Abstract
Background: β-Lapachone is a quinone-containing compound found in red lapacho (Tabebuia impetiginosa, syn. T avellanedae) trees. Lapacho has been used in traditional medicine by several South and Central American indigenous people to treat various types of cancer. The purpose of this study was to investigate the antimetastatic properties of β-lapachone and the underlying mechanisms using colon cancer cells. Methods: This research used metastatic murine colon cancer cell lines, colon 26 (CT26) and colon 38 (MC38). A WST assay, annexin V assay, cell cycle analysis, wound healing assay, invasion assay, western blot analysis, and real-time reverse transcription–polymerase chain reaction were performed to examine the effects of β-lapachone on metastatic phenotypes and molecular mechanisms. The effect of β-lapachone on lung metastasis was assessed in a mouse experimental metastasis model. Results: We found that the inhibition of proliferation of the colon cancer cell lines by β-lapachone was due to the induction of apoptosis and cell cycle arrest. β-Lapachone induced the apoptosis of CT26 cells through caspase-3, -8, and -9 activation; poly(ADP-ribose) polymerase cleavage; and downregulation of the Bcl-2 family in a dose- and time-dependent manner. In addition, a low concentration of β-lapachone decreased the cell migration and invasion by decreasing the expression of matrix metalloproteinases-2 and -9, and increased the expression of tissue inhibitors of metalloproteinases-1 and -2. Moreover, β-lapachone treatment regulated the expression of epithelial-mesenchymal transition markers such as E- and N-cadherin, vimentin, β-catenin, and Snail in CT26 cells. In the mouse experimental metastasis model, β-lapachone significantly inhibited the lung metastasis of CT26 cells. Conclusions: Our results demonstrated the inhibitory effect of β-lapachone on colorectal lung metastasis. This compound may be useful for developing therapeutic agents to treat metastatic cancer.
Keywords: β-lapachone, colorectal cancer, apoptosis, lung metastasis, CT26 cells
Introduction
Colorectal cancer (CRC) is the most commonly diagnosed cancer worldwide, and metastasis develops in 50% of the patients.1,2 The most frequent sites of metastasis are the liver and lungs. CRC patients with untreated metastatic cancer have a median survival period of less than 10 months and a 5-year survival rate of less than 5%.3,4 In particular, lung metastasis is seen in 10% to 20% of patients with CRC, and studies have reported 5-year survival rates of 24% to 64% in patients who have undergone resection.5,6 In the past 13 years (1999-2011), the age-standardized incidence rates of CRC have increased from 20.4 to 37.8% in Korea. Along with the significant increases in the incidence rates of CRC, mortality rates have also continued to increase.7
Metastasis is the spread of cancer cells from the primary lesion to remote organs, and abnormally fast growth of metastases is the most harmful aspect of cancer.8 Metastasis is a complex process of cancer cells spreading from a primary site and forming tumors at distant sites. It occurs through a multistep process by which primary tumor cells lose cellular adhesion and increase cellular motility. To metastasize into distant tissues, cancer cells need to acquire special properties that would enable them to invade the extracellular matrix (ECM), migrate to and invade lymph nodes and blood vessels, and then adhere and survive in target organs.9
Matrix metalloproteinases (MMPs) are important extracellular proteases that can trigger the degradation of ECM and provide a pathway for cancer cells to migrate, invade, and spread to distant organs.10 Tissue inhibitors of metalloproteinases (TIMPs) are endogenous inhibitors of the hydrolytic activities of MMPs and are consequently important regulators of ECM turnover, tissue remodeling, and cellular behavior.11,12 Therefore, the role of MMPs and TIMPs is important during the migration and invasion steps of the metastatic process.
Epithelial-mesenchymal transition (EMT) is a cellular process in which epithelial cells appear as mesenchymal cells during cancer progression and metastasis.13,14 Advanced tumor cells frequently exhibit marked downregulation of epithelial markers and a loss of intercellular junctions, resulting in a loss of epithelial polarity and reduced intercellular adhesion.15 The loss of epithelial features is often accompanied by increased cell motility and expression of mesenchymal genes. EMT regulatory transcription factors, including β-catenin, Snail, and Slug, change the epithelial gene expression profile, decrease the expression of genes encoding epithelial cell-cell adhesion complexes and cytokeratins, and increase the expression of genes encoding mesenchymal markers.16,17 These factors are expressed in various cancer types such as breast cancer, colon cancer, and melanoma.18-20
Induction of apoptosis or cell cycle arrest is important for tumor suppression.21 Synthetic inhibitors of cyclins and cyclin-dependent kinases (CDKs) induce cell cycle arrest and could be therefore useful as anticancer agents.22 Apoptosis is the process of programmed cell death, which changes the cell morphology, induces nuclear and DNA fragmentation and chromatin condensation, and ultimately leads to cell death. Apoptotic cells are eliminated by phagocytes such as macrophages, without eliciting inflammation, whereas necrotic cells release products of cell death, which promote an inflammatory response.23 Therefore, induction of cancer cell apoptosis is considered a therapeutic method against the progression of cancer. Various natural products and compounds can induce apoptosis by regulating the expression of apoptotic or cell cycle–related factors in cancer cells.24
Lapacho trees (Tabebuia impetiginosa, syn. T avellanedae), native to South America, have been used in traditional medicine by South and Central Americans for thousands of years. This plant has been used to treat bacterial infections, fever, malaria, and stomach diseases, as well as several types of cancer.25 β-Lapachone, a natural quinone compound, is the main bioactive compound obtained from lapacho trees. It shows various pharmacological effects, including antibacterial, antifungal, antiviral, and anti-inflammatory activities.26-29 In particular, this compound has been considered one of the main antitumor compounds and investigated to elucidate its antitumor effects and mechanisms. β-Lapachone selectively induces apoptotic death in various cancer cells, including breast cancer, prostate cancer, and leukemia.30,31 Moreover, β-lapachone also shows antitumor effects in human colon cancer cells by inducing cell cycle arrest and apoptosis via a sirtuin 1–forkhead box O1 or a caspase-mediated pathway, along with the elevation of either quinone oxidoreductase 1 (NQO1) or death receptor 5 levels.25 However, the antimetastatic effects of β-lapachone have been rarely reported, and the effect of β-lapachone on colorectal metastasis has not been clarified.32,33 Therefore, we investigated the antimetastatic effect and mechanism of β-lapachone action using metastatic colon cancer cell lines and a mouse experimental metastasis model.
