Abstract
Background. Fatigue is a major problem in children with cancer. The objective was to examine the feasibility of performing a clinical trial of homeopathic treatment for fatigue in children receiving chemotherapy. Materials. This was a single-institution, open-label, pilot study. Children 2 to 18 years old, diagnosed with cancer, and receiving chemotherapy were eligible. Participants were given individualized homeopathic treatment for a maximum of 14 days. In-home or clinic assessments were conducted up to 3 times weekly. Feasibility was defined as the ability to recruit and administer homeopathy to 10 participants within 1 year. Fatigue was measured using the Symptom Distress Scale daily and the PedsQL Multidimensional Fatigue Module weekly. Results. Between April 2012 and April 2014, 155 potential participants were identified. There were 45 eligible and contacted patients; 36 declined participation, 30 because they were not interested; 9 agreed to participate, but 1 participant withdrew prior to treatment initiation. Median length of homeopathic treatment was 10.5 (range = 6 to 14) days. All parents found homeopathic treatment to be easy or very easy to follow. Conclusions. Trials of individualized homeopathy for fatigue reduction in pediatric cancer are not feasible in this context; lack of interest was a primary reason. Alternative approaches to evaluating homeopathy efficacy are needed.
Keywords: feasibility, pilot, homeopathy, children, cancer, fatigue
Introduction
Cancer-related fatigue is frequently identified as one of the most troublesome symptoms in pediatric cancer patients.1 However, interventions for cancer-related fatigue have been largely unsuccessful. In a systematic review, Minton et al2 identified only 1 pharmacological intervention, methylphenidate, as a promising treatment for cancer-related fatigue in adults. The study of agents such as methylphenidate and modafinil are in progress, but these agents are often less acceptable in children because of concerns about the use of stimulants and their side effects. Thus, the study of agents to treat fatigue that have little to no toxicity in children with cancer is a priority.
Homeopathic treatments are the form of complementary and alternative medicine most widely used to treat side effects of chemotherapy in pediatric oncology in a number of European jurisdictions.3-5 It involves the treatment of patients with diluted natural substances aimed at stimulating the body’s healing system.6 In general, individualized homeopathy shows more promise compared with other forms of homeopathic intervention in which the homeopathic remedy does not change depending on symptoms. With individualized homeopathy, the decision to choose one homeopathic medicine over another is based on the patient’s presentation of mental, emotional, and physical symptoms.
Several studies examining individualized homeopathic treatment of fatigue in adults have shown promising results in diverse populations7-9 in addition to cancer patients.10,11 If effective, individualized homeopathy may have advantages compared with other interventions. Given the extremely diluted nature of homeopathic substances, there is little chance of drug interaction with chemotherapy. A systematic review evaluating homeopathic medicines used to prevent or treat side effects of cancer treatments concluded that no serious adverse events or interactions with conventional treatment were noted.12 Previous studies have shown that homeopathic treatments are well tolerated in adults and in children.13,14
A randomized trial is the optimal approach to determine whether individualized homeopathy is effective in reducing fatigue in children with cancer. However, it is unknown whether such a trial is feasible, and thus, a pilot study of individualized homeopathy would be informative. The primary objective was to determine the feasibility of recruiting pediatric cancer patients to a study of individualized homeopathy. Secondary objectives were to describe the proportion of participants who completed at least 10 days of treatment, to assess the acceptability of individualized homeopathy and to describe changes in fatigue scores according to 2 fatigue scales: the fatigue Symptom Distress Scale (SDS) and the PedsQL Multidimensional Fatigue Module (MFM).
Material and Methods
This was an open-label, pilot study of homeopathic treatment for fatigue in pediatric cancer patients treated at The Hospital for Sick Children (SickKids) in Toronto, Canada. The study followed guidelines of the Declaration of Helsinki and Tokyo for humans and was approved by the Research Ethics Board at SickKids. All patients/guardians provided informed consent or assent as appropriate. This trial was registered on clinicaltrials.gov (NCT01662076).
