Abstract
Introduction. Surgical resection in patients with non–small cell lung cancer (NSCLC) may be associated with significant morbidity, functional limitations, and decreased quality of life. Objectives. The safety and feasibility of a preoperative and early postoperative rehabilitation program in patients operated for NSCLC was determined in a nonhospital setting, with focus on high-intensity interval exercise. Methods. Forty patients with biopsy-proven NSCLC stages I to IIIa referred for surgical resection at the Department of Cardiothoracic Surgery RT, Rigshospitalet, University of Copenhagen, were randomly assigned to 1 of 4 groups (3 intervention groups and 1 control group). The preoperative intervention consisted of a home-based exercise program, while the postoperative exercise program comprised a supervised group exercise program involving resistance and high-intensity interval cardiorespiratory exercise 2 hours weekly for 12 weeks combined with individual counseling. The study endpoints were inclusion rate, adherence, and number of adverse events. Results. Forty patients (of 124 screened; 32%) were included and randomized into the 4 groups. The postoperative exercise was completed by 73% of the patients randomized to this intervention. No adverse events were observed, indicating that the early postoperative exercise program is safe. The preoperative home-based exercise program was not feasible due to interfering diagnostic procedures and fast-track surgery that left only 1 to 2 weeks between diagnosis and surgery. Conclusion. The early postoperative exercise program for patients with NSCLC was safe and feasible, but in a fast-track set up, a preoperative home-based exercise program was not feasible for this population.
Keywords: lung cancer, exercise, rehabilitation, NSCLC, perioperative intervention
Introduction
Among the most common cancers, lung cancer has the highest mortality rate of cancer worldwide.1 Most cases are non–small cell lung cancer (NSCLC). Accurate staging of NSCLC is crucial for allocation to surgical treatment, which may be curative in cases of localized disease (stages I and II) and for selected patients with locally advanced disease (stage IIIA).2 The recommended treatment of disseminated NSCLC and small cell lung cancer involves chemotherapy and radiation therapy.2
The number of long-term survivors after treatment of NSCLC is increasing.3 Surgical resection is still associated with potentially significant morbidity, functional limitations, and decreased quality of life. Therefore, evidence-based rehabilitation may be an important tool to improve outcome and quality of life in this group of patients.4,5
Systematic reviews suggest that pre- and postsurgical exercise in patients with NSCLC, compared with usual care, is associated with improved cardiopulmonary exercise capacity, increased muscle strength, as well as reduced fatigue, postoperative complications, and length of hospital stay.6-8 These reviews emphasize that an optimal exercise program is still to be determined and that prospective research in this area is needed.6-8
Preoperative exercise offered to NSCLC patients attracts attention because it may improve longevity and decrease risk of postoperative complications, but research in this field differs in design, type of intervention, and dose of exercise. The research is primarily based on case studies and studies with few and heterogeneous patients.9-11 In addition, recent research highlights the need for psychosocial support during the period from diagnosis to surgery.12
Evidence shows that postoperative exercise for NSCLC patients is both safe and associated with improvement of cardiorespiratory capacity and self-reported outcomes such as health-related quality of life and fatigue.13 Still more research is required to understand the potential effect of exercise on NSCLC patients and to determine how individual components such as mode, intensity, frequency, duration, and timing may contribute.7
Barriers for participating in rehabilitation and maintaining lifestyle changes are, for example, high symptom burden, such as side effects to the adjuvant treatment, and high prevalence of comorbidity, especially chronic obstructive pulmonary disease.14 The timing of rehabilitation is important when it comes to motivating patients to perform and sustain lifestyle changes.15 The teachable moment is a term used to describe a health event that motivates individuals to positive health behavior change. The time of diagnosis is a health event that can modify barriers and motivate patients to adopt to a healthier lifestyle.15
Silver et al defined cancer rehabilitation as
medical care that should be integrated throughout the oncology care continuum and delivered by trained rehabilitation professionals who have it within their scope of practice to diagnose and treat patients’ physical, psychological and cognitive impairments in an effort to maintain or restore function, reduce symptom burden, maximize independence and improve quality of life in this medically complex population.16(p3636)
Thus, the focus of this article is the evaluation of the exercise part in a rehabilitation program to patients with operable lung cancer.
The advantages of performing exercise during adjuvant treatment are better physical and mental status and a reduction of side effects to the adjuvant chemotherapy. These advantages are found in a variety of cancer patients.17-19
To our knowledge, no published research has studied whether initiating high-intensity interval exercise is safe in a nonhospital setting as early as 2 weeks after an operation for NSCLC. Our assumption is that introducing exercise before initiation of adjuvant chemotherapy, thereby laying the groundwork for better adherence to the exercise program, is advantageous compared to an exercise program initiated during adjuvant chemotherapy. We assume that NSCLC patients are willing and able to participate in home-based exercise prior to surgery and also hypothesize that patients attending preoperative home-based exercise are more prepared to participate in high-intensity interval exercise after surgery.
Aim of the Study
The overall aim of this feasibility study was to investigate the safety and feasibility of preoperative and early postoperative rehabilitation in a nonhospital setting, with focus on exercise, in patients undergoing surgery for lung cancer.
Methods
Patients and Settings
The PROLUCA feasibility study included 40 patients (age ≥18 years) with biopsy-proven NSCLC, stages I to IIIa20 assigned for curative surgery at the Department of Cardiothoracic Surgery, Rigshospitalet, University of Copenhagen. The inclusion criteria were the following: assigned for curative lung cancer surgery, at least 18 years old, performance status 0 to 2 (World Health Organization),21 resident of the City of Copenhagen or a surrounding municipality, able to read and understand Danish, and approval by primary surgeon. The exclusion criteria were the following: the presence of metastatic disease or surgical inoperability, diagnosis of lung cancer not verified by biopsy, severe cardiac disease, and contraindications to maximal exercise testing as recommended by the American Thoracic Society and by exercise testing guidelines for cancer patients.22
Procedure
The study was conducted in accordance with the Consolidated Standards of Reporting Trials (CONSORT) statement for nonpharmacologic interventions and the World Medical Association Declaration of Helsinki.23,24 Written informed consent was obtained from all patients prior to initiation of any study procedures. The study was approved by the Danish National Committee on Health Research Ethics (File No. H-3-2012-028) and the Danish Data Protection Agency (File No. 2007-58-0015).
