Abstract
Background. Phyllanthus emblica L (PEL), a well-known medical plant, has been used in Asian countries for a long time. Increasing evidence suggests that it can prevent the tumorigenesis of cancer associated with nonresolving inflammation. However, the possible anti-inflammatory mechanism responsible for preventing tumorigenesis of precancerous lung lesions is not well elucidated. Materials and methods. Male A/J mice were randomly divided into 5 groups with 10 mice in each group: (1) blank group (saline), (2) benzo(a)pyrene [B(a)P] group, (3) and (4) B(a)P + PEL (5 g/kg/d, 10 g/kg/d, administered by gavage), (5) B(a)P + celecoxib (30 mg/kg/d, administered by gavage). Nodes on the lung surface were observed and calculated. The levels of macrophage inflammatory protein (MIP-2), tumor necrosis factor-α (TNF-α), interleukin (IL)-6, and IL-1β were detected by enzyme-linked immunosorbent assay (ELISA) kits. Cyclo-oxygenase-2 (COX-2), hypoxia-inducible factor-1 (HIF-α), IL-1β, miR-101, and Lin28B protein levels were evaluated by immunohistochemistry and Western blotting. Results. PEL extract treatment significantly reduced the number of nodes on the lung surface and attenuated B(a)P-induced levels of proinflammatory cytokines MIP-2, TNF-α, IL-6, and IL-1β in lung tissue. The protein expressions of COX-2 and HIF-α were significantly decreased by the treatment of PEL. In addition, both PEL extract and celecoxib markedly upregulate the expression of miR-101 while downregulating IL-1β and Lin28B levels. Conclusion. Our study indicated that treatment with PEL extract can not only protect the lung from inflammatory injury but effectively prevent precancerous lung lesions through regulating the IL-1β/miR-i101/Lin28B signaling pathway.
Keywords: Phyllanthus emblica L, anti-inflammatory, precancerous lung lesion, IL-1β/miR-101/Lin28B signaling pathway
Introduction
Inflammation has been regarded as an essential immune response for a malignant tumor and consists of 4 basic components: inflammatory inducers, sensors, inflammatory mediators, and target tissues.1 The role of endogenous and exogenous inflammatory inducers in tumorigenesis of tissue has become an important contributor to such pathological lesions. Under long-term exposure or stimulation, the acute inflammatory responses to an inflammatory trigger can shift to a chronic one with nonresolving inflammation.2 In fact, this inflammation is not a primary cause of cancer, it is an essential adaptive response that aims to restore homeostasis and is also a significant contributor to their pathogenesis. That is, continuous stimulation for this homeostasis causes a microenvironment of unbalanced inflammatory mediators and cytokines such as cyclo-oxygenase (COX-2), hypoxia-inducible factor (HIF)-α, tumor necrosis factor (TNF)-α, and interleukin (IL)-1β resulting in the pathogenesis, invasion, and metastasis of tumor. Therefore, regulation of these mediators could become effective therapy in inflammation-related cancer, and treatment would be more efficient if the mechanism responsible for the mediators regulating nonresolving inflammation is elucidated.
Non-small-cell lung cancer (NSCLC) remains the leading cause of cancer-related mortality worldwide.3 Evidence suggest that the proinflammatory cytokine IL-1β is dramatically elevated in the serum of patients with NSCLC. In vitro studies indicated that IL-1β promoted the proliferation and migration of NSCLC cells. Mechanistically, IL-1β represses expression of a tumor inhibitor miR-101 through regulating COX-2 and HIF-α. Furthermore, the suppression of miR-101 downregulates its efficient target Lin28B, which is another critical mediator of tumor repression.4 A recent study has reported that the IL-1β/miR-101/Lin28B pathway is a novel regulatory axis of pathogenic inflammatory signaling in NSCLC.
Phyllanthus emblica L (PEL), commonly known as amla, has been used extensively in Asian countries such as India and Thailand, and collected in Chinese pharmacopoeia (2010). Phytochemical studies show that it is rich in tannins, polyphenols, gallic acid, flavonoids, vitamin C, and emblicol.5 It has been extensively used to treat various diseases, especially aspects of inflammation such as pneumonia, hepatitis, and even cancer. There is a wealth of information indicating that PEL incorporates both cancer-preventive and antitumor properties.6 Sultana et al7 have suggested that PEL extract shows strong chemopreventive potential for hepatocarcinogenesis with combined activity of the reported tannins and flavonoids through modulating the detoxification armory and inhibiting the expression of several cell proliferation markers.7 Progallin A from PEL fruit induces apoptosis of human hepatocellular carcinoma BEL-7404 cells, which is related to G1/M and G2/M arrest, and it exerts its apoptotic effect by upregulation of Bax expression and downregulation of Bcl-2 expression.8 Corilagin, a major component of the phenolic family of PEL, shows high biological activity against chromosome alterations and DNA damage as well as anti-inflammatory effects.9 In addition, PEL could significantly suppress proinflammatory genes, including COX-2, iNOS, IL-16, and IL-6.10,11 PEL extract also efficiently reduced TNF-α and IL-1β levels with immunomodulatory effects on NSAID-induced ulcers.12 Reported outcomes have strongly suggested that the anticarcinogenic activity of PEL might be attributed to its anti-inflammatory property. However, no research has been conducted to explore this relationship between the anticarcinogenic effects and anti-inflammatory properties of PEL. The aim of this study was to provide a possible mechanism for the effect of PEL on benzo(a)pyrene [B(a)P]-induced precancerous lung lesions related to the regulation of the inflammatory signaling pathway.