Participants
We included children 2 to 18 years of age diagnosed with any type of cancer receiving chemotherapy administered discontinuously in courses or cycles, with or without radiation treatment. Discharge from hospital had to have been anticipated following completion of the current chemotherapy treatment (or the regimen could have been given on an outpatient basis). Patients also had to have a score on the SDS of 2 or higher, be able to ingest medications in lactose/sucrose globule or liquid form, reside within the Greater Toronto area (or proximal enough to allow home visits by the homeopath), and have a guardian who could read and write in English. We excluded patients with a history of allergy to homeopathic products.
Patients had to be discharged from hospital because the SickKids hospital policy does not allow a homeopath to meet the patient within the hospital for the first time. However, if the patient did consent to the study and the patient was subsequently admitted, hospital policy allows for a family to invite the homeopath to provide in-hospital treatment once a relationship had been established.
Procedure
Potentially eligible families were approached in the inpatient or outpatient setting by a research nurse or clinical research associate. A member of the health care team had to approve the patient being approached by the study team. A member of the health care team was defined as an individual who is directly responsible for the clinical care of the patient. Such individuals could include physicians or nurses.
For consenting families, patients underwent screening for fatigue severity. Those experiencing a fatigue score on the SDS of 2 or higher (2 = there are periods when my child is rather tired or fatigued on a scale that ranges from 1 to 5) were eligible to continue with the study and receive homeopathy. Initial consultation and receipt of individualized homeopathy occurred either in the patient’s home or at the homeopath’s clinic (Riverdale Homeopathy Clinic, Toronto, Canada). Homeopathic treatment began within 5 days of chemotherapy cycle completion and was given orally or sublingually for a maximum of 14 days. Treatment could be continued concurrent with the next cycle of chemotherapy and could be stopped earlier if symptoms of fatigue resolved completely.
The homeopath (DB) was trained in individualized homeopathic methods, had more than 10 years of experience, and practiced greater than 20 clinic hours per week. For the homeopathic consultation, the method of case taking was classical, and the case analysis was performed using repertory software,15 Synthesis 9.1 repertory,16 and various materia medica.17,18 The consultation emulated normal practice and varied in length (baseline approximately 45 minutes and follow-up 5 to 30 minutes) and content according to the individual patient. The homeopath practiced usual homeopathic care based on the principles of Hahnemann’s Organon of Medicine.6 This approach involves a verbal interview with the patient and a guardian assessing the child’s physical and emotional symptoms as well as the history and course of the symptoms. Participants were informed of the potential for homeopathic aggravations. Homeopathic aggravations are defined as a mild and self-limited increase in symptoms occurring usually at the beginning of treatment; homeopathic aggravations occur in up to 20% of patients treated with homeopathy.6,19 No laboratory tests were incorporated into these evaluations. Based on the consultation results, the practitioner chose and administered a homeopathic remedy that focused on the reduction of fatigue. Patients and their guardians were taught how to administer the homeopathic medicines. There were no consultation or medicine fees for the participants.
The study medication was administered in 1 of 2 forms: (1) homeopathic medicine embedded in 2.5-mm-diameter lactose/sucrose globules or (2) 30% ethanol-based liquid homeopathic remedy. The homeopathic remedy was administered as either 1 globule sublingually or 0.2 mL orally (depending on the chosen formulation) up to 3 times per day. The homeopathic remedy or the homeopathic remedy potency could be changed on a daily basis during the course of treatment. However, only 1 homeopathic remedy and potency was administered at a given time.
Participants were asked to take the homeopathic medicine at least a half hour before or after food, beverages, or strong smelling substances. All conventional treatments, including counselling and exercise, and the use of other natural health products (other than homeopathy) were permitted. Participants were asked to refrain from taking other homeopathic substances while receiving study treatments and for 2 weeks following completion of homeopathic treatment.
While patients were receiving homeopathic treatments, an in-home or clinic assessment was performed by the study homeopath up to 3 times per week. A phone consultation was performed on all days when an assessment was not performed in clinic or at home. There was also 1 phone call or visit between the last dose of homeopathy and 14 days later. The following details were tracked by the study homeopath at the initial consultation and subsequent visits: adverse events, dietary changes, study medication use, conventional medication use, and other natural health product use. Adverse events were graded using the Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. The homeopath also recorded whether adverse events may represent homeopathic aggravation based on his clinical experience; the number of visits required at the Riverdale Homeopathic Clinic, home, or hospital; and the number of phone calls conducted with the family.
