Abstract
Introduction: After allogeneic hematopoietic stem cell transplantation (HSCT), NK cell reconstitution, which is crucial for positive outcomes, is dominated by the CD56bright subset with low NK cell cytotoxicity (NKCC) activity. Moderate exercise has been described as a potent NK cell stimulus in adults with cancer. Purpose: To determine the effects of a moderate-intensity exercise program on NK cell recovery early after HSCT and the feasibility of this intervention. Methods: Six children undergoing allogeneic HSCT were randomized to an exercise program (EP) or control (CT) group. The EP group performed a 10-week training combining in-hospital and home-based EP. Results: We observed a significant increase in the posttraining/pretraining ratio of the CD56dim subset (EP = 1.27 ± 0.07; CT = 0.99 ± 0.08; P < .005) of the EP group. The ratio of NKCC was 8 times greater in the EP group. Conclusion: Data suggest that a moderate-intensity EP program performed early after HSCT is feasible and might redistribute the CD56dim/CD56brigh NK cell subset, improving NKCC. The results are still preliminary and must be interpreted with caution.
Keywords: natural killer cells, moderate-intensity exercise, allogeneic stem cell transplantation, natural killer cell cytotoxicity
Introduction
Allogeneic hematopoietic stem cell transplantation (HSCT) is the only curative treatment option for some pediatric malignancies, as well as for several hereditary immunodeficiencies and metabolic disorders. However, conditioning regimen-related toxicities, graft-versus-host disease (GvHD), life-threatening infections, and relapse determine HSCT success.1 T-cell adaptive immunity and innate immunity mediated by natural killer (NK) cells are crucial elements for guaranteeing a successful posttransplantation outcome. Early immune reconstitution after HSCT is led by NK cells.2 These cells play an important role in the host defense against tumors and are also able to lyse some virus-infected target cells.3,4 Human NK cells can be subdivided into 2 major subsets based on the relative expression of the surface markers CD16 and CD56. These subsets are CD56bright (CD56bright CD16−) and CD56dim (CD56dim CD16+). The CD56bright subset might be an immediate precursor of CD56dim, which is the most cytotoxic NK cell subtype.3 In peripheral blood, approximately 90% of human NK cells are CD56dim, with the remainder characterized as CD56bright. The CD56dim NK cells express high levels of CD16, produce low levels of cytokines, and are the dominant subset in mediating natural cytotoxicity.5 The CD56bright NK cells have limited cytotoxic ability but they produce high levels of immunoregulatory cytokines.6,7 Early immune NK cell reconstitution after HSCT is dominated by the CD56bright subset.8 The increment in the CD56bright population has been associated with a decrease in NK cell cytotoxicity (NKCC), which is one of the primary functional measures of NK cell activity.3 Brief bouts of aerobic exercise can lead to large (4- to 5-fold) increases in the numbers of NK cells in human circulation.9 A moderate-intensity exercise program (EP) has been associated with increased survival rates and a reduced risk of cancer due to several factors, such as its potential beneficial effects on immunostimulation.10,11 However, this association depends on the type of EP and its duration. Specifically, the “inverted J hypothesis” suggests a moderate-intensity EP enhances immune function, whereas sedentary behavior and exhaustive EP suppress immune function and raise susceptibility to infection.12,13 During the recovery from acute high-intensity EP, the ratio of CD56bright to CD56dim cells favors the CD56bright subset, and NKCC is thus reduced.14 However, a moderate-intensity EP has been associated with an increase in NKCC in healthy and adult cancer populations.15 In populations with pediatric cancer, 3 studies to date have investigated the effect of EP on the immune system.16-18 Of these, only one assessed NKCC after a moderate- to high-intensity EP. The sensitivity of NK cells to physiological stress, combined with their important role in early immune reconstitution after HSCT, suggest that a regular EP could be a beneficial strategy free of any detrimental effect on NK cell recovery and NKCC after HSCT.19
Based on these data, it was hypothesized that a moderate-intensity EP could enhance NKCC and promote positive HSCT outcomes. The primary purpose of this pilot study was to determine the effects of a moderate-intensity EP on the NK cell phenotype, NKCC, and cytokine environment during the early phase after HSCT and the feasibility of this intervention. The secondary purpose was to determine the effects of this EP intervention on health-related fitness (HRF) variables.