Materials and Methods
Antibodies and Reagents
Anti-poly(ADP-ribose) polymerase (PARP), caspase-3, and caspase-8 antibodies were purchased from Cell Signaling Technology, Inc (Danvers, MA). Caspase-9 antibody was purchased from Enzo Life Sciences (Farmingdale, NY). B-cell lymphoma 2 (Bcl-2) antibody was purchased from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Bcl-xL, Bcl-2-associated X protein (Bax), and α-tubulin antibodies were purchased from Bioworld Technology (Louis Park, MN). β-Lapachone was chemically synthesized by KT&G Life Science (Suwon, Korea).
Cell Culture
The mouse colon carcinoma cell lines colon 26 (CT26) and colon 38 (MC38) were maintained in Dulbecco’s modified Eagle’s medium (DMEM; Gibco BRL, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin and incubated at 37°C in a 5% CO2 incubator.
Animals
BALB/c female mice (5 weeks old, 19-20 g) were purchased from Da-Mool Science (Daejeon, Korea). They were housed, 6 per cage, in a laminar airflow room maintained at a temperature of 22 ± 1°C and a relative humidity of 55 ± 1% throughout the study. The research was conducted in accordance with the internationally accepted principles for laboratory animal use and care as stated in the Wonkwang University guidelines (WKU14-15).
Cell Viability Assay
Cell viability was quantified using the cell proliferation reagent WST-8 [2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt] (Enzo Life Sciences). Cells were seeded in 96-well microplates at 3 × 103 cells/well, and a β-lapachone-containing medium was added to the wells. After the incubation, the medium was changed, followed by the addition of a WST-8 solution, and absorbance was measured at 450 nm.
Annexin V Assay
Annexin V assay was carried out using FITC Annexin V apoptosis detection kit I (BD Biosciences, San Diego, CA). Harvested cells were washed twice with cold phosphate-buffered saline (PBS) and then resuspended in 1× Annexin V binding buffer (1 × 106 cells/mL). Then, 100 µL of the suspension (1 × 105 cells) was transferred to a 5-mL culture tube and labeled with 3 µL of a titrated fluorescein isothiocyanate (FITC)-labeled annexin V and propidium iodide (PI) staining solution. The cells were vortexed and incubated for 15 minutes at room temperature in the dark. The volume was then adjusted to 500 µL, and the cells were analyzed using a FACS-Calibur flow cytometer (BD Biosciences).
Western Blot Analysis
CT26 cells (1 × 106 cells/well) were incubated with various concentrations of β-lapachone. The stimulated cells were rinsed with PBS and then lysed in lysis buffer (iNtRon Biotech, Seoul, Korea) for 1 hour. The cell lysates were centrifuged 13 000 rpm for 10 minutes, and the quantity of protein in the cytoplasmic and nuclear fractions was evaluated using a bicinchoninic acid protein assay. The supernatant was mixed with 2× sample buffer, then proteins were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to an Immobilon-P nylon membrane (Millipore, Bedford, MA). The membranes were blocked with 5% skim milk for 1.5 hours and incubated for 3 hours with primary antibodies. The bound antibodies were detected with horseradish peroxidase-conjugated anti-rabbit, anti-mouse, and IgG antibodies (Dako, Glostrup, Denmark) using an enhanced chemiluminescence system (Santa Cruz Biotechnology).
Cell Cycle Analysis
The cell cycle distribution was assessed using the Muse cell cycle kit (Millipore) according to the manufacturer’s instructions. CT26 cells (1 × 106 cells/well) were plated in 6-well plates and incubated with various concentrations of β-lapachone for 24 hours. The cells were harvested and fixed with 70% ice-cold ethanol at −20°C for at least 3 hours. After washing with PBS, the cell pellets were resuspended in 200 µL of the cell cycle reagent and incubated for 30 minutes at room temperature in the dark. The cells were analyzed using a Muse cell analyzer, and the cell cycle phase distribution was quantified using the Muse analysis software (Millipore).
Real-Time Reverse Transcription (RT)–Polymerase Chain Reaction (PCR)
Total RNA was extracted using an RNA-spin total RNA extraction kit (iNtRon Biotech) according to the manufacturer’s directions. First-strand cDNA was prepared from an RNA template (2 µg) using an oligo(dT)18 primer and a Power cDNA synthesis kit (iNtRon Biotech). RT was performed at 42°C for 50 minutes and then at 70°C for 15 minutes. Real-time quantitative PCR was performed using Power SYBR Green PCR master mix and a StepOnePlus real-time PCR system (Applied Biosystems, Foster City, CA). All data were normalized to GAPDH mRNA. The primer sequences are summarized in Table 1.
Table 1.
Sequences of Real-Time Reverse Transcription–Polymerase Chain Reaction Primers.
| Gene | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|
| MMP-2 | CCCCATGAAGCCTTGTTTACC | TTGTAGGAGGTGCCCTGGAA |
| MMP-9 | AGACCAAGGGTACAGCCTGTTC | GGCACGCTGGAATGATCTAAG |
| TIMP-1 | GCCTACACCCCAGTCATGGA | GGCCCGTGATGAGAAACTCTT |
| TIMP-2 | AGGAGATGTAGCAAGGGATCA | GAGCCTGAACCACAGGTACCA |
| E-cadherin | AATGGCGGCAATGCAATCCCAAGA | TGCCACAGACCGATTGTGGAGATA |
| N-cadherin | TGGAGAACCCCATTGACATT | TGATCCCTCAGGAACTGTCC |
| Snail | TCCAAACCCACTCGGATGTGAAGA | TTGGTGCTTGTGGAGCAAGGACAT |
| β-Catenin | ACTGCTGGGACTCTG | TGATGGCGTAGAACAG |
| Vimentin | CGGAAAGTGGAATCCTTGCA | CACATCGATCTGGACATGCTG |
| GAPDH | GACATGCCGCCTGGAGAAAC | AGCCCAGGATGCCCTTTAGT |
Wound Healing Assay
CT26 cells were seeded in a 6-well plate (2 × 105 cells/well) to form a monolayer. Using a 200 µL pipet tip, a scratch of ~1-mm width was made in triplicate. The detached cells were removed, and the attached cells were incubated in serum-free medium containing β-lapachone at the indicated concentrations. The scratches were monitored at regular intervals over a course of 0 to 24 hours, and images were acquired under phase-contrast microscopy (Leica, Wetzlar, Germany).