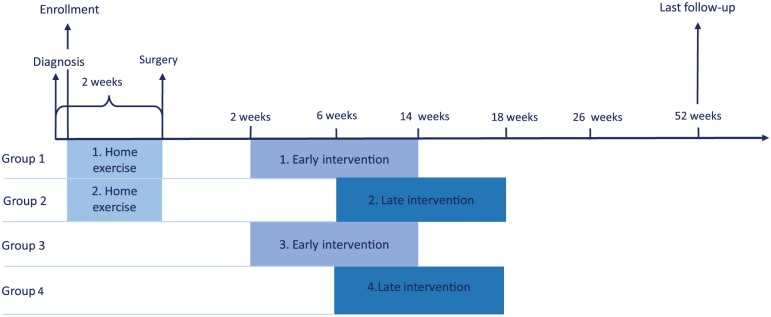
The study design is presented in Figure 1. Using a 4-arm, randomized design, potential subjects were identified and screened for eligibility and contacted by the study research coordinators at the referral departments at Bispebjerg and Gentofte Hospitals. After referral to surgery, the subjects were contacted by telephone and informed of the purpose and design of the study. Next, written informed consent was obtained and baseline assessment was performed at the Copenhagen Centre for Cancer and Health. At baseline the following assessments were performed (Table 1): (1) anthropometric data and tumor node metastasis (TNM) stage, (2) cardiorespiratory capacity expressed as maximal oxygen uptake (VO2peak) evaluated by an incremental test using an electromagnetically braked cycle ergometer (Lode Corival Ergometer, Groningen, Netherlands) where inspired and expired gases were analyzed breath-by-breath by a metabolic cart (JAEGER MasterScreen CPX, Care Fusion, San Diego, CA), (3) 6-minute walk distance (6MWD), (4) muscle strength measured by a 1 repetition maximum (1RM) in chest and leg press, (5) pulmonary function test (spirometry), and (6) patient-reported outcomes. All baseline assessments were completed as close to the time of diagnosis as possible and were repeated the day before surgery, postintervention, and at follow-up 6 months and 1 year after surgery. Assessments at pre-intervention were 6MWD, pulmonary function, and Functional Assessment of Cancer Therapy–Lung (FACT-L).
Figure 1.
Timeline for the PROLUCA feasibility study.
Table 1.
Baseline Characteristics.
| Variables | Total (N = 40) | Early Exercise (n = 18), Groups 1 + 3 | Late Exercise (n = 22), Groups 2 + 4 |
|---|---|---|---|
| Age (years), median (range) | 68 (36-85) | 67 (36-79) | 71 (56-86) |
| Female, n (%) | 24 (60%) | 10 (56%) | 14 (64%) |
| Body mass index (kg/m2), mean (SD) | 25 (5) | 25 (5) | 25 (4) |
| Academy professional degree <3 years, n (%) | 17 (43%) | 6 (33%) | 11 (50%) |
| Smoking history, N = 40 (groups 1 and 3, n = 18; groups 2 and 4, n = 22) | |||
| Currently smoking, n (%) | 10 (25%) | 7 (39%) | 3 (14%) |
| Never smoked, n (%) | 2 (5%) | 1 (6%) | 1 (5%) |
| Ex-smoker, n (%) | 28 (70%) | 10 (55%) | 18 (81%) |
| Years smoking, mean (SD) | 41 (15) | 44 (12) | 38 (13) |
| Presence of comorbidity (5 patients had none of the comorbidities mentioned below) | |||
| Hypertension, n (%) | 15 (38%) | 6 (33%) | 9 (41%) |
| Dyslipidemia, n (%) | 9 (23%) | 4 (22%) | 5 (23%) |
| Diabetes, n (%) | 6 (15%) | 3 (17%) | 3 (14%) |
| Atrial fibrillation, n (%) | 2 (5%) | 0 | 2 (9%) |
| COPD, n (%) | 8 (20%) | 2 (11%) | 6 (27%) |
| Rheumatic diseases, n (%) | 12 (30%) | 5 (28%) | 7 (32%) |
| Other type of cancer, n (%) | 6 (15%) | 4 (22%) | 2 (9%) |
| Depression, n (%) | 4 (10%) | 1 (6%) | 3 (14%) |
| Medication, number of drugs, median (range) | 3 (1-6) | 2 (1-5) | 3 (2-6) |
| Pulmonary function | |||
| FEV1 (L/s), mean (SD) | 2.4 (0.6) | 2.5 (0.5) | 2.3 (0.6) |
| FEV1 (L/s), % predicted (SD) | 94 (23.7) | 95 (26.1) | 93 (22.2) |
| FEV1/VC (%), mean (SD) | 67.4 (8.7) | 68 (6) | 68 (10) |
| Cardiorespiratory capacity | |||
| Fitness (mL/kg/min), mean (SD) | 19.4 (5) | 21.5 (6) | 17.6 (4) |
| Peak oxygen uptake (L/min), mean (SD) | 1.40 (0.39) | 1.53 (0.33)* | 1.28(0.40)* |
| 6MWD, mean (SD) | 477 (81) | 497 (92) | 461 (70) |
| TNM stage | |||
| Stage I (a + b), n (%) | 11 (27%) | 5 (28%) | 6 (27%) |
| Stage II (a + b), n (%) | 24 (60%) | 12 (67%) | 12 (55%) |
| Stage IIIa, n (%) | 5 (13%) | 1 (5%) | 4 (18%) |
Abbreviations: n, number; SD, standard deviation; COPD, chronic obstructive lung disease; FEV1, forced expiratory volume in 1 second; VC, vital capacity; 6MWD, 6-minute walk distance.