Outcomes
The primary outcome was feasibility, defined as the ability to enroll and initiate individualized homeopathy in 10 participants within 1 year. We used this threshold to define feasibility because this rate of enrollment would have allowed a definitive trial to complete enrollment with a reasonable number of sites in a timely fashion. Secondary outcomes were the number of participants who completed at least 10 days of individualized homeopathy, acceptability among enrolled participants, and measures of fatigue—namely, the fatigue SDS and the PedsQL MFM.
Acceptability was reported by guardians and children/adolescents ≥8 years of age using 5-point Likert scales evaluating how easy or difficult it was to follow the homeopathic treatment (1 = very difficult to follow to 5 = very easy to follow) and how easy or difficult it was to participate in the study (1 = very difficult to participate to 5 = very easy to participate). We also evaluated whether or not the guardian or patient would be interested in participating in a future randomized controlled study testing a similar homeopathic regimen compared with placebo. This information was obtained in person or by phone between end of treatment and 14 days later.
Measures of fatigue were collected in a daily diary. The SDS asks about symptoms experienced “lately” as opposed to defining a specific recall period. The SDS ranges from 1 to 5, in which 5 represents the worst fatigue. The PedsQL MFM is an 18-item instrument that assesses general fatigue, sleep/rest fatigue, and cognitive fatigue. The MFM is reliable and valid in children with cancer.20 We also described generic and cancer-specific quality of life (QoL) as measured by the PedsQL 4.0 Generic Core Scales and PedsQL 3.0 Acute Cancer Module. All PedsQL instruments used a 1-week recall period for this study.
The fatigue SDS was recorded daily for a 14-day period, starting at homeopathy treatment initiation, whereas the PedsQL MFM, Generic Core Scales, and Acute Cancer Module were completed on days 0, 7, and 14. The primary respondent for the fatigue and QoL outcomes was a guardian. Children ≥8 years of age were invited to self-report these symptoms along with their guardian. The guardian also recorded adverse events in the daily diary. To facilitate interpretation of the fatigue scores, we also determined whether the participant had received a red blood cell transfusion within 7 days of treatment initiation.
Homeopathic Remedies and Concomitant Medications
All products and potencies carried a DIN-HM number designating approval for over-the-counter use by Health Canada. Potencies were limited to 6CH, 15CH, 30CH, and 200CH. The homeopathic medicines were procured from Boiron Canada Inc. Remedies manufactured by Boiron Canada are prepared using the Hahnemannian multivial method and prepared according to the European Pharmacopoeia, the Pharmacopée Française, and the Homeopathic Pharmacopeia of the United States.
Statistics
We considered the ability to administer individualized homeopathy to 10 participants within 1 year as evidence of feasibility of a future randomized trial. We also examined the proportion of patients who completed at least 10 days of treatments. Descriptive data were reported for acceptability, adverse events, fatigue, and QoL. In an exploratory analysis, we also evaluated the change in fatigue scores over time according to the SDS and PedsQL MFM. We evaluated change over time using a repeated-measures linear mixed model, in which days since starting homeopathy treatment was the primary covariate for each participant. All analyses were conducted with SAS software (version 9.3; SAS Institute Inc, Cary, NC).
Results
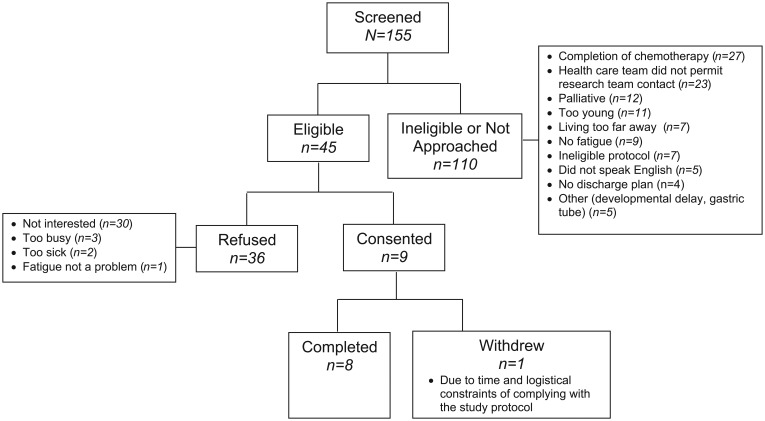
Between April 2012 and April 2014, we assessed 155 potential participants for this study. Figure 1 illustrates the flow diagram of patient identification and enrollment. Of 110 patients who were not further evaluated, there were 23 in which the primary health care team requested that the patient and family not be approached. The health care provider type in these cases were nurses (n = 13), doctors (n = 8), and both (n = 2). Of the 45 identified eligible patients who were approached, 36 refused; the most common reason for refusal was that the family was not interested in homeopathy (n = 30); 9 consented to participate. Three were approached as inpatients, and 6 were approached as outpatients.