Materials and Methods
Reagents
The dried fruit of PEL was collected from the actual area where it is produced: Tibet, China. It was authenticated as PEL by professor De-kang Wu from Nanjing University of Chinese Medicine. B(a)P was offered by Sigma Chemical Co (St Louis, MO). Macrophage inflammatory protein (MIP-2), TNF-α, IL-6, and IL-1β enzyme-linked immunosorbent assay (ELISA) kits were purchased from Key GEN Biotech Co, Ltd (Nanjing, P R China). Antibodies of COX-2, HIF-α, IL-1β, Lin28B, and miR-101 were obtained from Boster (Wuhan P R China). Other chemicals were all reagent grade.
Preparation of Plant Extracts
The dried fruit of PEL were ground into powder. The powder (1 kg) was weighed and extracted with double-distilled water (1000 mL) by reflux extraction for 1.5 hours every time (2 times). Then, the extract was filtered to remove the impurities and was concentrated in a rotary evaporator at 50°C under reduced pressure. The yields of dried residues were 21.5%, and the sample was stored at 4°C. Finally, the supernatant of the samples was filtered through a 0.45-µm cellulose acetate membrane for Liquid chromatography electrospray ionisation tandem mass spectrometry (LC-electrospray ionization (ESI)-MS/MS) analysis. The doses given to animals (5 and 10 g/kg/d) of distilled extract of PEL were expressed as grams of the original dry materials per kilogram body weight.
LC/ESI/MS/MS Analysis for PEL Components
The sample was separated with an Agilent C18 column (4.6 mm × 250 mm, 5 µm) by Agilent 1100 series. The mobile phase consisted of (A) water–acetic acid (100:2, v/v) and (B) methanol using a gradient program of 95% to 85% (B) in 0 to 10 minutes, 85% to 75% (B) in 10 to 15 minutes, 75% to 70% (B) in 15 to 30 minutes, 70% to 60% (B) in 30 to 50 minutes, 60% to 40% (B) in 50 to 70 minutes, 40% to 20% (B) in 70 to 90 minutes, and 20% to 10% (B) in 90 to 120 minutes. The flow rate was 1.0 mL/min, and the column temperature was maintained at 30°C. The detection wavelength was set at 280 nm. LC/MS/MS analysis was performed on Thermo Fisher Scientific ion trap mass spectrometer, equipped with an electrospray ionization (ESI) interface (Bremen, Germany). This analysis was operated under negative-ion mode. The optimized operating parameters were as follows: ion spray voltage, 4 kV; nebulizer gas (GS1): 0.50 L/min; and curtain gas (CUR): 0.15 L/min. The mass spectrometer was detected over a range of 80 to 1500 in the full scan mode.
Animal Treatment
Healthy male A/J mice (18-22 g) at 8 weeks of age were purchased from SLAC Experimental Animals Co, Ltd (Shanghai, China). The animal experiment protocol was reviewed and approved by the Institutional Animal Care and Use Committee of the Jiangsu Provincial Academy of Chinese Medicine. First, mice were injected with corn oil (0.2 mL) containing B(a)P 100 mg/kg for 2 weeks. Then, the mice were divided into 5 groups randomly, with 10 mice in each group, and they were administered for 24 consecutive weeks: blank group, model group, PEL (5 and 10 g/kg/d), and positive celecoxib (30 mg/kg/d) group. Blank and model mice were treated orally with saline solution (0.9%) by gavage administration each day. All the mice were put to death by suffocation applying CO2 gas after 24 weeks. Lung tissues of mice were fixed in Tellyesniczky’s solution (70% ethanol, 5% glacial acetic acid, and 5% formalin) overnight and stored in 70% ethanol. The lung surface nodes were calculated by counting under a dissecting microscope.
Determination of MIP-2, TNF-α, IL-6, and IL-1β by ELISA Kits
Blood samples were collected before sacrifice via the orbital sinus and centrifuged at 3500g at 4°C for 15 minutes, and the supernatant was collected for analysis with ELISA kits. The specific procedures were followed by the manufacturer’s instructions (Key GEN Biotech Co, Ltd, Nanjing, P R China). Finally, the absorbance was measured at 450 nm using a microplate reader (SPECTRAmax19.0, Molecular Devices, USA). The levels of inflammatory cytokines MIP-2, TNF-α, IL-6, and IL-1β were calculated according to the standard curve.
Immunohistochemical Analysis
All the tissues from lung lobes were cut to 4-µm thickness for further immunohistochemical analysis. Sections of paraffin-embedded samples from mice were prepared following the standard protocols. Briefly, sections were dewaxed for 20 minutes at 60°C and incubated in citrate buffer (pH = 6.0) for 30 minutes at 100°C. Then, the sections were washed 3 times with phosphate-buffered saline (PBS) each for 3 minutes and treated with 5% fetal bovine serum at room temperature for 25 minutes. Primary antibodies anti-COX-2 (1:200), anti-HIF-α (1:200), anti-IL-1β (1:200), anti-miR-101, and anti-Lin28B (1:200) were diluted for application in the incubation of tissues, and the sections were incubated overnight at 4°C. After washing with PBS, the sections were incubated at 37°C for 1 hour with the secondary antibodies and then stained with 3, 3-diaminobenzidine. An optical microscope was used to take photographs of slides.