Invasion Assay
The invasion ability of cancer cells was estimated using a BD BioCoat GFR matrigel invasion chamber (BD Biosciences). CT26 cells (5 × 104 cells) were suspended in serum-free DMEM with β-lapachone (10 and 100 nM) and added to the upper part of the transwell chamber. For the invasion assay, the lower part of the transwell was filled with DMEM containing 10% FBS as a chemoattractant. After 24 hours of incubation, the membrane inserts were washed twice with PBS and fixed in 3.7% paraformaldehyde in PBS for 5 minutes. After washing twice with PBS, the fixed cells were treated with 100% methanol for 20 minutes and stained with Giemsa stain for 15 minutes. The inner side of the chambers was wiped with a cotton swab. The membrane inserts were dried and observed under a microscope (Leica).
Gelatin Zymography
Activity of MMPs was assayed by gelatin zymography using a zymogram buffer kit (Koma Biotech, Seoul, Korea). Cells (5 × 105 cells) were seeded in 6-well plates and stabilized in serum-free medium overnight. After β-lapachone treatment for 24 hours, the conditioned medium was collected. Samples were mixed with 2× sample buffer and electrophoresed on 8% SDS-PAGE gels containing 0.1% gelatin. The gels were washed with renaturing buffer for 15 minutes and incubated in developing buffer at 37°C for 24 hours. The gel was stained with a Coomassie blue R-250 solution for 30 minutes and destained with destaining buffer (50% methanol, 10% acetic acid, and 40% distilled water) for 30 minutes. The gelatinolytic activity of MMPs was photographed using a digital imaging system.
Experimental Lung Metastasis Model
For experimental lung metastasis, CT26 cells were harvested and resuspended in PBS. Mice were intravenously inoculated with CT26 (3 × 104 cells) in 200 µL of PBS via the lateral tail vein. β-Lapachone and dimethyl sulfoxide (DMSO) were administered by intraperitoneal injection 2 hours prior to the injection of the cancer cells and then injected once every 2 days. The mice were sacrificed 17 days later, and the lungs were removed and fixed in Bouin’s solution (Sigma, St Louis, MO). The increase in lung weight and the number of tumor colonies in the lungs were measured to evaluate tumor metastasis.
Statistical Analyses
Data were analyzed for statistical significance using the Student’s t test. P values of <.05 were considered to indicate statistically significant differences. The mean and standard deviation (SD) were calculated for all variables.
Results
Effect of β-Lapachone on Viability of Colon Cancer Cells
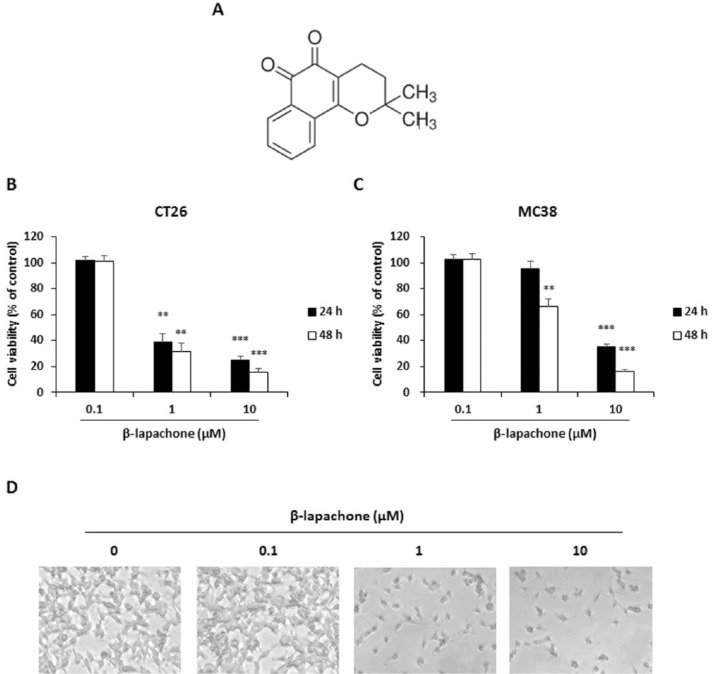
The chemical structure of β-lapachone is shown in Figure 1A. To determine whether β-lapachone is cytotoxic to the metastatic colon cancer cells, we evaluated their cell viability using the WST assay. Figure 1 shows that the treatment with β-lapachone decreased the viability of CT26 and MC38 cells in a dose- and time-dependent manner (Figure 1B and C). Moreover, apoptotic morphological changes were detected in β-lapachone-treated CT26 cells by microscopic observation (Figure 1D).
Figure 1.
β-Lapachone decreases viability of metastatic murine colon carcinoma cells. (A) Chemical structure of β-lapachone. (B and C) Viability of β-lapachone-treated CT26 and MC38 cells. Cells were seeded at a density of 3 × 103 cells/well in 96-well microplates and then treated with various concentrations of β-lapachone for 24 and 48 hours. After incubation at 37°C, cell viability was determined using the WST assay. Results are expressed as the mean ± SD of 3 independent experiments. **P < .01, ***P < .001. (D) Morphology of β-lapachone-treated CT26 cells. After 24 hours of incubation with β-lapachone, photographs were acquired by microscopy. The photographs are representative of 3 independent experiments.