Figure 1.
Flow diagram of patient identification and enrollment.
Demographic characteristics are illustrated in Table 1. Four patients had used homeopathy in the past, and 3 had used other complementary or alternative therapies. No patient had received a red blood cell transfusion within 7 days of initiating homeopathy.
Table 1.
Demographics of the Study Cohort.
| Characteristics | Value, n = 9 |
|---|---|
| Male (%) | 9 (100.0) |
| Median child age at participation (range) | 13.4 (3.6-16.7) |
| Diagnosis (%)a | |
| Leukemia/Lymphoma | 1 (11.1) |
| Solid tumor | 6 (66.7) |
| Brain tumor | 2 (22.2) |
| Median months since diagnosis (IQR) | 3.3 (2.0, 4.7) |
| Relapsed (%) | 1 (11.1) |
| Extent of disease (%) | |
| Nonmetastatic | 4 (44.4) |
| Metastatic | 5 (55.6) |
| Median baseline hemoglobin (g/L) (IQR) | 117.0 (97.0, 125.0) |
| Median baseline Symptom Distress Scale (IQR) | 3.0 (2.0, 4.0) |
Abbreviation: IQR, interquartile range.
Diagnoses included Hodgkin’s lymphoma (n = 1), medulloblastoma (n = 2), hepatoblastoma (n = 1), osteosarcoma (n = 2), rhabdomyosarcoma (n = 1), and Ewing sarcoma (n = 2).
In terms of the primary outcome, the study was open for patient accrual for 2 years, and 9 participants were accrued. One 4-year-old child withdrew from the study prior to receiving any homeopathy treatment because the family felt that the study protocol (treatment administration and follow-up visits) was too onerous and that they could not comply with it. Consequently, the primary outcome of feasibility of a study of homeopathy for fatigue in pediatric cancer was not met. All 8 patients who received individualized homeopathy began treatment within 5 days of chemotherapy completion. The median number of visits conducted at the Riverdale Clinic, home, or hospital was 6 (range = 5-7), and the median number of phone calls conducted with the family was 10 (range = 9-11).
In terms of the secondary end points, the number of children who received at least 10 days of homeopathy was 6. In 2 children, the homeopath stopped the treatment remedy before 10 days because of resolution of symptoms but continued to follow the participant. For those who initiated homeopathy, the median number of days participants were taking a homeopathic remedy was 10.5 (range = 6-14) days, and all participants were followed for exactly 14 days. Acceptability was reported by 8 parents and 4 patients who were ≥8 years of age and agreed to provide this data. The results were as follows: 8 parents and 4 patients found it was easy or very easy to follow the homeopathic treatment plan; 6 parents and 4 patients found that it was easy or very easy to participate in the study; and 6 parents and 4 patients were interested in participating in a future randomized placebo-controlled study of homeopathy.
Figure 2 shows the median and interquartile ranges for SDS scores by days on homeopathy treatment. SDS scores significantly improved during the observation time frame (β = −0.08, standard error [SE] = 0.02; P = .0005). The proxy-report fatigue scores according to the PedsQL MFM and the generic and cancer-specific QoL scores are illustrated in Table 2. There was a significant improvement in general fatigue (β = 1.7, SE = 0.8; P = .038) and sleep/rest fatigue (β = 2.4, SE = 0.7; P = .004) over time. Conversely, there was no change in cognitive fatigue with time (P = .611). Five children self-reported fatigue and QoL scores; these are illustrated in Table 3.
Figure 2.