Western Blotting Analysis
Lung tissues were collected from all 5 groups and washed twice with ice-cold PBS. Sequentially, tissues were lysed for 35 minutes at 4°C with radio-immunoprecipitation assay, and the lysates were obtained by centrifuging with 13 000g for 15 minutes. The concentration of protein was quantified by the BCA method according to the manufacturer’s instructions. Then, the equalized amounts of proteins from each sample were resolved in SDS-PAGE (sodium dodecyl polyacrylamide gel electrophoresis) and transferred to a polyvinylidene fluoride membrane. Additionally, membranes were blocked with blocking buffer before being incubated overnight with a 1:250 dilution of specific primary antibodies COX-2 (1:400), HIF-α (1:400), IL-1β (1:400), miR-101 (1:400), Lin28B (1:400), and β-actin (1:400) at 4°C. Finally, membranes were washed with tris-buffered saline and TWEEN 20 4 times (10 minutes each time), and then chemoluminescence reagents were added for the visualization of the protein bands. The quantification of proteins was analyzed. All experiments were performed at least 3 times.
Statistical Analysis
Experimental data in each group were analyzed using SPSS 11.5 software. (IBM, Armonk, NY). Statistical analyses were performed using a 1-way analysis of variance. P <.05 was considered to indicate a statistically significant difference. All values were presented as means ± SD.
Results
Identification of Chemical Components
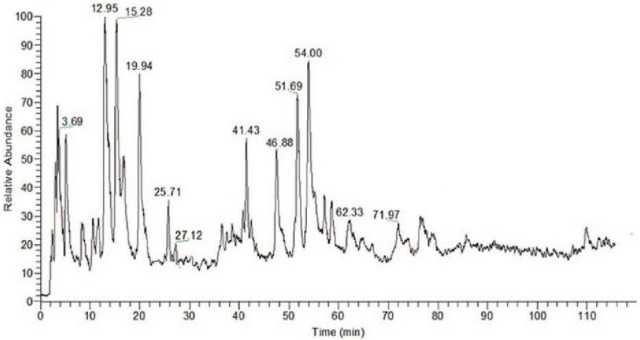
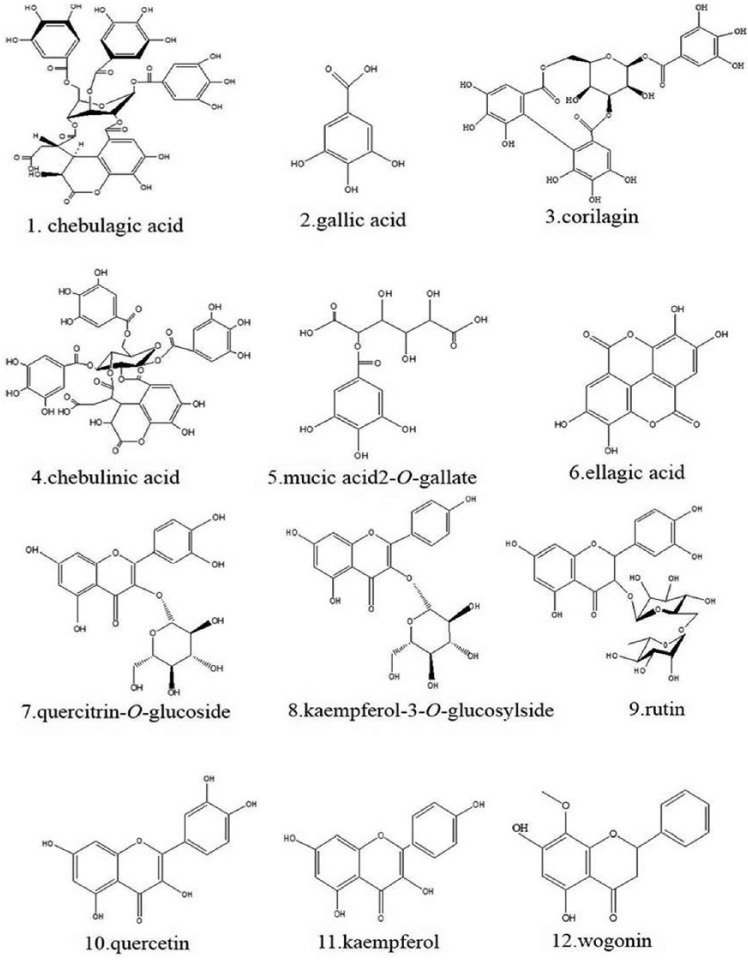
HPLC-ESI-MS/MS method was conducted to identify PEL compounds. Tannins, organic acids, and flavonoids of PEL could be eluted under the proper conditions within 120 minutes. The peaks whose retention time ranges from 3 to 100 minutes might consist primarily of tannins, organic acids, and flavonoids, compared with these reported data. Furthermore, as is shown in Figures 1 and 2 and Table 1, according to fragment ion information, these compounds of PEL were identified as chebulagic acid, gallic acid, chebulinic acid, mucic acid 2-O-gallate, corilagin, ellagic acid, quercitrin-O-glucoside, kaempferol-3-O-glucosylside, rutin, quercetin, kaempferol, and wogonin. Our established HPLC-ESI-MS/MS methods could make it possible for experiments with replications.
Figure 1.
LC/ESI/MS/MS Chromatogram of Phyllanthus emblica L.
Figure 2.
Chemical Components of Phyllanthus emblica L.
Table 1.
