Abstract
Purpose. Acute myeloid leukemia (AML) is the most deadly subtype of leukemia, and many patients with this disease seek other complementary therapies, one of which is Chinese medicine. We set out to provide reliable data regarding the benefit of Chinese herbal medicine (CHM) for AML patients, using mortality as the main outcome measure. We also characterized the herbal prescriptions of patients. Methods. Using the Taiwanese National Health Insurance Research Database, we performed a nationwide population-based cohort study among AML patients from 1997 to 2010. The Cox regression model was used to adjust for comorbidities and other variables, and the hazard ratios (HRs) of CHM users and non–CHM users were compared. Results. After 1:1 matching, 498 patients were included into the study. The HR of the CHM group was 0.41 (95% CI = 0.26-0.65; P = .0001) compared with the non-CHM group. This decrease in HR was also shown to be dose dependent (P < .001). The 3 single-herbs most commonly prescribed were Salvia miltiorrhiza (Dan Shen), Astragalus membranaceus (Huang Qi), and Spatholobus suberectus (Ji Xue Teng). The 3 mutli-herb products most commonly prescribed were Jia Wei Xiao Yao San, Gui Pi Tang, and Qi Ju Di Huang Wan. Conclusion. Prospective controlled clinical data is still needed, however, this study provides real-world data regarding the benefit AML patients may have from CHM. This study suggests that all AML patients, regardless of age or other prognostic factors, may achieve longer survival times when receiving CHM in addition to standard therapy.
Keywords: leukemia, acute myeloid leukemia, Chinese medicine, NHIRD, Taiwan
Introduction
Acute myeloid leukemia (AML) is a blood malignancy that mainly affects patients 55 to 84 years old, with an average 5-year survival rate of 25%.1,2 Since the late 1970s, a common first-line chemotherapy (C/T) regimen for the treatment of AML has been the so-called “7+3,” composed of cytarabine and daunorubicin.3-5 Induction and consolidation therapies have been developed throughout the past 4 decades, with additions such as idarubicin and cyclophosphamide as well as target therapies such as sorafenib, together with many other fine-tunings for different risk groups.6 Complete remission rates of C/T vary between 60% and 80%, and the advent of hematopoietic stem cell transplantation (HSCT) has provided a treatment option for patients who are C/T refractory or present with unfavorable karyotypes.7,8 However, studies show that whether it is C/T or HSCT, long-term survival rates for adult AML patients are grim and stand at 20% to 40%.9 All these make AML the deadliest subtype of leukemia, accounting for 42% of deaths among leukemia patients in the United States, testifying to the need for additional ways to manage this disorder.10
Although Chinese medicine (CM) is growing in popularity as a treatment for various conditions, there is still a long way to go before it is accepted by the mainstream biomedical community. Consequently, there is little interest among researchers to challenge CM in a clinical setting, and the sparse material available regarding CM in the context of AML consists of basic cell-line experiments.11,12 A systematic review published in 2013 retrieved nearly 3000 reported Chinese clinical trials using CM for cancer. Many of these did not use international standards such as CONSORT or TREND and were not peer reviewed, rendering the few studies that might relate to leukemia and AML dubious.13 This, in turn, creates a situation where primary caretakers are unable to provide educated advice to the patients seeking further complementary therapies.
Fortunately, real-world data are available on this topic. According to past data from Taiwan, for example, where CM is available as part of the National Health Insurance (NHI) since 1996, at least 60% of the population had utilized CM services.14 This is a substantial amount of data considering that 99% of Taiwanese are covered by the NHI.15 The NHI recognizes 3 major modalities of CM treatment: (1) Chinese herbal medicine (CHM) products, which come in the form of single-herb or multiherb products; (2) acupuncture, which also encompasses moxibustion and cupping; and (3) manual therapy, including acupressure, Tuina, and chiropractic.16 Since some of these modalities are harder to accurately reproduce than others, in this study we chose to focus on CHM.
All treatments reimbursed as part of the NHI are registered in the Taiwanese NHI Research Database (NHIRD). This dataset provides a nationwide population-based claims database with long-term follow-up, thus reducing the potential for sampling bias. We have previously investigated the use of CHM therapy in diseases such as chronic myeloid leukemia,17 diabetes mellitus,18 rheumatoid arthritis,19 asthma,20 and peptic ulcer disease.21 In the current study, we sought to provide insight into the potential of integration of CHM therapy for patients with AML, through a nationwide population-based retrospective cohort study, comparing CHM users and nonusers, using mortality as the outcome measure.
Methods
Database
The NHIRD contains information regarding each clinical visit and hospitalization incident of all its beneficiaries as well as treatment received and drugs or CHM prescribed. All diseases in the NHIRD are classified using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). In this study, we made use of the Registry for Catastrophic Illness Patients file for collection of data. This file is linked to all inpatient and outpatient claims for catastrophic illness patients. This file included all available NHI records of AML patients in Taiwan as well as all the treatments reimbursed for both standard and CM therapies. For an AML patient to be registered in this file, their diagnosis must be reviewed by a licensed hematologist or oncologist, ensuring the confirmation of disease. After identifying patients through this data set we searched these patients’ corresponding information, including comorbidities, in the NHIRD inpatient and outpatient files. It is important to note that only treatments administered by trained and licensed CM physicians are recorded in the NHIRD.