P > .05.
Group Allocation (Randomization)
Following the successful completion of baseline assessments, patients were randomized and allocated, on an individual basis, to 1 of the 4 exercise intervention groups:
Preoperative and postoperative exercise initiated 2 weeks after surgery
Preoperative and postoperative exercise initiated 6 weeks after surgery
Postoperative exercise initiated 2 weeks after surgery
Current standard care, postoperative exercise initiated 6 weeks after surgery
The random allocation sequences were concealed from all study personnel and performed by Copenhagen Trial Unit, Centre for Clinical Intervention Research. Randomly allocated patients remained in the same group for the entire duration of the intervention.
The intention-to-treat analysis included all randomized participants in their randomly assigned allocations. The intervention group assignment was not altered based on the participant’s adherence to the randomly allocated study arm. Patients who were lost to follow-up were included in the analysis (intention-to-treat).
Exercise Training Protocols
Preoperative Exercise
Individually designed according to functional status and comorbidity for each patient randomized to the preoperative intervention, the home-based exercise program consisted of 30 minutes of cardiorespiratory exercise daily until surgery. The exercise period varied in length due to the time available before surgery. The preoperative exercise was monitored by a heart rate monitor and software (Polar Team2, Polar Electro Oy, Kempele, Finland) and an exercise diary logbook.
Postoperative Exercise
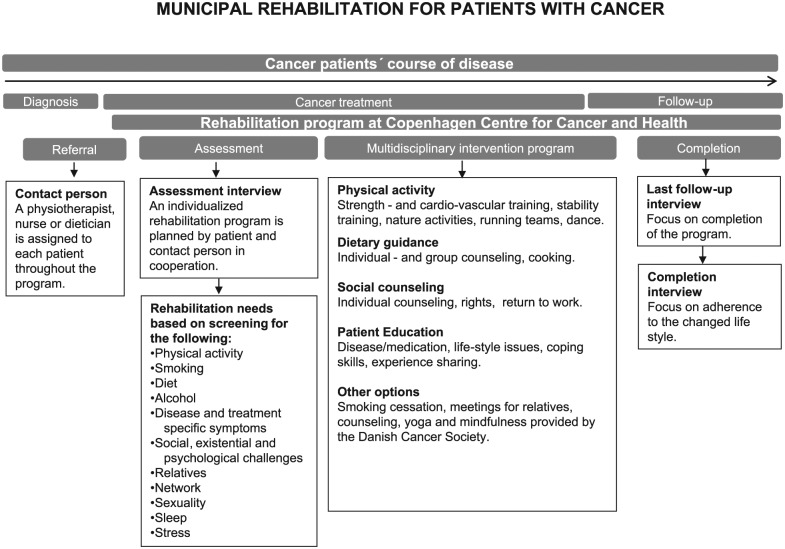
The postoperative exercise intervention was a part of the rehabilitation services available at a rehabilitation center described in Figure 2. Every participant was initially screened for rehabilitation needs following a professional rehabilitation guide covering the following topics: disease specific, social network, relatives, psychological, existential, diet, smoking, alcohol, physical activity, sexuality, sleep, and stress. The rehabilitation guide was based on the theoretical framework by the World Health Organization on International Classification of Functioning,25 the “self-efficacy theory” by Bandura,26 and motivational interviewing by Miller.27 The exercise intervention consisted of 24 group-based exercise sessions combined with 3 individual counseling sessions and 3 group-based lessons in health-promoting behavior. If the patients had special needs in terms of smoking cessation, nutritional counseling, or patient education, this was also offered as part of the rehabilitation. The postoperative exercise consisted of individually tailored, supervised strength exercise and group-based cardiorespiratory exercise twice a week (60 minute/session) on nonconsecutive days for 12 weeks, for a total of 24 sessions. It included the following exercises.
Figure 2.
Rehabilitation services available between diagnosis and follow-up at the rehabilitation center.
Warm-up (5 minutes) and cardiorespiratory exercise (25 minutes) on an ergometer bike (BODY BIKE Classic Supreme, TKO, Houston, TX), individually tailored strength exercise (25 minutes) carried out using 5 machines (Technogym, Cesena, Italy), leg press, chest press, leg extension, pull to chest, and pull down (upper body). Trained physiotherapists and cancer nurse specialists supervised the training program following principles recommended by the American College of Sports Medicine.28 All exercise sessions included supervised breathing exercises combined with stretching and tension-release techniques (5 minutes). All interventions were individually tailored to each patient and followed the principles of aerobic or resistance training prescription guidelines for adults as recommended by the American College of Sports Medicine.28 The high-intensity interval exercise consisted of a warmup period where the participants aimed at reaching a level at 85% of individually determined HRmax (5 minutes) followed by a short rest (1 minute). The duration of the high-intensity interval exercises was 25 minutes. In each interval (1-2 minutes), the participants aimed at reaching a level of 85% to 100% of individually determined HRmax in each interval followed by a short rest (1 minute). The high-intensity interval exercise was followed by a cool down period (2 minutes).
