Abstract
Purpose. So-called spontaneous remissions in cancer often seem to occur after febrile events. Mistletoe preparations (MPs) are used off-label intravenously to induce fever within concepts of integrative oncology. We wanted to investigate the frequency of febrile reactions and safety related to intravenously applied MPs (IAMPs). Methods. This was a retrospective analysis of data from consecutive cancer patients who were treated in 2 anthroposophic hospitals with IAMPs. The main outcome parameter was the rate of core temperature increase to ≥38.5°C within 24 hours after IAMPs. Secondary outcome parameters were Common Toxicity Criteria for Adverse Events (CTCAE; version 4.0). Results. 59 patients, with in total 567 IAMPs, were analyzed; 45 patients (76%, 95% CI = 65%-87%) had an increase of core temperature to ≥38.5°C after at least 1 treatment. Mean increase in temperature was 1.5°C ± 0.8°C. Adverse events were mostly fever-related symptoms (headache, joint pain, shivering). Grade 1 allergic reactions were documented in 0.6% of treatments. CTCAEs grade 3 to 5 did not occur; 38/59 patients had advanced and/or metastatic disease. Conclusion. IAMPs resulted in febrile reactions to >38.5°C in the majority of patients and can be considered as safe. Adverse events were mostly related to fever and were not severe.
Keywords: Viscum album, anthroposophic medicine, adverse events, safety
Introduction
Subcutaneous injection of preparations from the European mistletoe (Viscum album L) is widely used among cancer patients in Germany and Switzerland.1 The preparations from different manufacturers are officially approved as drugs by the state health departments and, when prescribed by physicians, reimbursed from the health insurances in these countries. They all contain substances like mistletoe lectins or viscotoxins with cytotoxic and immunodulatory properties.2,3 In some hospitals and outpatient clinics in Germany and Switzerland, mistletoe preparations (MPs) are also used off-label intravenously in cancer patients within treatment concepts of anthroposophic medicine.4 Since the 1920s, induction of fever is regarded as favorable for cancer patients from the viewpoint of anthroposophic medicine, and infusions of MPs are often used to induce fever.5 The view that fever might be beneficial is supported by reports from the end of the 19th and the first half of the 20th century when fever was induced by bacterial toxins, leading to remarkable response rates in various tumor entities.6,7 Furthermore, so called spontaneous remissions seem to occur more frequently after febrile events.8-11 Systemic hyperthermia as such has the potential to directly damage tumor cells, is known to be a potent sensitizer for radiotherapy and chemotherapy, and can activate natural killer cells and dendritic cells, which play a major role in the antitumor defense of the body.12-14 It has been hypothesized that pathogen-associated molecular patterns (PAMPs) such as lipopolysaccharides or lectins from plants (eg, mistletoe species15), which bind to pattern recognition receptors such as toll-like receptors, play a major role in fever related anticancer effects because fever and PAMPs themselves can activate cytotoxic T-cells.7 Even though cancer treatment with PAMPs is not established, it currently has a sound theoretical basis. Subcutaneous MP treatment also can cause fever in principle, but only in dosages that cause strong local reactions at the site of injection, which are limiting.16
In an integrative oncology meeting of clinicians and researchers in 2011 (http://www.integrative-oncology.ch/archiv/symposium-2011), it turned out that numerous off-label intravenous applications of MPs had been performed in the Ita Wegman Klinik, CH-Arlesheim, and Lukasklinik, CH-Arlesheim, to induce fever. However, little is known about the safety, tolerability, and clinical effects of intravenous MP application. It was, therefore, decided to analyze the available data retrospectively. From clinical experience, the following hypotheses about intravenous application of MPs were proposed:
it can induce an increase in body temperature to ≥38.5°C within 24 hours;
it is safe—no organ toxicity and no >grade 2 toxicity according to Common Toxicity Criteria for Adverse Events (CTCAE Version 4.0); and
it is tolerable, despite occasionally nausea, chills, muscle soreness, vomiting, and other fever-related symptoms may be experienced by the patients.