Methods
Clinical Protocol
The ethics committee of the Children’s University Hospital Niño Jesús, Madrid, Spain, approved this parallel randomized control study. Before inclusion in the study, pediatric verbal consent and parental written informed consent were obtained. Children were eligible for participation if they met the following criteria: (1) age from 5 to 19 years; (2) having no condition that could contraindicate a moderate-intensity EP, such as severe anemia (hemoglobin <8 g/dL), fever (>38°C), severe cachexia (loss of 35% premorbid body mass) or anthracycline-induced cardiotoxicity; (3) currently living with their parents in Madrid; (4) discharged from HSCT no later than day +30; and (5) physician clearance to participate. A total of 6 children participated in the study. They were randomized into an EP or control (CT) group (n = 3 per group; Table 1). A sequence to randomly allocate participants was generated by a statistics software research randomizer, a research tool provided by the Social Psychology Network (www.socialpsychology.org). Allocation concealment was ensured with an allocation ratio of 1:1. After baseline assessments, each participant chose a sealed, opaque envelope that contained a number allocating the participant to the EP or CT group. The sample size was based on the availability and voluntary participation of eligible children. A total of 7 patients met the selection criteria. One refused to participate due to concerns about fatigue.
Table 1.
Patient Characteristics.
| Group | Sex | Age (Years), Patient/Donor | Diagnosis | Donor Type |
|---|---|---|---|---|
| Training | ||||
| 1 | Male | 9/50 | NC | HP |
| 2 | Female | 17/38 | ID | MUD |
| 3 | Female | 12/12 | AML | MRD |
| Control | ||||
| 1 | Female | 13/46 | ALL | HP |
| 2 | Female | 19/33 | AML | MUD |
| 3 | Female | 8/47 | MS | MRD |
Abbreviations: NC, nasopharyngeal carcinoma; HP, haploidentical donor; ID, immunodeficiency; MUD, match unrelated donor; AML, acute myeloblastic leukemia; MRD, matched related donor; ALL, acute lymphoblastic leukemia; MS, myelodysplastic syndrome.
Allogeneic Stem Cell Transplantation Procedure and Intervention
All the patients received a non-myeloablative conditioning regimen and a T-cell depletion graft. The graft consisted of a mean CD34+ stem cell number of 4.8 ± 0.86 × 106/kg, a mean CD3+ T-cell number of 12 ± 5 × 104/kg, and a median CD3−/CD56+ NK cell number of 39.9 ± 9 × 106/kg, with no between-group differences (data not shown). GvHD prophylaxis consisted of cyclosporine and methotrexate scheduled on days 1, 3, and 6 posttransplantation. A 10-week EP intervention consisting of three 60-minute sessions per week (2 home-based and 1 in-hospital) was performed by the EP group. The week after discharge, the participants in the intervention group performed a supervised session in which they (patients and parents) were instructed on the home-based EP and were familiarized with the baseline HRF assessment. The week following this instruction session and familiarization, in which participants came to the hospital for their regular checkup with their oncologist, we performed the baseline assessment. After this assessment, the participants began the 10-week EP intervention. The CT group did not perform any specific EP intervention and received care as usual.
The in-hospital EP intervention was performed in the intrahospital gymnasium and was supervised by an expert in physical activity and pediatric cancer. The program was created based on previous research in pediatric cancer populations16,20,21 and followed the guidelines for strength training and aerobic exercise prescription in children and preadolescents.22-24 Participants started and ended with a low-intensity 10-minute warm-up and cool-down period, consisting of cycle-ergometer pedaling at very light workloads and stretching exercises. The core portion of the training session was divided into strength and aerobic exercises. Strength training included 11 exercises engaging the major muscle groups (bench press, shoulder press, leg extension, leg press, leg curl, abdominal crunch, low-back extension, arm curl, elbow extension, seated row, and lateral pull-down). For each exercise, the children performed one set of 8 to 12 maximal repetitions at moderate velocity. Rest periods of 1 to 2 minutes separated the exercises. Stretching of the muscles involved in the previous exercise was performed during the rest periods between exercises. The load was gradually increased as the strength of each child improved. When children were able to perform more than 12 repetitions, the load was increased approximately 5% in upper body and 10% in lower body exercises. The aerobic exercises consisted of pedaling on a cycle-ergometer, walking, and “aerobic games” involving large muscle groups, for example, jumping exercises, ball games, and group games to maintain the children’s adherence to the training program. The intensity of the aerobic exercise was set at 50% to 70% of the age-predicted maximum heart rate (HRmax, estimated as 220 minus age), with children wearing a portable HR monitor (Xtrainer plus, Polar Electro OY, Kempele, Finland).25
The home-based EP program included 20 to 30 minutes of aerobic exercises and 30 minutes of strengthening and stretching exercises using Nintendo’s Wii console with Wii Fit/Wii Sports games. Nintendo supported the consoles and the games during the study. The children’s parents supervised these sessions. The exercises were organized as circuit training, alternating between 10 minutes of aerobic exercises and 10 minutes of strengthening/stretching exercises engaging the major muscle groups. The intensity of aerobic exercises was set at 50% to 70% of HRmax, using the aforementioned HR monitor. The strength training was performed using mainly body weight and following Wii fit strength training instructions. The Wii fit provided clear technique instructions to perform the exercises. Once patients mastered the exercise technique they were instructed to skip the exercise explanation in order to shorten rest periods. Rest periods of 1 to 2 minutes separated the exercises. The intensity of these exercises was moderate (between 70% and 80% of 1 RM). A progressive variation in exercise selection was performed in order to maintain the moderate intensity of training. Advanced exercises were selected when children reported they easily performed strength training.