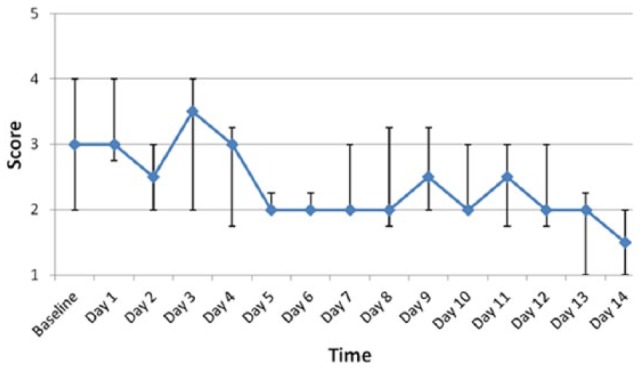
Median Fatigue Symptom Distress Scale scores by time. Circles indicate median values, and bars indicate interquartile range for fatigue Symptom Distress Scale scores.
Table 2.
Median Scores on Parent Proxy-Report PedsQL Outcomes.
| n | Day 0 (IQR) | n | Day 7 (IQR) | n | Day 14 (IQR) | |
|---|---|---|---|---|---|---|
| Multidimensional Fatigue Scale scores | ||||||
| General fatigue | 8 | 31.3 (18.8, 47.9) | 8 | 54.2 (31.3, 75.0) | 8 | 60.4 (47.9, 75.0) |
| Sleep/Rest fatigue | 8 | 33.3 (12.5, 43.8) | 8 | 52.1 (43.8, 66.7) | 8 | 62.5 (50.0, 77.1) |
| Cognitive fatigue | 8 | 64.6 (50.0, 70.8) | 8 | 68.8 (60.4, 75.0) | 8 | 75.0 (54.2, 75.0) |
| Cancer Module scores | ||||||
| Pain and hurt | 8 | 43.8 (31.3, 62.5) | 8 | 62.5 (50.0, 68.8) | 8 | 62.5 (56.3, 81.3) |
| Nausea | 8 | 37.5 (27.5, 52.5) | 8 | 60.0 (40.0, 65.0) | 8 | 67.5 (45.0, 77.5) |
| Procedural anxiety | 8 | 33.3 (8.3, 66.7) | 8 | 58.3 (45.8, 79.2) | 7 | 58.3 (50.0, 83.3) |
| Treatment anxiety | 8 | 50.0 (33.3, 66.7) | 8 | 62.5 (45.8, 83.3) | 7 | 75.0 (50.0, 91.7) |
| Worry | 8 | 54.2 (45.8, 75.0) | 8 | 62.5 (50.0, 87.5) | 8 | 62.5 (50.0, 87.5) |
| Cognitive problems | 8 | 64.2 (45.0, 78.1) | 8 | 65.8 (50.0, 78.1) | 8 | 70.0 (50.0, 85.4) |
| Perceived physical appearance | 8 | 66.7 (50.0, 83.3) | 8 | 70.8 (41.7, 91.7) | 8 | 70.8 (45.8, 91.7) |
| Communication | 8 | 62.5 (54.2, 87.5) | 8 | 58.3 (54.2, 87.5) | 7 | 66.7 (58.3, 100.0) |
| Generic Core Scales scores | ||||||
| Total score | 8 | 35.5 (29.6, 52.8) | 8 | 58.7 (40.2, 77.5) | 8 | 64.6 (42.6, 79.3) |
| Physical health summary | 8 | 15.6 (6.3, 43.8) | 8 | 51.6 (20.3, 73.4) | 8 | 62.5 (21.9, 76.6) |
| Psychosocial summary | 8 | 49.0 (45.0, 60.9) | 8 | 62.5 (52.5, 80.4) | 8 | 65.4 (55.9, 80.8) |
| Emotional functioning | 8 | 50.0 (40.0, 60.0) | 8 | 57.5 (50.0, 67.5) | 8 | 67.5 (57.5, 75.0) |
| Social functioning | 8 | 62.5 (42.5, 77.5) | 8 | 80.0 (62.5, 87.5) | 8 | 70.0 (52.5, 82.5) |
| School functioning | 5 | 30.0 (25.0, 45.0) | 6 | 55.0 (35.0, 100.0) | 5 | 55.0 (55.0, 70.0) |
Abbreviation: IQR, interquartile range.
Table 3.