Patients and Methods
Hospital patient charts of the 2 hospitals (Ita Wegman Klinik, Lukasklinik) actively performing post–radiotherapy and chemotherapy intravenous application of MPs to induce fever were studied to test the hypotheses. Both hospitals had official general allowance of the Kanton Basel-Land to collect anonymized data. The study was approved by the ethical committee of University Medical Center Freiburg, Germany (104/12, March 15, 2012). Inclusion criteria were the following: age ≥18 years, any type of histology-confirmed malignancy, first admission to the hospital after January 1, 2006, in the Lukasklinik and December 1, 2011, in the Ita Wegman Klinik, and at least 1 treatment with any type of intravenous MP during hospital stay. There were no exclusion criteria. The time points for the start of the evaluation (January 1, 2006, in the Lukasklinik and December 1, 2011, in the Ita Wegman Klinik) were set by the representatives of the respective hospitals. The patient files were selected from the archives of the 2 hospitals. All patient files were screened in the order of admission of the patients, beginning from the time points mentioned above. Files of included patients were studied, and the following information was, if available, manually transferred into a case report form: patient age; sex; Eastern Cooperative Oncology Group (ECOG) and Karnofsky performance state (0% and 100% = normal activity and 4% and 10% = very high level of care needed, respectively); date of admission and discharge; cancer type; stage at first diagnosis and at date of admission; time interval between first diagnosis and admission; date and type of first-, second-, and third-line therapies; date, type, dosage, frequency, and duration of MP infusions; course of body temperature during and 24 hours after MP infusions; all available data about side effects, adverse events, quality of life, immunological parameters, and tumor response; and date of last contact or death of the patient. All MP infusions from the included patients were analyzed. Follow-up period was until March 2013.
The primary objective of this study was to analyze the frequency of fever (increase of body temperature to ≥38.5°C)17 after infusion of MPs. Secondary objectives were time course and peak of temperature, safety, tolerability, course of immunological parameters, and response rate and survival.
Statistical Considerations
An increase in body temperature to ≥38.5°C within 24 hours after start of intravenous MP administration in ≥50% of the patients was regarded as relevant, and a rate of ≤25% uninteresting. Hence, an exact binomial test is done for the test problem. H0: p0 ≤25%; H1: p0 >25%. The above test with a nominal 2.5% one-sided significance level had at least 90% power to detect a significant increase when the alternative proportion of 50% is assumed and the sample size is 42. A total number of at least 300 intravenous MP administrations was expected and assumed to detect relevant safety risks.
Statistical Analysis
Descriptive statistics (including medians, means, SDs, and 95% CIs) were determined for all patients, infusions, and specific subgroups. Univariate regression analysis and multivariate regression analysis with stepwise selection of variables was performed for predictive factors associated with induction of elevated body temperature. To estimate dose effects, the different MPs were cumulated and standardized to a Z-value according to the formula Z = X – µ/σ, where X is a random variable with expectation value E(X) = µ, variance Var(X) = σ2, and the respective SD is σ. Data on case report forms were entered in an electronic database and analyzed with SPSS 21.0 (IBM, Armonk, NY).
Results
A total of 59 patients (9 in the Ita Wegman Klinik, 50 in the Lukasklinik) who fulfilled the inclusion criteria were consecutively included in the retrospective analysis out of 371 patient records screened. They were admitted between January 1, 2006, and July 12, 2006, in the Lukasklinik and between December 1, 2011, and May 31, 2012, in the Ita Wegman Klinik. Table 1 shows patient- and tumor-related characteristics. The majority of the patients had advanced and/or metastatic disease without option for curative treatment. The most common types of cancer (breast, colorectal, and lung) were the most frequent diagnosis in the collective.
Table 1.