The ultimate goal for the postoperative exercise was 2 group-based exercise sessions per week, with a cardiorespiratory intensity for the first 4 weeks of approximately 50% to 60% of individual HRmax. In the next 8 weeks, the intensity was increased to moderate-high intensity at approximately 70% to 90% of individually determined HRmax. The ultimate goal of the strength exercise program was to exercise with an intensity of approximately 60% to 80% of 1RM 2 times a week for 12 weeks. To ensure progression, every other week the load was progressively increased and the number of repetitions reduced, starting out at 12 repetitions in 3 sets, progressing to 10 repetitions in 3 sets, to a final of 8 repetitions in 3 sets. The protocol study by Sommer et al describes the pre- and postoperative interventions in further detail.29
Adherence Considerations
To maximize adherence, several strategies were employed: telephone-based follow-up, free parking in front of the center, and remuneration for transport expenses. A high degree of scheduling flexibility allowed patients to perform tests at a convenient time to allow space for competing demands such as medical appointments, work, and family commitments.
Study Endpoint and Assessment
Tracking and Monitoring of Adverse Events
Tracking and monitoring of adverse events took place as follows: before every intervention and test session, all patients received face-to-face supervision by a specialist cancer nurse to discuss any potential negative side effects of the intervention tasks. All injuries and adverse events (eg, knee pain, back pain) were recorded as unintended events. In addition, heart rate and blood pressure were recorded prior to every intervention session and repeated if any adverse events occurred during exercise.
Adherence to the Program
Reasons for not attending the program were assessed immediately after the participants decided not to participate or decided to drop out of the program. The assessment was performed by specially trained cancer staff and conducted either by phone or face-to-face. Adherence to exercise sessions was monitored by trained staff, and reasons for not attending a session were assessed immediately after absence from exercise.
Statistical Analysis
Descriptive statistics and paired t tests were calculated using SAS/STAT software. Statistical significance was set at P < .05. Baseline values of the study populations were compared with values measured at postintervention and 1-year follow-up. The values are expressed as mean ± standard deviation (SD). Pared t tests were also performed to reveal tendencies in patients who exercised for more or less than 70% of the exercise sessions. Cardiac rehabilitation normally sets the cutoff for adherence to both the number of training sessions prescribed and the duration of the prescribed program as at least 80%.30 In cancer rehabilitation, there is no standard practice on how to set adherence to an exercise intervention. Based on the small number of participants in this study, we chose a cutoff value for adherence as at least 70% of the exercise sessions.
Results
Study Population and Characteristics
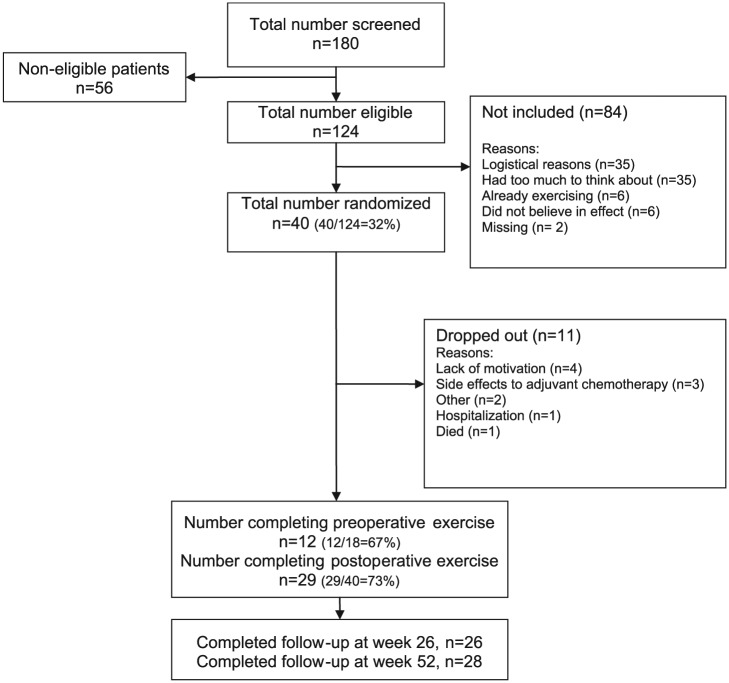
A total of 180 patients were screened for eligibility, 124 of whom were eligible. Forty patients (32%) were included and randomized. Table 1 presents the baseline characteristics of the 40 patients included and of the pooled subgroups with either early (groups 1 + 3) or late (groups 2 + 4) exercise intervention. The 2 most frequent reasons given by patients for not attending the study were either logistical ones or that the patients had too much to think about prior to surgery (Figure 3). The 40 patients included in the study had a mean age of 68 years, and the majority were retired (Table 1). The most frequent comorbidities (registered from the medical records) were hypertension, dyslipidemia, rheumatic disease, and chronic obstructive pulmonary disease. Five patients had no comorbidity, and 16 patients were categorized as having multiple morbidities. At baseline, 70% were ex-smokers, and 25% currently smoked. The baseline cardiopulmonary capacity of the included patients was 19.4 mL/kg/min and a forced expiratory volume in 1 second (FEV1 at 2.4 L/s and a FEV1/vital capacity [VC] at 67.4%; Table 1).
Figure 3.
Flow PROLUCA feasibility study.
The VO2peak and fitness measured at baseline was significant higher in the early exercise group compared to the late exercise group (Table 1).
Nine patients (22%) underwent open thoracotomy surgery, of which 3 participated in early exercise group and 6 participated in late exercise group. Thirty-one (78%) patients received video-assisted thoracic surgery and 13 (33%) received adjuvant chemotherapy (9 in early exercise groups and 4 in late exercise groups).
The types of operation that were performed were primarily lobectomy (83%); only 1 pneumonectomy was performed (2%). The patient that had a pneumonectomy was randomized to the early intervention group and had an adherence to the exercise program of 80%. Other types of operations performed were bilobectomy (5%), wedge resection (8%), and video-assisted thoracic surgery segmental resection (2%).
Safety and Adherence to the Perioperative Exercise
Safety
There were no adverse events reported or observed, and no patients showed spontaneous or unexpected reactions to initiating exercise 2 weeks after surgery.