MS Analysis of Compositions of Phyllanthus emblica L.
| No. | Component Category | Chemical Component | TR (min) | (-)ESI-MS m/z | UVmax (nm) | (-)ESI-MS m/z (Percentage Base Peak) | Formula |
|---|---|---|---|---|---|---|---|
| 1 | Chebulagic acid | 3.69 | 955.18 | 220, 272 | 817, 665, 513, 339, 241 | C41H32O27 | |
| 2 | Tannins and organic acids | Gallic acid | 12.95 | 169.02 | 212, 270 | 167, 123 | C7H6O5 |
| 3 | Chebulinic acid | 15.28 | 369.47 | 220, 271 | 253, 208, 161 | C15H14O11 | |
| 4 | Mucic acid2-O-gallate | 19.94 | 361.05 | 210, 275 | 300, 227, 169, 139 | C13H14O12 | |
| 5 | Corilagin | 54.00 | 633.45 | 220, 274 | 525, 357, 153 | C27H22O18 | |
| 6 | Ellagic acid | 71.97 | 301.01 | 210, 225 | 241, 237, 181 | C14H6O8 | |
| 7 | Quercitrin-O-glucoside | 25.71 | 463.08 | 210, 250, 334 | 315, 283, 207, 191 | C21H20O12 | |
| 8 | Kaempferol-3-O-glucosylside | 27.12 | 447.38 | 210, 252, 335 | 299, 283, 207, 175 | C21H20O11 | |
| 9 | Flavonoids | Rutin | 41.43 | 609.15 | 310, 333 | 447, 300, 269, 237, 161 | C27H30O16 |
| 10 | Quercetin | 46.88 | 300.24 | 210, 255, 375 | 282, 241, 172 | C15H10O7 | |
| 11 | Kaempferol | 51.69 | 285.23 | 266, 369 | 269, 193, 177, 145 | C15H10O6 | |
| 12 | Wogonin | 62.33 | 283.07 | 210, 275 | 265, 237, 221 | C16H12O5 |
Abbreviations: ESI, electrospray ionization; UV, ultraviolet; MS, mass spectrometry.
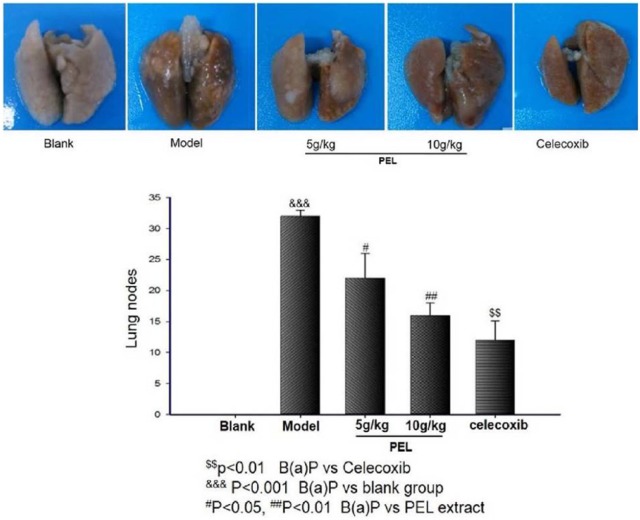
PEL Extract Reduces Nodes on Lung Surface
Compared with the normal, the nodes on lung surface in A/J mice were obviously increased by an induction of B(a)P (P < .001). However, the number of nodes was reduced by PEL extracts, especially by the treatment with a dose of 10 g/kg/d, compared with model group (P < .01 or P < .05; Figure 3). Results showed that administration of PEL did have a significant protective effect on lung tumorigenesis.
Figure 3.
PEL extract reduced nodes on the lung surface of A/J mice induced by B(a)P. Data are presented as means ± SD (n = 9).
Abbreviations: PEL, Phyllanthus emblica L; B(a)P, benzo(a)pyrene.
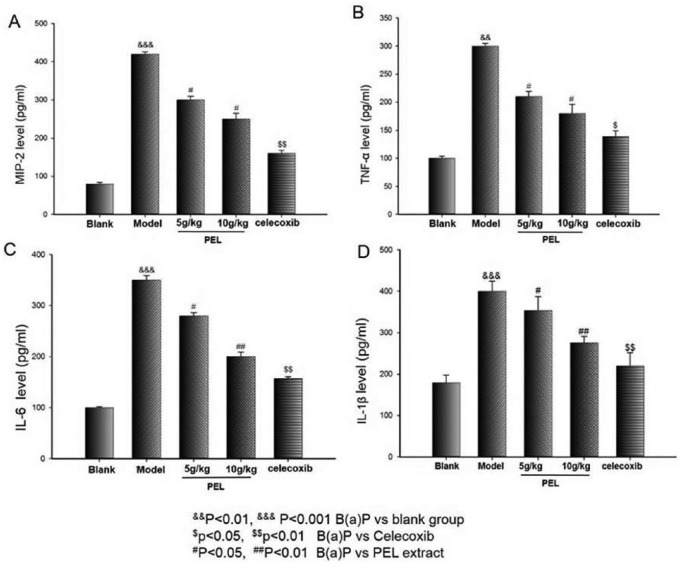
PEL Extract Reduces the Levels of Inflammatory Cytokines
ELISA analyses for several proinflammatory mediators were carried out. As shown in Figure 4, a remarkable increase of MIP-2, TNF-α, IL-6, and IL-1β appears in the model mice serum compared with the blank one (P < .001 or P < .01). However, an evident decrease in these protein levels could be observed by the treatment with celecoxib as well as in the PEL extract group compared with B(a)P. In addition, the high dosage of PEL extract (10 g/kg/d) could significantly reverse the levels of these cytokines to normal levels (P < .05 or P < .01). The results suggest that PEL extract reducing the odds of precancerous lung lesion may be related to the suppression of proinflammatory cytokines.
