the apoptotic induction of drugs in PC-3 and DU145 cells. Following the treatment of lavender essential oil (0.5%, v/v), linalool (2.5 µM), or linalyl acetate (2.5 µM) for 24 hours, the proportion of apoptotic cells was significantly increased in both PC-3 and DU145 cells compared to the control group (Figure 2). Apoptotic cell population observed in PC-3 cells treated with PBS (control), lavender essential oil, linalool, and linalyl acetate was 2.11%, 74.76%, 67.11%, and 56.14%, respectively. In DU145, the apoptotic cell population of control, lavender essential oil, linalool, and linalyl acetate treatment was 1.96%, 10.64%, 21.47%, and 12.15%, respectively.
Figure 2.
The effects of lavender essential oil (0.05%, v/v), linalool (2.5 µM), or linalyl acetate (2.5 µM) treatment on apoptosis induction in PC-3 (a) and DU145 (b) cells.
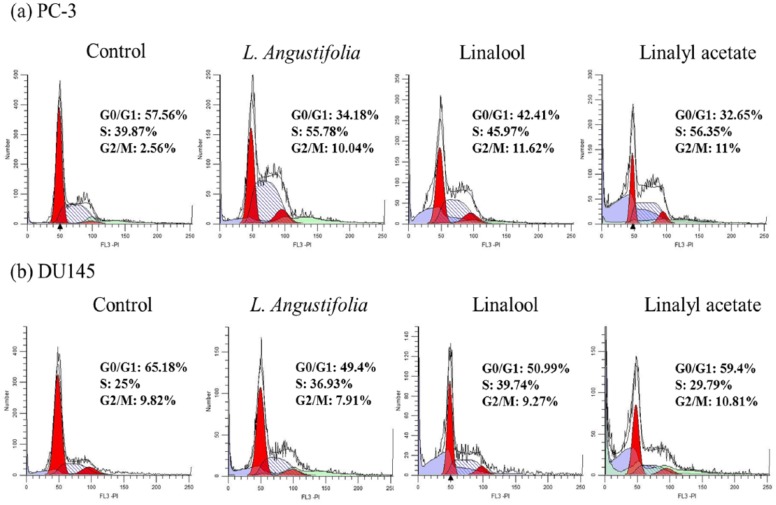
To investigate the effects of the drugs on cell cycle regulation, cell cycle distribution was studied by flow cytometry after PI staining. The distributions of PC-3 and DU145 cells with a 24-hour treatment of lavender essential oil (0.05%, v/v), linalool (2.5 µM), or linalyl acetate (2.5 µM) in cell cycle are shown in Figure 3a. The percentage of cells in G2/M phase was significantly increased after the treatment in PC-3 cells. The percentage of cells in G2/M phase was 2.56%, 10.04%, 11.62%, and 11% in control, lavender essential oil, linalool, and linalyl acetate treatment groups, respectively. However, in DU145 cell line, cells were arrested in the S phase instead of the G2/M phase (Figure 3b). The S phase cell population of control, lavender essential oil, linalool, or linalyl acetate treatment was 25%, 36.93%, 39.74%, and 29.79%, respectively.
Figure 3.
The effects of lavender essential oil (0.05%, v/v), linalool (2.5 µM), or linalyl acetate (2.5 µM) treatment on cell cycle progression in PC-3 (a) and DU145 (b) cells.