Adherence to Home-Based Preoperative Exercise
Twelve out of 18 patients randomized to preoperative exercise were instructed in the home-based exercise program. The combination of medical procedures and lack of time prior to operation meant 6 patients did not receive instruction, and 1 patient who had received instruction failed to start due to lack of motivation. The average number of days possible for exercise before surgery was 8 days, with a range between 2 and 15 days. It was only possible for 3 out of the 12 patients to accomplish exercises daily prior to surgery (Table 2).
Table 2.
Adherence to perioperative exercise.
| Preoperative exercise (home-based) (n = 18) | |
| Instruction given to, n (%) | 12 (67%) |
| Possible days for exercise, mean (range) | 8 (2-15) |
| Days of self-reported exercise out of days possible (%) (n = 12) | |
| Exercise ≥70%, n (%) | 8 (67%) |
| Exercise <70%, n (%) | 4 (33%) |
| Postoperative exercise adherence to 24 sessions (group exercise) (n = 29) | |
| Exercise ≥70%, n (%) | 15 (52%) |
| Exercise <70%, n (%) | 14 (48%) |
| Participation ≥70% in early and late postoperative interventions (n = 15) | |
| Early exercise, n (%) | 7 (47%) |
| Late exercise, n (%) | 8 (53%) |
| Dropouts in early and late postoperative interventions (n = 11) | |
| Early exercise, n (%) | 5 (45%) |
| Late exercise, n (%) | 6 (55%) |
| Initiation of exercise: early (n = 15) and late (n = 16) intervention groups | |
| Early, number of days after surgery, mean (range) | 18 (13-29) |
| Late, number of days after surgery, mean (range) | 47 (22-80) |
Adherence to Supervised Group Exercise After Surgery
The supervised group exercise after surgery was completed by 73% of the patients. Postoperative exercise adherence to 24 group-based sessions was ≥70% in 15 patients out of 29 (Table 2). Four out of the 29 never exercised, but as the study is designed as an intention-to-treat study, results from all 29 are provided according to the objectives of the study. For further in-depth analysis of the potential efficacy of exercise, analysis of the 25 completers was also carried out. The results did not change the significance of the results presented in Table 5, which is why results for the completers alone are not presented.
Table 5.
Physiological Capacity Change Scores (Evaluated in Relation to Adherence to Exercise).
| Variable | Baseline (N = 40) | Postintervention (n = 29) | One Year (n = 28) | Difference Between Baseline and Postintervention | Difference Between Baseline and 1-Year Follow-up | ||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (95% CI) | P Value | Mean (95% CI) | P Value | |
| VO2peak (L/min) | 1.40 (0.39) | 1.35 (0.54) | 1.38 (0.40) | −0.12 (−0.27 to 0.03) | .1038 | −0.09 (−0.21 to 0.02) | .1125 |
| Exercise ≥70% | 1.45 (0.45) | 1.45 (0.55) | 1.35 (0.44) | −0.08 (−0.26 to 0.11) | .3809 | −0.07 (−0.25 to 0.11) | .4124 |
| Exercise <70% | 1.37 (0.35) | 1.21 (0.48) | 1.41 (0.37) | −0.19 (−0.50 to 0.12) | .1876 | −0.12 (−0.30 to 0.06) | .1714 |
| Fitness (mL/kg/min) | 19.4 (4.8) | 18.8 (6.4) | 18.4 (2.9) | −1.9 (−3.9 to 0.2) | .0741 | −1.8 (−3.7 to 0.11) | .0637 |
| Exercise ≥70% | 19.2 (5.3) | 18.1(5.7) | 17.7 (3.1) | −1.3 (−3.3 to 0.7) | .1870 | −1.3 (−4.35 to 1.7) | .3676 |
| Exercise <70% | 19.6 (4.5) | 19.8 (7.7) | 19.2 (2.5) | −2.8 (−8 to 2.4) | .2393 | −2.4 (−5.2 to 0.3) | .0782 |
| 6MWD (m) | 477 (81) | 512 (80) | 517 (92) | 16 (−3 to 35) | .0947 | 22 (−9 to 53) | .1615 |
| Exercise ≥70% | 495 (63) | 525 (67) | 507 (113) | 28 (4 to 51) | .0229 | 20 (−30 to 69) | .4093 |
| Exercise <70% | 465 (90) | 486 (101) | 531 (59) | −7 (−43 to 29) | .6659 | 25 (−16 to 67) | .1977 |
| 1RM Leg (kg) | 107 (39) | 121 (46) | 130 (58) | 9 (−3 to 22) | .1441 | 21 (5 to 37) | .0136 |
| Exercise ≥70% | 112 (41) | 140 (67) | 133 (51) | 30 (5 to 55) | .0220 | 18 (1 to 36) | .0443 |
| Exercise <70% | 104 (39) | 100 (28) | 118 (47) | −6 (−21 to 9) | .3526 | 10 (−12 to 33) | .3431 |
| 1RM Chest (kg) | 34 (13) | 36 (13) | 37 (16) | 3 (0 to 6) | .0499 | 3 (0 to 6) | .0249 |
| Exercise ≥70% | 33 (12) | 39 (15) | 35 (14) | 5 (2 to 8) | .0029 | 4 (1 to 7) | .0129 |
| Exercise <70% | 34 (15) | 30 (8) | 40 (18) | −2 (−7 to 4) | .4746 | 2 (−4 to 7) | .4703 |
| FEV1, L/s (SD) | 2.4 (0.6) | 2.2 (0.5) | 2.2 (0.4) | −0.2 (−0.4 to 0) | .0830 | −0.2 (−0.3 to 0) | .0140 |
| FEV1/VC, % (SD) | 67.4 (8.7) | 66.7 (10.9) | 63.9 (8.4) | −1.3 (−3.8 to 1.2) | .2861 | −6.3 (−9.5 to −3) | .0006 |
Abbreviations: SD, standard deviation; CI, confidence interval; 6MWD, 6-minute walk distance; FEV1, forced expiratory volume in 1 second; VC, vital capacity.
