Abstract
Docetaxel (DTX) is an effective commercial anticancer agent for chemotherapy in non–small cell lung cancer (NSCLC), breast cancer, gastric cancer, and prostate cancer, but its adverse effects including edema, neurotoxicity, and hair loss limit its application. To improve the chemotherapeutic efficacy of DTX and reduce adverse effects, combination therapy is one of the alternative methods. So chrysin, which has various biological activities including anticancer effects, was considered. In vitro, the combination of chrysin and DTX was investigated in A549 cells. Increased cytotoxicity, suppressed cellular proliferation, and induced apoptosis were observed with posttreatment of chrysin following DTX treatment. In vivo, chrysin enhanced the tumor growth delay of DTX and increased DTX-induced apoptosis in the A549-derived xenograft model. Furthermore, chrysin prevented DTX-induced edema in ICR mouse. These results indicated that chrysin strengthened the therapeutic efficacy of DTX and diminished the adverse effect of DTX, suggesting chrysin could be exploited as an adjuvant therapy for NSCLC.
Keywords: chrysin, docetaxel, lung cancer, therapeutic efficacy, edema
Introduction
Lung cancer is the leading cause of cancer mortality in the world. Non–small cell lung cancer (NSCLC) comprises 85% of lung cancer and easily obtains resistance to chemotherapy. Thus, enhancement of chemotherapeutic efficacy is needed in NSCLC to reduce mortality. Docetaxel (DTX) is a chemical therapeutic agent in the taxane group and is commercially used to remedy NSCLC as well as breast cancer, gastric cancer, and prostate cancer. It leads to mitotic arrest and apoptosis by binding β-tubulin in microtubules and abrogating tumor progression.1 Although DTX is an effective chemotherapeutic agent, application of DTX is limited by its various side effects such as hair loss, low blood pressure, heart attack, neurosensory and neuromotor symptoms, edema, low white blood cell level, muscle pain, and so on.2 These adverse effects cause a decline in the patient’s quality of life. To solve these complications, DTX is often combined with a sensitizer. Therefore, good sensitizer candidates of anticancer drugs are needed to enhance chemotherapeutic efficacy and minimize adverse effects.
Natural products are good sources for adjuvant therapy because they have various biological activities and low toxicity. Various flavonoids have been studied for complementary medicines.3,4 Especially, chrysin (5,7-dihydroxyflavone) is a natural flavonoid from propolis, honey, fruits, Pleurotus ostreatus, Oroxylum indicum, Matricaria chamomilla, and passion flower.5-9 The major pharmacological properties of chrysin reportedly include antioxidant,10,11 anti-inflammatory,4 antiviral,12 and antitumor13-16 activities. Reactive oxygen species induced by oxidative stress cause cellular damage, but superoxide dismutase, glutathione peroxidase, and catalase scavenge reactive oxygen species and protect the cells. Chrysin increases superoxide dismutase, glutathione peroxidase, and catalase and thus exhibits an antioxidant effect. Furthermore, chrysin inhibits inflammation because it decreases cyclooxygenase-2 levels.17 These mechanisms of chrysin could prevent allergy, Parkinson disease, cerebral ischemia, and cancer. In vitro and in vivo studies of chrysin have suggested various mechanisms for its anticancer effect. Chrysin affects the PI3K/Akt pathway, caspase-3 and -8 activation, the tumor necrosis factor (TNF)-α pathway, and mitochondrial membrane depolarization. Additionally, chrysin inhibits histone deacetylase and aromatase, thus suppressing cellular proliferation. Therefore, these results indicate that chrysin could be a good candidate for a chemosensitizer.
In this study, we investigated whether chrysin enhances therapeutic efficacy of DTX in NSCLC A549 cells and A549-derived xenograft models. Furthermore, we found that chrysin extirpates DTX-induced edema as an adverse effect.
Materials and Methods
Cell Cultures
A549 (human NSCLC cells, ATCC No. CCL-185) cells were incubated in F-12K nutrient mixture (Gibco, Grand Island, NY) with 1% penicillin-streptomycin (GenDEPOT, Barker, TX) and 10% fetal bovine serum (GenDEPOT) in a 95% air/5% CO2 incubator.
Cytotoxicity of Combination Treatment
A549 cells were seeded in 96-well plates at a density of 2000 cells/well. After 48 hours, DTX (Taxotere, Sanofi Aventis, London, UK) was added into the media. DTX was serially diluted, and its final concentration ranged from 10 to 0.625 ng/mL. Chrysin (Aldrich, St Louis, MO) was added 24 hours before or after or immediately after DTX treatment. The concentration of chrysin ranged from 100 to 10 µM. Forty-eight hours after DTX treatment, 10 µL of cell counting kit-8 reagent (DOJINDO, Kumamoto, Japan) was added to each well and cells were incubated for 3 hours at 37°C.18 Absorbance at 450 nm was measured using a microplate reader (Infinite M200 PRO, TECAN, Grodig, Austria). To evaluate the combination effect, combination index (CI) was calculated with Equation (1)19:
where C is the concentration, A and B are tested drugs, X is the percentage of effect, ICx is the concentration to achieve x % drug effect, and CI of ≤1 indicates synergic or additive effect.
