Abstract
Five different crude polysaccharides from guava seed (GSPS), bitter buckwheat (BBPS), common buckwheat (CBPS), red Formosa lambsquarters (RFLPS), and yellow Formosa lambsquarters (YFLPS) were isolated to treat human prostate cancer PC-3 cells via direct action or tumor immunotherapy. The splenocyte- and macrophage-conditioned media (SCM and MCM) were prepared using individual selected polysaccharides, and then SCM or MCM was further collected to treat PC-3 cells. The relationship between PC-3 cell growth and Th1/Th2 cytokines in SCM as well as proinflammatory/anti-inflammatory cytokine secretion profiles in MCM were delineated. The results showed that all 5 selected polysaccharides did not significantly inhibit PC-3 cell growth via direct action. However, SCM or MCM cultured in the absence or presence of 5 selected polysaccharides significantly (P < .05) inhibited PC-3 cell growth. MCM cultured with 5 polysaccharides dose dependently enhanced their inhibitory effects on the viabilities of PC-3 cells than those cultured without polysaccharides. There was a significant (P < .05) negative correlation between PC-3 cell viabilities and (interleukin [IL]-6 + tumor necrosis factor [TNF]-α)/IL-10 level ratios in the corresponding MCM, implying that macrophages suppress PC-3 cell growth through decreasing secretion ratios of proinflammatory/anti-inflammatory cytokines in a tumor microenvironment.
Keywords: human prostate cancer PC-3 cells, macrophages, polysaccharides, splenocytes, tumor immunotherapy
Introduction
Prostate cancer is the most common intrinsic male organ malignancy in the world and the second leading cause of cancer death in American men, behind only lung cancer, with about 233 000 new cases per year; among these patients, about 29 480 men will die of prostate cancer.1 The incidence of prostate cancer in Taiwan is not as high as in the United States, but it is increasing every year. To date, surgery, radiation therapy, chemotherapy, targeted therapy, and/or immunotherapy are used to treat cancers. Immunotherapy that promotes tumor eradication through activating innate and adaptive immune responses, including nonspecific stimulators, cytokines, monoclonal antibodies, radiolabeled antibodies, immunotoxins, and cell-based therapy, is still a relatively new and promising method in cancer treatments.2 During the tumor development process, tumor cells may escape immune surveillance, implying that the restoration of the immune surveillance ability in the host may improve cancer treatments.3,4 Accumulative clinical results have indicated that immune-based therapies may play a role in the treatment of patients with prostate and other malignancies.5
Among cancer immunotherapy investigators, the growing interest over the past 2 decades in controlling the immune system to get rid of cancer has heightened characterization of cytokines to exploit their vast signaling networks to develop cancer treatments.6 Modulating cytokine secretion profiles by immune cells using active immunomodulatory components may be a smart and effective strategy to treat cancer via changing the tumor microenvironment in the host. The growing evidence indicates that diets rich in vegetables and fruits reduce the risk of numerous chronic diseases, including cancer. In addition, different fruits and different parts of one fruit could be used in different ways for the prevention and treatment of different cancers.7 The combination of immunotherapy and plant-derived effective components may provide a chance to treat prostate cancer.
Among active immunomodulatory components, particular polysaccharides are recognized as biological response modifiers and may enhance the immune surveillance of tumor cells. Recently, PS-F2, a polysaccharide purified from Ganoderma formosanum, was shown to exert significant antitumor activity via its immunological competence to the host but not direct cytotoxicity to the tumor cells. Both cellular and humoral immune responses in the host contributed to PS-F2–induced tumor rejection, and PS-F2 can function as a vaccine adjuvant to stimulate the production of antigen-specific antibodies.8 Particular polysaccharides may exist in other different sources. Food materials such as grains and fruit seeds that are rich in polysaccharides deserve to be further studied for their anti–prostate cancer potential.
Curative therapies for advanced or recurrent forms of prostate cancer are still absent, mandating continued development of novel, more effective treatment regimens. Recently, natural ingredients have been found to have vast potential for treating cancer, with potentially fewer side effects than most of today’s cancer therapies. Five selected polysaccharides from guava seed, common buckwheat, bitter buckwheat, red Formosa lambsquarters, and yellow Formosa lambsquarters cultivated in Taiwan were found to have diverse immunomodulatory potential. However, the effects of these selected polysaccharides on human prostate cancer PC-3 cells remain unclear. To unravel the puzzle, these 5 polysaccharides were isolated to treat PC-3 cells via direct action or tumor immunotherapy. Splenocyte- and macrophage-conditioned media (SCM and MCM) were prepared using selected polysaccharides and further collected to treat PC-3 cells. The relationship between PC-3 cell growth and Th1/Th2 cytokine secretion profile in their corresponding SCM as well as proinflammatory/anti-inflammatory cytokine secretion profile in their corresponding MCM were delineated.
Materials and Methods
Isolation of 5 Selected Polysaccharides
To isolate polysaccharides, seeds of fresh guava (Psidium guajava Linn) fruit were first carefully collected and then air dried at 40°C overnight for use. The air-dried seeds of guava, common buckwheat (Fagopyrum esculentum), bitter buckwheat (Fagopyrum leptopodum), red Formosa lambsquarters (Chenopodium formosanum), and yellow Formosa lambsquarters (Chenopodium formosanum) were individually ground to powder using a grinding machine and then passed through a 60-mesh sieve. To avoid disturbance in the subsequent polysaccharide extraction procedure, the lipids in the powder samples were removed using n-hexane (1:4, weight/volume). The mixtures were shaken at room temperature for 4 hours. After settling, the liquid phase containing n-hexane and oil was removed. The remaining solid sample was air dried at room temperature to evaporate the trace hexane. The de-fatted powder samples were stored at −20°C until use.