The primary reasons for cancelling exercise sessions were hospitalization/appointments at the hospital, lack of motivation, or lack of time. There was no difference in adherence between the groups that initiated exercise 2 weeks after surgery (groups 1 and 3) compared to those who started exercise 6 weeks after surgery (groups 2 and 4; Table 2). Patients who performed preoperative exercise were evenly distributed between patients who did at least 70% of the sessions or who did less than 70% of them.
In the early intervention group (groups 1 and 3), the average onset of exercise was 18 days postsurgery, with a range of 13 to 29 days, and in the late intervention group the average onset of exercise was 47 days, with a range of 22 to 80 days. One patient, in the early intervention group, started exercise 49 days after surgery, which was around the time the late intervention (groups 2 and 4) was initiated. As a result, this patient could no longer be categorized in the early initiation of exercise group.
The distribution of patients who performed preoperative exercise was even evenly distributed between patients who exercised for at least 70% or for less than 70% of the postoperative sessions. The mean intensity of the strength exercise was for the chest press 67% (SD 20) of 1RM and for leg press 69% (SD 22) of 1RM during the 24 exercise sessions. The intensity of the cardiorespiratory exercise for the first 4 weeks was at 74% (SD 8) of individual determined maximum heart rate and for the last 8 weeks at 77% (SD 4) of individual determined maximum heart rate.
Dropouts
Eleven patients dropped out during the intervention, primarily due to either lack of motivation to complete or side effects to the adjuvant chemotherapy. Patients receiving adjuvant chemotherapy were evenly distributed between completers and dropouts (Table 3). The prevalence of patients receiving adjuvant chemotherapy was lower in patients who exercised for 70% to 100% of the 24 exercise sessions compared to patients who exercised 0% to 69% (Table 3). There was no difference between completers and dropouts regarding demographic data, stage of disease, or type of surgery. Dropouts were evenly distributed between the groups who initiated exercise 2 and 6 weeks after surgery (Table 2).
Table 3.
Distribution of Patients Receiving Adjuvant Chemotherapy in Relation to Postoperative Exercise Adherence (24 Sessions).
| Patients Included | Completed Exercise | Exercise ≥70% | Exercise <70% | Dropout | |
|---|---|---|---|---|---|
| Total number, n | 40 | 29 | 15 | 14 | 11 |
| Numbers receiving chemotherapy, n (%) | 13 (33%) | 9 (31%) | 2 (13%) | 7 (50%) | 4 (36%) |
Postoperative Complications, Recurrence, and Mortality
Out of all of the registered pulmonary and cardiac complications, only one occurred during the exercise intervention and it involved pulmonary pneumatocele and was not evaluated as an adverse event caused by the exercise. All other pulmonary and cardiac complications occurred before the patients initiated the exercise intervention. The overall prevalence of pulmonary postoperative complications within 30 days after surgery was 23% and was highest in the early exercise group (groups 1 and 3; Table 4). The prevalence of cardiac complications was 13%, and the distribution of cardiac complications was evenly distributed between early and late exercise. Two patients experienced recurrence and 3 patients died within the first year after surgery (Table 4).
Table 4.
Postoperative Complications, Recurrence, and Mortality.
| Pulmonary complications, N = 40, n (%)a | 9 (23%) |
| Early, n = 18, n (%) | 6 (33%) |
| Late, n = 22, n (%) | 3 (14%) |
| Cardiac complications, N = 40, n (%)a | 5 (13%) |
| Early, n = 18, n (%) | 2 (11%) |
| Late, n = 22, n (%) | 3 (14%) |
| Recurrenceb, N = 40, n (%) | 2 (5%) |
| Mortalityb, N = 40, n (%) | 3 (8%) |
Data collected 30 days after surgery.
Data collected at 1-year follow-up.
Changes in Physiological Capacity
Table 5 shows the results of physiological capacity change scores from baseline to postintervention and from baseline to 1-year follow-up (VO2peak, fitness, 6MWD, 1RM, FEV1, and FEV1/VC).
There was a significant increase postintervention in walking distance (P = .0229) for patients who participated in at least 70% of the sessions. This effect on walking distance was not reproduced at the 1-year follow-up. There was a significant improvement in strength for patients who participated in at least 70% of the sessions. This improvement was found for leg press in both the postintervention and at the 1-year follow-up (P = .0220 and P = .0443). Correspondingly, the improvement for chest press was also significant (P = .0029 and P = .0129).
Independent of adherence to exercise, there was a trend in mean decrease in fitness of 1.9 mL/kg/min from baseline to postintervention (P = .0741). This trend was retained at the 1-year follow-up (P = .0637).
Discussion
This feasibility study showed that rehabilitation with high-intensity interval exercise initiated 2 weeks after surgery in NSCLC patients in a nonhospital setting was safe and feasible. The preoperative home-based exercise was inconsistent and not feasible in the present setup due to the short time interval between referral and surgery (fast-track surgical program).
Preoperative Home-Based Exercise
To our knowledge only one study has investigated the effect of a home-based exercise training program prior to surgery in patients who are potentially candidates for lung resection, but the patients were younger and diagnosed with early-stage lung cancer compared to the patients in the present study.31 Coats et al found good adherence to a 4-week home-based exercise intervention as all of the included patients (n = 16) completed more than 75% of the prescribed exercise sessions.31 In Denmark, the maximum waiting time for surgery after a diagnosis of lung cancer is, by law, specified not to exceed 2 weeks. Therefore, a 4-week preoperative training program would not be possible.