Study Population
All patients 18 years old and above, diagnosed with AML (ICD-9-CM: 205.0), between January 1997 and December 2010, were included. End of follow-up time was defined as December 31, 2011. Both groups were matched according to age, sex, and index year.
In this study, we excluded patients who had not received any form of standard therapy. During this process of exclusion, we discovered that many patients had begun a course of treatment prior to their AML diagnosis being entered into the NHIRD. We, therefore, also included any patient who had received C/T or radiotherapy within 6 months prior to their formal AML diagnosis.
Statistical Analysis
In this study, SAS 9.4 (SAS Institute Inc, Cary, NC) was used for statistical analysis of all information retrieved from the NHIRD. The statistical differences between the two groups were determined through the χ2 or Fisher exact test for categorical variables, and the independent t test was used for continuous variables. Exploratory analyses of hazard ratios (HRs) were performed through a Cox proportional hazard model, taking into account age, gender, urbanization level, and Charlson comorbidity index (CCI), with a 95% CI. Kaplan-Meier and log-rank tests were used for categorical covariates. Only P values <.05 were considered statistically significant.
Ethical Considerations
This study was conducted in compliance with the Helsinki Declaration guidelines. The data sets obtained from the NHIRD were encrypted and deidentified for the protection of patient privacy, and the identification of individuals was impossible by any means. The Research Ethics Committee of China Medical University and Hospital approved this study (CMUH104-REC2-115).
Results
A total of 4825 patients were diagnosed with AML within the years 1997-2010, and after excluding those who did not meet the study’s inclusion criteria, 2757 patients remained. These patients were grouped according to a cut-point of a minimal 30 days of accumulated use of CHM. In our initial results, the non-CHM group contained significantly more patients older than 60 years. Because the majority of patients older than 65 years do not receive C/T in Taiwan, this posed a substantial risk for statistical a artifact. We were, thus, obliged to randomly match the patients by age (by 5-year intervals) at the cost of sample size. In addition, both groups were matched according to sex and index year, resulting in 249 patients in both the CHM group and the non-CHM group (Figure 1).
Figure 1.
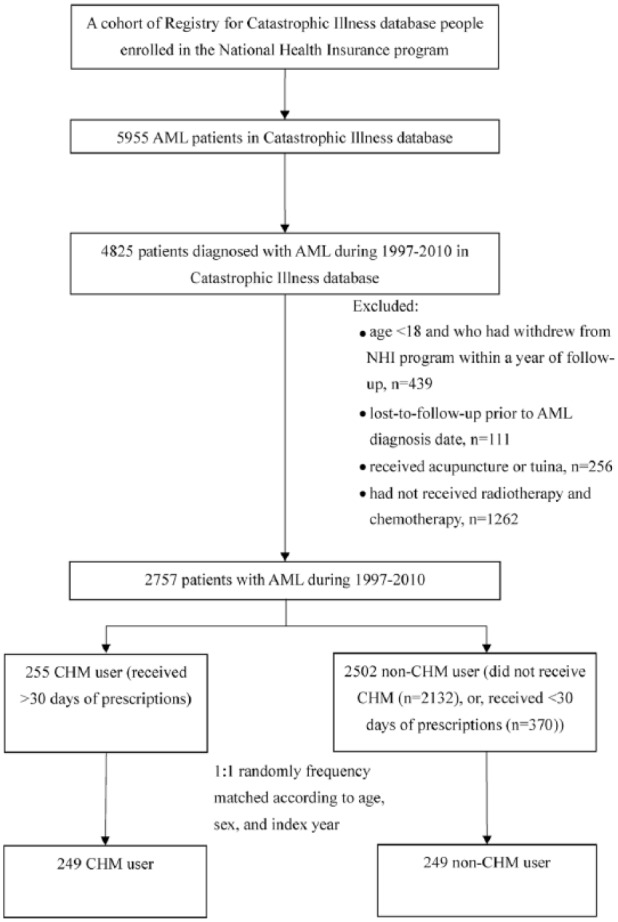
Study population flowchart diagram. Of the total number of AML patients registered in the NHIRD (n = 5955), 4825 patients were diagnosed within the years 1997-2010. After the exclusion process, as well as matching 1:1 by age, sex, and index year, both groups contained 249 patients.
Abbreviations: AML, acute myeloid leukemia; CHM, Chinese herbal medicine; NHIRD, National Health Insurance Research Database.
Characteristics of patients in both groups can be seen in Table 1. The frequency of women was higher in the two groups (56%). The mean age of patients was 46 years, with 51.8% of the patients between the ages 40 and 59 years, 36.9% between 18 and 39 years, and 11.2% aged 60 to 85 years. The NHIRD records the urbanization level of the location where treatment occurred. As the level of urbanization may correlate with socioeconomic status, adding this item bears meaning, and no statistical difference (P < .05) was found to exist between the CHM and non-CHM groups in this respect. Also, no statistical differences were found between the two groups in terms of type of treatment received (radiotherapy, C/T, or stem cell transplant) and CCI score, which has been shown to be related to survival time in AML patients.22 Mean follow-up times were 1.99 and 4.23 years for the non-CHM and CHM groups, respectively.