To extract polysaccharides, aliquots of de-fatted powder of guava seed, common buckwheat, bitter buckwheat, red Formosa lambsquarters, and yellow Formosa lambsquarters were added to 5 to 10 volumes of deionized water and shaken at room temperature for 4 hours. The resultant mixtures were centrifuged at 5000 ×g for 30 minutes at room temperature. The supernatant was collected, measured, and volumetrically added with 3 volumes of 95% ethyl alcohol. The mixtures were slowly shaken at 4°C for 12 hours to precipitate the polysaccharides and centrifuged at 5000 ×g for 30 minutes at room temperature to separate the insoluble polysaccharides from the supernatant. The insoluble polysaccharide pellet was collected to evaporate any trace ethyl alcohol. The polysaccharide pellet was lyophilized and stored at −30°C until use. Five isolated polysaccharides, including guava seed polysaccharides (GSPS), common buckwheat polysaccharides (CBPS), bitter buckwheat polysaccharides (BBPS), red Formosa lambsquarters polysaccharides (RFLPS), and yellow Formosa lambsquarters polysaccharides (YFLPS) were obtained for the following experiments. All 5 isolated polysaccharides have been partially characterized. All 5 isolated polysaccharide fractions had maximal absorption peaks around 210 to 230 nm, with a minor peak around 260 to 280 nm. Total carbohydrate and protein contents in these isolated polysaccharides indicated that the isolated polysaccharides were proteopolysaccharides or glycoproteins. Generally, crude polysaccharides were contaminated by coextracted proteins. Unfortunately, the step to remove proteins in the purification procedure was skipped in this study, although the removal of proteins may also discard particular proteopolysaccharides or glycoproteins in the crude polysaccharides. Among these isolated polysaccharides, GSPS presented the highest sugar content (60.7%), whereas BBPS had the highest protein content (85.6%). Five isolated polysaccharides were further purified and identified using Sepharose 6B gel filtration column. Each isolated polysaccharide separated into 2 to 3 subfractions. The molecular weight (MW) of each subfraction was calibrated with a standard compound (blue dextran, MW = 2000 kDa, Sigma–Aldrich Co, MO) or protein MW standards kit (MWGF 1000 kit, MW: 6500-670 000 Da, Sigma, MO) using the Sepharose 6B gel filtration column. MWs of guava seed polysaccharide subfraction 1 (coded as GSF1), GSF2, and GSF3 in GSPS were distributed at 6.1 × 105 kDa, 3.3 × 104 kDa, and 6.8 kDa; common buckwheat polysaccharide subfraction 1 (coded as CBF1), CBF2, and CBF3 in CBPS were distributed at 4.5 × 104 kDa, 1.8 × 102 kDa, and 9.4 kDa; bitter buckwheat polysaccharide subfraction 1 (coded as BBF1), BBF2, and BBF3 in BBPS were distributed at 4.5 × 104 kDa, 2.5 × 102 kDa, and 13 kDa; red Formosa lambsquarters polysaccharide subfraction 1 (coded as RFLF1) and RFLF2 in RFLPS were distributed at 6.2 × 104 kDa and 9.4 kDa; yellow Formosa lambsquarters polysaccharide subfraction 1 (coded as YFLF1) and YFLF2 in YFLPS were distributed at 3.3 × 104 kDa and 9.4 kDa, respectively.
Polysaccharides have highly complex structures and species specificity. In generalized definition, polysaccharides may include simple polysaccharides, glycoproteins, and proteopolysaccharides. In general, polysaccharides are soluble in water but insoluble in alcohol. However, the seed samples are rich in starch, which is highly soluble in hot water but insoluble in cold water. Hot water may extract a great deal of starch from the samples and destroy active ingredients. Therefore, a standard protocol with a slight modification was used to extract the polysaccharides. The samples were extracted using cold water (room temperature) rather than hot water (70°C-100°C) in this study. Because all 5 isolated polysaccharides contain sugar and proteins, they may be glycoproteins or mixtures of polysaccharides and proteins. Because the characteristics of 5 isolated polysaccharides consisting of sugars and proteins have not been fully determined, they can be called crude polysaccharides according to the generalized definition.
Isolation of Primary Immune Cells
Female BALB/cByJNarl mice (8 weeks old) were purchased from the National Applied Research Laboratories, Ministry of Science and Technology in Taipei, Taiwan, ROC. The experimental mice were maintained in the Department of Food Science and Biotechnology at National Chung Hsing University, College of Agriculture and Natural Resources in Taichung, Taiwan, ROC. The animals were provided chow diet (laboratory standard diet) and water ad libitum. All mice were housed in stainless steel cages and kept at a controlled temperature (23°C ± 2°C) and ambient humidity (50%-75%). Lights were maintained on a 12-hour dark-light cycle. After acclimation for 2 weeks, the experimental mice were killed humanely for isolating primary immune cells, including splenocytes and peritoneal macrophages. The animal use protocol listed in this study was reviewed and approved by the Institutional Animal care and Use Committee (IACUC Approval No: 101-95R), National Chung Hsing University, Taiwan, ROC.
The protocol was performed as described previously.9-11 Briefly, the experimental mice (10 weeks old) were anesthetized with diethyl ether and immediately bled using retro-orbital venous plexus puncture to collect blood. Immediately after blood collection, the animals were killed humanely using CO2 inhalation. Peritoneal macrophages were prepared by lavaging the peritoneal cavity with 2 aliquots of 5-mL sterile Hank’s balanced salts solution (HBSS)—50 mL of 10× HBSS (Hyclone Laboratories Inc, South Logan, UT), 2.5 mL of antibiotic-antimycotic solution (100× antibiotic mixture containing 10 000 units penicillin, 10 mg streptomycin, and 25 µg amphotericin B per mL in 0.85% saline; Atlanta Biologicals Inc, Norcross, GA), 20 mL of 3% bovine serum albumin (Sigma-Aldrich Co, St Louis, MO) in phosphate-buffered saline (PBS; 137 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7.4, 0.22 µm filtered), 2.5 mL of 7.5% NaHCO3 (Wako, Osaka, Japan), 425 mL sterile water—for a total of 10 mL through the peritoneum. The peritoneal lavage fluid was centrifuged at 400 ×g for 10 minutes at 4°C. The cell pellets were collected and resuspended in tissue culture medium (TCM; a serum replacement, Celox Laboratories Inc, Lake Zurich, IL), suspended in a medium consisting of 10 mL TCM, 500 mL Roswell Park Memorial Institute 1640 medium (Atlanta Biologicals Inc, Norcross, GA), and 2.5 mL of antibiotic-antimycotic solution (100× PSA). The peritoneal adherent cells (>90% of macrophages) from each animal were adjusted to 2 × 106 cells/mL in TCM medium with a hemocytometer, using the trypan blue dye exclusion method.9 Isolated mouse primary peritoneal macrophages were used for subsequent experiments.