Some possible advantages of home-based preoperative programs are greater flexibility and convenience for patients, low time consumption, and more manageable financially compared to preoperative interventions in an outpatient setting.32,33 In our study, 28% of eligible patients found physical activity before surgery unmanageable in the fast-track setting. In the present feasibility study, patients who performed preoperative exercise were evenly distributed between patients who exercised for at least 70% or for less than 70% of the postoperative sessions, which indicates that preoperative home-based exercise had no influence.
Preoperative exercise is a component in the emerging medical discipline called Prehabilitation.34 Silver and Baima define prehabilitation
as a process on the cancer continuum of care that occurs between the time of cancer diagnosis and the beginning of acute treatment and includes physical and psychological assessments that establish a baseline functional level, identify impairments, and provide interventions that promote physical and psychological health to reduce the incidence and/or severity of future impairments.34(p716)
The potential benefit of prehabilitation to cancer patients and the research within this area seems promising in terms of reducing morbidity, improving physical and psychological function, and decreasing hospital readmissions.34
Supervised Group Exercise After Surgery
In a nonhospital setting, the present study is the first to demonstrate that supervised, group-based high-intensity interval exercise initiated 2 weeks after surgery is safe in NSCLC patients. The present study also demonstrates that the patients could exercise with the average intensity we have prescribed. The intensity of the cardiorespiratory exercise was for the first 4 weeks at 73% of individual determined HRmax. These results indicate that the patients in the present study were able to exercise with a higher intensity than the 50% to 60% we prescribed.
Previous research concerning early postoperative exercise in operable lung cancer patients is based on studies with limited intensity and duration of exercise. In the majority of the studies, the exercise is initiated the day after operation and carried out during hospitalization. These studies find that exercising shortly after an operation for NSCLC is safe, but the studies are characterized by having small sample sizes.35-37 Recently published research investigated the efficacy of home-based postoperative exercise, but the interventions in these studies are characterized by low-intensity exercise interventions.38,39 The present study found a higher prevalence of pulmonary complications in the group that initiated exercise 2 weeks after surgery, but since the complications occurred before exercise was initiated there was no causal relation. The prevalence of pulmonary and cardiovascular postoperative complications in the present study is comparable to other findings in a cohort study by Boffa et al.40 Edvardsen et al carried out a randomized clinical trial (RCT) in NSCLC patients, where the intervention was initiated 4 to 6 weeks after surgery and carried out in a fitness center near the patients’ home. In addition to demonstrating significant improvements in both physical performance and health-related quality of life, the study found the intervention to be safe, with only one adverse event reported (a hip fracture).41 Thus, with regard to safety their study supports our results.
Missel et al interviewed patients with operable NSCLC and found that motivation for participation in an exercise program depended on patient expectations concerning the physical benefits and the comfort of having health care professionals present.12 This underlines the importance of having specialized cancer nurses and physiotherapists to manage the exercise instead of attending a public fitness center.
Seventy-three percent of patients in the present study completed the supervised group exercise and adherence to the program exceeded 70% in half of the patients. In the study by Edvardsen et al, mean adherence to the exercise intervention was 88%, but technically some participants could exceed 100%. Other studies reported an adherence of around 50% to 55%.42,43 The present study found no difference between early and late intervention in terms of adherence or dropouts. Among the patients receiving adjuvant chemotherapy, only 2 patients (n = 13) managed to exercise for at least 70% of the sessions, indicating the difficulty of attending exercise during adjuvant chemotherapy.
In the present study, 33% of the patients received adjuvant chemotherapy, which is comparable to the NSCLC population in Denmark.3 Edvardsen et al41 found that patients receiving the last courses of chemotherapy had to postpone their training sessions until they had completed the adjuvant treatment. In contrast, Jones et al44 found good adherence in the same group of patients receiving chemotherapy, but the effect of the intervention was inferior to the findings in the study by Edvardsen et al,41 and the second most frequent reason for dropping out of the present study was side effects of the adjuvant treatment, reported by 27% of the total number of dropouts. The prevalence of patients receiving adjuvant chemotherapy was highest in the group with early exercise. These results show that exercising during adjuvant treatment is feasible and supported by findings in inoperable lung cancer as well as other cancer diagnoses.17,19
The present study found a significant improvement in strength for patients who exercised for at least 70% of the sessions. This improvement was found for leg and chest press, both postintervention and at the 1-year follow-up and is comparable to the findings of the RCT by Edvardsen et al,41 where the same significant improvement in leg press was found (mean difference at 29.5 kg P > .001). This improvement is of great importance as muscle strength is inversely associated with all-cause mortality.45
Our feasibility study also found a trend toward a mean decrease in fitness of 1.9 mL/kg/min from baseline to postintervention. These findings are not supported in other studies published in NSCLC patients. Since the study is underpowered, the results must be interpreted with caution.
Strength and Limitations
The strength of this study is the precise surveillance of adverse events, the reported reasons for dropping out, and the precise detection of postoperative complications. Additional strengths are the use of well-validated objective measurements, blinded professionals collecting data, and the statistical analysis.
The fact that only 32% of the eligible patients participated in the present study can result in a selection bias because the present population might not be representative of the population operated on for NSCLC in Denmark. The low recruitment rate could also affect possibilities to implement these results in a clinical setting. It might be that the patients choosing to participate in the present study represents a group with better physical fitness than the group that did not want to participate in the study. A comparison of the patients in the present study with cohort studies in patients with NSCLC reveals similarities regarding age, sex, pulmonary function, and comorbidities.40,46
A methodological weakness of this study is that blinding participants to their actual treatment allocation was not possible since participants were aware of whether they initiated preoperative exercise and whether their postoperative exercise started 2 or 6 weeks after surgery. Another limitation in the present study is the low number of participants and thereby the risk of finding or not finding statistically significant results when using a t test to analyze the mean difference from baseline to postintervention and from baseline to 1 year after surgery. Therefore, the result from the present study must be interpreted with caution. It is also very important to emphasize that the t test only allows us to investigate the effect on a certain time point and we cannot conclude anything about the effect over time.