Training adherence was reported in a training diary in which children reported training days and if they omitted any exercise. A training session was declared valid if 80% of the planned session was performed. Follow-up phone calls were performed weekly by the research coordinator. Adherence to the training was 80% across subjects. No adverse effects and no major health problems were reported during the EP (Table 2).
Table 2.
Training Characteristics of Each Intervention Group Patient.
| Patient | Total Number of Sessions |
Number of FSS | Adherence (% Total Planned Sessions) | Mean Duration of NS Session (Minutes) | Mean Intensity of NS Session (% HRmax) | |
|---|---|---|---|---|---|---|
| NS | S | |||||
| 1 | 22 | 8 | 1 | 80 | 61 | 62 |
| 2 | 20 | 10 | 1 | 83 | 56 | 65 |
| 3 | 12 | 6 | 1 | 78 | 58 | 61 |
| Mean ± ED | 26 ± 4 | 1 ± 0 | 80.3 ± 1.4 | 58 ± 1.4 | 62.6 ± 1.2 | |
Abbreviations: NS, not supervised; S, supervised at hospital gym; FSS, familiarization supervised session; HRmax, theoretical maximal heart rate.
Outcome and Assessments
Assessments were performed for all the participants at baseline (a week before the EP started) and after the 10-week EP intervention was completed. The assessment included the following: (1) adherence to training and possible adverse effects, (2) immunological recovery, (3) demographic and health characteristics, and (4) HRF assessment (only the EP group). Adherence to the program was recorded and measured by the number of days participants engaged in the planned sessions out of the total (attendance). Any unexpected or adverse incidents were recorded, as well as any adverse effects deemed to be provoked by the EP.
Flow Cytometry Analysis, NKCC, and Cytokine Immunoassay
Immunological studies were performed on peripheral blood samples collected at approximately the same time of day (8–10 am) after abstinence of vigorous EP for at least 36 hours. We were not interested in acute changes mediated by EP, but in long-term effects.13,26 The blood samples were collected coinciding with other blood samples needed during regular checkups. NK cell phenotyping was performed on fresh samples of peripheral blood mononuclear cells by multiparametric flow-cytometry (Becton Dickinson, FACSCanto II). NK cells were defined as CD3 negative, CD56 positive cells. NK bright subset cells were defined as CD3 negative, CD56bright positive, and CD16dim negative cells. NK dim subset cells were defined as CD3 negative and CD56dim and CD16dim positive cells. NK cell phenotyping was determined by direct measurement of the surface expression of NCRs (NKp44 and NKp30) and NKG2D. NK cell natural cytotoxicity was monitored in a conventional 2h europium-TDA release assay (Perkin-Elmer Wallac, Turku, Finland) in a ratio 2.5:1 effectors–target. Because of the small sample size, we compared the NKCC ratio to avoid individual differences. We used the Bio-Plex human cytokine immunoassay (Bio-Rad Laboratories Inc, Hercules, CA) for simultaneous quantification of the following cytokines in serum: interleukin (IL)-2, IL-4, IL-6, IL-8, IL-10, interferon-γ (INF-γ), tumor necrosis factor-α (TNF-α), and granulocyte macrophage colony-stimulating factor (GM-CSF).
HRF Assessment
The secondary objective was HRF assessment. Only the EP group completed the assessment, at the in-hospital gym. The control group did not receive a HRF assessment because it was not considered ethical to perform a familiarization period if they would not be benefiting from the training. At least one familiarization session for all tasks was performed before the HRF assessments. In this session, the correct technique for each test was taught. Dynamic upper- and lower-body muscle strength endurance were measured using a seated bench, seated lateral row, and seated leg-press machine (Strive Inc, Canonsburg, PA). The 6 repetition maximum test (6RM) was used (recorded in kilograms); it determines the maximum strength capacity to perform 6 repetitions until momentary muscular exhaustion. The testing protocol is fully described in a previous intervention.21 To measure the children’s functional mobility, the timed up and go (TUG) 3-m test and time up and down stairs (TUDS) test were used. Both tests, previously used in pediatric cancer populations, have been shown to be reliable and valid in healthy children and also in children with various diseases or disabilities.27 For the TUDS, all the children used the handrail to avoid falls.