Immediately after the peritoneal cavity of the experimental mice was lavaged for preparing peritoneal macrophages, the spleen was aseptically removed from the experimental mice and homogenized in TCM medium. Single splenocytes were collected and treated with red blood cell (RBC) lysis buffer (0.017 M Trizma base [Sigma-Aldrich Co], 0.144 M ammonium chloride [Sigma-Aldrich Co], pH 7.4, 0.20 µm filtered) to lyse the RBCs. Splenocytes isolated from each animal were adjusted to a concentration of 1 × 107 cells/mL in TCM medium with a hemocytometer, using the trypan blue dye for subsequent experiments.10,11
Preparation of SCM and MCM in the Absence or Presence of the 5 Selected Polysaccharides
To achieve noncytotoxic optimal concentrations of 5 selected polysaccharide fractions, the cell viabilities of macrophages treated with individual polysaccharides at different concentrations were determined using 3-(4,5-dimethylthiazol-2-diphenyl)-2,5-tetrazolium bromide (MTT, Sigma, MO) assay. All polysaccharide stock solutions were aseptically diluted into working solutions using TCM medium before use. The splenocytes or macrophages (50 µL/well) in the absence or presence of polysaccharides (50 µL/well) at the indicated final concentrations of 0, 1.6, 8, 40, 200, 500, and 1000 µg/mL in 96-well plates were incubated at 37°C in a humidified incubator with 5% CO2 and 95% air for 72 and 48 hours, respectively. The cell viability was determined using MTT assay. GSPS, CBPS, BBPS, RFLPS, and YFLPS at the same concentrations of 1.6, 8, 40, and 200 µg/mL were found to be noncytotoxic doses for primary immune cells (data not shown); thus, these noncytotoxic optimal doses at 1.6, 8, 40, and 200 µg/mL were selected to treat PC-3 cancer cells through direct action or tumor immunotherapy via preparing immune cell–conditioned media (CMs).
To prepare an immune cell CM, isolated splenocytes (1 × 107 cells/mL TCM medium, 0.5 mL/well) or peritoneal macrophages (2 × 106 cells/mL TCM medium, 0.5 mL/well) were cocultured with GSPS, CBPS, BBPS, RFLPS, and YFLPS at the indicated noncytotoxic concentrations of 0, 8, 40, and 200 µg/mL TCM medium (0.5 mL/well) in 24-well plates, respectively. The plates were incubated at 37°C in a humidified incubator with 5% CO2 and 95% air for 48 hours. The cultured plate was centrifuged at 400 ×g for 10 minutes to collect the supernatant (ca 1.0 mL/well) in the cell cultures—namely, SCM or MCM. The supernatant of immune cell cultures was collected and lyophilized. The lyophilized SCM or MCM was dissolved in 0.5 mL F-12K medium: F12 Kaighn’s modification medium supplemented with 7% fetal bovine serum, penicillin (100 units/mL), streptomycin (100 µg/mL), and amphotericin B (0.25 µg/mL). The 2-fold concentrated CM was stored at −80°C until use.
Additionally, to clarify the relationship between cytokine secretion profiles by immune cells and PC-3 cell viability, levels of Th1 (interleukin [IL]-2) and Th2 (IL-10) cytokines in SCM, and proinflammatory (IL-1β, IL-6, and tumor necrosis factor [TNF]-α) and anti-inflammatory (IL-10) cytokines in MCM were measured using a sandwich ELISA according to the cytokine ELISA protocol from the manufacturer’s instructions (mouse DuoSet ELISA Development system, R&D Systems, Minneapolis, MN).12
Effects of the 5 Selected Polysaccharides on the Growth of Human Prostate Cancer PC-3 Cells
The human prostate cancer PC-3 cell line was purchased from Bioresource Collection and Research Center (Food Industry Research and Development Institute, Hsinchu, Taiwan, ROC). The PC-3 cells were maintained in F-12K medium at 37°C in a humidified incubator with 95% air and 5% CO2. After they had grown to 90% confluence in a 75T tissue culture flask (TPP Biochrom AG, Trasadingen, Switzerland), the PC-3 cells were plated at a density of 2 × 105 cells/mL in 96-well plates to perform the following bioassay.
To evaluate the direct action of 5 isolated polysaccharides on PC-3 cell growth, PC-3 cells (2 × 105 cells/mL, 50 µL/well) were treated with GSPS, CBPS, BBPS, RFLPS, and YFLPS (50 µL/well) at the indicated final concentrations of 0, 1.6, 8, 40, and 200 µg/mL F-12K medium as well as paclitaxel (as a positive control) at 2.5 µΜ for 24 and 48 hours, respectively. The cell viability of PC-3 cells was determined using MTT assay. After incubation, aliquots of 10 µL of MTT (5 mg/mL in PBS) were added to each well in the 96-well plate. The plates were incubated at 37°C in a humidified incubator with 5% CO2 and 95% air for another 4 hours. The plates were centrifuged at 400 ×g for 10 minutes, and the culture supernatant was carefully discarded. The cell pellet was carefully washed with PBS buffer twice. Aliquots of 100 µL dimethyl sulfoxide were added to each well and oscillated for 30 minutes to lyse cell membrane to extract formed insoluble formazan in mitochondria. The absorbance (A) was measured at 550 nm on a plate reader (ELISA reader, ASYS Hitech, GmbH, Austria). The cell viability was expressed as the relative percentage compared with the mean absorbency of the control. The cell viability percentage in each biological determination was calculated using the following equation: Cell viability (percentage of control) = [(Asample − Ablank)/(Acontrol − Ablank)] × 100.12
To evaluate the effects of cytokine immunotherapy on PC-3 cell growth, PC-3 cells (2 × 105 cells/mL; 50 µL/well) were treated with SCM (50 µL/well) or MCM (50 µL/well) as well as paclitaxel (as a positive control) at 2.5 µΜ, respectively, for 24 and 48 hours. To evaluate the possible interference of different media used in the cell line (F-12K medium) and primary cells (TCM medium), PC-3 cells cultured in TCM medium alone were also selected as a negative control. The remaining survival cells were measured using MTT assay. PC-3 cells cultured in F-12K medium alone (a negative control) were selected as a control to calculate the effects of SCM or MCM treatments. The cell viability (%) in each biological determination was calculated using the following equation: Cell viability (percentage of control) = [(Asample − Ablank)/(Acontrol,F-12K − Ablank)] × 100.12
Statistical Analysis
Results are expressed as the mean ± SD. Differences between means were compared using 1-way ANOVA, followed by Duncan’s multiple range test using the SPSS system 19.0. The association between cytokine levels in CM and the viabilities of PC-3 cells was described as the Pearson product-moment correlation coefficient (r). P values <.05 were considered significant.