Characteristics of All Patients and Patients With Advanced and/or Metastatic Disease.a
| All Patients (n = 59) | Patients With Advanced and/or Metastatic Disease (n = 38) | |
|---|---|---|
| Female/Male | 34 (58%)/25 (42%) | 19 (50%)/19 (50%) |
| Age (years) | 57 ± 11 | 59 ± 10 |
| Time interval from first diagnosis to admission (months) | 30 ± 50 | 37 ± 59 |
| Tumor entity | ||
| Breast cancer | 17 (29%) | 7 (18%) |
| Colorectal cancer | 9 (15%) | 7 (18%) |
| Lung cancer | 5 (8%) | 5 (13%) |
| Ovarian cancer | 4 (7%) | 4 (11%) |
| Prostate cancer | 4 (7%) | 2 (5%) |
| Head and neck cancer | 4 (7%) | 2 (5%) |
| Pancreatic cancer | 2 (3%) | 2 (5%) |
| Other types of cancer | 14 (24%) | 9 (24%) |
n (%) Or mean ± SD.
Pretreatment was documented in 58 of the 59 patients: 51 (86%) had received surgery, 35 (59%) chemotherapy, 19 (32%) radiation therapy, and 16 (27%) other therapies such as hormone or immune therapy. During hospital stay for MP infusions, no concomitant chemotherapy or radiation therapy was performed. Also, 567 infusions with MP were documented in the 59 patients. Table 2 shows documented parameters in relation to the MP infusion: 112 infusions (20%) were performed within 3 weeks after surgery, chemotherapy, or radiation therapy, and 428 infusions had a time interval of >3 weeks between these therapies (no documentation for 27 infusions). Most frequently, Iscador M had been used. Dosages and time intervals between the infusions varied considerably (Table 2 and Figure 1).
Table 2.
Parameters in Relation to the Infusions of Mistletoe Preparations (MPs).a
| Parameter | Mean ± SD | Median/Range | Documented Infusions (n) |
|---|---|---|---|
| Number of MP infusions per patient | 10 ± 11 | 6/1-66 | 567 |
| Dosage of mistletoe preparation (in mg) | 69 ± 160 | 25/2-1000 | 567 |
| Dosage Iscador P | 39 ± 27 | 30/10-140 | 20 |
| Dosage Iscador M | 25 ± 24 | 20/3-140 | 268 |
| Dosage Iscador Q | 27 ± 22 | 20/2-120 | 141 |
| Dosage Iscador A | 38 ± 19 | 36/14-80 | 47 |
| Dosage Iscador U | 28 ± 11 | 36/12-40 | 11 |
| abnobaVISCUM Fraxini | 141 ± 35 | 160/40-200 | 55 |
| Helixor P | 712 ± 335 | 900/50-1000 | 25 |
| Duration of infusion (hours) | 2.8 ± 0.8 | 3/0.5-6 | 456 |
| Time interval between infusions (days) | 26 ± 58 | 14/1-868 | 504 |
Abbreviations: A, Abietes (fir tree); Fraxini, ash tree; M, Mali (apple tree); P, Pini (pine tree); Q, Quercus (oak tree); U, Ulmus (elm tree).
n = 59 Patients. Iscador preparations are fermented with lactobacilli and sterile filtered; abnobaVISCUM and Helixor are unfermented, sterile-filtered preparations.
Figure 1.
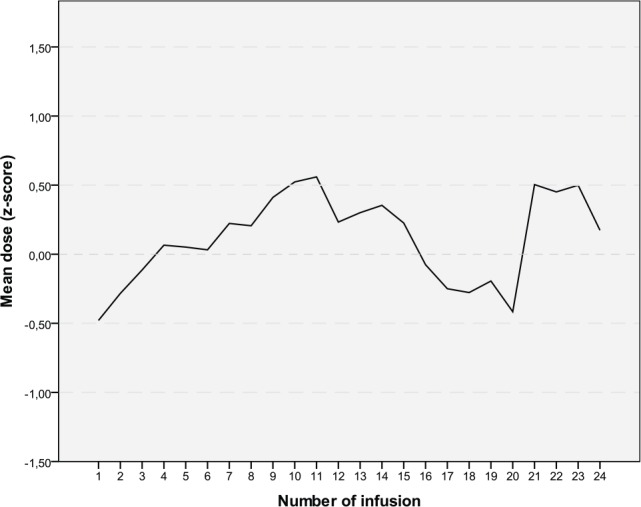
Dose given as Z-score in relation to infusions 1 to 24; 59 patients received at least 1 infusion, and 5 patients received 24 infusions.