DR4 and DR5 Regulation
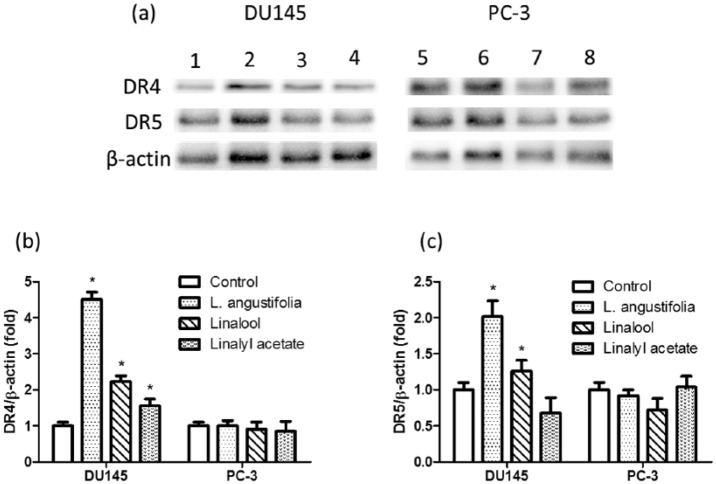
The expression levels of DR4 and DR5 in DU145 and PC-3 prostate cancer cells subjected to treatments were assessed by Western blotting (Figure 4). The normalized expression of DR4/β-actin and DR5/β-actin are shown in Figure 4b and c. In DU145 cell line, both DR4 and DR5 were significantly upregulated in the essential oil and linalool treatment groups. However, in PC-3 cell line, there was no significant difference in the expressions of DR4 and DR5 in any of the treatment groups compared to the control group.
Figure 4.
The expression of DR4 and DR5 in DU145 and PC3 cells was assessed by Western blot. Lanes 1 and 5: control; lanes 2 and 6: essential oil; lanes 3 and 7: linalool; lanes 4 and 8: linalyl acetate.
Xenograft Tumor Growth in Nude Mice
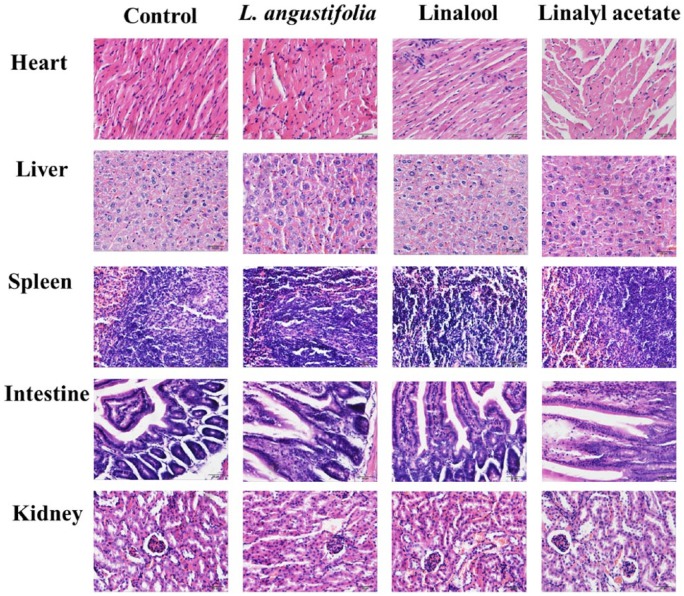
Before the in vivo efficacy studies, the maximum tolerable dose was determined in nude mice bearing xenograft tumors established from prostate cancer PC-3 cell line (Figure 5). At the maximum dose of 200 mg/kg body weight, mild edema in heart was observed in animal treated with L angustifolia essential oil. However, there was no significant differences of histological finding between the control group and the treatment groups (Table 1). These results suggest the treatments to the heart, liver, spleen, intestine and kidney of the tested animals do not cause obvious toxic effect. Therefore, the dose of 200 mg/kg body weight was safe to animals and used for the subsequent experiment.
Figure 5.
Microscopic observations of major mice organs after the treatment of lavender essential oil, linalool, or linalyl acetate at a concentration of 200 mg/kg.
Table 1.