Figure 4.
Effects of PEL on B(a)P-induced inflammation response in A/J mice. ELISA was performed for MIP-2 (A), TNF-α (B), IL-6 (C), and IL-1β (D) levels in A/J mice induced by B(a)P. Data are presented as means ± SD (n = 6).
Abbreviations: PEL, Phyllanthus emblica L; B(a)P, benzo(a)pyrene; ELISA, enzyme-linked immunosorbent assay; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor; IL, interleukin.
PEL Extract Downregulates the Expressions of COX-2 and HIF-α
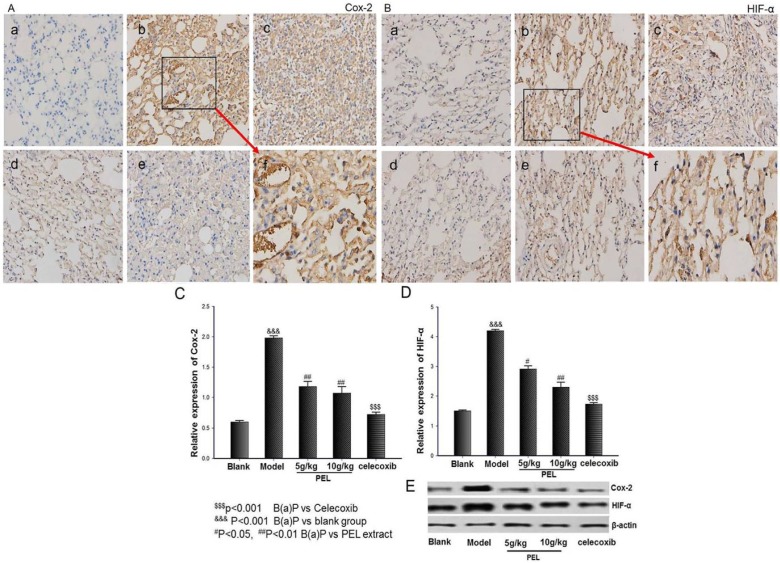
As shown in Figure 5, COX-2 protein expression could be markedly increased by treatment with B(a)P in vivo when compared with that in the blank group (P < .001). However, the treatment with celecoxib distinctly decreased the high level of COX-2 protein (P < .001). High-dose (10 g/kg/d) and low-dose (5 g/kg/d) PEL extracts also remarkably downregulated COX-2 protein expression in vivo (P < .01). Interestingly, the same tendency was seen in HIF-α expression. This outcome indicated that PEL extract downregulated the expressions in both COX-2 and HIF-α factors and also demonstrated that there is a certain link between these 2 vital mediators in tumor, which has been reported often.
Figure 5.
Effect of PEL on COX-2 and HIF-α in lung tissue. (a), (b), (c), (d), and (e) represent blank group, model group, low dose (5 g/kg/d) and high dose (10 g/kg/d) respectively; (f) represents an amplification of model group. Immunohistochemistry (IHC) as well as Western blot analysis indicated that PEL extracts significantly downregulated the expressions in both COX-2 and HIF-α factors in lung tissue. A. IHC for COX-2. B. IHC for HIF-α. C. Relative expression of COX-2 by Western blot. D. Relative expression of HIF-α by Western blot. E. Western blot for COX-2 and HIF-α. Data are presented as means ± SD (n = 6).
Abbreviations: PEL, Phyllanthus emblica L; COX-2, cyclo-oxygenase-2; HIF-α, hypoxia inducible factor-1; B(a)P, benzo(a)pyrene.
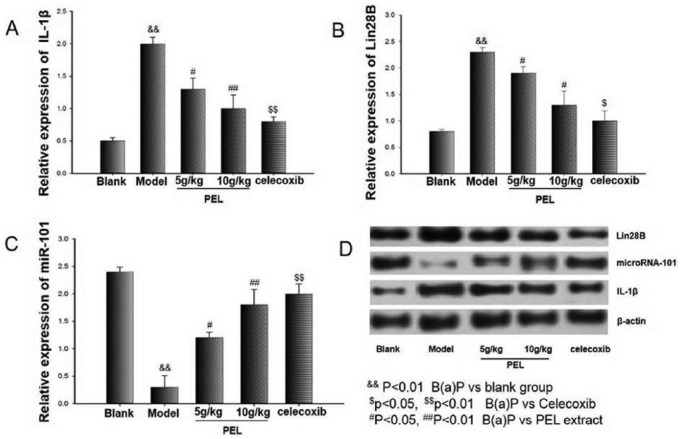
Effects of PEL Extract on the IL-1β/miR-101/Lin28B Signaling Pathway
Evidence indicates that the IL-1β/miR-101/Lin28B signaling pathway plays an important role in the pathogenesis of NSCLC. Thus, Western blot analysis was conducted to determine the expression of IL-1β, miR-101, and Lin28B, and it showed that celecoxib as well as PEL extract significantly decreased the overexpression of IL-1β and Lin28B compared with the blank group (Figure 6). Data indicated that PEL extract might provide protection from precancerous lung lesions through regulation of the IL-1β/miR-101/Lin28B signaling pathway.
Figure 6.
Regulation of PEL on B(a)P-induced Lin28B, IL-1β, and miR-101 in lung tissue. Western blotting was performed for Lin28B, IL-1β, and miR-101 protein expressions. Data are presented as means ± SD (n = 3).
Abbreviations: PEL, Phyllanthus emblica L; B(a)P, benzo(a)pyrene; IL, interleukin.