Clonogenic Assay
A549 cells were seeded in 6-well plates at a density of 100 cells/well for the control group, 500 cells/well for DTX or chrysin only treatment group, and 1000 cells/well for combination treatment of DTX and chrysin (n = 3). After 24 hours, DTX (2.5 ng/mL finally) was added into the media for 2 hours and media were changed. On the next day, working solution of chrysin (in 5% dimethyl sulfoxide [DMSO]) was diluted to 25 µM (0.5% DMSO) in the media. Then, the media in the 6-well plates were changed to media containing chrysin. The cells were incubated until colonies formed (10 days). The colonies were stained with 0.5% crystal violet in 10% methanol for 15 minutes and washed with tap water several times. Colonies consisting of over 50 cells were counted by FluorChem E (San Jose, CA). Plating efficiency (PE) and survival fraction (SF) from the counted colonies were calculated according to Equations (2) and (3), respectively20:
Apoptosis Assay
A549 cells were seeded in 6-well plates at a density of 40 000 cells/well and incubated for 24 hours (n = 3). DTX (2.5 ng/mL, final) and chrysin (25 µM, final) were added to cells at an interval of 24 hours and then cells were incubated for 24 hours. The same volume of media was added to the other cells. To elucidate the apoptotic pathway, expression of apoptosis-related proteins was measured by human apoptosis antibody array-membrane kit (Abcam, Cambridge, UK). Cells were lysed with lysis buffer including protease inhibitor, and extracted proteins were quantified by BCA assay (Thermo Scientific, Waltham, MA). According to kit protocol, 42 apoptosis-related proteins were detected using the antibody-printed membrane and their expression was observed by FluorChem E. The intensity of their expression was analyzed by Image J.
Animals
For the edema study, female ICR mice (4 weeks old) were purchased from SAMTACO (Osan, Korea). For tumor growth examination, female BALB/c nude mice (4 weeks old) were purchased from SLC (Shizuoka, Japan). Mice were handled using protocols approved by Duksung Women’s University Animal Care Committee (No. 2013-004 and No. 2013-005) in accordance with the guidelines of the care and use of laboratory animals, Duksung Women’s University.
Drug Administration and Measurement of Paw Edema
Chrysin was dissolved in DMSO before use. DTX and chrysin were diluted in normal saline solution before administration. Paw volume was measured by an Ugo Basile plethysmometer (model 7140). Before treatment with samples, the mouse’s paw volume was recorded as initial volume (V0). Chrysin (25 and 50 mg/kg) was orally administered 2 hours before DTX treatment, and DTX (25 mg/kg) was intravenously injected. The same volume of saline was injected into the control group. The mouse’s paw volume (Vt) was measured after 30 minutes. Edema (%) was calculated by Equation (4):
Drug Administration and Measurement of Tumor Volume
A549 cells (1 × 106 cells/100 µL) were subcutaneously implanted in the right leg of mice. When tumor volume was about 100 mm3, the mice were randomly divided into 4 groups. Chrysin (50 mg/kg) was orally administered once a day, 5 days a week, during the experimental period, and DTX (10 mg/kg) was intravenously injected once on the first day. Control group was injected with the same volume of saline. The length and width of tumor was measured by caliper every 3 days for 4 weeks and tumor volume was calculated as in Equation (5):
To assess change of tumor growth, a percent T/C was calculated as in Equation (6):
Delta T is changes in tumor volume for each treatment group, and delta C is changes in tumor volume for control group. The value of delta T or delta C is measured by subtracting the tumor volume on the first day from the tumor volume on the final observation day.
TUNEL Assay
After measurement of final tumor growth, tumors were isolated, fixed with 4% formalin (JUNSEI Chemical Co, Ltd, Tokyo, Japan), and protected with 30% saccharose (DUKSAN Reagents, Gyeonggi, Korea) in distilled water. Each tumor tissue was molded with OCT compound (Leica Biosystems, Nussolch, Germany) at a very low temperature. The frozen tissues were sliced at 5-µm thickness by cryostat (Leica Biosystems), and the sections were placed on a glass slide (MUTO Pure Chemicals Co, Ltd, Tokyo, Japan). The sections were hydrated with 70% ethanol, bathed in 3% H2O2 in distilled water, and washed with distilled water twice. The tissue sections were incubated in warm distilled water at 60°C for 1 hour and cooled to room temperature. The tissue slides were treated with TdT-labeling buffer and incubated in TdT (Sigma)/biotinylated deoxyuridine (Roche Diagnostics, Mannheim, Germany) diluted in TdT-labeling buffer for 1 hour at 37°C in a humid chamber. The reaction was stopped with terminating buffer, and the slides were washed with distilled water. The sliced tissues were blocked with 2% BSA (bioWORLD, Dublin, OH) in phosphate-buffered saline, and washed in phosphate-buffered saline. Then, the sections were incubated with ABC complex, washed with 0.05 M Tris buffer, and colorized with DAB (Vector Laboratories, Inc, Burlingame, CA). The mounted slides were observed using Nikon eclipse microscope (Nikon Instruments Inc, Melville, NY).