Animal Major Organ Pathological Biopsy Examination Records.
| Organ | Histology | Control | Lavender angustifolia | Linalool | Linalyl Acetate |
|---|---|---|---|---|---|
| Heart | Karyopyknosis | ||||
| Waviness of fibers at border | |||||
| Neutrophil infiltration | |||||
| Edema | + | ||||
| Liver | Necrosis | ||||
| Infiltration of inflammatory cells | |||||
| Cellular degeneration | |||||
| Spleen | Splenic corpuscle | ||||
| Megakaryocyte | |||||
| Intestine | Epithelial degeneration | ||||
| Villi edema | |||||
| Submucosal inflammation | + | + | + | + | |
| Kidney | Glomerulosclerosis | ||||
| Hydropic degeneration | |||||
| Tubular atrophy | |||||
| Inflammation | |||||
| Overall | + | ++ | + | + |
The antitumor effects of lavender essential oil, linalool, and linalyl acetate were examined in mouse xenograft model. Human PC-3 cell line was used to establish subcutaneous xenograft tumors in nude mice. As shown in Figure 6, at the end of the treatment, compared to the control group, lavender essential oil (P < .001), linalool (P < .001), and linalyl acetate (P = .016) significantly suppressed xenograft tumor growth.
Figure 6.
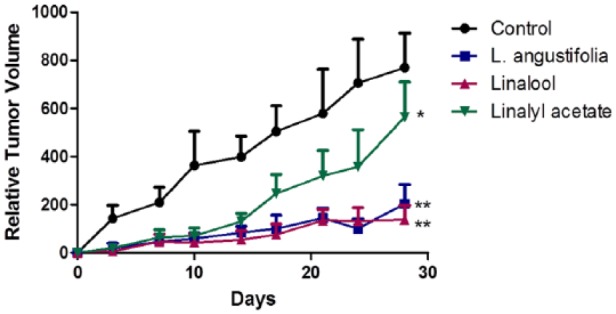
Lavender essential oil, linalool, and linalyl acetate significantly suppressed tumor growth in nude mice transplanted with PC-3 prostate cancer cells (mean ± SD; n = 6; *P< .05; **P< .001).
Cell Proliferation and Apoptosis in Xenograft Tumor
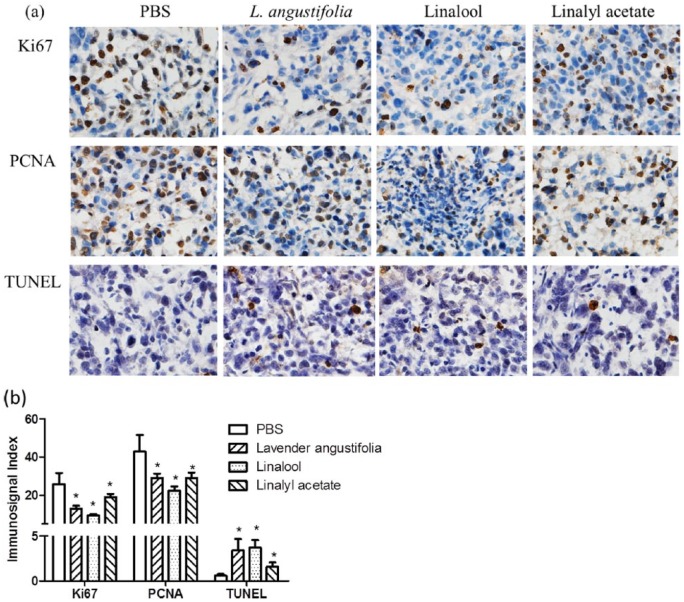
Tumor cell proliferation indexes were assessed by detecting PCNA and Ki67 immunosignals in xenograft tissues. As shown in Figure 7a, the expressions of PCNA and Ki67 were dramatically reduced in xenograft tumors from mice that received treatment of lavender essential oil, linalool, or linalyl acetate compared to that of the control group. These data are consistent with the tumor growth curve as measured by tumor size, indicating that lavender essential oil and linalool suppressed tumor growth in vivo by inhibiting tumor cell proliferation.
Figure 7.
Lavender essential oil, linalool, and linalyl acetate significantly suppressed cell proliferation (Ki67 and PCNA) and induced apoptosis (TUNEL) (mean ± SD; n = 5; *P < .05).