Discussion
B(a)P, a procarcinogen, has been a complete carcinogen in animal models during the whole process from tumor initiation to tumor promotion. Lung tumors generated by B(a)P in mouse share many genetic, biochemical, and histopathological characteristics similar to those observed in human lung cancer.13 Thus, in this study, we established the mouse model induced by B(a)P following recognized principle.
Currently, lung cancer has been shown to be closely associated with nonresolving inflammation in the process of malignant transformation.3 Different phytochemicals of PEL extract are reported to possess desirable cytotoxicity against tumor cells and reverse the status of antioxidants to normal level in cancer-bearing animals.14,15 They not only supplement the conventional treatment that can act at almost each step of carcinogenesis but offer better conditions with minor side effects.5,13 In the present study, we explored the primary chemical components, tannins, organic acids, and flavonoids by implementing LC/MS/MS analysis. In general, phenolic compounds of PEL may serve as potential herbal candidates for amelioration of acute and chronic inflammation because of the modulatory action of free radicals.16 The rutin in PEL, which is a flavonoid, is known for its anti-inflammatory properties and can provide protective effects to the pancreas.17 Compounds with a galloyl moiety showed higher antiproliferative activity than the flavonoid and are worth investigating as potential cancer chemopreventive agents.18 Ascorbic acid (vitamin C), which is abundantly present in PEL fruit, has the potential to shelter the body from oxidative cascades leading to inflammatory injury.19 In addition to these constituents, other substances such as chebulaginic acid, kaempferol, and quercetin are also responsible for the multiple effects on every stage of carcinogenesis by serving as carcinogen inactivators, enzyme inducers, and scavengers or antioxidants.
Continuous stimulus by inflammatory cytokines such as IL-6 and TNF-α may activate multiple signaling pathways.20 IL-6 secreted from the activated monocyte, macrophage, or tumor cell is a pleiotropic inflammatory cytokine with demonstrated tumor stimulatory and inhibitory properties. Moreover, patients with increased IL-6 levels in advanced metastatic cancer have poor prognosis.21,22 In addition, TNF-α has multiple antitumor effects including lysis, hemorrhagic necrosis of tumor cells, and tissue damage through adhesive molecules.23,24 It is also the primary inducer of MIP-2, which mainly has chemotaxis effects on neutrophils and involves inflammatory response. Furthermore, a significant pathological feature in acute lung injury is that there are a large number gathered, along with expression of MIP-2 mRNA, in lung tissue.25,26 In the present study, we found that PEL significantly reduced the levels of TNF-α, IL-6, and MIP-2, which definitely alleviated the inflammation in acute lung injury induced by B(a)P and could be the reason why PEL helped restore the production of anti-inflammatory factors and prevented the formation of precancerous lung lesions.
Almost all NSCLC preinvasive precursor lesions have higher expression levels of COX-2 than normal lung tissue.27,28 Thus, COX-2 has become an important target of chemoprevention, and celecoxib is one of its inhibitors. Besides, COX-2 can be dramatically upregulated by various stimuli such as IL, TNF-α, and other tumor promoters.29 Furthermore, because tumor cells lacking oxygen is a common phenomenon in solid tumors, HIF-α is the primary transcription factor that makes sure the organism adapts to the hypoxic condition and is comprehensively involved in metabolism as well as apoptosis of cells.30,31 It ensures normal function of the organism and cells under hypoxic conditions and plays a critical role in tumor metastasis and invasion because it promotes angiogenesis.32,33 Also, it is directly regulated by COX-2 at the transcriptional level. Our findings have shown that COX-2 and HIF-α levels increased significantly in B(a)P-induced mice, whereas they were evidently decreased by treatment with PEL extract; this means that PEL extract can protect normal lung tissue from damage not only in protein expression of COX-2 but at the transcriptional level.
IL-1β is a key proinflammatory cytokine associated with chronic inflammation and is dramatically elevated in the serum of NSCLC patients.34 A previous study suggested that sustained induction of IL-1β can create an inflammatory microenvironment to the advantage of tumor initiation or metastasis.35 Moreover, IL-1β signaling acts to repress microR-101, a well-known tumor-suppressive miRNA, which is negatively regulated in lung cancer and repressed by HIF-α at the transcriptional level in prostate cancer cells.36 Previous studies have demonstrated that IL-1β activates HIF-α through the NF-kB/COX-2 pathway.37,38 More important, Lin28 is an RNA-binding protein correlated with tumorigenesis and tissue inflammatory response.39,40 It can regulate the biogenesis of the let-7 family.41,42 Interestingly, overexpression of let-7 significantly reduced IL-6 expression, which was measured to gauge the influence on tumorigenesis of inflammatory factors.43,44 Overall, IL-1β upregulated Lin28B by downregulating miR-101, within which the inhibition of COX-2 abrogated IL-1β-mediated repression of miR-101 and IL-1β-mediated activation of Lin28B along with their stimulatory effects on NSCLC cell proliferation and migration. To investigate the anti-inflammatory mechanism responsible for preventing tumorigenesis of precancerous lung lesions, we examined the expression of IL-1β, Lin28B, and miR-101. As expected, the results showed that PEL extract effectively prevented precancerous lung lesions by relieving the inflammatory response through downregulating IL-1β and Lin28B expression and upregulating the level of miR-101 as well as by inhibiting unfavorable inflammatory factors that are involved in carcinogenesis. These findings suggest that PEL could inhibit the B(a)P-induced precancerous lung lesion by alleviating inflammation through regulating the IL-1β/miR-101/Lin28B signaling pathway and is, therefore, expected to be a promising chemopreventive agent.