Table 1.
Characteristics of Acute Myeloid Leukemia (AML) Patients According to Use of CHM.
| Variable | Patients of AML |
P Value | |||
|---|---|---|---|---|---|
| CHM | |||||
| No (n = 249) |
Yes (n = 249) |
||||
| n | Percentage | n | Percentage | ||
| Gendera | |||||
| Female | 140 | 56.22 | 140 | 56.22 | .99 |
| Male | 109 | 43.78 | 109 | 43.78 | |
| Age groupa (years) | |||||
| 18-39 | 92 | 36.95 | 92 | 36.95 | .99 |
| 40-59 | 129 | 51.81 | 129 | 51.81 | |
| 60-85 | 28 | 11.24 | 28 | 11.24 | |
| Age meanb ± SD (years) | 46.67(14.30) | 46.74(14.16) | .9594 | ||
| Urbanization levela,c | |||||
| 1 (highest) | 57 | 22.89 | 55 | 22.09 | .5168 |
| 2 | 74 | 29.72 | 88 | 35.34 | |
| 3 | 55 | 22.09 | 45 | 18.07 | |
| 4 (lowest) | 63 | 25.3 | 61 | 24.5 | |
| CCI scorea | |||||
| 0 | 195 | 78.31 | 214 | 85.94 | .0826 |
| 1 | 28 | 11.24 | 19 | 7.63 | |
| ≥2 | 26 | 10.44 | 16 | 6.43 | |
| Treatmentd | |||||
| Only radiotherapy | 1 | 0.4 | 3 | 1.2 | .589 |
| Only chemotherapy | 213 | 85.54 | 213 | 85.54 | |
| Radiotherapy+Chemotherapy | 35 | 14.06 | 33 | 13.25 | |
| HSCTa | |||||
| No | 223 | 89.56 | 226 | 90.76 | .6517 |
| Yes | 26 | 10.44 | 23 | 9.24 | |
| Follow-up time (mean, median) | 1.76 (0.84) | 4.50 (3.12) | |||
Abbreviations: CHM, Chinese herbal medicine; CCI, Charlson comorbidity index; HSCT, hematopoietic stem cell transplantation.
χ2 Test.
t Test.
The urbanization level was categorized by the population density of the residential area into 4 levels, with level 1 as the most urbanized and level 4 as the least urbanized.
Fischer’s exact test.
Table 2 presents the related HR of the items listed in Table 1, as determined through a Cox proportional hazard model. After adjusting for age, gender, urbanization level, CCI, and treatment received, the HR of morbidity in the CHM group was 0.29 (95% CI = 0.23-0.37; P < .0001) compared with that of the non-CHM group. An additional factor that may influence the efficacy of CHM is the delay between the time of diagnosis and initiation of CHM treatment, defined by some as “lag time to treatment.” The lag time to treatment may differ from patient to patient, and its influence on the improvement of prognosis has been discussed in previous studies.23 We, therefore, took lag time to CHM treatment into account in Table 2, and the adjusted HR was 0.41 (95% CI = 0.26-0.65, P = .0001). Through the records in the NHIRD we were able to track the cumulative days of CHM administered to patients, and thus in Table 3, we investigated a further aspect of reduction in HR. It is shown that patients who took CHM for 30 to 89 days had a HR of 0.39 (95% CI = 0.29-0.52, P < .001) compared with patients from the non-CHM group; patients who took CHM for 90 to 179 days had a HR of 0.30 (95% CI = 0.19-0.47; P < .001), and patients who took CHM for more than 180 days had a HR of 0.13 (95% CI = 0.08-0.22; P < .001). In addition, we analyzed the average time from AML diagnosis to commencement of CHM treatment. Following AML diagnosis, median times for patients from the CHM group to begin herbal therapy were 220, 222, and 197.5 days for the first group (30-89 days), second group (90-179 days), and third group (>180 days), respectively. This is also illustrated in Figure 2.
Table 2.
Cox Model With HRs and 95% CIs of Mortality Associated With CHM and Covariates Among AML Patients.
| Variable | Patients of AML |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Frequency of Death (n = 274) | Crudea |
Adjustedb |
Adjustedc (Plus Lag-Time) |
|||||||
| HR | (95% CI) | P Value | HR | (95%CI) | P Value | HR | (95%CI) | P Value | ||
| CHM use | ||||||||||
| Non-CHM | 173 | 1.00 | Reference | 1.00 | Reference | 1.00 | Reference | |||
| CHM | 101 | 0.30 | (0.23-0.39) | <.0001 | 0.29 | (0.22-0.37) | <.0001 | 0.41 | (0.26-0.65) | 0.0001 |
Abbreviations: HR, hazard ratio; CHM, Chinese Herbal Medicine; AML, acute myeloid leukemia; CCI, Charlson Comorbidity Index.
Relative hazard ratio.
Represented adjusted hazard ratio: mutually adjusted for CHM use, age, gender, urbanization level, CCI score and treatment in Cox proportional hazard regression.