Finally, a TUNEL assay was performed to evaluate if the drug induced apoptotic cell death in xenograft tumor. Positive immunosignals were observed on xenograft tumor sections of mice treated by essential oil, linalool, and linalyl acetate. Quantitative analysis of the images (Figure 7b) indicated a moderate apoptosis rate in lavender essential oil and linalool treatment groups, but a relative low level in linalyl acetate–treated tumor specimens.
Discussion
Cancer is the second leading cause of death, and prostate cancer is the most commonly diagnosed cancer in men. Due to improved early detection and chemotherapy, cancer is no longer considered an incurable disease. Since natural products played an important role in drug discovery32,33 and studies of natural products have led to the development of many potent anticancer agents, such as vincristine, paclitaxel, and silibilin,34-36 it is desirable to continue the efforts aiming at discovering new anticancer agents based on naturally occurring compounds. In the past decade, much effort and progress has been made in prostate cancer research.1,37,38 The effects of green tea polyphenols for prostate cancer prevention and treatment have been studied in epidemiological, preclinical, and early clinical investigations.39
Owing to the possession of antibacterial and anti-cancer activities, plant essential oil has become the focus of phytomedicine research40,41 and drug delivery system formulation development.42 In recent times, lavender is widely cultivated around the world, and L angustifolia is the most common species. Steam distillation yields lavender essential oil, which has enjoyed a long history of folk and traditional therapeutic use for a wide range of modalities including aromatherapy, cosmetics, massage oils, and perfumes. Since the component of L angustifolia essential oil may display some variation,22,43 the major content of the essential oil was examined by LC/MS/MS. The content of linalool and linalyl acetate in the essential oil used in this study is similar to previous reports.44,45 In this research, we investigated the therapeutic effect of L angustifolia essential oil and its major constituents (linalool and linalyl acetate) on human prostate cancer cells.
Invasion and metastasis are the most significant biological characteristics of advanced metastatic prostate cancer. Invasion and metastasis of cancer cells involve many factors. Deriving from different origins, with DU145 cells from brain metastasis and PC-3 cells from bone metastasis,46 DU145 and PC-3 often exhibit different metastatic potentials. Studies have suggested that DU145, but not PC-3, depends on cathepsin L + B activity for invasion.47 In addition, DU145 cell line was found to be more resistant to the inhibition of S100A3 expression than PC-3 cells. In PC-3 cell line, MMP-2 and MMP-9 were downregulated, whereas in DU145 cells, only MMP-9 was downregulated after the inhibition of S100A3.48 In addition, TMPRSS2/ERG gene fusions showed differing effects on radiosensitivity and sensitivity to paclitaxel, a phytochemical extracted from the bark of pacific yew, in PC-3 and DU145 cell lines.49 There are variations in ERG signaling between PC-3 and DU145 containing TMPRSS2/ERG fusions.49 Furthermore, PC-3 is PTEN deficient, and DU145 is PTEN functional. In conjunction with the presence of TMPRSS2/ERG fusion, loss of PTEN expression has been shown to promote prostate carcinogenesis.50 Therefore, cell line variations and discrepancies in the molecular characteristics (ie, the fusing type of TMPRSS2/ERG) may explain why PC-3 cell line is more sensitive to the phytochemicals compared to the DU145 cells.
The suppression of tumorigenesis involves regulation of signal transduction pathways that lead to gene expression alteration, cell cycle arrest, and apoptosis. The induction of apoptosis (Type I programmed cell death) is considered as a crucial mechanism in cancer prevention and treatment. The apoptosis signaling pathway has become a promising target for novel anticancer agent development.51 Flow cytometry with FITC-Annexin V/PI staining suggested that the essential oil, linalool, and linalyl acetate could induce cell death via the apoptotic pathway. This indicates that lavender essential oil and its major constituents have the potential to be developed as chemopreventive agents. Furthermore, uncontrolled cell division or proliferation of mutated cells can contribute to tumorigenesis. Both cell proliferation inhibition and apoptosis induction are highly correlated with the intracellular signaling regulations, which lead to cell cycle arrest in the G0/G1, G2/M, or S phase.52 Blocking passage through cell cycle check points also can inhibit cancer cell growth. Lavender essential oil, linalool, and linalyl acetate induced G2/M and S phase in PC-3 and DU145 cells, respectively, indicating that the natural products targeted differently in these cell lines.