Data Analysis
All statistical analyses were performed using the Statistical Package for Social Sciences (SPSS) 21.0 software (SPSS Inc, Chicago, IL). The immunological variable assessors were blinded to the participant’s condition (EP or CT), and all the statistical analyses were performed maintaining blindness to condition. Nonparametric testing (Mann-Whitney U test) was used to compare the change over time in the aforementioned variables between the EP and CT groups. To evaluate the combined effect of group and time on the different subpopulations of the immune system (NKCC, NK cell phenotype, and cytokine profile), the Mann-Whitney U test was used to compare mean ratio (posttraining mean/baseline mean). The mean ratio was calculated for the following variables: (1) NK cell subtypes (expressed as a percentage of total NK cells), (2) NKCC (expressed as percentage of K562 lysis), and (3) cytokines (expressed in pg/µL). Additionally, given the small sample size (and thus to avoid an inflated type I error), we used the nonparametric Wilcoxon signed-rank test to compare the mean values of all the HRF variables, measured at baseline and posttraining, for the participants in the EP group. The level of significance was set at .05. The results are expressed as means ± standard deviation (SD).
Results
Transplantation Outcome
All the patients were engrafted and achieved full donor whole blood chimerism after transplantation. Neutrophil and platelet engraftment was achieved on day 12 (range 11–14) and day 13 (range 9–16), respectively, with no between-group differences (data not shown). Three patients had grade I-II acute GvHD (1 in the control group and 2 in the training group), which resolved with topical steroid treatment. No severe GvHD was observed. No cytomegalovirus or other viral reactivations were reported. To date, 2 patients (both from the control group) have died; one because of relapse and the other due to thrombotic microangiopathy.
Immune Reconstitution
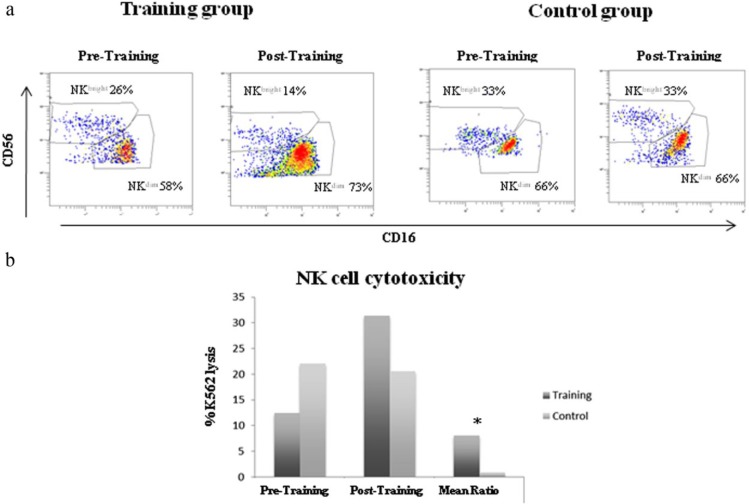
Early immune recovery was dominated by NK cells, as expected. When comparing the percentage of CD56dim at pre- and posttraining, no statistically significant differences were found. However, a significant increase was observed in the mean ratio (posttraining/pretraining) of the EP group of the CD56dim NK cell subset (EP = 1.27 ± 0.07; CT = 0.99 ± 0.08; P < .03). The EP group compared with the CT group showed less of an increase in CD56bright over time in the percentages of NK cells and also in the mean ratio (EP = 0.69 ± 0.27; CT = 1.08 ± 0.47; Table 3 and Figure 1A). However, none of the changes described for CD56bright were statistically significant. No other differences were found in the percentage of NK cell markers. The NKCC ratio (posttraining/pretraining) was 8 times greater in the EP group compared with the CT group (EP = 8.02 ± 3.9; CT = 0.91 ± 0.2, P < .05; Figure 1B). No significant differences were found in the mean values for the EP and CT groups in any of the 8 evaluated cytokine concentrations (IL-2, IL-4, IL-6, IL-8, IL-10, GM-CSF, IFN-γ, and TNF-α) over the course of the intervention (Table 3).
Table 3.