Adjusted for CHM use, age, gender, urbanization level, CCI score, and treatment as well as the lag time for each patient, which was defined as the time from AML diagnosis to initial CHM treatment.
Table 3.
HRs and 95% CIs of Mortality Risk Associated With Cumulative Use Day of CHM Among AML Patients.a
| n | Frequency of Death (n = 274) | Median Lag Time to CHM Treatment (Day) | HR (95% CI) |
||
|---|---|---|---|---|---|
| Crudeb | Adjustedc | ||||
| Non-CHM user (included < 30 days) | 249 | 173 | — | 1 (Reference) | 1 (Reference) |
| CHM user | |||||
| 30-89 days | 133 | 65 | 220 | 0.39 (0.29-0.52)*** | 0.39 (0.29-0.52)*** |
| 90-179 days | 50 | 21 | 222 | 0.31 (0.20-0.49)*** | 0.30 (0.19-0.47)*** |
| >180 days | 66 | 15 | 197.5 | 0.14 (0.08-0.24)*** | 0.13 (0.08-0.22)*** |
Abbreviations: HR, hazard ratio; CHM, Chinese Herbal Medicine; AML, acute myeloid leukemia; CCI, Charlson Comorbidity Index.
*P < .05; **P < .01; ***P < .001.
Relative hazard ratio.
Represented adjusted hazard ratio: mutually adjusted for CHM use, age, gender, urbanization level, CCI score and treatment in Cox proportional hazard regression.
Figure 2.
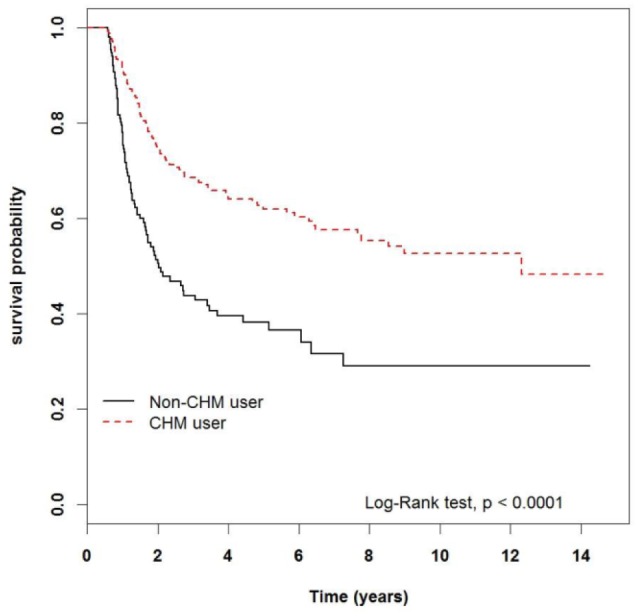
Kaplan-Meier curves of overall survival of patients according to length of consumption of Chinese herbal medicine (CHM): non-CHM user (included < 30 days), 30-89 days, 90-179 days, and >180 days (see Table 3). The curves have been adjusted according to the median lag time to treatment (213 days), as described in Table 3.
The longer survival time of the CHM group can be seen in 2 Kaplan-Meier plots. Figure 3 illustrates the difference in survival time between the two groups, with the CHM group displaying superior outcomes (P < .0001). Figure 4 stratifies the groups according to patient age. It is clear that each age subgroup from the CHM group achieved longer survival time compared with the respective non-CHM subgroup (P < .0001).
Figure 3.
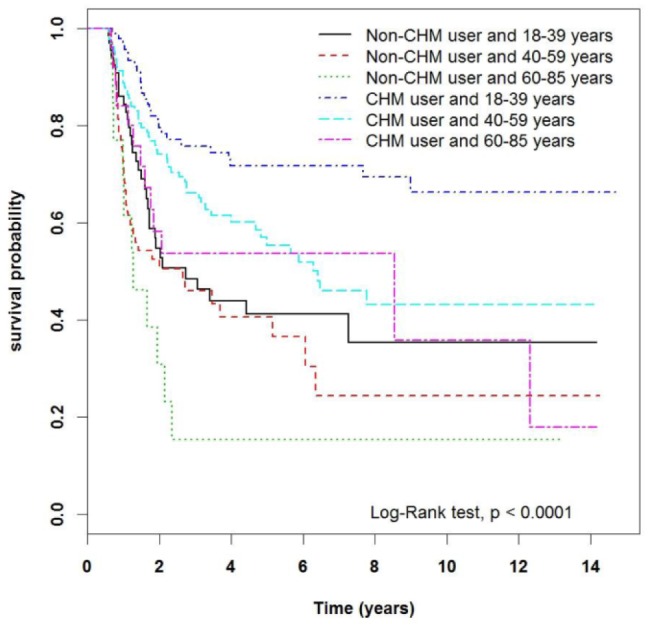
Kaplan-Meier curves of overall survival in patients with acute myeloid leukemia according to Chinese herbal medicine (CHM) use. The curves have been adjusted according to the median lag time to treatment (213 days), as described in Table 3.
Figure 4.