Although there was statistically significant alleviation of malignant transformations in lungs after treatment with PEL extract in vivo, we still believe that more in vitro mechanism studies need to be done. There definitely exist several inflammatory pathways leading to cancer, and these roads connect with as well as affect each other closely. Exploration of all the mysteries of the relationship between chronic inflammation and cancer is definitely full of both challenges and rewards.
Conclusion
PEL extract not only eliminates the risk of nonresolving inflammation but also prevents precancerous lung lesions by the IL-1β /miR-101/Lin28B signaling pathway. This study underscores the potential benefits and applications of PEL in controlling inflammation-related precancerous lung lesions.
Acknowledgments
The authors acknowledge financial support of the Suzhou Science and Technology Program (ZXY2012009) and National Natural Science Foundation of China (No. 81202906, 81473394) and Natural Science Foundation of Jiangsu Province (BK2012491) for this investigation.
Footnotes
Authors’ Note: Cheng-cheng Wang and Jia-rui Yuan contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors acknowledge the financial support of Suzhou Science and Technology program (ZXY2012009) and National Natural Science Foundation of China (No. 81202906, 81473394) and Natural Science Foundation of Jiangsu Province (BK2012491) for this investigation.
References
- 1. Nathan C, Ding A. Nonresolving inflammation. Cell. 2010;140:871-882. [DOI] [PubMed] [Google Scholar]
- 2. Medzhitov R. Inflammation 2010: new adventures of an old flame. Cell. 2010;140:771-776. [DOI] [PubMed] [Google Scholar]
- 3. Yoon SH. Immunotherapy for non-small cell lung cancer. Tuberc Respir Dis (Seoul). 2014;77:111-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang L, Zhang LF, Wu J, et al. IL-2beta-mediated repression of microRNA-101 is crucial for inflammation-promoted lung tumorigenesis. Cancer Res. 2014;74:4720-4730. [DOI] [PubMed] [Google Scholar]
- 5. Sarin B, Verma N, Martín JP, et al. An overview of important ethnomedicinal herbs of Phyllanthus species: present status and future prospects. ScientificWorldJournal. 2014;2014:839172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhao T, Sun Q, Marques M, et al. Anticancer properties of Phyllanthus emblica (Indian gooseberry). Oxid Med Cell Longev. 2015;2015:950890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sultana S, Ahmed S, Jahangir T. Emblica officinalis and hepatocarcinogenesis: a chemopreventive study in Wistar rats. J Ethnopharmacol. 2008;118:1-6. [DOI] [PubMed] [Google Scholar]
- 8. Zhong Z, Wu D, Huang J, et al. Progallin A isolated from the acetic ether part of the leaves of Phyllanthus emblica L. induces apoptosis of human hepatocellular carcinoma BEL-7404 cells by up-regulation of Bax expression and down-regulation of Bcl-2 expression. J Ethnopharmacol. 2011;133:765-772. [DOI] [PubMed] [Google Scholar]
- 9. Chen Y, Chen C. Corilagin prevents tert-butyl hydroperoxide-induced oxidative stress injury in cultured N9 murine microglia cells. Neurochem Int. 2011;59:290-296. [DOI] [PubMed] [Google Scholar]
- 10. Sripanidkulchai B, Junlatat J. Bioactivities of alcohol based extracts of Phyllanthus emblica branches: antioxidation, antimelanogenesis and anti-inflammation. J Nat Med. 2014;68:615-622. [DOI] [PubMed] [Google Scholar]
- 11. Colucci R, Dragoni F, Conti R, et al. Evaluation of an oral supplement containing Phyllanthus emblica fruit extracts, vitamin E, and carotenoids in vitiligo treatment. Dermatol Ther. 2015;28:17-21. [DOI] [PubMed] [Google Scholar]
- 12. Chatterjee A, Chattopadhyay S, Bandyopadhyay S. Biphasic effect of Phyllanthus emblica L. extract on NSAID-induced ulcer: an antioxidative trail weaved with immunomodulatory effect. Evid Based Complement Alternat Med. 2011;2011:146808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jeong SJ. Are there new therapeutic options for treating lung cancer based on herbal medicines and their metabolites? J Ethnopharmacol. 2011;138:652-661. [DOI] [PubMed] [Google Scholar]
- 14. Vaithiyanathan V, Mirunalini S. Chemo preventive potential of fruit juice of Phyllanthus emblica Linn. (amla) against mammary cancer by altering oxidant/antioxidant status, lipid profile levels and estrogen/progesterone receptor status in female Sprague–Dawley rats. Biomed Prev Nutr. 2013;3:357-366. [Google Scholar]
- 15. Iamsaard S. Phenolic contents and antioxidant capacities of Thai-Makham Pom (Phyllanthus emblica L.) aqueous extracts. J Zhejiang Univ Sci. 2014;B15:405-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muthuraman A, Sood S, Singla S. The antiinflammatory potential of phenolic compounds from Emblica officinalis L. in rat. Inflammopharmacology. 2011;19:327-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aruna R, Geetha A, Suguna P, et al. Rutin rich Emblica officinalis Gaert. fruit extract ameliorates inflammation. J Complement Integr Med. 2014;11:9-18. [DOI] [PubMed] [Google Scholar]