Comparison of NK Cells, NK Cell Subtypes, and Serum Cytokines Between Groups Before and After a Training Intervention.
| Population | Group | Mean, Pre/Post | SD, Pre/Post | P |
|---|---|---|---|---|
| NK (cells/µL) | Training | 167/368 | 35/75 | ns |
| Control | 133/357 | 25/86 | ||
| NK (%) | Training | 1.7/8.6 | 0.9/3.9 | ns |
| Control | 5.2/10.7 | 2.2/5.9 | ||
| CD56dim (%) | Training | 58/73 | 6.4/3.7 | ns |
| Control | 66/66 | 8.9/11.6 | ||
| CD56bright (%) | Training | 26/14 | 7.2/5.2 | ns |
| Control | 31/31 | 3.4/11.3 | ||
| NKG2D (%) | Training | 75.6/71.9 | 5.3/4.5 | ns |
| Control | 75.4/71.5 | 10.5/2.8 | ||
| NKp30 (%) | Training | 1.7/2.1 | 0.7/1.2 | ns |
| Control | 4.2/2.3 | 2.3/1.6 | ||
| NKp44 (%) | Training | 47.4/17.4 | 17.1/7.6 | ns |
| Control | 17.9/35.2 | 5.4/22 | ||
| Mean ratio CD56dim | Training | 1.27 | 0.07 | .03 |
| Control | 0.99 | 0.08 | ||
| Mean ratio CD56bright | Training | 0.69 | 0.27 | ns |
| Control | 1.08 | 0.47 | ||
| IL-2 (pg/µL) | Training | 38.3/77.4 | 6.3/37.9 | ns |
| Control | 41.9/42.4 | 2.4/1.0 | ||
| IL-4 (pg/µL) | Training | 30.9/81.8 | 3.9/37.4 | ns |
| Control | 39.2/30.9 | 4.6/6.0 | ||
| IL-6 (pg/µL) | Training | 46.7/163.9 | 17.9/13.0 | ns |
| Control | 44.0/101.4 | 16.6/50.1 | ||
| IL-8 (pg/µL) | Training | 90.8/436.8 | 14.3/172.4 | ns |
| Control | 81.6/332.5 | 22.9/94.8 | ||
| IL-10 (pg/µL) | Training | 99.8/220.5 | 17.4/59.9 | ns |
| Control | 178.2/202.3 | 74.6/51.8 | ||
| INF-γ (pg/µL) | Training | 30.8/86.4 | 3.8/40.8 | ns |
| Control | 37.8/29.3 | 4.9/4.9 | ||
| TNF-α (pg/µL) | Training | 33.0/82.6 | 6.5/30.1 | ns |
| Control | 38.9/34.0 | 3.1/4.8 | ||
| GMC-SF (pg/µL) | Training | 47.6/87.6 | 2.9/33.1 | ns |
| Control | 83.8/45.3 | 32.4/8.1 |
Figure 1.
(A) Flow charts of NK cell subsets and (B) percentage of NK cell cytotoxicity (NKCC) against the K562 cell line at effector/target ratio 2.5:1 in the training (EP) and control (CT) groups before and after the EP.
HRF Assessment
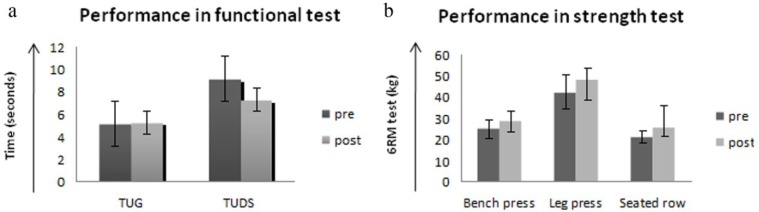
Performance in the TUDS, TUG 3-m, and strength endurance tests (eg, seated bench press, seated row, seated leg press) improved nonsignificantly over the course of the EP intervention (Figure 2A and B).
Figure 2.
(A) Performance in the functional test and (B) performance in the 6 repetition maximum test (6RM) in the training (EP) and control (CT) groups before and after the EP.