Effect of β-Lapachone on Apoptosis of CT26 Cells
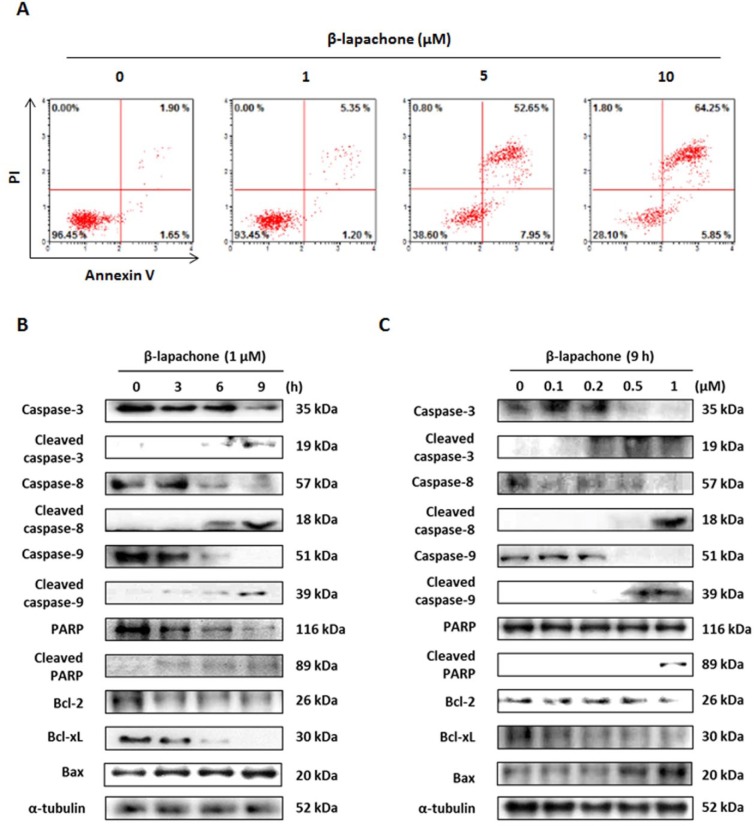
To determine whether the inhibition of cell proliferation by β-lapachone was due to cell apoptosis, CT26 cells were treated with β-lapachone (0, 1, or 10 µM) for 9 hours, and the annexin V assay was conducted. As shown in Figure 2A, β-lapachone increased both early (lower right of Figure 2A) and late (upper right of Figure 2A) apoptosis of CT26 cells. Because β-lapachone increased the annexin V–positive CT26 cell population, the mechanism underlying β-lapachone-induced apoptosis was investigated by western blot analysis. Exposure of CT26 cells to β-lapachone (1 µM) for 0 to 9 hours or to various concentrations (0, 0.1, 0.2, 0.5, or 1 µM) of β-lapachone for 9 hours caused cleavage of caspases-3, -8, -9, and PARP. In addition, β-lapachone decreased the truncation of Bcl-2 and Bcl-xL and increased the expression level of Bax in a time- and dose-dependent manner in CT26 cells in an intrinsic pathway (Figure 2B and C).
Figure 2.
β-Lapachone induces apoptosis through extrinsic and intrinsic signaling pathways in CT26 cells. (A) CT26 cells were incubated with the indicated concentrations of β-lapachone for 9 hours and stained with annexin V and PI. The figure is representative of 3 independent experiments. (B) CT26 cells were treated with β-lapachone (1 µM) for 0 to 9 hours. (C) CT26 cells were treated with various concentrations of β-lapachone for 9 hours and subjected to western blotting with antibodies against PARP, caspase-3, -8, -9, Bcl-2, Bcl-xL, and Bax.
Effect of β-Lapachone on Cell Cycle Arrest in CT26 Cells
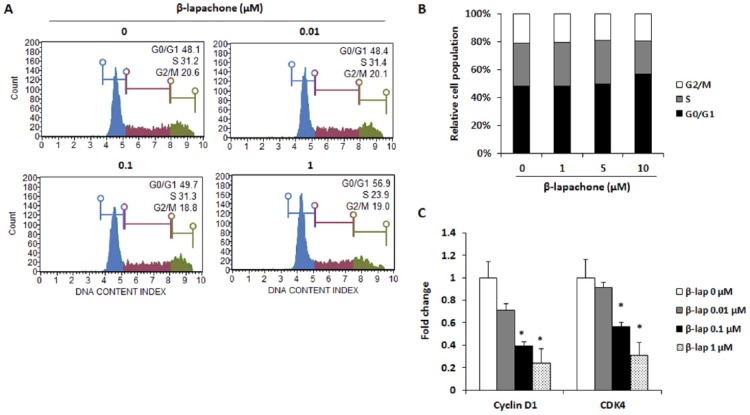
To investigate whether β-lapachone induces the cell cycle arrest, flow cytometry was used to analyze the changes in the cell cycle. CT26 cells were treated with various concentrations of β-lapachone for 24 hours, and its DNA content was measured. It was found that, on treatment with a high concentration (1 µM) of β-lapachone, the percentage of CT26 cells entering the S phase was decreased and the cells were blocked in the G0/G1 phase (Figure 3A and B). Moreover, downregulation of the mRNA expression of cyclin D1 and CDK4 by β-lapachone was also observed in CT26 cells (Figure 3C).
Figure 3.
β-Lapachone induces G0/G1 phase cell cycle arrest through inhibition of cyclin D1 and CDK4 expression. (A) Cell cycle analysis of CT26 cells after treatment with β-lapachone for 24 hours. Data are representative of 3 independent experiments. (B) Percentages of cells with the DNA content consistent with each phase of the cell cycle were plotted. (C) mRNA expression of cyclin D1 and CDK4. CT26 cells were treated with various concentrations of β-lapachone for 24 hours. Results are expressed as the mean ± SD of 3 independent experiments. *P < .05.
Effect of β-Lapachone on EMT Markers in CT26 Cells
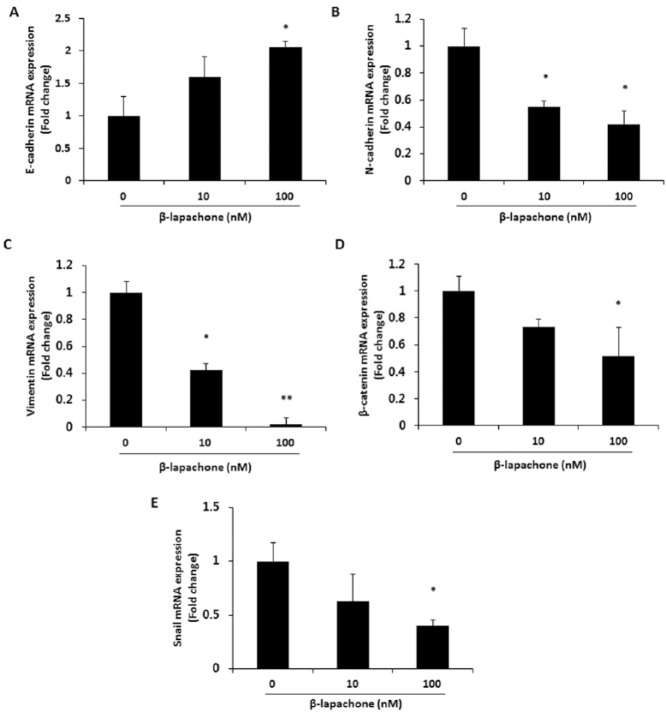
To determine whether β-lapachone affects the expression of EMT markers typical for metastatic phenotypes, mRNA expression of EMT-related molecules was determined. As shown in Figure 4, the expression of the epithelial phenotypic marker E-cadherin was increased (Figure 4A), while that of the mesenchymal phenotypic markers N-cadherin, vimentin, β-catenin, and Snail were decreased in β-lapachone-treated CT26 cells (Figure 4B-E).
Figure 4.