Temperature was measured rectally (75% continuously with a rectal digital probe), with an ear infrared thermometer (21%), or sublingually, with a digital thermometer (4%). Mean temperature increase within 24 hours after infusion was 1.5°C ± 0.8°C and was significantly different from baseline temperature (P < .001). Fever ≥38.5°C was documented in 54% of infusions. After at least 1 infusion with MP, 45 patients (76%, 95% CI = 65%-87%) had an increase in core temperature to ≥38.5°C. Fever most frequently occurred in Iscador preparations (39%-75%) but also after infusion of abnobaVISCUM Fraxini (35%) and Helixor P (29%). The Z-value of the dose is correlated with the increase in core temperature (P = .002, 2-tailed; Figure 2). Higher dosages of MPs resulted in a higher increase in core temperature.
Figure 2.
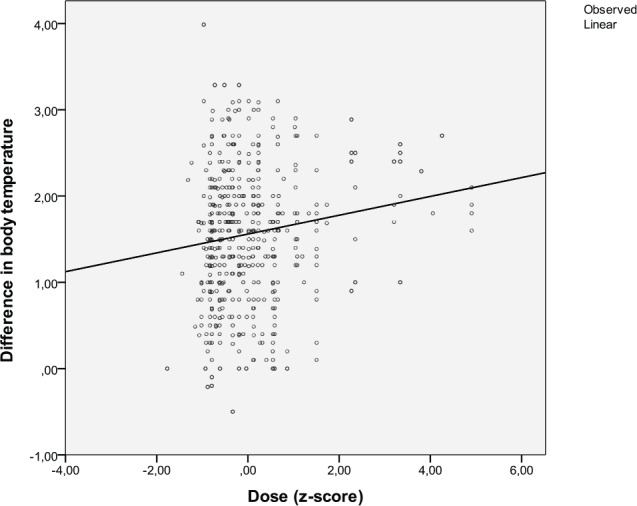
Concentration-effect curve: Z-scores of the respective doses given are plotted against maximum difference in body temperature after infusion of the mistletoe preparation. Circles represent the single infusions. Linear regression (r = 0.137) fitted best to the data.
The mean number of fever episodes was 4.75 per patient and varied depending on the number of MP infusions applied (Table 3). Patients with higher numbers of infusions had more episodes of fever. The increase in body temperature after MP infusions was, however, not significantly different between patients who received only 1 to 5 and patients who received >20 infusions (Table 3). Mean duration between start of infusion and peak temperature was 5.15 ± 1.5 hours (median = 5, range = 1-14 hours).
Table 3.
Frequency of Fever and Increase in Body Temperature in Relation to the Number of MP Infusions and Number of Patients.
| Number of Infusions (Mean, SD), Range | Frequency of Temperature >38.5°C Achieved (Mean, SD), Range | Increase in Body Temperature Per Infusion (Mean, SD), Range | |
|---|---|---|---|
| Patients with 1-5 infusions (n = 29) | 2.90 (1.59), 1-5 | 1.54 (1.45), 0-5 | 1.49 (0.63), 0.30-2.53 |
| Patients with 6-10 infusions (n = 9) | 7.56 (1.51), 6-10 | 1.75 (1.75), 0-5 | 0.96 (0.42), 0.05-1.51 |
| Patients with 11-20 infusions (n = 15) | 14.53 (3.82), 11-20 | 5.80 (4.89), 0-16 | 1.33 (0.57), 0.23-2.16 |
| Patients with >20 infusions (n = 6) | 32.83 (16.80), 21-66 | 21.17 (12.40), 10-44 | 1.76 (0.30), 1.48-2.29 |
| Patient total (n = 59) | 9.61 (10.71), 1-66 | 4.75 (7.53), 0-44 | 1.40 (0.59), 0.05-2.53 |
Abbreviation: MP, mistletoe preparations.