Results and Discussion
Direct Action of the 5 Selected Polysaccharides on PC-3 Cell Growth
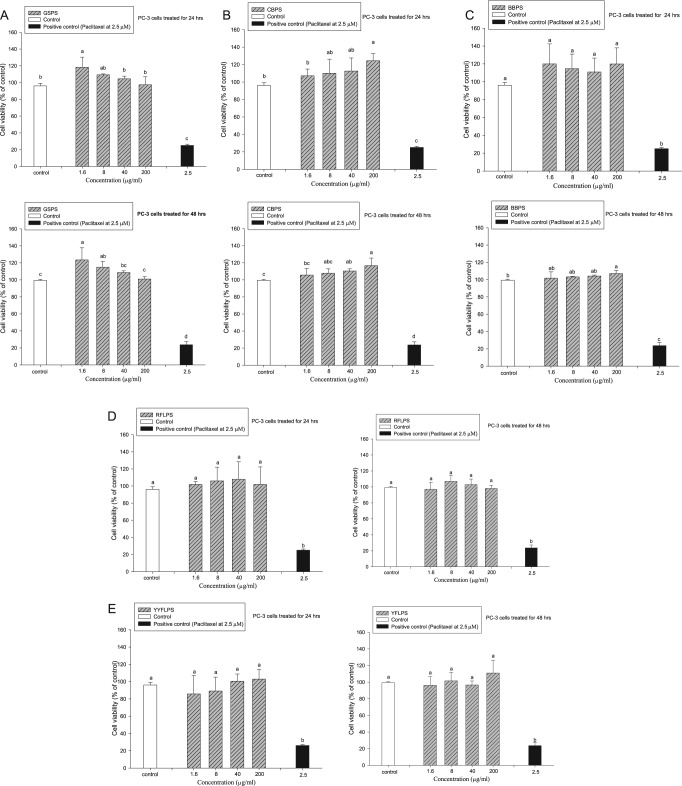
In our previous study, administration of GSPS, CBPS, BBPS, RFLPS, or YFLPS at the indicated concentrations of 1.6, 8, 40, and 200 µg/mL was found to be noncytotoxic to primary immune cells. To evaluate direct action of 5 selected polysaccharides on PC-3 cell growth, the 5 isolated polysaccharides at the indicated concentrations of 1.6, 8, 40, and 200 µg/mL were added to PC-3 cells for 24 or 48 hours, respectively. Paclitaxel administration at 2.5 µM was selected as a positive control. The results showed that administration of GSPS, BBPS, CBPS, RFLPS, and YFLPS at the indicated concentrations could not significantly (P > .05) inhibit the PC-3 cell viability as compared with that of the control (Figure 1). Contrary to our anticipation, direct administrations with 5 selected polysaccharides, particularly CBPS and BBPS, slightly increased PC-3 cell viabilities. Polysaccharides such as β-glucans were found to have cytoprotective and genoprotective effects on lymphocytes.13 Therefore, an indirect action of polysaccharides to treat tumor cells through immunotherapy shows a promising pathway. We assumed that direct administration of certain polysaccharides might enhance the growth of PC-3 cancer cells via stimulating particular receptors on the cancer cells in vitro. However, it remains to be further studied. Importantly, paclitaxel administration at 2.5 µM significantly (P < .05) decreased the PC-3 cell viability, indicating that paclitaxel may be applied to the treatment of PC-3 cancer cells via direct action to the cancer cells (Figure 1).
Figure 1.
Effects of treatments with guava seed (GSPS) (A), common buckwheat (CBPS) (B), bitter buckwheat (BBPS) (C), red Formosa lambsquarters (RFLPS) (D) and yellow Formosa lambsquarters (YFLPS) (E) polysaccharides on human prostate cancer PC-3 cell growth. Values are means ± SD (n = 6). Bars not sharing a common letter are significantly different (P < .05) from each other, analyzed by 1-way ANOVA, followed by Duncan’s multiple range test. Each cell population (2 × 105 cells/mL) was treated with the polysaccharides at the indicated concentrations of 1.6, 8, 40, and 200 µg/mL as well as paclitaxel (a positive control) at 2.5 µM for 24 and 48 hours, respectively. The cell viability was determined using MTT assay.
Effects of SCM or MCM on PC-3 Cell Growth
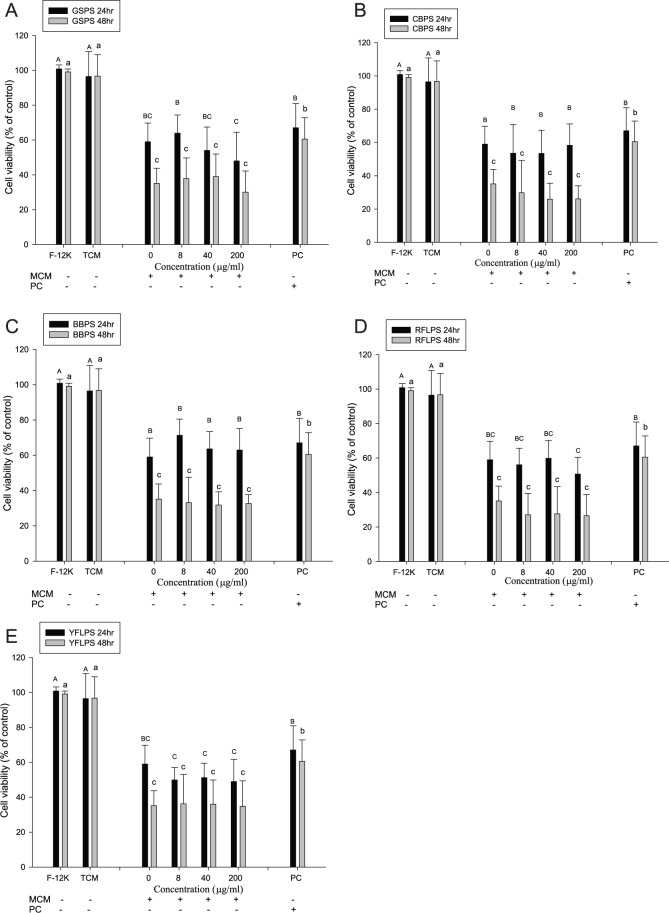
To evaluate the effect of cytokine immunotherapy using immune cell CMs in the absence or presence of 5 polysaccharides on PC-3 cell growth, PC-3 cells were treated with SCM or MCM for 24 or 48 hours, respectively. PC-3 cells cultured in F-12 K medium alone for 24 or 48 hours were selected as a control to calculate the cell viability. The results showed that there are no significant differences of PC-3 cell viabilities between F-12 K medium and TCM medium alone. Identically, paclitaxel (a positive control) at 2.5 µM significantly inhibited (P < .05) PC-3 cell growth either through 24 or 48 hours of incubation compared with the negative controls (Figure 2). Importantly, SCM treatments significantly (P < .05) inhibited PC-3 cell viability either through 24 or 48 hours of incubation as compared with the negative controls. However, SCM cultured with different concentrations of the 5 polysaccharides did not show enhanced, but rather slightly decreased, inhibitory effects on the viabilities of PC-3 cells relative to those cultured without the 5 polysaccharides. Because these 5 polysaccharides were not removed from the SCM, the remaining polysaccharides in the SCM might slightly increase the growth of PC-3 cells via direct action (Figure 1). Most important, SCM cultured without the 5 polysaccharides exhibited a better inhibitory effect on PC-3 cell viability than paclitaxel administration at 2.5 µM either through 24 or 48 hours of incubation (Figure 2). Our results indicate that treatments of PC-3 cancer cells through tumor immunotherapy using splenocytes might have the better inhibitory effect than that of chemotherapy using paclitaxel (Figure 2).