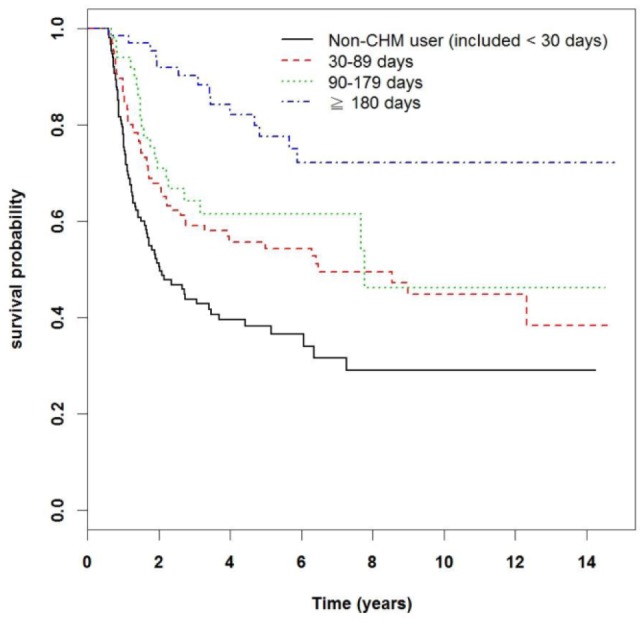
Kaplan-Meier curves of overall survival in patients with acute myeloid leukemia according to Chinese herbal medicine (CHM) use, stratified by age group. The curves have been adjusted according to the median lag time to treatment (213 days), as described in Table 3.
CHM is available to all patients of the NHI as concentrated granulated powders manufactured by pharmaceutical companies with GMP certification, which ensures the quality and consistency of product batches. Single-herb products are produced by extracting 1 single substance, whereas multiherb products are formulae composed of several single herbs. In Table 4, we analyze the HR of the 10 single- and multiherb products most commonly prescribed. All were found to be statistically significant (P < .001). The 3 single-herb products most commonly prescribed were Salvia miltiorrhiza (Dan Shen), Astragalus membranaceus (Huang Qi), and Spatholobus suberectus (Ji Xue Teng). The 3 most commonly prescribed multiherb products were Jia Wei Xiao Yao San, Gui Pi Tang, and Qi Ju Di Huang Wan.
Table 4.
HRs and 95% CIs of Mortality Risk Associated With the Most-Used Herbal Products Among AML Patients.a
| Pin yin Nomenclature | Scientific Name | Accumulated Person-Days | n | Frequency of Mortality | Hazard Ratio (95% CI) |
|
|---|---|---|---|---|---|---|
| Crudeb | Adjustedc | |||||
| Non-Chinese herbal Medicine group | 249 | 173 | 1 (Reference) | 1 (Reference) | ||
| Single-herb products | ||||||
| Dan Shen | Salvia miltiorrhiza | 8711 | 67 | 24 | 0.29 (0.19-0.45)*** | 0.29 (0.19-0.45)*** |
| Huang Qi | Astragalus membranaceus | 7507 | 79 | 35 | 0.35 (0.24-0.50)*** | 0.37 (0.26-0.54)*** |
| Ji Xue Teng | Spatholobus suberectus | 6414 | 57 | 27 | 0.38 (0.25-0.57)*** | 0.36 (0.24-0.54)*** |
| Ge Gen | Pueraria lobata | 5791 | 47 | 14 | 0.20 (0.11-0.34)*** | 0.19 (0.11-0.33)*** |
| Bai Hua She She Cao | Hedyotis diffusa | 5122 | 26 | 13 | 0.40 (0.23-0.71)** | 0.38 (0.22-0.68)*** |
| He Shou Wu | Fallopia multiflora | 4864 | 34 | 10 | 0.21 (0.11-0.41)*** | 0.23 (0.12-0.43)*** |
| Mai Men Dong | Ophiopogon japonicus | 4670 | 57 | 19 | 0.24 (0.15-0.39)*** | 0.25 (0.15-0.41)*** |
| Bei Mu | Fritillariae cirrhosa | 4650 | 64 | 23 | 0.27 (0.17-0.42)*** | 0.26 (0.17-0.40)*** |
| Gan Cao | Glycyrrhiza glabra | 4569 | 60 | 23 | 0.29 (0.19-0.45)*** | 0.29 (0.18-0.45)*** |
| Du Zhong | Eucommia ulmoides | 4417 | 25 | 4 | 0.11 (0.04-0.30)*** | 0.11 (0.04-0.30)*** |
| Multiherb products | ||||||
| Pin yin nomenclature | Scientific name | |||||
| Jia Wei Xiao Yao San | — | 11 822 | 67 | 15 | 0.15 (0.09-0.26)*** | 0.15 (0.09-0.26)*** |
| Gui Pi Tang | — | 7937 | 65 | 30 | 0.37 (0.25-0.55)*** | 0.39 (0.27-0.58)*** |
| Qi Ju Di Huang Wan | — | 5933 | 22 | 3 | 0.09 (0.03-0.28)*** | 0.08 (0.03-0.26)*** |
| Zhi Bo Di Huang Wan | — | 5241 | 40 | 15 | 0.28 (0.16-0.47)*** | 0.26 (0.15-0.44)*** |
| Bu Zhong Yi Qi Tang | — | 5234 | 51 | 27 | 0.45 (0.30-0.68)*** | 0.41 (0.27-0.61)*** |
| Tian Wang Bu Xin Dan | — | 4285 | 36 | 13 | 0.25 (0.14-0.44)*** | 0.21 (0.12-0.38)*** |
| Suan Zao Ren Tang | — | 4278 | 34 | 11 | 0.23 (0.13-0.43)*** | 0.22 (0.12-0.41)*** |
| Xiang Sha Liu Jun Zi Tang | — | 4143 | 46 | 21 | 0.38 (0.24-0.60)*** | 0.34 (0.22-0.54)*** |
| Shu Jing Huo Xue Tang | — | 3940 | 35 | 10 | 0.21 (0.11-0.39)*** | 0.20 (0.10-0.37)*** |
| Shen Ling Bai Zhu San | — | 3846 | 33 | 15 | 0.38 (0.23-0.65)*** | 0.36 (0.21-0.61)*** |
Abbreviations: HR, hazard ratio; CHM, Chinese Herbal Medicine; AML, acute myeloid leukemia.