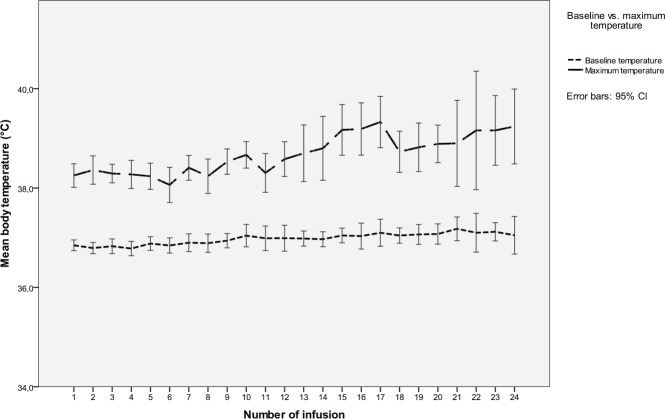
During repeated infusions, there was no loss of the temperature-effect (Figure 3). The higher peak temperature after infusion 12 was neither related to the dose (compare with Figure 1) nor number of infusions but to the individual reactivity of the patients who had more than 12 infusions (data not shown). The baseline temperature remained unchanged. The type of cancer (Table 4) or age (P = .960) did not affect the probability of reacting with fever.
Figure 3.
Mean temperature before and maximum temperature after 1 to 24 infusions of mistletoe preparations (n = 59 patients).
Table 4.
Increase in Body Temperature After Infusion of Mistletoe Preparations in Relation to the Type of Cancer.
| Number of Infusions (n) | Increase in Body Temperature (Mean, SD) | Increase in Body Temperature (Range) | |
|---|---|---|---|
| Breast cancer | 140 | 1.41 (0.73) | −0.10 to 3.10 |
| Colorectal cancer | 86 | 1.78 (0.87) | 0.10 to 3.99 |
| Ovarian cancer | 41 | 1.99 (0.67) | 0.40 to 2.60 |
| Prostate cancer | 45 | 1.80 (0.63) | 0.10 to 2.80 |
| Pancreatic cancer | 22 | 1.78 (0.75) | 0.40 to 3.00 |
| Lung cancer | 31 | 1.54 (0.86) | −0.50 to 3.10 |
| Head/Neck cancer | 35 | 1.48 (0.72) | 0.00 to 3.00 |
| Other types of cancer | 113 | 1.31 (0.75) | −0.21 to 2.90 |
| Total | 513 | 1.56 (0.78) | −0.50 to 3.99 |
Side effects and adverse events were documented after 527 of the 567 MP infusions (Table 5). They consisted mainly of fever-related symptoms and were highly correlated with the peak temperature (r = 0.386; P < .0001; Spearman’s ρ). No side effects or adverse events were documented more than 12 hours after the start of MP infusion. Grade 3 to 5 adverse events did not occur.
Table 5.
Side Effects and Adverse Events According to Common Toxicity Criteria (CTCAE), Version 4, Within 12 Hours After Start of Infusion of Mistletoe Preparation (n = 527 Infusions).a
| Adverse Event | Number (%) | Grade 1 | Grade 2 |
|---|---|---|---|
| Fever-related symptoms (headache, shivering) | 253/48 | 177/34 | 76/14 |
| Nausea | 77/15 | 67/13 | 10/2 |
| Allergic reaction | 3/0.6 | 3/0.6 | 0 |
| Others | 12/2.3 | 9/1.7 | 3/0.6 |
Grade 3 and 4 toxicities did not occur.