Discussion
We have previously demonstrated that a moderate-intensity EP benefits the physical condition and overall health status of children both during and ≤1 year after HSCT.16,20 In this pilot study, we extended our intervention postdischarge and into the early period after HSCT to assess the EP effects on NK cell reconstitution. Results showed the feasibility of this intervention. Seven participants were eligible and 6 agreed to participate. All the children in the EP group completed the intervention and had high rates of completion for the program (80%), which is similar to other supervised exercise programs21 and is higher than other home-based exercise programs.22 We agree that supervised EP promotes greater results than home-based or mixed programs. However, in this study the supervised exercise programs 3 times/week would not be possible due to 2 of the 3 participants living more than 80 km from the hospital. The high adherence to the home-based EP showed that the use of the Wii games could be a useful tool to promote EP through play, providing the children with enjoyment and fun, key factors for obtaining high adherence in the pediatric population. Promising results discussed below show the importance of conducting this study with larger populations. We recommend performing the same protocol, including the cytokine analysis due to the importance of some of them, such as IL-6.23 We recommend including the assessment of aerobic capacity and a control group in the HRF assessment as well to better control the HRF due to an exercise intervention.
The primary finding was that a moderate-intensity EP increases the mean ratio of CD56dim cells and NKCC in immune-compromised children who receive allogeneic HSCT ≤30 days before starting EP intervention. It has been noted that the early posttransplant period is dominated by a greater increase in the CD56bright count compared with CD56dim.8 In our study, both groups (EP and CT) showed at pretraining an elevated percentage of CD56bright compared with the healthy population.14 The EP group, at almost 100 days posttransplant, showed a percentage of CD56bright similar to the donors in the study performed by Dulphy et al,8 whereas the CT showed levels similar to those of the recipients. Although we did not find the decrease of CD56bright to be statistically significant between the EP and CT groups, we did find a statistically significant increase in the mean ratio of the CD56dim count, which is the most cytotoxic NK cell subtype. This exploratory study suggests that EP might help reverse the imbalance in NK subpopulations. Therefore, our results might support and expand on previous research that has shown the differential effect of EP on the 2 major NK subsets, CD56bright and CD56dim, with an inhibiting effect on the immature, less cytotoxic NK cell subset.8,14 The study results are in agreement with previous research in adults with cancer in which an EP improved the NK function.10,11,15 The redistribution of CD56dim and CD56bright in response to EP could potentially be a therapeutic tool for children recently receiving an HSCT.11,13,15 NKCC is positively associated with the HSCT outcome.2 Medium to high NKCC is associated with reduced risk of cancer and leukemia relapse, whereas low activity is associated with increased risk, suggesting a role for NK cell defense mechanisms against cancer.4 Kruijsen-Jaarsma et al also showed that treated patients with cancer who showed high peripheral blood NKCC had a significantly longer metastasis-free survival time than those with low NKCC.24 In the present study, the NKCC ratio (posttraining/pretraining) was 8 times greater in the EP group compared with the CT group. Concerns have been expressed that excessive exercise training could suppress the immune response, increasing vulnerability to both viral infection and tumor cells.17,25 However, we did not see any detrimental effect in a moderate exercise intervention on the immune system reconstitution in our population. Our data support the only previous study that assessed NKCC in a population with pediatric cancer receiving an exercise program.17 Nonsignificant differences were found between the EP and CT groups in the leukocyte subpopulation. Also, nonsignificant differences were found between the EP and CT groups in the cytokine profile in the plasma of IL-2, IL-4, IL-6, IL-8, IL-10, TNF-γ, TNF-α, and GM-CSF.
Altogether, these findings could support the hypothesis that a moderate-intensity EP might be a promising adjuvant therapeutic intervention during early recovery after HSCT, taking into account the benefits incurred, including an increment in the CD56dim mean ratio and NKCC, and the improvement in HRF, quality of life, and motor performance previously shown. Besides, no significant detrimental effects due to a moderate-intensity EP were described.26 Keeping in mind the small sample size, the safety of an EP intervention in this population has yet to be completely determined, and future studies with larger sample sizes will more adequately address this issue.
As a secondary outcome, we assessed HRF in the EP group. The TUG test, TUDS test, and strength test improved over time, but the change was statistically nonsignificant. Clinically, however, patients noted an improvement in their strength and functional mobility, 2 factors that are usually negatively affected in the early stages of treatment due to adverse effects of antineoplastic treatment.27,28 We did not assess aerobic capacity in this intervention. However, we observed that children at the end of the intervention were able to increase the cadence (from 45 to 70) and complete 30 minutes of aerobic training with fewer pauses, staying within the target HR zone (50% to 70% HRmax). Regarding strength training, every 4 weeks we had to changes some strength exercise prescribed to do at home by the Wii because children reported that were so easy. Despite this, we did not prescribe advanced Wii exercise because we could not check for proper execution at home. In the supervised EP we also increased the loads every 4 weeks because patients were able to execute 12 repetitions with ease. When comparing the pre-post strength test results, participants in the EP group increased 15.5% in the chest press, 16.7% in the leg press test, and 49% in the rowing test. The Canadian Society for Exercise Physiology position paper for children and adolescents states that an increase of between 13% and 20% in strength should be expected after 8 to 20 weeks of training,29 consistent with our results. Thus, even though the improvement was not statistically significant it was clinically significant and within what is expected for strength improvement in children. Children gain strength through neural adaptations, not muscle hypertrophy. Strength training in children likely improves the number and coordination of activated motor neurons, as well as the firing rate and pattern. Also, the percentage of improvement in the strength test and TUG test was similar to a previous study performed by our team in which the improvements were significant.30,31 The limitations include the small population sample size, mixed types of disease, lack of follow-up after the training period, and the lack of evaluation of HRF in the control group. In particular, the small sample size hindered our ability to find statistical significance, providing further support for conducting larger, multi-site trials in this population in the future.