*P < .05; **P < .01; ***P < .001.
Relative HR.
Represented adjusted HR: mutually adjusted for CHM use, age, gender, urbanization level, Charlson Comorbidity Index score, and treatment in Cox proportional hazard regression.
Discussion
Whether one is a proponent of CM or not, there is no ignoring the fact that patients, including cancer patients, seek these treatments when faced with health issues. A small Canadian cohort study showed that among breast cancer patients, the use of CM had doubled between 1998 and 2005.24 In Australia, 5% of these patients had sought CM, whereas in Germany, a study from 2007 reported that among 1030 patients with gynecological and breast malignancies, 42% had used CM.25,26 One can only guess what the statistics are among leukemia patients because no studies have been conducted. Nevertheless, the above data demonstrate that although we should eventually strive for evidence ranked high on the evidence-based medicine pyramid, such as meta-analyses and randomized clinical trials, we must in the meanwhile embrace any reliable data.
Our most significant finding was that the use of CHM was associated with a significant decrease in HR. Patients from the CHM group had a 49% lower HR than that of the non-CHM group. Moreover, it was shown that patients who took CHM for longer periods of time experienced a more substantial decrease in HR. Certain influences, such as motivation of patients, cannot be ruled out in this type of retrospective study; however, this dose-dependence supports the possibility of a causal relationship between the two.
In the past, there have also been conflicting reports as to whether or not the so-called lag time to treatment predicts outcome in certain populations in AML.27,28 To test the possibility that decrease in HR was a result of this phenomenon and not a result of dose dependence, we analyzed the median lag time to treatment of each one of the subgroups in Table 3. We found that all 3 groups commenced CHM treatment roughly 7 months following AML diagnosis, and this did not seem to be a significant factor.
There are different types of factors that may lead to favorable or unfavorable outcomes in AML, most significant of which are various molecular mutations. A recent study performed in Taiwan showed that out of 378 AML patients diagnosed between 2000 and 2008, 16.9% were diagnosed with unfavorable chromosomal abnormalities, and 13.2% presented positive ones.29 Yet one of the most substantial prognostic factors, associated with poor survival in AML, is the genetic mutation of Fms-like tyrosine kinase 3 (FLT3).30,31 Roughly 30% of AML patients are found to possess mutated FLT3 and receive a target therapy named sorafenib, which induces growth arrest and apoptosis of cells fostering this mutation.32 However, cytogenetic abnormalities, such as the favorable t(8;21) or the unfavorable 11q23 and the FLT3 gene mutation mentioned above, all go unregistered in the NHIRD, and we were, therefore, unable to conclusively rule out the influence of these on our results.
We sought to address the lack of FTL3 status of patients in the NHIRD and adjusted our data for the use of sorafenib. Unfortunately, only in recent years has it become standard to prescribe this drug to FLT3-positive patients in Taiwan, and consequently, this attempt did not yield results. Despite the lack of these data, we did manage to challenge the notion that the improvement in HR of the CHM group was a result of sampling bias. In many cases of high-risk or refractory AML, patients opted for HSCT. HSCT patients accounted for roughly 10% of the cohort and were evenly distributed among the two groups. Moreover, HSCT patients from the CHM group had a 38% lower HR compared with HSCT patients who did not receive CHM (P = .04). This suggests that not only were high-risk patients evenly distributed between the two groups, but also that they might benefit from CHM treatment.
An additional important aspect of our results is shown in Figure 4. Not only does the incidence of AML increase with age, but the latter is also an important prognostic factor.33 One population-based study from Sweden stratified more than 2700 AML patients according to 5-year age groups and showed a near perfect correlation between this stratification and survival time.34 In the Kaplan-Meier curve presented in Figure 3, we performed a similar stratification according to age and discovered that in this Taiwanese cohort, there was also a strong correlation between age and survival time. Correspondingly, there was a proportional improvement of survival time for patients in the CHM group over the non-CHM group. This finding once again challenges the notion that improvement of HR was due to an uneven distribution of low-risk and high-risk patients among the two groups. It is known that the incidence of AML with favorable cytogenetic abnormalities decreases with age.35,36 Following this rationale, if improvement in HR was a result of favorable prognostic factors, and not CHM, plot trends would have appeared differently in Figure 4.