Immunological parameters (mostly differential blood count) were documented from 23 patients. No baseline tests were performed in any of them. The time interval between laboratory tests varied considerably (median = 14 days and range = 2-371 days for the first 12 measuring times). Because no comparison with baseline could be performed and because the collection was not systematic, no conclusions regarding effects of MP infusions on immunological parameters can be drawn (results not shown).
Tumor response was documented in 32 patients. Complete remission was described in 9% (n = 3), partial remission in 6% (n = 2), no change in 22% (n = 7), and progression in 63% (n = 20). In all patients with complete or partial response, the files were studied in more detail—for example, whether they had received concomitant therapies. It was found that all patients with documented complete or partial remission had received chemotherapy or radiation therapy in coincidence with the response. Therefore, the impact of MP infusions on tumor response could not be analyzed. A total of 37 patients died during the observation period (until March 2013), 4.9 ± 5.5 (median = 3) years after initial diagnosis and 0.8 ± 1.2 (median = 0) years after the first MP infusion; 89% (n = 33) of them had been diagnosed with advanced and/or metastatic disease.
Discussion
Our retrospective study analyzed 567 MP infusions in 59 cancer patients. Peak core temperature after MP infusion was documented in 90%, and adverse events and side effects of infusions were documented in 93%. Because a sufficient number of MP infusions were analyzed and data quality was good, firm conclusions about the frequency of fever ≥38.5°C and side effects after MP infusion can be drawn. Our hypothesis regarding these parameters could be confirmed: MP infusions can induce an increase in body temperature ≥38.5°C within 24 hours; they are safe (no >grade 2 toxicity according to CTCAE, version 4.0), despite frequent fever-related symptoms experienced by the patients. Duration of the fever was not recorded in the files, but because peak temperature occurred 5.15 hours after the start of the infusion and because no side effects were documented later than 12 hours after the start of MP infusions, it can be concluded that the fever episodes lasted for less than 12 hours. The number of infusions was in proportion to the frequency of the different cancer types (Tables 1 and 4). For infusion, the respective doses of MPs were diluted in 250 mL physiological saline solution. The substances responsible for the induction of fever have not yet been clarified in detail. Apart from mistletoe lectins, which can act as PAMPs, bacterial products might be present in very low concentrations in fermented MPs.18 They are difficult to determine in MPs because of compounds that interact with the detection assays.18 Safety of MPs given intravenously has recently also been investigated in an observational study.4 In this study, 4.6% of patients only reported side effects, and 1.7% only had fever. Probable reasons for these differences to our study are different MP preparations, different dosages, and different settings. Accordingly, fermented Iscador preparations were used in the majority of patients in our study and were the less-frequently used preparations in the study of Steele et al.4 In >50% of patients, MP infusions were given in parallel to chemotherapy and corticosteroids, compared with zero in our study. It seems that the reason for giving MP intravenously was different in the 2 studies and that the induction of fever is related to the choice of preparation and dose. Also, unfermented preparations, which did not cause fever in the study by Steele et al,4 such as Helixor P or abnobaVISCUM Fraxini, induced fever in our study, with the dose given.
Limitations of our study are a result of the retrospective design. The question of whether MP infusions can induce specific immunological reactions could not be answered by our investigations. Because baseline values were missing and a differential blood count was not regularly taken from the patients, no conclusions can be drawn.
Partial or complete remissions, which could be associated with sole MP infusions, were not found in our study. In all patients who had documented remissions, conventional therapies had also been given at different times (data not shown). Conclusions on survival related to MPs, therefore, cannot be drawn from this retrospective study. To address these issues with adequate methodology, a prospective controlled study would be necessary.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was financially supported by the Kelm Foundation.