There are 2 typical caspase-dependent pathways involved in apoptosis: the intrinsic mitochondria-dependent apoptosis and the extrinsic death receptor-dependent apoptosis. There is accumulating evidence that upregulation of DR4 and DR5 is related to sensitization of TRAIL-induced apoptosis.53 In this study, we showed that the expression levels of DR4 and DR5 were increased by essential oil and linalool only in DU145, but not PC-3, cell line. This result is consistent with previous experiments, which indicated that the phytochemicals involve different mechanisms in the treatment of DU145 and PC-3 cells. Essential oil and linalool may augment the TRAIL-induced apoptosis by increasing the expression of DR4 and DR5 in DU145 but not PC-3 cell line. However, the study of the mechanism of TRAIL mediated signaling in TMPRSS/ERG fusion-positive prostate cancer is incomplete. Therefore, in order to understand the effect of TRAIL-mediated signaling restoration by phytochemicals, further study on the death receptors in TMPRSS/ERG fusion positive prostate cancers is necessary.
The antitumor activities of essential oils and their components have been reported. For example, the aldehyde compounds isolated from Citrus paradisi essential oil was found to induce apoptosis in HL-60 cells54; jasmine essential oil has cytotoxic effects in A549 and MCF-7 cell lines25; lavender essential oil showed both cytotoxic and apoptotic activities on MCF-7 and HeLa cell lines.55 However, most of the studies were performed only in cell-based assays. In this work, the antiproliferative effects were also studied in in vivo model. We showed that the lavender essential oil and linalool treatment significantly suppressed the growth of xenograft mouse tumors established from PC-3 cell line. Linalyl acetate suppressed xenograft tumor growth by approximately 27% compared to the control group. Although further work is needed for further elucidating the mechanisms of the antitumor activity, these data provide preliminary evidence for the implication of lavender essential oil and its active constituents in treating human prostate cancer and other similar conditions.
In conclusion, we demonstrated that lavender essential oil was effective in inhibiting tumor growth of human prostate cancer xenografts in nude mice. Linalool, but not linalyl acetate, mainly contributed to this effect. This antitumor effect was associated with apoptosis induction and cell proliferation inhibition. Our data suggest that the lavender essential oil and linalool have the potential to be developed as a novel therapeutic agent for prostate cancer treatment.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by Open Research Foundation of Yunnan Key Laboratory of Pharmacology for Natural Products 2014G006 and Scientific Research Foundation for the Returned Overseas Chinese Scholars (PI: Yunqi Zhao). Also, Dr. Yixin Yang spilted the APC fee with us.