Figure 2.
Effects of treatments with splenocyte-conditioned media (SCM) using polysaccharides GSPS (A), CBPS (B), BBPS (C), RFLPS (D), and YFLPS (E) on human prostate cancer PC-3 cell growth. Values are means ± SD (n = 6 biological determinations). Bars at the same incubation time not sharing a common letter are significantly different (P < .05) from each other, assayed by 1-way ANOVA, followed by Duncan’s multiple range test. Each PC-3 cell population (2 × 105 cells/mL) was treated with SCM for 24 and 48 hours, respectively. The mean number of PC3 cells cultured in F-12K medium alone for 24 and 48 hours was selected as a control to calculate the cell viability, respectively. F-12K PC-3 cells were cultured in F-12K medium alone (a negative control); TCM PC-3 cells were cultured in TCM medium alone (a negative control); PC PC-3 cells were treated with paclitaxel at 2.5 µΜ (a positive control).
Abbreviations: GSPS, guava seed; CBPS, common buckwheat; BBPS, bitter buckwheat; RFLPS, red Formosa lambsquarters; YFLPS, yellow Formosa lambsquarters; TCM, tissue culture medium.
Figure 3 shows the effects of MCM treatments on the growth of PC-3 cells. The results showed that MCM treatment significantly (P < .05) inhibited PC-3 cell viability either through 24 or 48 hours of incubation as compared with the negative controls (Figure 3). Importantly, MCM cultured with different concentrations of the 5 polysaccharides, particularly GSPS, CBPS, and YFLPS, showed slightly and dose-dependently, although not significantly (P > .05), enhanced inhibitory effects on the viabilities of PC-3 cells relative to those cultured without the 5 polysaccharides (Figure 3). Because these 5 polysaccharides were not removed from MCM, the remaining polysaccharides in MCM might slightly increase the growth of PC-3 cells via direct action (Figure 1), interfering with the net effect of MCM. In addition, MCM cultured without the 5 polysaccharides exhibited a better inhibitory effect on PC-3 cell viability than paclitaxel administration at 2.5 µM, particularly through 48 hours of incubation (Figure 3). Our results indicate that treatments of PC-3 cancer cells through tumor immunotherapy using immune cells might have better inhibitory effect than that of chemotherapy.
Figure 3.
Effects of treatments with macrophage-conditioned media (MCM) using polysaccharides GSPS (A), CBPS (B), BBPS (C), RFLPS (D), and YFLPS (E) on human prostate cancer PC-3 cell growth. Values are means ± SD (n = 6 biological determinations). Bars at the same incubation time not sharing a common letter are significantly different (P < .05) from each other assayed by 1-way ANOVA, followed by Duncan’s multiple range test. Each PC-3 cell population (2 × 105 cells/mL) was treated with MCM for 24 and 48 hours, respectively. The mean number of PC3 cells cultured in F-12K medium alone for 24 and 48 hours was selected as a control to calculate the cell viability. F-12K PC-3 cells were cultured in F-12K medium alone (a negative control); TCM PC-3 cells were cultured in TCM medium alone (a negative control); PC PC-3 cells were treated with paclitaxel at 2.5 µΜ (a positive control).
Abbreviations: GSPS, guava seed; CBPS, common buckwheat; BBPS, bitter buckwheat; RFLPS, red Formosa lambsquarters; YFLPS, yellow Formosa lambsquarters; TCM, tissue culture medium.
In comparison with the effects of SCM and MCM administrations, both SCM and MCM presented inhibitory effects on PC-3 cell viability, implying that immune cells, including splenocytes and macrophages, might be involved in anticancer activities in vivo. We hypothesized that cytokines secreted by these immune cells are key contributors to inhibiting the growth of PC-3 cells through cytokine chemotherapy. To clarify the puzzle, associations between cytokine secretion levels in the corresponding CM and viabilities of PC-3 cells were further analyzed.