Statistical Analysis
Data were evaluated using Student’s t test. All data are shown as the mean ± standard deviation. All P values <.05 were considered statistically significant.
Results
Chrysin Modifies DTX-Induced Cytotoxicity
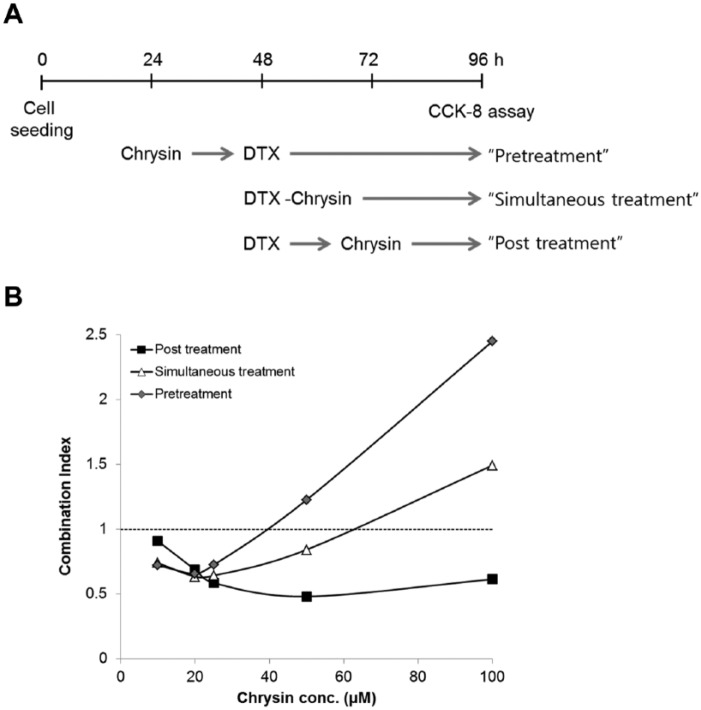
DTX or chrysin showed dose-dependent cytotoxicity as described previously.21,22 As a result, the concentrations of chrysin and DTX for combination treatment were fixed from 100 to 10 µM and from 10 to 0.625 ng/mL, respectively. To observe the combination effect of chrysin on DTX-induced cytotoxicity, chrysin was treated before or after or immediately after DTX treatment (Figure 1A). As shown in Figure 1B, combination index (CI) of chrysin increased with dose escalation. Interestingly, posttreatment of chrysin enhanced DTX-induced cytotoxicity more than other treatments. The lowest CI value was 0.48 at 50 µM chrysin (ratio chrysin–DTX = 1:4.6 × 10−5). So a standard method of chrysin treatment after DTX treatment was used in subsequent experiments.
Figure 1.
Chrysin increased DTX-induced cytotoxicity in A549 cells.
(A) Flowchart of drug treatment for cytotoxicity. (B) Combination effect of chrysin on DTX-induced cytotoxicity. Combination index below 1 showed synergic effect.
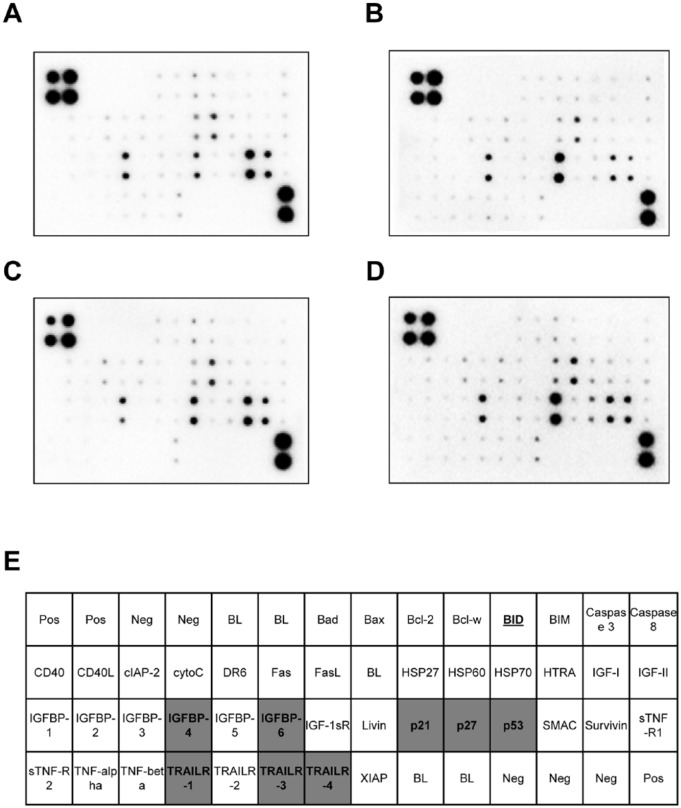
A549 cells were treated with (A) control, (B) DTX, (C) chrysin, (D) combination of chrysin and DTX. The lysates were applied on apoptosis-related protein antibody-printed membrane according to Materials and Methods. (E) Each spot represented apoptosis-related proteins (n = 2). Gray block showed synergic effect in the combination of chrysin and DTX. Underline represented inhibition of expression in the combination of chrysin and DTX comparing with control, chrysin, or DTX. Abbreviations: Pos, positive control; Neg, negative control; BL, blank.