Median Scores on Child Self-Report PedsQL Outcomes.
| n | Day 0 (IQR) | n | Day 7 (IQR) | n | Day 14 (IQR) | |
|---|---|---|---|---|---|---|
| Multidimensional Fatigue Scale scores | ||||||
| General fatigue | 5 | 29.2 (25.0, 45.8) | 4 | 50.0 (45.8, 52.1) | 5 | 50.0 (33.3, 54.2) |
| Sleep/Rest fatigue | 5 | 29.2 (25.0, 29.2) | 4 | 35.4 (25.0, 47.9) | 5 | 45.8 (41.7, 50.0) |
| Cognitive fatigue | 5 | 62.5 (50.0, 75.0) | 4 | 70.8 (63.3, 79.2) | 5 | 70.8 (54.2, 70.8) |
| Cancer Module scores | ||||||
| Pain and hurt | 5 | 50.0 (50.0, 50.0) | 5 | 62.5 (62.5, 87.5) | 5 | 50.0 (50.0, 62.5) |
| Nausea | 5 | 40.0 (25.0, 50.0) | 5 | 50.0 (40.0, 50.0) | 5 | 65.0 (45.0, 65.0) |
| Procedural anxiety | 5 | 66.7 (33.3, 91.7) | 5 | 58.3 (50.0, 100.0) | 5 | 66.7 (50.0, 100.0) |
| Treatment anxiety | 5 | 91.7 (83.3, 100.0) | 5 | 75.0 (66.7, 91.7) | 5 | 83.3 (66.7, 100.0) |
| Worry | 5 | 66.7 (50.0, 83.3) | 5 | 66.7 (66.7, 83.3) | 5 | 75.0 (66.7, 91.7) |
| Cognitive problems | 5 | 55.0 (50.0, 75.0) | 5 | 65.0 (45.0, 85.0) | 5 | 55.0 (35.0, 90.0) |
| Perceived physical appearance | 5 | 75.0 (66.7, 75.0) | 5 | 75.0 (66.7, 91.7) | 5 | 75.0 (75.0, 75.0) |
| Communication | 5 | 75.0 (41.7, 75.0) | 5 | 58.3 (41.7, 75.0) | 5 | 66.7 (41.7, 83.3) |
| Generic Core Scales scores | ||||||
| Total score | 5 | 41.3 (40.9, 62.0) | 5 | 44.3 (38.0, 60.9) | 5 | 47.8 (47.7, 57.6) |
| Physical health summary | 5 | 25.0 (15.6, 28.1) | 5 | 21.9 (15.6, 28.1) | 5 | 25.0 (25.0, 34.4) |
| Psychosocial summary | 5 | 57.1 (55.0, 78.3) | 5 | 57.1 (51.7, 78.3) | 5 | 61.7 (60.7, 70.0) |
| Emotional functioning | 5 | 65.0 (60.0, 85.0) | 5 | 70.0 (60.0, 85.0) | 5 | 85.0 (70.0, 90.0) |
| Social functioning | 5 | 75.0 (70.0, 80.0) | 5 | 70.0 (60.0, 85.0) | 5 | 80.0 (65.0, 85.0) |
| School functioning | 5 | 37.5 (20.0, 55.0) | 5 | 40.0 (37.5, 60.0) | 5 | 40.0 (30.0, 43.8) |
Abbreviation: IQR, interquartile range.
The following homeopathic medicines were used: cadmium sulphuricum (n = 8), phosphorus (n = 1), Lycopodium clavatum (n = 2), Nux vomica (n = 1), and calcarea phosphorica (n = 1).
The homeopath identified 3 possible episodes of homeopathic aggravation in the clinical notes. One participant had increased fatigue within 4 hours of receiving a dose of homeopathic medicine; 1 participant experienced nausea within 12 hours of receiving a dose of homeopathic medicine; and 1 participant experienced an increase in mucositis within 24 hours of a dose of homeopathic medicine. According to the physician-based adverse event review, no patient had an adverse event thought to be possibly, probably, or definitely caused by homeopathy, and the possible homeopathic aggravations were expected toxicities associated with chemotherapy.