An investigation of the specific role played by the single- and multiherb preparations presented in the results is beyond the scope of this study. These were presented for 2 main reasons: (1) to summarize the collective experience of Taiwanese CM physicians and provide other CHM practitioners a reference point and (2) in hopes that future research, whether it be basic or clinical, may use these findings to assert a causal relation and therapeutic mechanism.
Conclusion
It is a reasonable assumption that a portion of AML patients seek complementary forms of therapies following their diagnosis. Although this study cannot replace a controlled clinical trial or prove a causal relation, it strongly suggests that adjunctive CHM therapy may benefit AML patients of all ages, including those with poor prognosis. We are unable to infer whether this is a direct result of the CHM consumed, a synergistic effect with standard agents, or even just a result of the former reducing the adverse effect of the latter and consequently leading to more successful treatments. We hope that this study may serve as a foundation for further future research. The quality of life and safety outcomes should also be included in future studies.
Acknowledgments
This study was supported by China Medical University under the Aim for Top University Plan of the Ministry of Education, Taiwan. It was also supported in part by China Medical University Hospital (DMR-105-005) and the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019). This study was based in part on data from the National Health Insurance Research Database, provided by the National Health Insurance Administration, and Ministry of Health and Welfare and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of the National Health Insurance Administration, Ministry of Health and Welfare, or National Health Research Institutes. The funding agencies had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by China Medical University under the Aim for Top University Plan of the Ministry of Education, Taiwan, and China Medical University Hospital, Taiwan (DMR-105-005). This study was also supported in part by the Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105-TDU-B-212-133019).
References
- 1. Dohner H, Lubbert M, Fiedler W, et al. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 2014;124:1426-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Comparative Study on Spleen with TCM and Western Medicine. Zhejiang J Tradit Chin Med. 2006;41:1-6. [Google Scholar]
- 3. Yates JW, Wallace HJ, Jr, Ellison RR, Holland JF. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep. 1973;57:485-488. [PubMed] [Google Scholar]
- 4. Yates J, Glidewell O, Wiernik P, et al. Cytosine arabinoside with daunorubicin or adriamycin for therapy of acute myelocytic leukemia: a CALGB study. Blood. 1982;60:454-462. [PubMed] [Google Scholar]
- 5. Rai KR, Holland JF, Glidewell OJ, et al. Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood. 1981;58:1203-1212. [PubMed] [Google Scholar]
- 6. Tefferi A, Letendre L. Going beyond 7 + 3 regimens in the treatment of adult acute myeloid leukemia. J Clin Oncol. 2012;30:2425-2428. [DOI] [PubMed] [Google Scholar]
- 7. Lowenberg B, Downing JR, Burnett A. Acute myeloid leukemia. N Engl J Med. 1999;341:1051-1062. [DOI] [PubMed] [Google Scholar]
- 8. Zittoun RA, Mandelli F, Willemze R, et al. Autologous or allogeneic bone marrow transplantation compared with intensive chemotherapy in acute myelogenous leukemia. European Organization for Research and Treatment of Cancer (EORTC) and the Gruppo Italiano Malattie Ematologiche Maligne dell’Adulto (GIMEMA) Leukemia Cooperative Groups. N Engl J Med. 1995;332:217-223. [DOI] [PubMed] [Google Scholar]
- 9. Mandelli F, Vignetti M, Suciu S, et al. Daunorubicin versus mitoxantrone versus idarubicin as induction and consolidation chemotherapy for adults with acute myeloid leukemia: the EORTC and GIMEMA Groups Study AML-10. J Clin Oncol. 2009;27:5397-5403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. American Cancer Society. Cancer Facts & Figures 2015. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 11. Lin SJ, Cheng YY, Chang CH, Lee CH, Huang YC, Su YC. Traditional Chinese medicine diagnosis “yang-xu zheng”: significant prognostic predictor for patients with severe sepsis and septic shock. Evid Based Complement Alternat Med. 2013;2013:759748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hui H, Chen Y, Yang H, et al. Oroxylin A has therapeutic potential in acute myelogenous leukemia by dual effects targeting PPARgamma and RXRalpha. Int J Cancer. 2014;134:1195-1206. [DOI] [PubMed] [Google Scholar]
- 13. Li X, Yang G, Li X, et al. Traditional Chinese medicine in cancer care: a review of controlled clinical studies published in Chinese. PLoS One. 2013;8:e60338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen FP, Chen TJ, Kung YY, et al. Use frequency of traditional Chinese medicine in Taiwan. BMC Health Serv Res. 2007;7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Office of Information Services, Executive Yuan. The Republc of China (Taiwan): ROC Yearbook 2013. http://http://www.ey.gov.tw/en/ [Accessed August 2016)]