References
- 1. Kienle GS, Kiene H. Review article: influence of Viscum album L (European mistletoe) extracts on quality of life in cancer patients: a systematic review of controlled clinical studies. Integr Cancer Ther. 2010;9:142-157. [DOI] [PubMed] [Google Scholar]
- 2. Franz H. Mistletoe lectins and their A and B chains. Oncology. 1986;43(suppl 1):23-34. [DOI] [PubMed] [Google Scholar]
- 3. Huber R, Classen K, Werner M, Klein R. In vitro immunoreactivity towards lectin-rich or viscotoxin-rich mistletoe (Viscum album L.) extracts Iscador applied to healthy individuals. Arzneimittelforschung. 2006;56:447-456. [DOI] [PubMed] [Google Scholar]
- 4. Steele ML, Axtner J, Happe A, Kröz M, Matthes H, Schad F. Safety of intravenous application of mistletoe (Viscum album L.) preparations in oncology: an observational study. Evid Based Complement Alternat Med. 2014;2014:236310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kienle GS, Albonico HU, Baars E, Hamre HJ, Zimmermann P, Kiene H. Anthroposophic medicine: an integrative medical system originating in Europe. Glob Adv Health Med. 2013;2(6):20-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Coley-Nauts HC, Fowler G, Bogatko FH. A review of the influence of bacterial infection and of bacterial products (Coley’s toxins) on malignant tumors in man. Acta Med Scand. 1953;145:5-102. [PubMed] [Google Scholar]
- 7. Hobohm U. Toward general prophylactic cancer vaccination. Bioessays. 2009;31:1071-1079. [DOI] [PubMed] [Google Scholar]
- 8. Abel U. Spontanremissionen und fieberhafte Erkrankungen. In: Heim ME, Schwarz R. eds. Spontanremissionen in der Onkologie. Stuttgart, Germany: Schattauer; 1998:68-75. [Google Scholar]
- 9. Abel U. Die antineoplastische Wirkung pyrogener Bakterientoxine. In: Hager ED, Abel U. eds. Biomodulation und Biotherapie des Krebses—Endogene Fiebertherapie und exogene Hyperthermie in der Onkologie. Heidelberg, Germany: Verlag für Medizin Dr E. Fischer; 1987:21-85. [Google Scholar]
- 10. Hobohm U. Fever therapy revisited. Br J Cancer. 2005;92:421-425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kleef R, Jonas WB, Knogler W, Stenzinger W. Fever, cancer incidence and spontaneous remissions. Neuroimmunomodulation. 2001;9:55-64. [DOI] [PubMed] [Google Scholar]
- 12. Frey B, Weiss EM, Rubner Y, et al. Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia. 2012;28:528-542. [DOI] [PubMed] [Google Scholar]
- 13. Dayanc BE, Beachy SH, Ostberg JR, Repasky EA. Dissecting the role of hyperthermia in natural killer cell mediated anti-tumor responses. Int J Hyperthermia. 2008;24:41-56. [DOI] [PubMed] [Google Scholar]
- 14. Knippertz I, Stein MF, Dörrie J, et al. Mild hyperthermia enhances human monocyte-derived dendritic cell functions and offers potential for applications in vaccination strategies. Int J Hyperthermia. 2011;27:591-603. [DOI] [PubMed] [Google Scholar]
- 15. Park HJ, Hong JH, Kwon HJ, et al. TLR4-mediated activation of mouse macrophages by Korean mistletoe lectin-C (KML-C). Biochem Biophys Res Commun. 2010;396:721-725. [DOI] [PubMed] [Google Scholar]
- 16. Huber R, Eisenbraun J, Miletzki B, et al. Pharmacokinetics of natural mistletoe lectins after subcutaneous injection. Eur J Clin Pharmacol. 2010;66:889-897. [DOI] [PubMed] [Google Scholar]
- 17. Kallinich T, Lainka E, Berner R, Niehues T. Fieber unklarer Genese. http://www.awmf.org/uploads/tx_szleitlinien/027-053l_S1_Fieber_unklarer_Ursache_2013-01.pdf. Accessed June 23, 2016.
- 18. Becker KP, Ditter B, Nimsky C, Urbaschek R, Urbaschek B. Endotoxin contents of phytopharmaceuticals: correlation with clinically observed side effects [in German]. Dtsch Med Wochenschr. 1988;113:83-87. [DOI] [PubMed] [Google Scholar]