Discussion
We found that a future randomized trial of individualized homeopathy for fatigue reduction in pediatric cancer is not feasible in this context. In spite of the large number of identified, potentially eligible patients, major barriers included lack of interest and failure to obtain permission to approach the patient by the health care team while the patient was still receiving chemotherapy treatment. The hospital policy toward homeopathy meant that children had to be discharged from hospital to be enrolled and did not permit the homeopath to meet children and families initially while in hospital, thus presenting additional barriers to enrollment
We allowed the study to accrue patients over 2 years rather than the originally planned 1 year to ensure that unfamiliarity with the study and slow study start-up were not the reasons behind failure to accrue patients. Furthermore, our threshold for feasibility was likely too low. Even if we could have enrolled 10 participants over 1 year in this single-arm, open-label study, this would not have translated to enrollment of 10 participants in a randomized trial of homeopathy at a single center. Families enrolled in this study were likely the most enthusiastic about homeopathy. Only 75% of parents would have agreed to a future randomized trial, reflecting the lack of equipoise among potential participants and further highlighting the infeasibility of homeopathy randomized trials in this setting.
In contrast to the difficulty in accruing patients, among those enrolled in whom homeopathy was initiated, all found homeopathy easy to use and the study easy to complete. Interestingly, we showed a significant improvement in fatigue over the 14-day observation period, when we would have anticipated worsening of fatigue.21,22 These data may suggest that homeopathy is effective in improving fatigue in this population. Conversely, the results may be explained by the effect of the consultation process or placebo effect and highlight the need for randomized, placebo-controlled trials of interventions to reduce the subjective symptom of fatigue.
Our results are consistent with those of Rostock et al,11 who observed a similar significant reduction in fatigue symptoms in the homeopathic care of an adult cancer patient population. Our safety data are also consistent with other studies showing a low incidence of adverse events.12 There were no serious adverse events attributed to homeopathy.
We found that important reasons for failure to enroll patients in this feasibility trial were because the primary health care team did not permit access and because participants were not interested. To address these issues, future studies should consider the following. First, qualitative studies among health care providers are critically important. Such a perspective can identify whether there is enthusiasm for studying homeopathy and, if not, whether the barriers are addressable. Such factors are likely to differ greatly by region and culture. Second, similar qualitative studies among potential participants are important to gauge enthusiasm for participating in studies of homeopathy. A future study may be considered in a different population or setting such as an adult population or an inpatient setting.
The strength of this study is the careful evaluation of feasibility prior to the institution of a randomized trial and the inclusion of feasibility end points. However, the study is limited by its conduct at a single institution. It is possible that a study of homeopathy would have been feasible in other settings and especially in countries in which homeopathy use is prevalent. Another limitation is that given the single arm, open-label design of the trial, we do not have a comparison group in which to evaluate the effect of homeopathy on fatigue scores. Finally, the use of other natural health products was permitted in this study, and this approach may also have affected fatigue. We took this approach to maximize the acceptability of this feasibility study. However, results need to be interpreted in light of this limitation.
Conclusions
In conclusion, trials of individualized homeopathy for fatigue reduction in pediatric cancer are not feasible in this context. Alternative approaches to evaluating homeopathy efficacy are needed.
Acknowledgments
We would like to thank the Lotte and John Hecht Memorial Foundation for their generous support of the study. The foundation had no involvement in study design; the collection, analysis, and interpretation of data; writing of the report; and in the decision to submit the article for publication.
Footnotes
Authors’ Note: DB and LS were responsible for writing the manuscript. DB, BG, ML, and LS were involved in the acquisition of the data. All authors contributed to the analysis and interpretation of the data. All authors have critically reviewed and approved the manuscript and agree to be accountable for all aspects of the work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding was received from the Lotte and John Hecht Memorial Foundation to support this research.