β-Lapachone regulates mRNA expression levels of EMT markers. mRNA expression levels of EMT markers were analyzed by real-time RT-PCR after treatment of CT26 cells with β-lapachone (0-100 nM) for 24 hours. (A) Epithelial marker; E-cadherin. (B-E) Mesenchymal markers; N-cadherin, vimentin, β-catenin, and Snail. Results are expressed as the mean ± SD of 3 independent experiments. *P < .05 and **P < .01.
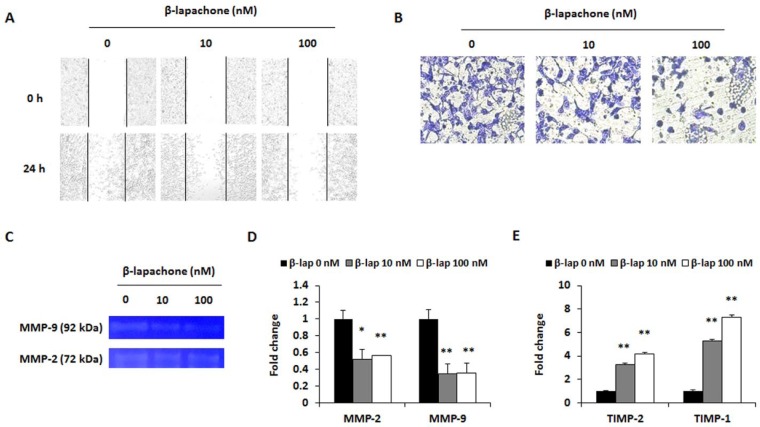
Effect of β-Lapachone on Migratory and Invasive Ability of CT26 Cells
Migration and invasion are the fundamental features of metastasis after the EMT process. Therefore, a wound healing assay was performed to determine whether β-lapachone suppresses the migration of CT26 cells. Cell movements were observed 24 hours after the treatment with β-lapachone. In the presence of β-lapachone, the cells showed a decrease in collective cell migration when compared with that of the control. The control cells migrated toward the scratched region in a dose-dependent manner, whereas the β-lapachone (10 and 100 nM) treated cells did not migrate (Figure 5A). The invasive ability of CT26 cells was measured by the matrigel invasion assay. As shown in Figure 5B, β-lapachone (10 and 100 nM) induced significant decreases in the invasion of CT26 cells. To determine the molecular mechanism of the anti-invasive ability of β-lapachone, mRNA expressions of MMP-2/9 and their inhibitor TIMP-1/2 were measured. Activities of MMP-2 and MMP-9 were also determined using gelatin zymography. β-Lapachone dose-dependently inhibited the activities of MMP-2 and MMP-9 (Figure 5C). The real-time RT-PCR results showed that β-lapachone treatment suppressed MMP-2 and MMP-9 expressions, and increased TIMP-1 and TIMP-2 expressions in a dose-dependent manner (Figure 5D and E).
Figure 5.
β-Lapachone reduces migration and invasion ability. (A) Wound healing assay. CT26 cells were cultured to 80% confluence in a 6-well plate, and the cell layer was scratched with a 200 µL micropipette tip. After washing with a serum-free medium, the cells were treated for 24 hours with the indicated concentrations of β-lapachone. After the incubation, images were photographed using a microscope (100× magnification). (B) Invasion assay. CT26 cells (5 × 104 cells/well) were cultured in a 24-well plate with a matrigel-coated transwell chamber. After 24 hours of incubation with β-lapachone, the inner side of the chamber was fixed and stained. Photographs are representative of 3 independent experiments. (C) MMP-2 and MMP-9 activities in β-lapachone-treated CT26 cells were determined by gelatin zymography. (D and E) mRNA expression levels of the cell invasion–related factors MMP-2, -9 (D) and TIMP-2, -1 (E) were analyzed by real-time RT-PCR after β-lapachone (10 or 100 nM) treatment for 24 hours. Results are expressed as the mean ± SD of 3 independent experiments. *P < .05 and **P < .01.
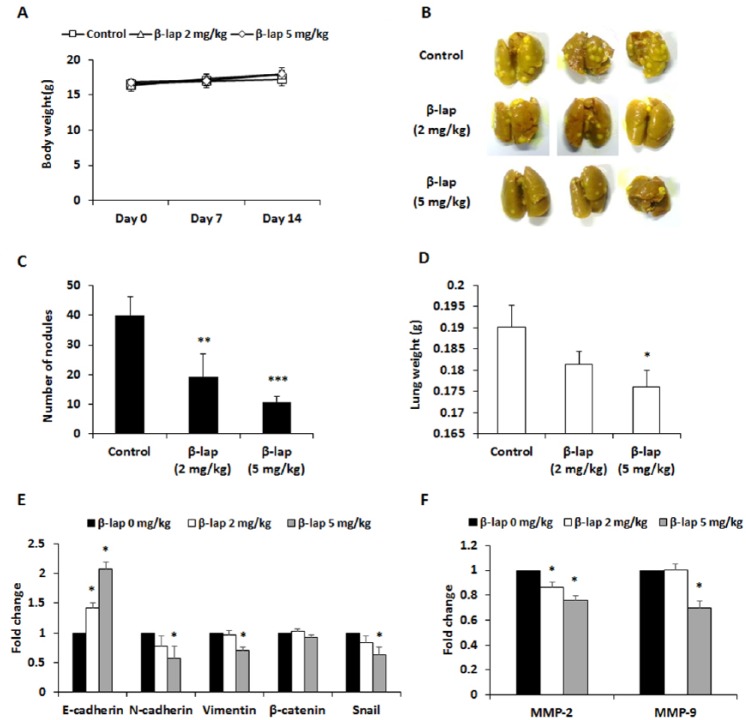
Effect of β-Lapachone on Lung Metastasis of CT26 Cells in an Experimental Metastasis Model
The present study also investigated whether β-lapachone could inhibit spontaneous lung metastasis of colon cancer cells in mice. Body weight, an indicator of toxicity, was not changed by the injection of β-lapachone (Figure 6A). As shown in Figure 6B and C, the number of pulmonary tumor nodules significantly was increased in the lungs of the mice intravenously injected with CT26 cells alone. However, the intraperitoneal injection of β-lapachone (2 or 5 mg/kg) for 2 weeks significantly decreased the formation of tumor nodules by about 50% or 55%, respectively. Compared to the control group, the β-lapachone-treated mice showed significantly smaller tumors (Figure 6D). Additionally, we investigated the expression of EMT markers and MMP-2/9 in lung tissues to determine whether the in vivo antimetastatic effect of β-lapachone is associated with the regulation of EMT-related genes and MMP-2/9. β-Lapachone administration increased the expression of E-cadherin, whereas it downregulated the expression of N-cadherin, vimentin, and Snail (Figure 6E). Moreover, the expressions of MMP-2 and MMP-9 were also decreased in lung tissues of the β-lapachone-administered group (Figure 6F). These results are consistent with those of the in vitro experiments.