References
- 1. Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet. 2016;387:70-82. [DOI] [PubMed] [Google Scholar]
- 2. Chen FZ, Zhao XK. Prostate cancer: current treatment and prevention strategies. Iran Red Crescent Med J. 2013;15:279-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wargovich MJ, Woods C, Hollis DM, Zander ME. Herbals, cancer prevention and health. J Nutr. 2001;131(11 suppl):3034S-3036S. [DOI] [PubMed] [Google Scholar]
- 4. Ho JW, Leung YK, Chan CP. Herbal medicine in the treatment of cancer. Curr Med Chem Anticancer Agents. 2002;2:209-214. [DOI] [PubMed] [Google Scholar]
- 5. Wong YN, Ferraldeschi R, Attard G, de Bono J. Evolution of androgen receptor targeted therapy for advanced prostate cancer. Nat Rev Clin Oncol. 2014;11:365-376. [DOI] [PubMed] [Google Scholar]
- 6. Chaturvedi S, Garcia JA. Novel agents in the management of castration resistant prostate cancer. J Carcinog. 2014;13:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Farooqi AA, Sarkar FH. Overview on the complexity of androgen receptor-targeted therapy for prostate cancer. Cancer Cell Int. 2015;15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Farooqi AA, Butt G, Yousaf G, et al. Making personalized prostate cancer medicine a reality: challenges and opportunities in the re-establishment of gold standards. Pak J Pharm Sci. 2013;26:831-840. [PubMed] [Google Scholar]
- 9. Demichelis F, Rubin MA. TMPRSS2-ETS fusion prostate cancer: biological and clinical implications. J Clin Pathol. 2007;60:1185-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hossain D, Bostwick DG. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. BJU Int. 2013;111:834-835. [DOI] [PubMed] [Google Scholar]
- 11. Yu J, Yu J, Mani RS, et al. An integrated network of androgen receptor, polycomb, and TMPRSS2-ERG gene fusions in prostate cancer progression. Cancer Cell. 2010;17:443-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Farooqi AA, Hou MF, Chen CC, Wang CL, Chang HW. Androgen receptor and gene network: micromechanics reassemble the signaling machinery of TMPRSS2-ERG positive prostate cancer cells. Cancer Cell Int. 2014;14:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fayyaz S, Farooqi AA. miRNA and TMPRSS2-ERG do not mind their own business in prostate cancer cells. Immunogenetics. 2013;65:315-332. [DOI] [PubMed] [Google Scholar]
- 14. Farooqi AA, Naqi A, Qureshi MZ, et al. Prostate cancer is known by the companionship with ATM and miRNA it keeps: craftsmen of translation have dual behaviour with tailors of life thread. Cell Biochem Funct. 2012;30:611-617. [DOI] [PubMed] [Google Scholar]
- 15. Voelkel-Johnson C, King DL, Norris JS. Resistance of prostate cancer cells to soluble TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) can be overcome by doxorubicin or adenoviral delivery of full-length TRAIL. Cancer Gene Ther. 2002;9:164-172. [DOI] [PubMed] [Google Scholar]
- 16. Mitterberger M, Neuwirt H, Cavarretta IT, Hobisch A, Culig Z. Increased resistance to trail-induced apoptosis in prostate cancer cells selected in the presence of bicalutamide. Prostate. 2007;67:1194-1201. [DOI] [PubMed] [Google Scholar]
- 17. Fandy TE, Srivastava RK. Trichostatin A sensitizes TRAIL-resistant myeloma cells by downregulation of the antiapoptotic Bcl-2 proteins. Cancer Chemother Pharmacol. 2006;58:471-477. [DOI] [PubMed] [Google Scholar]
- 18. Jung EM, Lim JH, Lee TJ, Park JW, Choi KS, Kwon TK. Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5). Carcinogenesis. 2005;26:1905-1913. [DOI] [PubMed] [Google Scholar]
- 19. Ashkenazi A. Targeting death and decoy receptors of the tumour-necrosis factor superfamily. Nat Rev Cancer. 2002;2:420-430. [DOI] [PubMed] [Google Scholar]