The authors recognize that, due to these limitations and the small sample size, conclusions should be interpreted with caution. Nevertheless, considering the difficulty of conducting clinical trials in this population, the findings of this exploratory study would contribute to the existing literature and could guide future research.
Conclusions
According to our preliminary data, a moderate-intensity combined EP (supervised and home-based) performed early after HSCT does not appear to impair immune system reconstitution and function, and therefore could be feasible even in this immunocompromised population. Moreover, the redistribution of CD56dim and CD56bright and the improvement in NKCC in response to a moderate-intensity EP might have an adjuvant therapeutic effect in children with cancer. However, the results are still preliminary and must be interpreted as such. Future research with larger patient samples will better elucidate these effects.
Acknowledgments
The authors wish to thank Jaime Valentín for helpful assistance during the conduct of the study and María de Miguel Gallo for writing assistance.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported in part by the National Health Service of Spain, Instituto de Salud Carlos III (ISCIII; FIS, Grant # PI15/00973), Cofinanciación con FONDOS FEDER, the Fundación Mutua Madrileña, and the CRIS Cancer Foundation (http://criscancer.org/en).
References
- 1. Miano M, Labopin M, Hartmann O, et al. Haematopoietic stem cell transplantation trends in children over the last three decades: a survey by the paediatric diseases working party of the European Group for Blood and Marrow Transplantation. Bone Marrow Transplant. 2007;39:89-99. [DOI] [PubMed] [Google Scholar]
- 2. Bosch M, Khan FM, Storek J. Immune reconstitution after hematopoietic cell transplantation. Curr Opin Hematol 2012;19:324-335. [DOI] [PubMed] [Google Scholar]
- 3. Crisafulli A, Tocco F, Melis F, Milia R, Concu A. Natural killer cells responsiveness to physical exercise: a brief review. Open J Immunol. 2013;3:190-200. [Google Scholar]
- 4. Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet 2000;356:1795-1799. [DOI] [PubMed] [Google Scholar]
- 5. Nagler A, Lanier LL, Cwirla S, Phillips JH. Comparative studies of human FcRIII-positive and negative natural killer cells. J Immunol. 1989;143:3183-3191. [PubMed] [Google Scholar]
- 6. Lanier LL, Le AM, Civin CI, Loken MR, Phillips JH. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480-4486. [PubMed] [Google Scholar]
- 7. Cooper MA, Fehniger TA, Turner SC, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146-3151. [DOI] [PubMed] [Google Scholar]
- 8. Dulphy N, Haas P, Busson M, et al. An unusual CD56(bright) CD16(low) NK cell subset dominates the early posttransplant period following HLA-matched hematopoietic stem cell transplantation. J Immunol. 2008;181:2227-2237. [DOI] [PubMed] [Google Scholar]
- 9. Radom-Aizik S, Zaldivar F, Haddad F, Cooper DM. Impact of brief exercise on peripheral blood NK cell gene and microRNA expression in young adults. J Appl Physiol. 2013;114:628-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fairey AS, Courneya KS, Field CJ, Bell GJ, Jones LW, Mackey JR. Randomized controlled trial of exercise and blood immune function in postmenopausal breast cancer survivors. J Appl Physiol. 2005;98:1534-1540. [DOI] [PubMed] [Google Scholar]
- 11. Na YM, Kim MY, Kim YK, Ha YR, Yoon DS. Exercise therapy effect on natural killer cell cytotoxic activity in stomach cancer patients after curative surgery. Arch Phys Med Rehabil. 2000;81:777-779. [DOI] [PubMed] [Google Scholar]
- 12. Timmons BW. Paediatric exercise immunology: health and clinical applications. Exerc Immunol Rev. 2005;11:108-144. [PubMed] [Google Scholar]