- 16. Huang TP, Liu PH, Lien AS, Yang SL, Chang HH, Yen HR. A nationwide population-based study of traditional Chinese medicine usage in children in Taiwan. Complement Ther Med. 2014;22:500-510. [DOI] [PubMed] [Google Scholar]
- 17. Fleischer T, Chang TT, Chiang JH, Chang CM, Hsieh CY, Yen HR. Adjunctive Chinese herbal medicine therapy improves survival of patients with chronic myeloid leukemia: a nationwide population-based cohort study. Cancer Med. 2016;5:640-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lee AL, Chen BC, Mou CH, Sun MF, Yen HR. Association of traditional Chinese medicine therapy and the risk of vascular complications in patients with type ii diabetes mellitus: a nationwide, retrospective, Taiwanese-registry, cohort study. Medicine (Baltimore). 2016;95:e2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Huang MC, Pai FT, Lin CC, et al. Characteristics of traditional Chinese medicine use in patients with rheumatoid arthritis in Taiwan: a nationwide population-based study. J Ethnopharmacol. 2015;176:9-16. [DOI] [PubMed] [Google Scholar]
- 20. Huang TP, Liu PH, Lien AS, Yang SL, Chang HH, Yen HR. Characteristics of traditional Chinese medicine use in children with asthma: a nationwide population-based study. Allergy. 2013;68:1610-1613. [DOI] [PubMed] [Google Scholar]
- 21. Huang CY, Lai WY, Sun MF, et al. Prescription patterns of traditional Chinese medicine for peptic ulcer disease in Taiwan: a nationwide population-based study. J Ethnopharmacol. 2015;176:311-320. [DOI] [PubMed] [Google Scholar]
- 22. Breccia M, Frustaci AM, Cannella L, et al. Comorbidities and FLT3-ITD abnormalities as independent prognostic indicators of survival in elderly acute myeloid leukaemia patients. Hematol Oncol. 2009;27:148-153. [DOI] [PubMed] [Google Scholar]
- 23. Guo H, Liu L, Baak JP. Is the improvement of prognosis of patients with metastatic pulmonary adenocarcinoma treated with TCM herbal medicine due to lag time to treatment bias? Integr Cancer Ther. 2011;10:234-239. [DOI] [PubMed] [Google Scholar]
- 24. Boon HS, Olatunde F, Zick SM. Trends in complementary/alternative medicine use by breast cancer survivors: comparing survey data from 1998 and 2005. BMC Womens Health. 2007;7:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fasching PA, Thiel F, Nicolaisen-Murmann K, et al. Association of complementary methods with quality of life and life satisfaction in patients with gynecologic and breast malignancies. Support Care Cancer. 2007;15:1277-1284. [DOI] [PubMed] [Google Scholar]
- 26. Kremser T, Evans A, Moore A, et al. Use of complementary therapies by Australian women with breast cancer. Breast. 2008;17:387-394. [DOI] [PubMed] [Google Scholar]
- 27. Bertoli S, Berard E, Huguet F, et al. Time from diagnosis to intensive chemotherapy initiation does not adversely impact the outcome of patients with acute myeloid leukemia. Blood. 2013;121:2618-2626. [DOI] [PubMed] [Google Scholar]
- 28. Sekeres MA, Elson P, Kalaycio ME, et al. Time from diagnosis to treatment initiation predicts survival in younger, but not older, acute myeloid leukemia patients. Blood. 2009;113:28-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cheng C-L, Li C-C, Hou H-A, et al. Risk factors and clinical outcomes of acute myeloid leukaemia with central nervous system involvement in adults. BMC Cancer. 2015;15:344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Levis M, Small D. FLT3: ITDoes matter in leukemia. Leukemia. 2003;17:1738-1752. [DOI] [PubMed] [Google Scholar]
- 31. Yanada M, Matsuo K, Suzuki T, Kiyoi H, Naoe T. Prognostic significance of FLT3 internal tandem duplication and tyrosine kinase domain mutations for acute myeloid leukemia: a meta-analysis. Leukemia. 2005;19:1345-1349. [DOI] [PubMed] [Google Scholar]
- 32. Zhang W, Konopleva M, Shi Y-X, et al. Mutant FLT3: a direct target of sorafenib in acute myelogenous leukemia. J Natl Cancer Inst. 2008;100:184-198. [DOI] [PubMed] [Google Scholar]
- 33. Deschler B, Lubbert M. Acute myeloid leukemia: epidemiology and etiology. Cancer. 2006;107:2099-2107. [DOI] [PubMed] [Google Scholar]
- 34. Juliusson G, Antunovic P, Derolf Å, et al. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113:4179-4187. [DOI] [PubMed] [Google Scholar]
- 35. Bacher U, Kern W, Schnittger S, Hiddemann W, Haferlach T, Schoch C. Population-based age-specific incidences of cytogenetic subgroups of acute myeloid leukemia. Haematologica. 2005;90:1502-1510. [PubMed] [Google Scholar]
- 36. Bruserud O, Hovland R, Wergeland L, Huang TS, Gjertsen BT. Flt3-mediated signaling in human acute myelogenous leukemia (AML) blasts: a functional characterization of Flt3-ligand effects in AML cell populations with and without genetic Flt3 abnormalities. Haematologica. 2003;88:416-428. [PubMed] [Google Scholar]