Associations Between Cytokine Secretion Levels in the CMs and Cell Viabilities of PC-3 Treated With Their Corresponding CMs
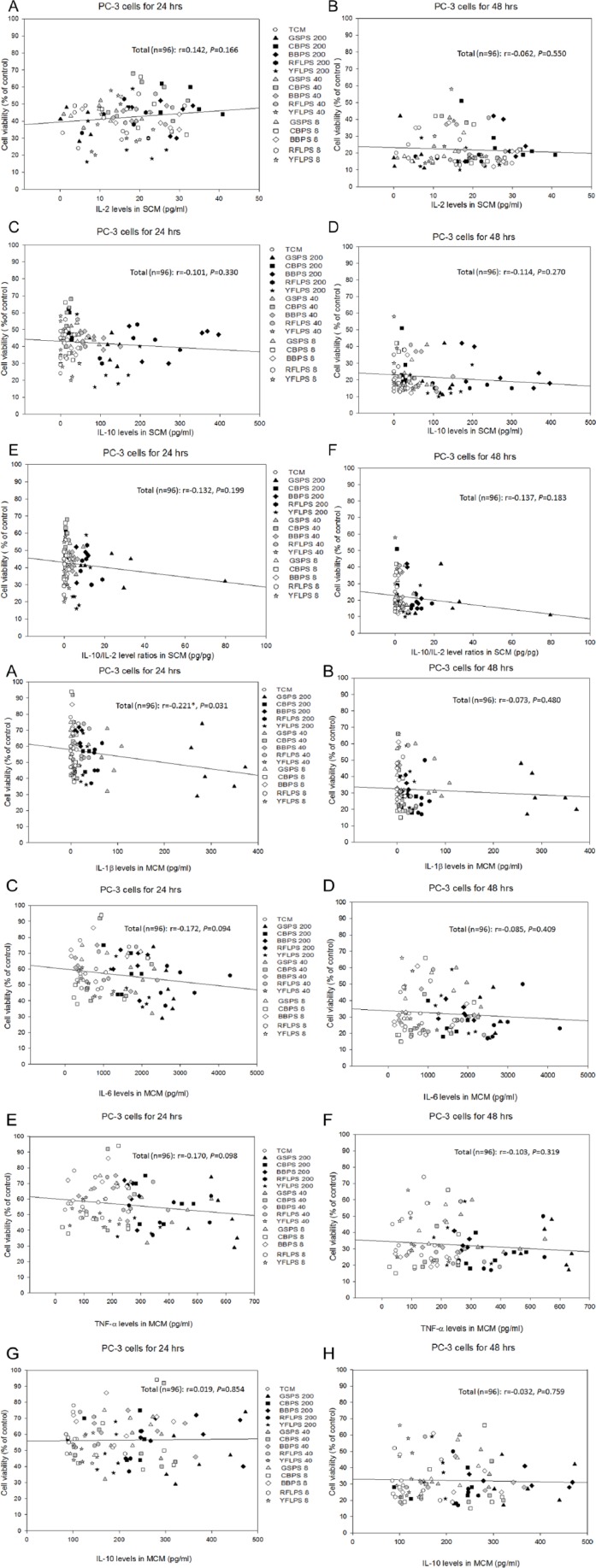
To clarify the correlation between cytokine secretion levels and cancer cell survival, the relationship between cytokine levels secreted by immune cells and viabilities of PC-3 cells treated with CM cultured without or with the 5 selected polysaccharides were determined using Pearson’s correlation coefficients (r). Figure 4 shows associations between the viabilities of PC-3 cells treated with SCM for 24 or 48 hours and IL-2, IL-10, or IL-10/IL-2 cytokine secretion profiles in their corresponding SCM. The results showed that the viability of PC-3 cells through 24 or 48 hours of incubation did not significantly (P > .05) correlate with IL-2 (Th1 cytokine), IL-10 (Th2 cytokine), and IL-10/IL-2 (Th2/Th1) secretion profiles by splenocytes, implying that there was a weak correlation between the PC-3 cell viability and Th1/Th2 immune balance. However, more Th1/Th2 cytokines secreted in the SCM should be further analyzed to provide stronger evidence.
Figure 4.
The associations between PC-3 cell viabilities treated with splenocyte-conditioned media (SCM) for 24 and 48 hours, respectively, and interleukin [IL]-2 (A, B), IL-10 (C, D), and IL-10/IL-2 (E, F) cytokine secretion profiles in corresponding SCM.
Figure 5 shows associations between viabilities of PC-3 cells treated with MCM for 24 or 48 hours and IL-1β, IL-6, TNF-α (proinflammatory cytokines), IL-10 (anti-inflammatory cytokine), or (IL-6 + TNF-α)/IL-10 cytokine secretion profiles in their corresponding MCM. Contrary to our expectedness, results showed that the PC-3 cell viability through 24 hours of incubation significantly (P = .031) and negatively (r = −0.221) correlated with IL-1β (proinflammatory cytokine) secretion by macrophages, demonstrating that IL-1β might play a role in the inhibition to PC-3 cell viability (Figure 5A). However, more evidence should be accumulated to clarify the phenomenon. Importantly, the results showed that the PC-3 cell viability through 24 hours of incubation significantly (P ≤ .0001) and negatively (r = −0.385) correlated with (IL-6 + TNF-α)/IL-10 (proinflammatory/anti-inflammatory) cytokine secretion ratios by macrophages, indicating that increasing anti-inflammatory cytokines but decreasing proinflammatory cytokines by macrophages might inhibit the PC-3 cell viability (Figure 5I). Unfortunately, the significant correlation disappeared after 48 hours of incubation (Figure 5J), showing that the cytokine chemotherapy might just be effective for a short period, resulting from the short half-life of cytokines.
Figure 5.
The associations between PC-3 cell viabilities treated with macrophage-conditioned media (MCM) for 24 and 48 hours, respectively, and interleukin [IL]-1β (A, B), IL-6 (C, D), TNF-α (E, F), IL-10 (G, H), and (IL-6 + TNF-α)/IL-10 (I, J) cytokine secretion profiles in corresponding MCM.
Currently, the prevalent choices for treating human cancer are surgical resection, general chemotherapy, and radiation therapy.14 Recently, the ability of the immune system to fight cancer has been recognized and partially understood. Tumor cells may be directly destroyed by immune cells, such as natural killer cells and cytotoxic T cells in vivo. However, tumor cells may escape immune surveillance, so that they cannot be killed in time.3,4 Tumor immunotherapy endeavors to promote tumor eradication through the activation of innate and adaptive immune responses, including nonspecific stimulators, cytokines, monoclonal antibodies, radiolabeled antibodies, immunotoxins, and cell-based therapy.2 Accumulative clinical results reported that immune-based therapies may play a role in the treatment of patients with prostate and other malignancies.5 In the present study, our results indicated that the PC-3 cell viability treated with MCM through 24 hours of incubation significantly (P ≤ .0001; r = −0.385) negatively correlated with (IL-6 + TNF-α)/IL-10 (proinflammatory/anti-inflammatory) cytokine secretion ratios by macrophages, implying that increasing anti-inflammatory cytokines but decreasing proinflammatory cytokines by macrophages might inhibit the PC-3 cell viability (Figure 5I). Furthermore, maintaining low inflammation status in vivo may inhibit the growth of prostate cancer. Adopting dietary changes, such as a diet rich in antioxidant or anti-inflammatory components, to regulate the immune system may promote anticancer properties. Our results showed that MCM cultured with different concentrations of the 5 polysaccharides slightly and dose-dependently, although not significantly (P > .05), enhanced their inhibitory effects on the viabilities of PC-3 cells relative to those cultured without the 5 polysaccharides (Figure 3), indicating that these selected polysaccharides might increase anticancer abilities of immune cells, particularly macrophages but not splenocytes (Figure 2), via their anti-inflammatory potential. However, the anticancer mechanism of these selected polysaccharides through tumor immunotherapy should be further clarified.