References
- 1. Gibson F, Garnett M, Richardson A, Edwards J, Sepion B. Heavy to carry: a survey of parents’ and healthcare professionals’ perceptions of cancer-related fatigue in children and young people. Cancer Nurs. 2005;28:27-35. [DOI] [PubMed] [Google Scholar]
- 2. Minton O, Richardson A, Sharpe M, Hotopf M, Stone P. Drug therapy for the management of cancer-related fatigue. Cochrane Database Syst Rev. 2010;(7):CD006704. [DOI] [PubMed] [Google Scholar]
- 3. Clerici CA, Veneroni L, Giacon B, Mariani L, Fossati-Bellani F. Complementary and alternative medical therapies used by children with cancer treated at an Italian pediatric oncology unit. Pediatr Blood Cancer. 2009;53:599-604. [DOI] [PubMed] [Google Scholar]
- 4. Langler A, Spix C, Edelhauser F, Kameda G, Kaatsch P, Seifert G. Use of homeopathy in pediatric oncology in Germany. Evid Based Complement Alternat Med. 2011;2011:867151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Simon L, Prebay D, Beretz A, et al. Complementary and alternative medicines taken by cancer patients. Bull Cancer. 2007;94:483-488. [PubMed] [Google Scholar]
- 6. Hahnemann S, ed. Organon of the Medical Art. 6th ed. Redmond, WA: Birdcage Books; 1996. [Google Scholar]
- 7. Weatherley-Jones E, Nicholl JP, Thomas KJ, et al. A randomised, controlled, triple-blind trial of the efficacy of homeopathic treatment for chronic fatigue syndrome. J Psychosom Res. 2004;56:189-197. [DOI] [PubMed] [Google Scholar]
- 8. Thompson EA, Reilly D. The homeopathic approach to the treatment of symptoms of oestrogen withdrawal in breast cancer patients: a prospective observational study. Homeopathy. 2003;92:131-134. [DOI] [PubMed] [Google Scholar]
- 9. Relton C, Weatherley-Jones E. Homeopathy service in a national health service community menopause clinic: audit of clinical outcomes. J Br Menopause Soc. 2005;11:72-73. [DOI] [PubMed] [Google Scholar]
- 10. Thompson EA, Reillly D. The homeopathic approach to symptom control in the cancer patient: a prospective observational study. Palliat Med. 2002;16:227-233. [DOI] [PubMed] [Google Scholar]
- 11. Rostock M, Naumann J, Guethlin C, Guenther L, Bartsch HH, Walach H. Classical homeopathy in the treatment of cancer patients: a prospective observational study of two independent cohorts. BMC Cancer. 2011;11:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kassab S, Cummings M, Berkovitz S, Van Haselen R, Fisher P. Homeopathic medicines for adverse effects of cancer treatments. Cochrane Database Syst Rev. 2009;(2):CD004845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brien S, Lachance L, Prescott P, Mcdermott C, Lewith G. Homeopathy has clinical benefits in rheumatoid arthritis patients that are attributable to the consultation process but not the homeopathic remedy: a randomized controlled clinical trial. Rheumatology. 2011;50:1070-1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frei H, Everts R, Von Ammon K, et al. Homeopathic treatment of children with attention deficit hyperactivity disorder: a randomised, double blind, placebo controlled crossover trial. Eur J Pediatr. 2005;164:758-767. [DOI] [PubMed] [Google Scholar]
- 15. Archibel. Radar homeopathic software. http://www.archibel.com/radar.html. Accessed October 30, 2015.
- 16. Schroyens F. Synthesis Repertorium Homeopathicum. 9.1th ed. London, UK: Homeopathic Book; 2004. [Google Scholar]
- 17. Vermeulen F. Concordent Materia Medica. 3rd ed. Haarlem, Netherlands: Emryss; 2000. [Google Scholar]
- 18. Murphy R. Nature’s Materia Medica. Blacksburg, VA: Lotus Health Institute; 2006. [Google Scholar]
- 19. Grabia S, Ernst E. Homeopathic aggravations: a systematic review of randomised, placebo-controlled clinical trials. Homeopathy. 2003;92:92-98. [DOI] [PubMed] [Google Scholar]
- 20. Varni JW, Burwinkle TM, Katz ER, Meeske K, Dickinson P. The PedsQL in pediatric cancer: reliability and validity of the Pediatric Quality of Life Inventory Generic Core Scales, Multidimensional Fatigue Scale, and Cancer Module. Cancer. 2002;94:2090-2106. [DOI] [PubMed] [Google Scholar]
- 21. Anderson KO, Giralt SA, Mendoza TR, et al. Symptom burden in patients undergoing autologous stem-cell transplantation. Bone Marrow Transplant. 2007;39:759-766. [DOI] [PubMed] [Google Scholar]
- 22. Meyers CA, Albitar M, Estey E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer. 2005;104:788-793. [DOI] [PubMed] [Google Scholar]