- 13. Timmons BW, Cieslak T. Human natural killer cell subsets and acute exercise: a brief review. Exerc Immunol Rev. 2008;14:8-23. [PubMed] [Google Scholar]
- 14. Suzui M, Kawai T, Kimura H, et al. Natural killer cell lytic activity and CD56(dim) and CD56(bright) cell distributions during and after intensive training. J Appl Physiol. 2004;96:2167-2173. [DOI] [PubMed] [Google Scholar]
- 15. Peters C, Lötzerich H, Niemeier B, Schüle K, Uhlenbruck G. Influence of a moderate exercise training on natural killer cytotoxicity and personality traits in cancer patients. Anticancer Res. 1994;14:1033-1036. [PubMed] [Google Scholar]
- 16. Chamorro-Viña C, Ruiz JR, Santana-Sosa E, et al. Exercise during hematopoietic stem cell transplant hospitalization in children. Med Sci Sports Exerc. 2010;42:1045-1053. [DOI] [PubMed] [Google Scholar]
- 17. Shore S, Shepard RJ. Immune responses to exercise in children treated for cancer. J Sports Med Phys Fitness. 1999;39:240-243. [PubMed] [Google Scholar]
- 18. Ladha AB, Courneya KS, Bell GJ, Field CJ, Grundy P. Effects of acute exercise on neutrophils in pediatric acute lymphoblastic leukemia survivors: a pilot study. J Pediatr Hematol Oncol. 2006;28:671-677. [DOI] [PubMed] [Google Scholar]
- 19. Wiskemann J, Huber G. Physical exercise as adjuvant therapy for patients undergoing hematopoietic stem cell transplantation. Bone Marrow Transplant. 2008;41:321-329. [DOI] [PubMed] [Google Scholar]
- 20. San Juan AF, Chamorro-Viña C, Moral S, et al. Benefits of intrahospital exercise training after pediatric bone marrow transplantation. Int J Sports Med. 2008;29:439-446. [DOI] [PubMed] [Google Scholar]
- 21. San Juan AF, Fleck SJ, Chamorro-Viña C, et al. Effects of an intrahospital exercise program intervention for children with leukemia. Med Sci Sports Exerc. 2007;39:13-21. [DOI] [PubMed] [Google Scholar]
- 22. Takken T, van der Torre P, Zwerink M, et al. Development, feasibility and efficacy of a community-based exercise training program in pediatric cancer survivors. Psychooncology. 2009;18:440-448. [DOI] [PubMed] [Google Scholar]
- 23. Lucia A, Ramírez M. Muscling in on cancer. N Engl J Med. 2016;375:892-894. [DOI] [PubMed] [Google Scholar]
- 24. Kruijsen-Jaarsma M, Révész D, Bierings MB, Buffart LM, Takken T. Effects of exercise on immune function in patients with cancer: a systematic review. Exerc Immunol Rev. 2013;19:120-143. [PubMed] [Google Scholar]
- 25. Walsh NP, Gleeson M, Shephard RJ, et al. Position statement. Part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6-63. [PubMed] [Google Scholar]
- 26. Braam KI, van der Torre P, Takken T, Veening MA, van Dulmen-den Broeder E, Kaspers GJ. Physical exercise training interventions for children and young adults during and after treatment for childhood cancer. Cochrane Database Syst Rev. 2013;(4):CD008796. [DOI] [PubMed] [Google Scholar]
- 27. Kelly AK. Physical activity prescription for childhood cancer survivors. Curr Sports Med Rep. 2011;10:352-359. [DOI] [PubMed] [Google Scholar]
- 28. San Juan AF, Wolin K, Lucia A. Physical activity and pediatric cancer survivorship. Recent Results Cancer Res. 2011;186: 319-347. [DOI] [PubMed] [Google Scholar]
- 29. Behm DG, Faigenbaum AD, Falk B, Klentrou P. Canadian Society for Exercise Physiology position paper: resistance training in children and adolescents. Appl Physiol Nutr Metab. 2008;33:547-561. [DOI] [PubMed] [Google Scholar]
- 30. San Juan AF, Fleck SJ, Chamorro-Viña C, et al. Early-phase adaptations to intrahospital training in strength and functional mobility of children with leukemia. J Strength Cond Res. 2007;21:173-177. [DOI] [PubMed] [Google Scholar]
- 31. Dahab KS, McCambridge TM. Strength training in children and adolescents: raising the bar for young athletes? Sports Health. 2009;1:223-226. [DOI] [PMC free article] [PubMed] [Google Scholar]