Combination of Chrysin and DTX Inhibits Cell Proliferation
To investigate the effect of chrysin on cell proliferation, colony formation was measured by clonogenic assay. Treatment time of DTX was limited to 2 hours because DTX powerfully inhibits cell proliferation. When the survival fraction (SF) of control was 1, DTX, chrysin, or the combination of chrysin and DTX was 0.13, 0.37, or 0.04, respectively (Figure 2). Chrysin synergized the antiproliferative effect of DTX.
Figure 2.
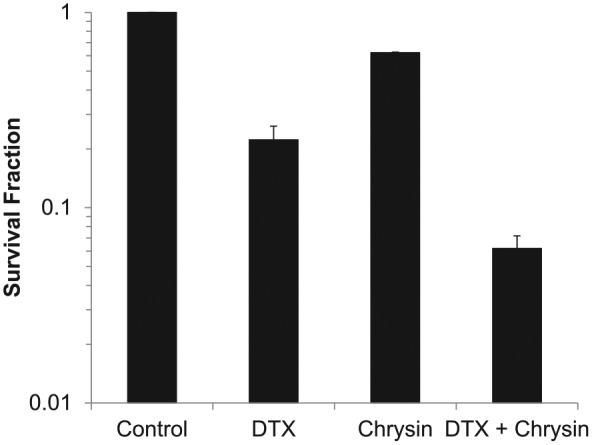
Combination of chrysin and DTX declines survival fraction in A549 cells.
A549 cells were treated with chrysin, DTX, or combination of chrysin and DTX and incubated until the formation of colonies as described Materials and Methods.
A549 cells were treated with (A) control, (B) DTX, (C) chrysin, (D) combination of chrysin and DTX. The lysates were applied on apoptosis-related protein antibody-printed membrane according to Materials and Methods. (E) Each spot represented apoptosis-related proteins (n = 2). Gray block showed synergic effect in the combination of chrysin and DTX. Underline represented inhibition of expression in the combination of chrysin and DTX comparing with control, chrysin, or DTX. Abbreviations: Pos, positive control; Neg, negative control; BL, blank.
Chrysin Upregulated Apoptosis-Related Proteins
Chrysin and DTX were reported to induce apoptosis in previous studies.22,23 In order to interpret chrysin-induced apoptosis, expression of apoptosis-related proteins (43 species) was assessed with the human apoptosis assay kit. As in previous reports,24,25 DTX or chrysin treatment alone increased expression of several proteins in cell lysates. DTX-treated cells showed the induction of BIM (Bcl-2 interacting protein), Bax (proapoptotic member), BID and Bad (BH-3 members), p53, and p21. Furthermore, insulin-like growth factor binding protein (IGFBP)-1 and -2, TNF-α and -β, Fas, Fas ligand (FasL), and TNF-related apoptosis-inducting ligand receptor (TRAILR) 1 and 2 as extrinsic pathway of apoptosis were increased by DTX (Figure 3B). Chrysin-treated cells also showed the increase of Bad, Bax, cytochrome C, p21, p27, and p53. Moreover, death receptor (DR) 6, IGFBP-3, and TNF-α and -β were inducted by chrysin (Figure 3C). We focused on the proteins upregulated by the combination of chrysin and DTX compared with chrysin or DTX alone. As shown in Figure 3D, IGFBP-4 and -6 and TRAILR-1, -3, and -4 were enhanced by the combination of chrysin and DTX. Apoptosis and cell proliferation-related p53, p21, and p27 were also induced. On the other hand, BID was decreased by the combination of chrysin and DTX comparing with control, chrysin, or DTX only.
Figure 3.
Combination of chrysin and DTX synergizes the expression of apoptosis-related proteins.
A549 cells were treated with (A) control, (B) DTX, (C) chrysin, (D) combination of chrysin and DTX. The lysates were applied on apoptosis-related protein antibody-printed membrane according to Materials and Methods. (E) Each spot represented apoptosis-related proteins (n = 2). Gray block showed synergic effect in the combination of chrysin and DTX. Underline represented inhibition of expression in the combination of chrysin and DTX comparing with control, chrysin, or DTX. Abbreviations: Pos, positive control; Neg, negative control; BL, blank.