- 20. Abou El, Naga RN, Azab SS, El-Demerdash E, Shaarawy S, El-Merzabani M, Ammar el SM. Sensitization of TRAIL-induced apoptosis in human hepatocellular carcinoma HepG2 cells by phytochemicals. Life Sci. 2013;92:555-561. [DOI] [PubMed] [Google Scholar]
- 21. Basch E, Foppa I, Liebowitz R, et al. Lavender (Lavandula angustifolia Miller). J Herb Pharmacother. 2004;4:63-78. [PubMed] [Google Scholar]
- 22. Denner SS. Lavandula angustifolia Miller: English lavender. Holist Nurs Pract. 2009;23:57-64. [DOI] [PubMed] [Google Scholar]
- 23. Cavanagh HM, Wilkinson JM. Biological activities of lavender essential oil. Phytother Res. 2002;16:301-308. [DOI] [PubMed] [Google Scholar]
- 24. Sokovic M, Glamoclija J, Marin PD, Brkic D, van Griensven LJ. Antibacterial effects of the essential oils of commonly consumed medicinal herbs using an in vitro model. Molecules. 2010;15:7532-7546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zu Y, Yu H, Liang L, et al. Activities of ten essential oils towards Propionibacterium acnes and PC-3, A-549 and MCF-7 cancer cells. Molecules. 2010;15:3200-3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Budzynska A, Wieckowska-Szakiel M, Sadowska B, Kalemba D, Rozalska B. Antibiofilm activity of selected plant essential oils and their major components. Pol J Microbiol. 2011;60:35-41. [PubMed] [Google Scholar]
- 27. Peana AT, D’Aquila PS, Panin F, Serra G, Pippia P, Moretti MD. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine. 2002;9:721-726. [DOI] [PubMed] [Google Scholar]
- 28. Chang MY, Shen YL. Linalool exhibits cytotoxic effects by activating antitumor immunity. Molecules. 2014;19:6694-6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jana S, Patra K, Sarkar S, et al. Antitumorigenic potential of linalool is accompanied by modulation of oxidative stress: an in vivo study in sarcoma-180 solid tumor model. Nutr Cancer. 2014;66:835-848. [DOI] [PubMed] [Google Scholar]
- 30. Kundakovic T, Stanojkovic T, Kolundzija B, et al. Cytotoxicity and antimicrobial activity of the essential oil from Satureja montana subsp. pisidica (Lamiceae). Nat Prod Commun. 2014;9:569-572. [PubMed] [Google Scholar]
- 31. Peng HY, Lin CC, Wang HY, Shih Y, Chou ST. The melanogenesis alteration effects of Achillea millefolium L. essential oil and linalyl acetate: involvement of oxidative stress and the JNK and ERK signaling pathways in melanoma cells. PLoS One. 2014;9:e95186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mishra BB, Tiwari VK. Natural products: an evolving role in future drug discovery. Eur J Med Chem. 2011;46:4769-4807. [DOI] [PubMed] [Google Scholar]
- 33. Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci. 2005;78:431-441. [DOI] [PubMed] [Google Scholar]
- 34. Hogan FS, Krishnegowda NK, Mikhailova M, Kahlenberg MS. Flavonoid, silibinin, inhibits proliferation and promotes cell-cycle arrest of human colon cancer. J Surg Res. 2007;143:58-65. [DOI] [PubMed] [Google Scholar]
- 35. Cragg GM, Newman DJ. Plants as a source of anti-cancer agents. J Ethnopharmacol. 2005;100:72-79. [DOI] [PubMed] [Google Scholar]
- 36. Singh RP, Agarwal R. Mechanisms and preclinical efficacy of silibinin in preventing skin cancer. Eur J Cancer. 2005;41:1969-1979. [DOI] [PubMed] [Google Scholar]
- 37. Zhao Y, Duan S, Zeng X, et al. Prodrug strategy for PSMA-targeted delivery of TGX-221 to prostate cancer cells. Mol Pharm. 2012;9:1705-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Miano R, Asimakopoulos A, Da Silva R, et al. Focal therapy for prostate cancer: current status and future perspectives. Minerva Urol Nefrol. 2015;67:263-280. [PubMed] [Google Scholar]