Fucoidan, a polysaccharide from Undaria pinnatifida, was found to induce the apoptosis of PC-3 human prostate cancer cells via the inactivation of the ERK1/2 MAPK signaling pathway, cell cycle arrest, and downregulation of the Wnt/β-catenin signaling pathway.15 Polysaccharide fractions F1 and F2 from Cymbopogon citratus induce apoptosis by reducing mitochondrial transmembrane potential and increasing pro-(Bax) apoptotic gene expression in Siha and LNCap cells.16 Glycoproteins from Cyclocarya paliurus leaves inhibit the growth of human gastric cancer HeLa cells through keeping cell cycle arrest in the S phase, which may further induce apoptosis.17 However, direct administrations of GSPS, CBPS, BBPS, RFLPS, or YFLPS to PC-3 cells at the indicated concentrations of 1.6, 8, 40, and 200 µg/mL did not significantly (P > .05) inhibit the growth of PC-3 cells (Figure 1), but MCM cultured with different concentrations of the 5 polysaccharides did dose dependently enhance their inhibitory effects on the viabilities of PC-3 cells (Figure 3). It is suggested that these selected polysaccharides might exert their anticancer abilities against PC-3 cells, via regulating particular immune cells such as macrophages to increase anti-inflammatory cytokine production but decrease pro-inflammatory cytokine production in vivo (Figure 5I). The anticancer properties of polysaccharides have been shown to be primarily mediated through 3 approaches: (1) direct cytotoxicity, (2) immunoenhancement, and (3) synergistic effects in combination treatment with conventional anticancer drugs.18 Based on our results from these 5 isolated polysaccharides, we uncover another possible anticancer property of polysaccharides—that is, to decrease secretion ratios of proinflammatory/anti-inflammatory cytokines in a tumor microenvironment. After digestion of particular polysaccharides, certain immune cells in Peyer’s patches in the small intestine may be regulated to secrete cytokines into the blood stream and constitute an immune environment in the body. An anti-inflammatory immune environment in the body may help inhibit the growth of human prostate cancers.
In the present study, we showed that SCM or MCM inhibited the growth of PC-3 cells, possibly via the pathway of cytokine immunotherapy (Figures 2, 3, and 5). Mammalian models, such as the mouse, have been preeminent in modeling human diseases, primarily because of the striking homology between mammalian genomes and the many similarities in aspects spanning from anatomy to cell biology and physiology.19 The overall structure of the immune system in mice and humans is quite similar.20,21 Therefore, mouse primary immune cell CMs were selected to treat PC-3 cells. SCM, that is, CM of splenocytes containing 41.54% B cells and 47.11% T cells, could reflect the status of the adaptive immune system,22 whereas MCM, that is, CM of macrophages containing higher than 90% macrophages, could reflect the status of the innate immune system. Our results further demonstrated that B lymphocytes, T lymphocytes, and macrophages might contribute to the inhibition of PC-3 cell growth. However, characterizations and anticancer mechanisms of the 5 selected polysaccharides remain to be further studied in the future.
Among the 5 selected polysaccharides, GSPS, CBPS, and YFLPS had the better anticancer activity through tumor immunotherapy (Figure 3). Guava (Psidium guajava) shows potential for the treatment of different diseases—for example, it has anti-inflammatory, antidiarrheal,23 antidiabetic,24 antihypertensive, antimicrobial,25 hepatoprotective,26 antiallergic,27 and anticancer/antitumor potential.28,29 Recently, guava leaf hexane fraction was found to modulate both PI3K/AKT/mTOR/S6K1 and MAPK signaling pathways, leading to apoptosis through downmodulating proteins that mediate tumor cell survival, proliferation, metastasis, and angiogenesis in PC-3 cells.30 The present study is the first to report an anticancer property of guava seed polysaccharides through tumor immunotherapy. Buckwheat, a herbaceous plant that belongs to the Polygonaceae family, has been used for foods and traditional medicine in Taiwan. Buckwheat proteins were reported to have anticancer activity.31 Buckwheat polysaccharide has the potential to stimulate cytokine secretion by peripheral blood mononuclear cells and to induce leukemic THP-1 cell differentiation by both direct and indirect treatments.32 The present study is the first to investigate the effect of buckwheat polysaccharides on the growth of human prostate cancer cells via tumor immunotherapy. Formosa lambsquarters, which was found to have diverse immunomodulatory potential, is an endemic species cultivated in Taiwan. The present study is the first to probe into the effect of Formosa lambsquarters polysaccharides on the growth of human prostate cancer cells via tumor immunotherapy. The application of particular polysaccharides using tumor immunotherapy to treat certain cancers may be promising and provide an alternative choice for curing cancers. The major purpose of this study was to evaluate and compare the immunomodulatory potential of different polysaccharides and their effects on human prostate cancer PC-3 cells to screen potent polysaccharides for the treatment of prostate cancer. The purified fractions of most potent polysaccharides will be further subjected to thorough study in the future.