Chrysin Stimulates the Therapeutic Efficacy of DTX
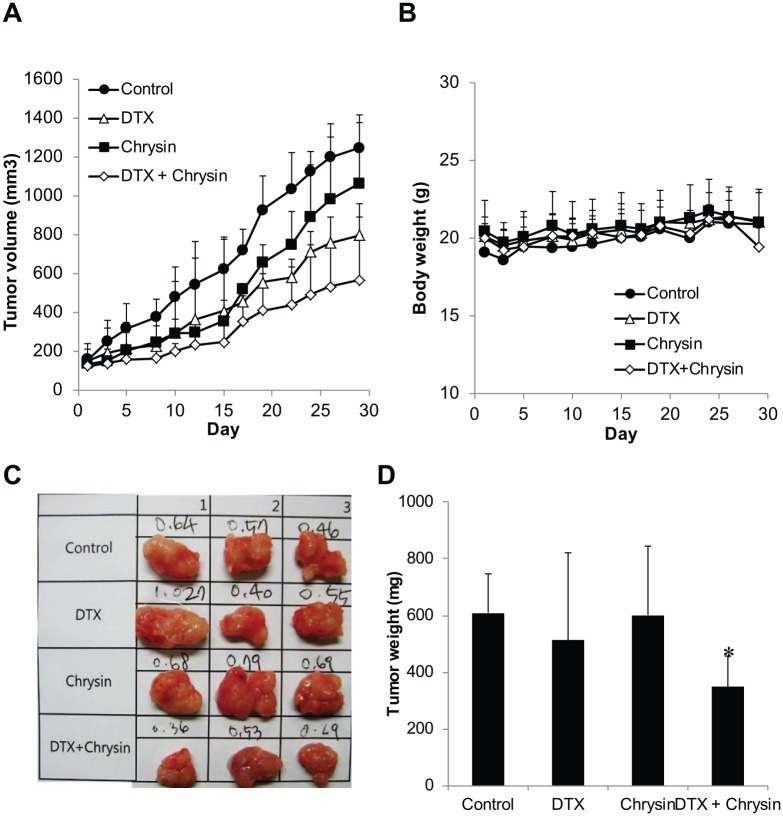
In the in vitro assays, chrysin enhanced DTX-induced cytotoxicity. For evaluation of the anticancer effect in A549-derived xenograft model, tumor growth was investigated. DTX was intravenously injected as a single dose on the first day. Chrysin was orally administered every day for 4 weeks. As shown in Figure 4, treatment with DTX only or chrysin only delayed the tumor growth compared to the control group. T/C (%) of DTX-treated group and chrysin-treated group was 59.6% and 85.6%, respectively. DTX as a commercial anticancer drug retained its chemotherapeutic efficacy for 4 weeks with a single treatment. The chrysin-treated group also showed an anticancer effect like the DTX-treated group, but after 2 weeks the anticancer effect of chrysin diminished and the chrysin-treated tumor increased quickly although chrysin was administered every day. However, the combination of chrysin with DTX (T/C, 40.6%) significantly delayed tumor growth more than control, chrysin, or DTX alone and decreased tumor weight. So the combination therapy (0.35 ± 0.1 g) reduced tumor weight of control by 57% (0.61 ± 0.1 g) as shown in Figure 4C and D. During the tumor growth assay, no groups showed a decrease of body weight (Figure 4B), indicating no toxicity. These results indicate that chrysin stimulates the anticancer effect of DTX.
Figure 4.
Combination of chrysin and DTX delayed tumor growth without loss of body weight in the A549-derived xenograft model.
(A) Tumor growth and (B) body weight of mice were determined for 30 days (n = 5). After the final measurement of tumor growth, tumors were isolated. (C) Tumor weights were determined (n = 3). (D) The data shown are mean ± standard deviation. *, The value of P < .05 was indicated to be statistically significant (t test).
Chrysin Intensifies the Chemotherapeutic Efficacy of DTX by Inducing Apoptosis In Vivo
Chrysin induced the expression of apoptosis-related proteins in A549 cells (Figure 3) and improved DTX-induced protein expression. So we investigated apoptosis by chrysin and DTX in the A549-derived xenograft model. As shown in Figure 5, brown spots indicated apoptotic cells in A549-derived tumor tissue. The combination of chrysin and DTX increased the number of brown spots more than the other groups. The results show that the combination of chrysin and DTX induces apoptosis, which increases the anticancer effect in vivo.
Figure 5.
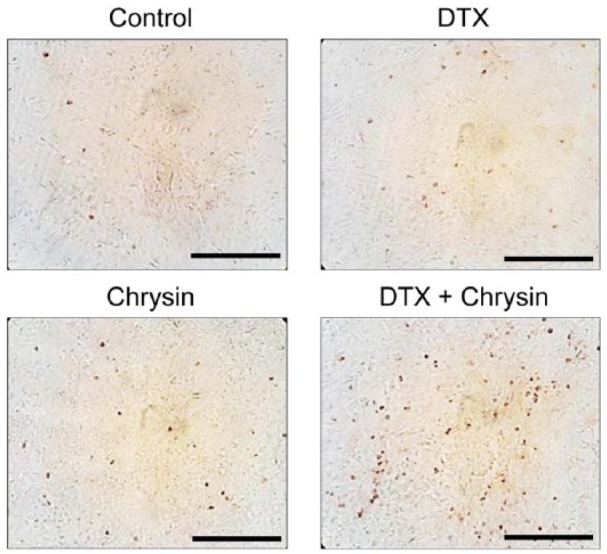
Combination of chrysin and DTX induced apoptosis in the A549-derived xenograft model.
Tumor tissues were isolated and subjected to TUNEL staining. Brown spot represented the apoptotic cells. Scale bar = 50 µm.