- 39. Johnson JJ, Bailey HH, Mukhtar H. Green tea polyphenols for prostate cancer chemoprevention: a translational perspective. Phytomedicine. 2010;17:3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hussain AI, Rathore HA, Sattar MZ, Chatha SA, Sarker SD, Gilani AH. Citrullus colocynthis (L.) Schrad (bitter apple fruit): a review of its phytochemistry, pharmacology, traditional uses and nutritional potential. J Ethnopharmacol. 2014;155:54-66. [DOI] [PubMed] [Google Scholar]
- 41. Aras A, Iqbal MJ, Naqvi SK, et al. Anticancer activity of essential oils: targeting of protein networks in cancer cells. Asian Pac J Cancer Prev. 2014;15:8047-8050. [DOI] [PubMed] [Google Scholar]
- 42. Bilia AR, Guccione C, Isacchi B, Righeschi C, Firenzuoli F, Bergonzi MC. Essential oils loaded in nanosystems: a developing strategy for a successful therapeutic approach. Evid Based Complement Alternat Med. 2014;2014:651593. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43. Loizzo MR, Tundis R, Menichini F, Saab AM, Statti GA, Menichini F. Antiproliferative effects of essential oils and their major constituents in human renal adenocarcinoma and amelanotic melanoma cells. Cell Prolif. 2008;41:1002-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Prashar A, Locke IC, Evans CS. Cytotoxicity of lavender oil and its major components to human skin cells. Cell Prolif. 2004;37:221-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Fakhari AR, Salehi P, Heydari R, Ebrahimi SN, Haddad PR. Hydrodistillation-headspace solvent microextraction, a new method for analysis of the essential oil components of Lavandula angustifolia Mill. J Chromatogr A. 2005;1098:14-18. [DOI] [PubMed] [Google Scholar]
- 46. van Bokhoven A, Varella-Garcia M, Korch C, et al. Molecular characterization of human prostate carcinoma cell lines. Prostate. 2003;57:205-225. [DOI] [PubMed] [Google Scholar]
- 47. Colella R, Jackson T, Goodwyn E. Matrigel invasion by the prostate cancer cell lines, PC3 and DU145, and cathepsin L+B activity. Biotech Histochem. 2004;79:121-127. [DOI] [PubMed] [Google Scholar]
- 48. Kang M, Lee HS, Lee YJ, et al. S100A3 suppression inhibits in vitro and in vivo tumor growth and invasion of human castration-resistant prostate cancer cells. Urology. 2015;85:273.e9-15. [DOI] [PubMed] [Google Scholar]
- 49. Swanson TA, Krueger SA, Galoforo S, et al. TMPRSS2/ERG fusion gene expression alters chemo- and radio-responsiveness in cell culture models of androgen independent prostate cancer. Prostate. 2011;71:1548-1558. [DOI] [PubMed] [Google Scholar]
- 50. Carver BS, Tran J, Gopalan A, et al. Aberrant ERG expression cooperates with loss of PTEN to promote cancer progression in the prostate. Nat Genet. 2009;41:619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hu W, Kavanagh JJ. Anticancer therapy targeting the apoptotic pathway. Lancet Oncol. 2003;4:721-729. [DOI] [PubMed] [Google Scholar]
- 52. Singh RP, Dhanalakshmi S, Agarwal R. Phytochemicals as cell cycle modulators—a less toxic approach in halting human cancers. Cell Cycle. 2002;1:156-161. [PubMed] [Google Scholar]
- 53. Kim SY, Kim JH, Song JJ. c-Cbl shRNA-expressing adenovirus sensitizes TRAIL-induced apoptosis in prostate cancer DU-145 through increases of DR4/5. Cancer Gene Ther. 2013;20:82-87. [DOI] [PubMed] [Google Scholar]
- 54. Hata T, Sakaguchi I, Mori M, et al. Induction of apoptosis by Citrus paradisi essential oil in human leukemic (HL-60) cells. In Vivo. 2003;17:553-559. [PubMed] [Google Scholar]
- 55. Tayarani-Najaran Z, Amiri A, Karimi G, Emami SA, Asili J, Mousavi SH. Comparative studies of cytotoxic and apoptotic properties of different extracts and the essential oil of Lavandula angustifolia on malignant and normal cells. Nutr Cancer. 2014;66:424-434. [DOI] [PubMed] [Google Scholar]