- 18. Zhang Y, Nagao T, Tanaka T, et al. Antiproliferative activity of the main constituents from Phyllanthus emblica. Biol Pharm Bull. 2004;27:251-255. [DOI] [PubMed] [Google Scholar]
- 19. Saito K, Kohno M, Yoshizaki F, et al. Extensive screening for edible herbal extracts with potent scavenging activity against superoxide anions. Plant Foods Hum Nutr. 2008;63:65-70. [DOI] [PubMed] [Google Scholar]
- 20. O’Callaghan DS, O’Donnell D, O’Connell F, et al. The role of inflammation in the pathogenesis of non-small cell lung cancer. J Thorac Oncol. 2010;5:2024-2036. [DOI] [PubMed] [Google Scholar]
- 21. Blay JY, Negrier S, Combaret V, et al. Serum level of interleukin -6 as a prognosis factor in metastatic renal cell carcinoma. Cancer Res. 1992;52:3317-3322. [PubMed] [Google Scholar]
- 22. Porta C, De Amici M, Quaglini S, et al. Circulating interleukin-6 as a tumor marker for hepatocellular carcinoma. Ann Oncol. 2008;19:353-358. [DOI] [PubMed] [Google Scholar]
- 23. Larric JW, Wright SC. Cytotoxic mechanism of tumor necrosis factor-alfa. FASEB J. 1990;4:3125-3223. [DOI] [PubMed] [Google Scholar]
- 24. Lejeune FJ, Ruegg C, Lienard D. Clinical applications of TNF-alpha in cancer. Curr Opin Immunol. 1998;10:573-580. [DOI] [PubMed] [Google Scholar]
- 25. Shanley TP, Schmal H, Wanrer RL. Requirement for CXC chemokines ((macrophage inflammatory Protein-2 and cytokine-induced and neutrophil chemoattractant) in lgG immune complex-induced lung injury. J Immunol. 1997;158:3439-3448. [PubMed] [Google Scholar]
- 26. Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827-872. [DOI] [PubMed] [Google Scholar]
- 27. Hida T, Yatabe Y, Achiwa H, et al. Increased expression of cyclooxygenase-2 occurs frequently in human lung cancers, specifically in adenocarcinomas. Cancer Res. 1998;58:3761-3764. [PubMed] [Google Scholar]
- 28. Wolff H, Saukkonen K, Anttila S, et al. Expression of cyclooxygenase-2 in human lung carcinoma. Cancer Res. 1998;58:4997-5001. [PubMed] [Google Scholar]
- 29. Brown JR, DuBois RN. Cyclooxygenase as a target in lung cancer. Clin Cancer Res. 2004;10:4266-4279. [DOI] [PubMed] [Google Scholar]
- 30. Chan DA, Suthin PD, Denko NC. Role of poly l hydroxylation in oncogenically stabilized hypoxia-inducible factor 1alpha. J Biol Chem. 2002;277:40112-40117. [DOI] [PubMed] [Google Scholar]
- 31. Lando D, Gorman JJ, Whltelaw ML. Oxygen-dependent regulation of hypoxia -inducible factors by proly l and asparaginy l hydroxylation. Eur J Biochem. 2003;70:781-790. [DOI] [PubMed] [Google Scholar]
- 32. Covello KL, Simon MC. HIFs, hypoxia, and vascular development. Curr Top Dev Biol. 2004;62:37-54. [DOI] [PubMed] [Google Scholar]
- 33. Lum JJ, Bui T, Gruber M, et al. The transcription factor HIF-1α plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21:1037-1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bartel DP. MicroRNAs genomics, biogenesis, mechanism, and function. Cell. 2004;116:281-297. [DOI] [PubMed] [Google Scholar]
- 35. Garofalo M, Croce CM. MicroRNAs: master regulators as potential therapeutics in cancer. Annu Rev Pharmacol Toxicol. 2005;51:25-43. [DOI] [PubMed] [Google Scholar]
- 36. Cao P, Deng Z, Wan M, et al. MicroRNA-101 negatively regulates Ezh2 and its expression is modulated by androgen receptor and HIF-1alpha/HIF-1beta. Mol Cancer. 2010;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jung Y, Isaacs J, Lee S, et al. IL-1beta-mediated up-regulation of HIF-1alpha via an NFkappaB/COX-2 pathway identifies HIF-1 as a critical link between inflammation and oncogenesis. FASEB J. 2003;17:2115-2127. [DOI] [PubMed] [Google Scholar]
- 38. Ji R, Chou C, Xu W, et al. EP1 prostanoid receptor coupling to G i/o up-regulates the expression of hypoxia-inducible factor-1 alpha through activation of aphosphoinositide-3 kinase signaling pathway. Mol Pharmacol. 2010;77:1025-1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Koki A, Khan NK, Woerner BM, et al. LIN28B promotes colon cancer progression and metastasis. Cancer Res. 2011;71:4260-4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wen J, Liu H, Wang Q, et al. Genetic variants of the LIN28B gene predict severe radiation pneumonitis in patients with non-small cell lung cancer treated with definitive radiation therapy. Eur J Cancer. 2014;50:1706-1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yang DH, Moss EG. Temporally regulated expression of Lin-28 in diverse tissues of the developing mouse. Gene Expr Patterns. 2003;3:719-726. [DOI] [PubMed] [Google Scholar]
- 42. Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL-6 links inflammation to cell transformation. Cell. 2009;139:693-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iliopoulos D, Jaeger SA, Hirsch HA, et al. STAT3 activation of miR-21 and miR-181b-1 via PTEN and CYLD are part of the epigenetic switch linking inflammation to cancer. Mol Cell. 2010;39:493-506. [DOI] [PMC free article] [PubMed] [Google Scholar]