Chrysin Prevents DTX-Induced Edema
So far, our results suggest that chrysin enhances the chemotherapeutic efficacy of DTX. We investigated whether chrysin attenuated an adverse effect of DTX. One of the side effects of DTX is edema, which reduces the patient’s quality of life. The DTX-induced edema model was established in our previous study.26 To elucidate the effect of chrysin on DTX-induced edema, chrysin was orally administered 2 hours before DTX treatment. Paw edema was measured before and after drug treatment. Paw of DTX-treated group swelled to twice that of the control group, but paw volume in the chrysin-treated group (50 µM) was the same as before treatment (Figure 6). Furthermore, DTX injection after chrysin treatment resulted in 25% edema of DTX only. These results suggested that chrysin prevented DTX-induced edema.
Figure 6.
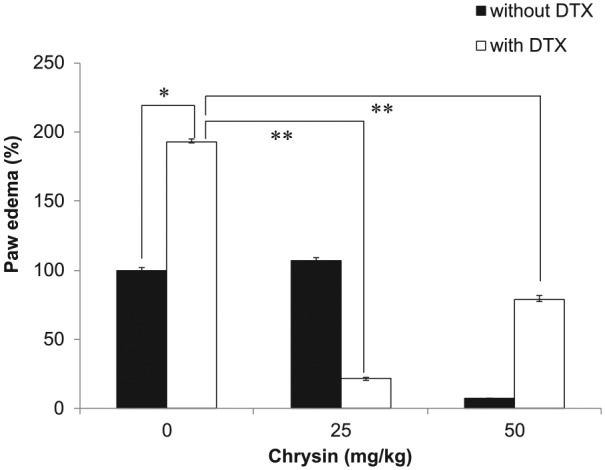
Chrysin prevented DTX-induced edema.
DTX injection induced paw edema (n = 6). Mice administered with chrysin did not show edema. The data shown are mean ± standard deviation (*, P < .05; **, P < .005; t test).
Discussion
In this study, we elucidated that chrysin improved the existing chemotherapy of DTX in vivo. The reports to date indicated that chrysin showed anticancer effects in various cancer cell lines, but the anticancer effect of chrysin was not thoroughly studied in in vivo models. Owing to poor solubility and bioavailability of chrysin, many researchers tried adjuvant therapy rather than chrysin alone for chemotherapy. The combination therapy could enhance the therapeutic efficacy. Chrysin increased cisplatin-induced p53 level and induced apoptosis in HepG2 cells.27 Doxorubicin- or cisplatin-induced cytotoxicity was augmented by chrysin, which depletes glutathione in lung cancer cells.28,29 However, the combinations of chrysin and commercial anticancer drugs were reported only in the therapeutic view without the toxicological view. Here, we have revealed that combination with chrysin enhanced the therapeutic efficacy and improved the adverse effects in DTX chemotherapy.
Drug interactions can have additive, synergic, or antagonistic effects in the combined condition. Sometimes, the combination can change the therapeutic efficacy even though the 2 combined materials or medicines are unchanged. For example, pretreatment or posttreatment of Shenfu in a gemcitabine-plus-cisplatin regimen produced significantly different results.30 Therefore, we defined the condition that maximized the effect in the combination of chrysin and DTX (Figure 1). In our results, pretreatment with chrysin had a dose-dependent antagonistic effect on DTX. On the other hand, chrysin following DTX synergized the anticancer effect in vitro and in vivo. Under this condition, the mechanism of cytotoxicity and antiproliferation was elucidated in this study.
In previous studies, chrysin was reported to induce apoptosis in various cancer cell lines via increase of p53, p21, JNK, p38, and Bax13,31 and suppression of XIAP.32 DTX also induced apoptosis via Bax, Bak, and JNK. In Figure 4, apoptosis-related proteins were increased by chrysin or DTX, which coincided with previous studies. In addition to these proteins, we found several proteins regarding extrinsic pathway of apoptosis were induced by chrysin or DTX in A549 cells (Figure 4). Moreover, the combination of chrysin and DTX potently enhanced the expression of p53, p21, p27, IGFBP-4 and -6, and TRAILR-1, -3, and -4. The tumor-suppressor protein p53 plays a critical part in apoptosis and cell proliferation. In response to DNA damage, p53 accumulates and activates the transcription of p21, IGFBP-6.33,34 As well as p21, p27 induced by the combination of chrysin and DTX triggered G1 arrest. TRAILR is one of the death receptors and is known to induce apoptosis and transformed cells without toxicity of normal cells.35 TRAILR induced by the combination of chrysin and DTX stimulated apoptosis via a p53-independent pathway. Similarly, our results coincided with that of quercetin, having similar structure to chrysin, which induced TRAILR and apoptosis in lung cancer cells.36 These results indicated that the combination of chrysin and DTX potentiated apoptosis and antiproliferation via multiple pathways.
In the xenograft model, daily treatment of chrysin delayed tumor growth, but its efficacy was weaker than DTX single treatment. The combination of chrysin and DTX increased apoptosis and enhanced therapeutic efficacy (Figures 4 and 5). The combination treatment reduced tumor weight, but not body weight of mice. Our results indicated that chrysin as an adjuvant agent in DTX therapy was effective in vivo as well as in vitro.