Figure 6.
β-Lapachone inhibits colorectal lung metastasis. BALB/c mice were intravenously transplanted with 1 × 105 cells of CT26 cancer cells into the tail vein. Then, the mice were divided into 3 groups (n = 6) and subjected to intraperitoneal injection of β-lapachone (2 or 5 mg/kg) once every 2 days until sacrifice. The control group mice were administered the same volume of DMSO. (A) The body weight of the mice was monitored for assessing toxicity. (B) Lungs were excised and stained with Bouin’s solution to compare the patterns of pulmonary tumor nodule formation among the experimental groups. (C and D) The number of tumor nodules (C) and lung weight (D) are expressed as the mean ± SD. (E and F) mRNA expression levels of EMT markers (E) and MMP-2/9 (F) were measured in lung tissues. *P < .05, **P < .01, and ***P < .001. Data are representative of 3 independent experiments.
Discussion
Because lapacho has been used by South Americans since old times for its improvement effects against cancer and pain caused by diseases, researchers have focused on antitumor effects and related mechanisms of this plant and its main active compounds. In particular, it has been demonstrated that β-lapachone shows a potent anticancer effect against colon cancer.25,34 However, the antimetastatic effects of β-lapachone have not been widely studied. Only a few studies have revealed that β-lapachone inhibits the migratory ability of HepG2 and Hep3B human hepatocarcinoma cells and reduces the spleen-to-liver tumor burden in a metastatic pancreatic cancer mouse model by inducing the NQO1-mediated apoptosis.32,33 In this study, we clarified that β-lapachone inhibits the colorectal lung metastasis by inducing apoptosis and reducing metastatic phenotypes such as EMT, migration, and invasion.
Cancer is a complex disease involving changes in multiple pathways and microenvironments. In particular, cancer cells that avoid cell death and continue to proliferate uncontrollably help in cancer progression. Apoptosis is a programmed cell death that occurs naturally in cells and can be beneficial for cancer therapy.35 Therefore, anticancer effects of natural products and compounds caused by induction of apoptosis have been extensively studied.24 According to recent studies, β-lapachone can induce apoptosis in human leukemia HL-60 cells and human prostate cancer cells.31 It can also promote the cell cycle arrest and caspase-mediated apoptosis in human colon cancer cell lines, including HCT-116, SW480, and SW620.34,36 In this study, the antiproliferative effects of β-lapachone were investigated in the metastatic colon cancer cell lines CT26 and MC38. We demonstrated that β-lapachone significantly inhibited the proliferation of both cell lines (Figure 1B-D), and this antiproliferative effect occurred through the induction of apoptosis (Figure 2).
Apoptosis occurs via 2 main pathways, extrinsic and intrinsic. The extrinsic pathway is a death receptor-dependent pathway, with death ligands involved or extracellular stimulations initiated through specific death receptors. In this pathway, caspase-8 is activated by binding of death ligands to the receptors, which subsequently activates caspase-3 and PARP.37 The intrinsic pathway, which is a mitochondria-dependent pathway, is activated by several signals, including DNA damage and loss of cell survival factors due to various types of cell stress. This pathway is maintained by balancing the activity of pro- and anti-apoptotic proteins of the Bcl-2 family. Among the Bcl-2 family members, the anti-apoptotic proteins such as Bcl-2 and Bcl-xL suppress Bax, which promotes apoptosis by release of cytochrome c, a pro-apoptotic protein. The release of cytochrome c from the mitochondria activates caspase-9 and subsequently activates caspase-3.38 In the present study, β-lapachone induced the apoptosis of CT26 cells through the cleavage of caspases-3, -8, -9, and PARP. Moreover, the expression of Bcl-2 and Bcl-xL, which are anti-apoptotic proteins, decreased, while that of the pro-apoptotic protein Bax increased after treatment with β-lapachone (Figure 2B and C). These results suggest that β-lapachone shows a strong apoptosis induction effect through inhibition of both extrinsic and intrinsic apoptotic pathways in CT26 cells.
Control of cell cycle progression is considered an effective strategy for tumor treatment. It has been reported that β-lapachone induces cell cycle arrest in diverse cancer cells.31 Our data showed that β-lapachone (1 µM) treatment for 24 hours induced G0/G1 phase arrest of cell cycle progression in CT26 cells (Figure 3), which indicates that the cell cycle arrest is one of the mechanisms of inhibition of CT26 cell proliferation. However, human colon cancer cells SW480, SW620, and DLD1 were arrested at different stages of the S phase by β-lapachone (5 µM) treatment for 4 hours.36 In other studies, it was shown that β-lapachone induces the S-phase blockage in human hepatoma cells (HepA2), and G0/G1 phase arrest in leukemia, prostate, and breast cancer cells.31,39 Additionally, it was found that low concentrations of another phytochemical berberine induce G0/G1 arrest, while higher concentrations induce G2/M arrest in prostate cancer cells.40 Therefore, these studies indicate that the mechanisms of β-lapachone-mediated cell cycle arrest is modulated by external conditions such as the treatment concentration or time.
Metastasis has been found to be accompanied by various physiological alterations, which contribute to the degradation of ECM, as well as invasion and migration of tumor cells via the blood stream or lymphatic system. This also includes subsequent transport of tumor cells to other tissues and organs.8 Because the EMT process contributes to a more stromal cellular adhesion profile, increased tumor cell motility, and invasive properties, this process is important for metastasis. The classical cadherins (E- and N-cadherin) are transmembrane adhesion glycoproteins linked to the actin cytoskeleton by different types of catenin molecules.19 The mobility and invasive ability of cancer cells increase with reduction in E-cadherin expression, which is a cell-cell adhesion molecule, and increase in the N-cadherin expression, which is inversely associated with the expression of E-cadherin. EMT regulators, including the transcriptional repressor Snail and transcriptional targets such as vimentin and β-catenin, are also associated with this response.41 Our results showed that β-lapachone at noncytotoxic concentrations increased the expression of the epithelial marker E-cadherin and decreased the expressions of the mesenchymal markers N-cadherin, vimentin, β-catenin, and Snail (Figure 4).