Conclusions
In the present study, GSPS, CBPS, BBPS, RFLPS, and YFLPS did not significantly inhibit PC-3 cell growth via direct action. However, SCM or MCM cultured in the absence or presence of the 5 selected polysaccharides significantly (P < .05) inhibited PC-3 cell growth. MCM cultured with 5 polysaccharides, particularly GSPS, CBPS, and YFLPS, dose dependently enhanced their inhibitory effects on the viabilities of PC-3 cells relative to those cultured without polysaccharides. There was a significant (P < .05) negative correlation between PC-3 cell viabilities and (IL-6 + TNF-α)/IL-10 level ratios in the corresponding MCM, implying that macrophages suppress PC-3 cell growth through decreasing secretion ratios of proinflammatory/anti-inflammatory cytokines in a tumor microenvironment.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was kindly supported by a research grant NSC102-2313-B-005-033-MY3 from the Ministry of Science and Technology, Taipei, Taiwan, ROC.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [DOI] [PubMed] [Google Scholar]
- 2. Dougan M, Dranoff G. Immunotherapy of cancer. In: Wang R.-F, ed. Innate Immune Regulation and Cancer Immunotherapy. New York, NY: Springer; 2012:391-414. [Google Scholar]
- 3. Fox BA, Schendel DJ, Butterfield LH, et al. Defining the critical hurdles in cancer immunotherapy. J Transl Med. 2011;9:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646-674. [DOI] [PubMed] [Google Scholar]
- 5. Vieweg J. Immunotherapy for advanced prostate cancer. Rev Urol. 2007;9(suppl 1):S29-S38. [PMC free article] [PubMed] [Google Scholar]
- 6. Lee S, Margolin K. Cytokines in cancer immunotherapy. Cancers. 2011;3:3856-3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li F, Li S, Li HB, et al. Antiproliferative activity of peels, pulps and seeds of 61 fruits. J Funct Foods. 2013;5:1298-1309. [Google Scholar]
- 8. Wang CL, Lu CY, Hsueh YC, Liu WH, Chen CJ. Activation of antitumor immune responses by Ganoderma formosanum polysaccharides in tumor-bearing mice. Appl Microbiol Biotechnol. 2014;98:9389-9398. [DOI] [PubMed] [Google Scholar]
- 9. Liao YR, Lin JY. Quercetin, but not its metabolite quercetin-3-glucuronide, exerts prophylactic immuno-stimulatory activity and therapeutic anti-inflammatory effect on lipopolysaccharide-treated mouse peritoneal macrophages ex vivo. J Agric Food Chem. 2014;62:2872-2880. [DOI] [PubMed] [Google Scholar]
- 10. Liao CH, Lin JY. Purified active lotus plumule (Nelumbo nucifera Gaertn) polysaccharides exert anti-inflammatory activity through decreasing toll-like receptor-2 and -4 expressions using mouse primary splenocytes. J Ethnopharmacol. 2013;147:164-173. [DOI] [PubMed] [Google Scholar]
- 11. Liu CJ, Lin JY. Protective effects of strawberry and mulberry fruit polysaccharides on inflammation and apoptosis in murine primary splenocytes. J Food Drug Anal. 2014;22:210-219. [Google Scholar]
- 12. Ku CM, Lin JY. Anti-inflammatory effects of 27 selected terpenoid compounds tested through modulating Th1/Th2 cytokine secretion profiles using murine primary splenocytes. Food Chem. 2013;141:1104-1113. [DOI] [PubMed] [Google Scholar]
- 13. Zimmermann CEP, Cruz IBM, Cadoná FC, et al. Cytoprotective and genoprotective effects of β-glucans against aflatoxin B1-induced DNA damage in broiler chicken lymphocytes. Toxicol Vitro. 2015;29:538-543. [DOI] [PubMed] [Google Scholar]
- 14. Wu JY, Chen CH, Chang WH, et al. Anti-cancer effects of protein extracts from Calvatia lilacina, Pleurotus ostreatus and Volvariella volvacea. Evid Based Complement Alternat Med. 2011;2011:982368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boo HJ, Hong JY, Kim SC, et al. The anticancer effect of fucoidan in PC-3 prostate cancer cells. Mar Drugs. 2013;11:2982-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thangam R, Sathuvan M, Poongodi A, et al. Activation of intrinsic apoptotic signaling pathway in cancer cells by Cymbopogon citratus polysaccharide fractions. Carbohydr Polym. 2014;107:138-150. [DOI] [PubMed] [Google Scholar]
- 17. Xie JH, Liu X, Shen MY, et al. Purification, physicochemical characterisation and anticancer activity of a polysaccharide from Cyclocarya paliurus leaves. Food Chem. 2013;136:1453-1460. [DOI] [PubMed] [Google Scholar]
- 18. Zong AZ, Cao HZ, Wang FS. Anticancer polysaccharides from natural resources: a review of recent research. Carbohydr Polym. 2012;90:1395-1410. [DOI] [PubMed] [Google Scholar]
- 19. Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353-367. [DOI] [PubMed] [Google Scholar]
- 20. Mestas J, Hughes CC. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172:2731-2738. [DOI] [PubMed] [Google Scholar]
- 21. Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2:151-161. [DOI] [PubMed] [Google Scholar]
- 22. Lin BF, Chiang BL, Lin JY. Amaranthus spinosus water extract directly stimulates proliferation of B lymphocyte in vitro. Int Immunopharmacol. 2005;5:711-722. [DOI] [PubMed] [Google Scholar]
- 23. Rishika D, Sharma R. An update of pharmacological activity Psidium guajava in the management of various disorders. Int J Pharm Sci Rev Res. 2012;3:3577-3584. [Google Scholar]
- 24. Huang CS, Yin MC, Chiu LC. Antihyperglycemic and antioxidative potential of Psidium guajava fruit in streptozotocin-induced diabetic rats. Food Chem Toxicol. 2011;49:2189-2195. [DOI] [PubMed] [Google Scholar]
- 25. Pelegrini PB, Murad AM, Silva LP, et al. Identification of a novel storage glycine-rich peptide from guava (Psidium guajava) seeds with activity against Gram-negative bacteria. Peptides. 2008;29:1271-1279. [DOI] [PubMed] [Google Scholar]
- 26. Chen H, Wu P, Lo D, Pan Y, Wu M. Hepatoprotective effect of guava (Psidium guajava L.) leaf extracts on ethanol-induced injury on clone 9 rat liver cells. Food Nutr Sci. 2011;2:983-988. [Google Scholar]
- 27. Seo N, Ito T, Wang N, et al. Anti-allergic Psidium guajava extracts exert an antitumor effect by inhibition of T regulatory cells and resultant augmentation of Th1 cells. Anticancer Res. 2005;25:3763-3770. [PubMed] [Google Scholar]
- 28. Bontempo P, Doto A, Miceli M, et al. Psidium guajava L. anti-neoplastic effects: induction of apoptosis and cell differentiation. Cell Prolif. 2012;45:22-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen KC, Hsieh CL, Peng CC, et al. Brain derived metastatic prostate cancer DU-145 cells are effectively inhibited in vitroby guava (Psidium gujava L.) leaf extracts. Nutr Cancer. 2007;58:93-106. [DOI] [PubMed] [Google Scholar]
- 30. Ryu NH, Park KR, Kim SM, et al. A hexane fraction of guava leaves (Psidium guajava L.) induces anticancer activity by suppressing AKT/mammalian target of rapamycin/ribosomal p70 S6 kinase in human prostate cancer cells. J Med Food. 2012;15:231-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Park SS, Ohba H. Suppressive activity of protease inhibitors from buckwheat seeds against human T-acute lymphoblastic leukemia cell lines. Appl Biochem Biotechnol. 2004;117:65-74. [DOI] [PubMed] [Google Scholar]
- 32. Wu SC, Lee BH. Buckwheat polysaccharide exerts antiproliferative effects in THP-1 human leukemia cells by inducing differentiation. J Med Food. 2011;14:26-33. [DOI] [PubMed] [Google Scholar]