Most of all, our results indicate that the advantage of chrysin is that it decreases DTX-induced edema. In combination therapy, the ideal goal is the reduction of adverse effects and the increase of therapeutic effects. The combination of chrysin and DTX could be applicable to the ideal therapeutic regimen. Edema, an adverse effect of DTX, is caused by obstruction of fluid, increased blood vessel permeability, and so on. It is one of the pathological symptoms of inflammation. On the other hand, chrysin is well known to have anti-inflammatory effects via inhibition of COX-2, prostaglandin-E2, and NF-κB.4,37 So we suggest that DTX-induced edema could be prevented by the biological activities of chrysin, although the mechanism of chrysin-stimulated attenuation of edema is not elucidated in this study.
In conclusion, chrysin as an adjuvant agent reinforced the therapeutic efficacy of DTX and mitigated edema. Further preclinical and potentially clinical studies are suggested to further assess the suitability of the combination of chrysin plus DTX for chemotherapy of NSCLC.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by a grant from the National Research Foundation of Korea (NRF-2014R1A1A3049498) and the Korean Health Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (HI06C0868).
References
- 1. Kodumudi KN, Woan K, Gilvary DL, Sahakian E, Wei S, Djeu JY. A novel chemoimmunomodulating property of docetaxel: suppression of myeloid-derived suppressor cells in tumor bearers. Clin Cancer Res. 2010;16:4583-4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verweij J, Clavel M, Chevalier B. Paclitaxel (Taxol) and docetaxel (Taxotere): not simply two of a kind. Ann Oncol. 1994;5:495-505. [DOI] [PubMed] [Google Scholar]
- 3. Li-Weber M. Targeting apoptosis pathways in cancer by Chinese medicine. Cancer Lett. 2013;332:304-312. [DOI] [PubMed] [Google Scholar]
- 4. O’Leary KA, de Pascual-Teresa S, Needs PW, Bao YP, O’Brien NM, Williamson G. Effect of flavonoids and vitamin E on cyclooxygenase-2 (COX-2) transcription. Mutat Res. 2004;551:245-254. [DOI] [PubMed] [Google Scholar]
- 5. Kasala ER, Bodduluru LN, Madana RM, Athira KV, Gogoi R, Barua CC. Chemopreventive and therapeutic potential of chrysin in cancer: mechanistic perspectives. Toxicol Lett. 2015;233:214-225. [DOI] [PubMed] [Google Scholar]
- 6. Patel S. Emerging adjuvant therapy for cancer: propolis and its constituents. J Diet Suppl. 2016;13:245-268. [DOI] [PubMed] [Google Scholar]
- 7. Chen LJ, Games DE, Jones J. Isolation and identification of four flavonoid constituents from the seeds of Oroxylum indicum by high-speed counter-current chromatography. J Chromatogr A. 2003;988:95-105. [DOI] [PubMed] [Google Scholar]
- 8. Zanoli P, Avallone R, Baraldi M. Behavioral characterisation of the flavonoids apigenin and chrysin. Fitoterapia. 2000;71(suppl 1):S117-S123. [DOI] [PubMed] [Google Scholar]
- 9. Martos I, Ferreres F, Tomas-Barberan FA. Identification of flavonoid markers for the botanical origin of eucalyptus honey. J Agric Food Chem. 2000;48:1498-1502. [DOI] [PubMed] [Google Scholar]
- 10. Blonska M, Bronikowska J, Pietsz G, Czuba ZP, Scheller S, Krol W. Effects of ethanol extract of propolis (EEP) and its flavones on inducible gene expression in J774A.1 macrophages. J Ethnopharmacol. 2004;91:25-30. [DOI] [PubMed] [Google Scholar]
- 11. Wu NL, Fang JY, Chen M, Wu CJ, Huang CC, Hung CF. Chrysin protects epidermal keratinocytes from UVA- and UVB-induced damage. J Agric Food Chem. 2011;59:8391-8400. [DOI] [PubMed] [Google Scholar]
- 12. De Clercq E. Current lead natural products for the chemotherapy of human immunodeficiency virus (HIV) infection. Med Res Rev. 2000;20:323-349. [DOI] [PubMed] [Google Scholar]
- 13. Sawicka D, Car H, Borawska MH, Niklinski J. The anticancer activity of propolis. Folia Histochem Cytobiol. 2012;50:25-37. [DOI] [PubMed] [Google Scholar]
- 14. Yu XM, Phan T, Patel PN, Jaskula-Sztul R, Chen H. Chrysin activates Notch1 signaling and suppresses tumor growth of anaplastic thyroid carcinoma in vitro and in vivo. Cancer. 2013;119:774-781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Samarghandian S, Nezhad MA, Mohammadi G. Role of caspases, Bax and Bcl-2 in chrysin-induced apoptosis in the A549 human lung adenocarcinoma epithelial cells. Anticancer Agents Med Chem. 2014;14:901-909. [DOI] [PubMed] [Google Scholar]
- 16. Beaumont DM, Mark TM, Jr, Hills R, Dixon P, Veit B, Garrett N. The effects of chrysin, a Passiflora incarnata extract, on natural killer cell activity in male Sprague-Dawley rats undergoing abdominal surgery. AANA J. 2008;76:113-117. [PubMed] [Google Scholar]