Characteristic changes associated with EMT enable the migration and invasion of cancer cells.15 Deregulated cell-cell or cell-matrix adhesion and increased cell migration are preconditions for the progression of metastatic cancer.42 MMPs have a crucial role in the cancer cell migration and invasion.43,44 In particular, MMP-2 and MMP-9 are known to degrade the ECM in a process associated with cancer progression, invasion, and metastasis, whereas their inhibitors TIMP-2 and TIMP-1 regulate these activities. Thus, many studies have focused on the levels of MMPs and TIMPs which are involved in the metastasis process.45 Based on these observations, we investigated the effects of β-lapachone on the invasive ability of CT26 cells. We confirmed that β-lapachone could decrease the migratory and invasive ability by inhibiting the activities and expression of MMP-2 and MMP-9, while increasing the expression of TIMP-1 and TIMP-2 in CT26 cells (Figure 5).
β-Lapachone induces cell cycle arrest and apoptosis in various cancer cells, and several mechanisms have been reported for the anti-proliferative effects of β-lapachone.46-49 β-Lapachone, known as an NADPH quinone oxidoreductase 1 (NQO1) regulator, triggered necroptosis through the NQO1-dependent, reactive oxygen species–mediated receptor-interacting protein kinase 1–PARP1–apoptosis-inducing factor pathway in human hepatic adenocarcinoma SK-Hep1 cells.46 Additionally, inactivation of the phosphoinositide 3-kinase/Akt signaling pathway was found to be related to β-lapachone-induced apoptosis and growth inhibition of human gastric carcinoma AGS cells.47 Specificity protein 1 (Sp1) is a constitutive transcription factor involved in the regulation of apoptosis in cancer cells.48,49 β-Lapachone modulated the transactivation of Sp1 and induced apoptosis through regulation of apoptosis- and cell cycle–related proteins in non–small cell lung cancer and melanoma cells. Therefore, further studies are needed to elucidate other mechanisms for the antiproliferative and antimetastatic effects of β-lapachone on CRC cells.
Conclusions
Our study demonstrated for the first time that the antimetastatic effect of β-lapachone against the metastatic colon cancer cell line CT26 in vitro and in vivo. β-Lapachone inhibited the lung metastasis of colon cancer cells by inducing apoptosis and suppressing metastatic phenotypes such as EMT, migration, and invasion. These results suggest that β-lapachone may serve as an effective therapeutic agent for colorectal lung metastasis treatment. Further studies on β-lapachone are necessary to investigate its antimetastatic effects and mechanisms in various cancer cell lines and clinical cases.
Footnotes
Authors’ Note: Authors Jae-Young Um and Seung-Heon Hong contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by grants from the Korea Government (MSIP) (NRF-2014R1A6A3A01056089, NRF-2015R1A4A1042399, and NRF-2011-0030130).
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [DOI] [PubMed] [Google Scholar]
- 2. Cidón EU. The challenge of metastatic colorectal cancer. Clin Med Insights Oncol. 2010;18:55-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Simmonds PC. Palliative chemotherapy for advanced colorectal cancer: systematic review and meta-analysis. Colorectal Cancer Collaborative Group. BMJ. 2000;321:531-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Seymour MT, Stenning SP, Cassidy J. Attitudes and practice in the management of metastatic colorectal cancer in Britain. Colorectal Cancer Working Party of the UK Medical Research Council. Clin Oncol (R Coll Radiol). 1997;9:248-251. [DOI] [PubMed] [Google Scholar]
- 5. Labianca R, Beretta GD, Kildani B, et al. Colon cancer. Crit Rev Oncol Hematol. 2010;74:106-133. [DOI] [PubMed] [Google Scholar]
- 6. Ashley AC, Deschamps C, Alberts SR. Impact of prognostic factors on clinical outcome after resection of colorectal pulmonary metastases. Clin Colorectal Cancer. 2006;6:32-37. [DOI] [PubMed] [Google Scholar]
- 7. Jung KW, Won YJ, Kong HJ, Oh CM, Lee DH, Lee JS. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2011. Cancer Res Treat. 2014;46:109-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fidler IJ. The pathogenesis of cancer metastasis: the “seed and soil” hypothesis revisited. Nat Rev Cancer. 2003;3:453-458. [DOI] [PubMed] [Google Scholar]
- 9. Gomes FG, Nedel F, Alves AM, Nör JE, Tarquinio SB. Tumor angiogenesis and lymphangiogenesis: tumor/endothelial crosstalk and cellular/microenvironmental signaling mechanisms. Life Sci. 2013;92:101-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nabeshima K, Inoue T, Shimao Y, Sameshima T. Matrix metalloproteinases in tumor invasion: role for cell migration. Pathol Int. 2002;52:255-264. [DOI] [PubMed] [Google Scholar]
- 11. Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): an ancient family with structural and functional diversity. Biochim Biophys Acta. 2010;1803:55-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bourboulia D, Stetler-Stevenson WG. Matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs): positive and negative regulators in tumor cell adhesion. Semin Cancer Biol. 2010;20:161-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871-890. [DOI] [PubMed] [Google Scholar]
- 14. Voulgari A, Pintzas A. Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta. 2009;1796:75-90. [DOI] [PubMed] [Google Scholar]
- 15. Christiansen JJ, Rajasekaran AK. Reassessing epithelial to mesenchymal transition as a prerequisite for carcinoma invasion and metastasis. Cancer Res. 2006;66:8319-8326. [DOI] [PubMed] [Google Scholar]
- 16. Thiery JP, Sleeman JP. Complex networks orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell Biol. 2006;7:131-142. [DOI] [PubMed] [Google Scholar]
- 17. Batlle E, Sancho E, Francí C, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84-89. [DOI] [PubMed] [Google Scholar]
- 18. Mendez MG, Kojima S, Goldman RD. Vimentin induces changes in cell shape, motility, and adhesion during the epithelial to mesenchymal transition. FASEB J. 2010;24:1838-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11-26. [DOI] [PubMed] [Google Scholar]
- 20. Medici D, Hay ED, Olsen BR. Snail and Slug promote epithelial-mesenchymal transition through beta-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol Biol Cell. 2008;19:4875-4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bruna P, Margaret K, Antonio G. Cell cycle and apoptosis. Neoplasia. 2000;2:291-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shapiro GI, Harper JW. Anticancer drug targets: cell cycle and checkpoint control. J Clin Invest. 1999;104:1645-1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kerr JF. History of the events leading to the formulation of the apoptosis concept. Toxicology. 2002;181-182:471-474. [DOI] [PubMed] [Google Scholar]
- 24. Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768-780. [DOI] [PubMed] [Google Scholar]
- 25. Gómez Castellanos JR, Prieto JM, Heinrich M. Red Lapacho (Tabebuia impetiginosa)—a global ethnopharmacological commodity? J Ethnopharmacol. 2009;121:1-13. [DOI] [PubMed] [Google Scholar]
- 26. Macedo L, Fernandes T, Silveira L, Mesquita A, Franchitti AA, Ximenes EA. β-Lapachone activity in synergy with conventional antimicrobials against methicillin resistant Staphylococcus aureus strains. Phytomedicine. 2013;21:25-29. [DOI] [PubMed] [Google Scholar]
- 27. Medeiros CS, Pontes-Filho NT, Camara CA. Antifungal activity of the naphthoquinone beta-lapachone against disseminated infection with Cryptococcus neoformans var. neoformans in dexamethasone-immunosuppressed Swiss mice. Braz J Med Biol Res. 2010;43:345-349. [DOI] [PubMed] [Google Scholar]
- 28. Li CJ, Zhang LJ, Dezube BJ, Crumpacker CS, Pardee AB. Three inhibitors of type 1 human immunodeficiency virus long terminal repeat-directed gene expression and virus replication. Proc Natl Acad Sci U S A. 1993;90:1839-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sitônio MM, Carvalho Júnior CH, Campos Ide A, et al. Anti-inflammatory and anti-arthritic activities of 3,4-dihydro-2,2-dimethyl-2H-naphthol[1,2-b]pyran-5,6-dione (β-lapachone). Inflamm Res. 2013;62:107-113. [DOI] [PubMed] [Google Scholar]
- 30. Bey EA, Reinicke KE, Srougi MC. Catalase abrogates β-lapachone-induced PARP1 hyperactivation-directed programmed necrosis in NQO1-positive breast cancers. Mol Cancer Ther. 2013;12:2110-2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Planchon SM, Wuerzberger S, Frydman B, et al. Beta-lapachone-mediated apoptosis in human promyelocytic leukemia (HL-60) and human prostate cancer cells: a p53-independent response. Cancer Res. 1995;55:3706-3711. [PMC free article] [PubMed] [Google Scholar]
- 32. Kim SO, Kwon JI, Jeong YK, Kim GY, Kim ND, Choi YH. Induction of Egr-1 is associated with anti-metastatic and anti-invasive ability of beta-lapachone in human hepatocarcinoma cells. Biosci Biotechnol Biochem. 2007;71:2169-2176. [DOI] [PubMed] [Google Scholar]
- 33. Li LS, Bey EA, Dong Y, et al. Modulating endogenous NQO1 levels identifies key regulatory mechanisms of action of β-lapachone for pancreatic cancer therapy. Clin Cancer Res. 2011;17:275-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Choi BT, Cheong J, Choi YH. β-Lapachone-induced apoptosis is associated with activation of caspase-3 and inactivation of NF-kappaB in human colon cancer HCT-116 cells. Anticancer Drugs. 2003;14:845-850. [DOI] [PubMed] [Google Scholar]
- 35. Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013-2026. [DOI] [PubMed] [Google Scholar]
- 36. Huang L, Pardee AB. Beta-lapachone induces cell cycle arrest and apoptosis in human colon cancer cells. Mol Med. 1999;5:711-720. [PMC free article] [PubMed] [Google Scholar]
- 37. Boatright KM, Salvesen GS. Mechanisms of caspase activation. Curr Opin Cell Biol. 2003;15:725-731. [DOI] [PubMed] [Google Scholar]
- 38. Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899-1911. [DOI] [PubMed] [Google Scholar]
- 39. Lai CC, Liu TJ, Ho LK, Don MJ, Chau YP. Beta-lapachone induced cell death in human hepatoma (HepA2) cells. Histol Histopathol. 1998;13:89-97. [DOI] [PubMed] [Google Scholar]
- 40. Barzegar E, Fouladdel S, Movahhed TK. Effects of berberine on proliferation, cell cycle distribution and apoptosis of human breast cancer T47D and MCF7 cell lines. Iran J Basic Med Sci. 2015;18:334-342. [PMC free article] [PubMed] [Google Scholar]
- 41. Cervantes-Arias A, Pang LY, Argyle DJ. Epithelial-mesenchymal transition as a fundamental mechanism underlying the cancer phenotype. Vet Comp Oncol. 2013;11:169-184. [DOI] [PubMed] [Google Scholar]
- 42. Mierke CT. The role of vinculin in the regulation of the mechanical properties of cells. Cell Biochem Biophys. 2009;53:115-126. [DOI] [PubMed] [Google Scholar]
- 43. Rajoria S, Suriano R, George A, et al. Estrogen induced metastatic modulators MMP-2 and MMP-9 are targets of 3,3′-diindolylmethane in thyroid cancer. PLoS One. 2011;6:e15879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yan L, Lin B, Gao L, et al. Lewis (y) antigen overexpression increases the expression of MMP-2 and MMP-9 and invasion of human ovarian cancer cells. Int J Mol Sci. 2010;11:4441-4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Eissa S, Ali-Labib R, Swellam M, Bassiony M, Tash F, El-Zayat TM. Noninvasive diagnosis of bladder cancer by detection of matrix metalloproteinases (MMP-2 and MMP-9) and their inhibitor (TIMP-2) in urine. Eur Urol. 2007;52:1388-1396. [DOI] [PubMed] [Google Scholar]
- 46. Park EJ, Min KJ, Lee TJ, Yoo YH, Kim YS, Kwon TK. β-Lapachone induces programmed necrosis through the RIP1-PARP-AIF-dependent pathway in human hepatocellular carcinoma SK-Hep1 cells. Cell Death Dis. 2014;5:e1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yu HY, Kim SO, Jin CY, et al. β-Lapachone-induced apoptosis of human gastric carcinoma AGS cells is caspase-dependent and regulated by the PI3K/Akt pathway. Biomol Ther (Seoul). 2014;22:184-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jeon YJ, Bang W, Choi YH, Shim JH, Chae JI. β-Lapachone suppresses NSCLC cancer proliferation through the regulation of specificity protein 1. Biol Pharm Bull. 2015;38:1302-1308. [DOI] [PubMed] [Google Scholar]
- 49. Bang W, Jeon YJ, Cho JH, et al. β-Lapachone suppresses the proliferation of human malignant melanoma cells by targeting specificity protein 1. Oncol Rep. 2016;35:1109-1116. [DOI] [PubMed] [Google Scholar]