- 17. Harris GK, Qian Y, Leonard SS, Sbarra DC, Shi X. Luteolin and chrysin differentially inhibit cyclooxygenase-2 expression and scavenge reactive oxygen species but similarly inhibit prostaglandin-E2 formation in RAW 264.7 cells. J Nutr. 2006;136:1517-1521. [DOI] [PubMed] [Google Scholar]
- 18. Reilly CA, Taylor JL, Lanza DL, Carr BA, Crouch DJ, Yost GS. Capsaicinoids cause inflammation and epithelial cell death through activation of vanilloid receptors. Toxicol Sci. 2003;73:170-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27-55. [DOI] [PubMed] [Google Scholar]
- 20. Bae W, Lim HK, Kim KM, et al. Apoptosis-inducing activity of marine sponge Haliclona sp. extracts collected from Kosrae in nonsmall cell lung cancer A549 Cells. Evid Based Complement Altern Med. 2015;2015:717959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jung J, Park SJ, Chung HK, et al. Polymeric nanoparticles containing taxanes enhance chemoradiotherapeutic efficacy in non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;84:e77-e83. [DOI] [PubMed] [Google Scholar]
- 22. Khoo BY, Chua SL, Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int J Mol Sci. 2010;11:2188-2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kocdor H, Ates H, Aydin S, et al. Zinc supplementation induces apoptosis and enhances antitumor efficacy of docetaxel in non-small-cell lung cancer. Drug Des Devel Ther. 2015;9:3899-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li X, Huang Q, Ong CN, Yang XF, Shen HM. Chrysin sensitizes tumor necrosis factor-alpha-induced apoptosis in human tumor cells via suppression of nuclear factor-kappaB. Cancer Lett. 2010;293:109-116. [DOI] [PubMed] [Google Scholar]
- 25. He X, Zhang T. Alteration in the balance of prosurvival and proapoptotic signalling pathways leads to sequence-dependent synergism between docetaxel and sorafenib in human non-small cell lung cancer cell lines. Cell Biochem Biophys. 2014;68:411-418. [DOI] [PubMed] [Google Scholar]
- 26. Choi J, Ko E, Chung HK, et al. Nanoparticulated docetaxel exerts enhanced anticancer efficacy and overcomes existing limitations of traditional drugs. Int J Nanomed. 2015;10:6121-6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li X, Huang JM, Wang JN, Xiong XK, Yang XF, Zou F. Combination of chrysin and cisplatin promotes the apoptosis of Hep G2 cells by up-regulating p53. Chem Biol Interact. 2015;232:12-20. [DOI] [PubMed] [Google Scholar]
- 28. Brechbuhl HM, Kachadourian R, Min E, Chan D, Day BJ. Chrysin enhances doxorubicin-induced cytotoxicity in human lung epithelial cancer cell lines: the role of glutathione. Toxicol Appl Pharmacol. 2012;258:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kachadourian R, Leitner HM, Day BJ. Selected flavonoids potentiate the toxicity of cisplatin in human lung adenocarcinoma cells: a role for glutathione depletion. Int J Oncol. 2007;31:161-168. [PMC free article] [PubMed] [Google Scholar]
- 30. Wu WY, Long SQ, Zhang HB, et al. Improvement of quality of life with Shenfu injection in non small cell lung cancer patients treated with gemcitabine plus cisplatin regimen. Chin J Integr Med. 2006;12:50-54. [DOI] [PubMed] [Google Scholar]
- 31. Pichichero E, Cicconi R, Mattei M, Canini A. Chrysin-induced apoptosis is mediated through p38 and Bax activation in B16-F1 and A375 melanoma cells. Int J Oncol. 2011;38:473-483. [DOI] [PubMed] [Google Scholar]
- 32. Woo KJ, Jeong YJ, Park JW, Kwon TK. Chrysin-induced apoptosis is mediated through caspase activation and Akt inactivation in U937 leukemia cells. Biochem Biophys Res Commun. 2004;325:1215-1222. [DOI] [PubMed] [Google Scholar]
- 33. Vanlandingham JW, Tassabehji NM, Somers RC, Levenson CW. Expression profiling of p53-target genes in copper-mediated neuronal apoptosis. Neuromolecular Med. 2005;7:311-324. [DOI] [PubMed] [Google Scholar]
- 34. Chua MW, Lin MZ, Martin JL, Baxter RC. Involvement of the insulin-like growth factor binding proteins in the cancer cell response to DNA damage. J Cell Commun Signal. 2015;9:167-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mahmood Z, Shukla Y. Death receptors: targets for cancer therapy. Exp Cell Res. 2010;316:887-899. [DOI] [PubMed] [Google Scholar]
- 36. Youn H, Jeong JC, Jeong YS, Kim EJ, Um SJ. Quercetin potentiates apoptosis by inhibiting nuclear factor-kappaB signaling in H460 lung cancer cells. Biol Pharm Bull. 2013;36:944-951. [DOI] [PubMed] [Google Scholar]
- 37. Khan MS, Devaraj H, Devaraj N. Chrysin abrogates early hepatocarcinogenesis and induces apoptosis in N-nitrosodiethylamine-induced preneoplastic nodules in rats. Toxicol Appl Pharmacol. 2011;251:85-94. [DOI] [PubMed] [Google Scholar]