Conclusion
This study shows that patients with operable NSCLC are able to initiate high-intensity interval group exercise 2 weeks after lung resection in a nonhospital setting. Early, supervised, group-based high-intensity interval exercise is both safe and feasible. In the study setting, the preoperative home-based exercise was not feasible due to low recruitment rate and the short time interval between referral and surgery. Our findings are currently under investigation in an RCT study examining the effect of a postoperative exercise intervention initiated either 2 or 14 weeks after surgery. To ensure a higher inclusion rate, the preoperative exercise intervention has been omitted.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, suthorship, and/or publication of this article: This study was supported by grants from the Center for Intregrated Rehabilitation of Cancer Patients (CIRE), which was established by and receives support from the Danich Cancer Society and the Novo Nordisk Foundation.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2. Lim E, Baldwin D, Beckles M, et al. Guidelines on the radical management of patients with lung cancer. Thorax. 2010;65(suppl 3):iii1-27. doi: 10.1136/thx.2010.145938. [DOI] [PubMed] [Google Scholar]
- 3. Jakobsen E, Green A, Oesterlind K, Rasmussen TR, Iachina M, Palshof T. Nationwide quality improvement in lung cancer care: the role of the Danish Lung Cancer Group and Registry. J Thorac Oncol. 2013;8:1238-1247. doi: 10.1097/JTO.0b013e3182a4070f. [DOI] [PubMed] [Google Scholar]
- 4. Kenny PM, King MT, Viney RC, et al. Quality of life and survival in the 2 years after surgery for non small-cell lung cancer. J Clin Oncol. 2008;26:233-241. doi: 10.1200/JCO.2006.07.7230. [DOI] [PubMed] [Google Scholar]
- 5. Skokan L, Reed CE, Koh S, Ott GY, Silvestri GA. What happens to patients undergoing lung cancer surgery ? Surgery. 2002;1:21-30. [DOI] [PubMed] [Google Scholar]
- 6. Granger CL, McDonald CF, Berney S, Chao C, Denehy L. Exercise intervention to improve exercise capacity and health related quality of life for patients with non-small cell lung cancer: a systematic review. Lung Cancer. 2011;72:139-153. doi: 10.1016/j.lungcan.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 7. Cavalheri V, Tahirah F, Nonoyama M, Jenkins S, Hill K. Exercise training for people following lung resection for non-small cell lung cancer: a Cochrane systematic review. Cancer Treat Rev. 2014;40:585-594. doi: 10.1016/j.ctrv.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 8. Crandall K, Maguire R, Campbell A, Kearney N. Exercise intervention for patients surgically treated for non-small cell lung cancer (NSCLC): a systematic review. Surg Oncol. 2014;23:17-30. doi: 10.1016/j.suronc.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 9. Bobbio A, Chetta A, Ampollini L, et al. Preoperative pulmonary rehabilitation in patients undergoing lung resection for non-small cell lung cancer. Eur J Cardiothorac Surg. 2008;33:95-98. doi: 10.1016/j.ejcts.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 10. Divisi D, Di Francesco C, Di Leonardo G, Crisci R. Preoperative pulmonary rehabilitation in patients with lung cancer and chronic obstructive pulmonary disease. Eur J Cardiothorac Surg. 2013;43:293-296. doi: 10.1093/ejcts/ezs257. [DOI] [PubMed] [Google Scholar]
- 11. Benzo R, Kelley GA, Recchi L, Hofman A, Sciurba F. Complications of lung resection and exercise capacity: a meta-analysis. Respir Med. 2007;101:1790-1797. doi: 10.1016/j.rmed.2007.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Missel M, Pedersen JH, Hendriksen C, Tewes M, Adamsen L. Exercise intervention for patients diagnosed with operable non-small cell lung cancer: a qualitative longitudinal feasibility study. Support Care Cancer. 2015;23:2311-2318. doi: 10.1007/s00520-014-2579-3. [DOI] [PubMed] [Google Scholar]
- 13. Jones LW, Eves ND, Waner E, Joy AA. Exercise therapy across the lung cancer continuum. Curr Oncol Rep. 2009;11:255-262. [DOI] [PubMed] [Google Scholar]
- 14. Bade BC, Thomas DD, Scott JB, Silvestri GA. Increasing physical activity and exercise in lung cancer. J Thorac Oncol. 2015:10:861-871. doi: 10.1097/JTO.0000000000000536. [DOI] [PubMed] [Google Scholar]
- 15. Pinto BM, Maruyama NC, Clark MM, Cruess DG, Park E, Roberts M. Motivation to modify lifestyle risk behaviors in women treated for breast cancer. Mayo Clin Proc. 2002;77:122-129. doi: 10.4065/77.2.122. [DOI] [PubMed] [Google Scholar]
- 16. Silver JK, Raj VS, Fu JB, Wisotzky EM, Smith SR, Kirch RA. Cancer rehabilitation and palliative care : critical components in the delivery of high-quality oncology services. Support Care Cancer. 2015;23:3633-3643. doi: 10.1007/s00520-015-2916-1. [DOI] [PubMed] [Google Scholar]
- 17. Quist M, Adamsen L, Rorth M, Laursen JH, Christensen KB, Langer SW. The impact of a multidimensional exercise intervention on physical and functional capacity, anxiety, and depression in patients with advanced-stage lung cancer undergoing chemotherapy. Integr Cancer Ther. 2015;14:341-349. doi: 10.1177/1534735415572887. [DOI] [PubMed] [Google Scholar]
- 18. Adamsen L, Quist M, Andersen C, et al. Effect of a multimodal high intensity exercise intervention in cancer patients undergoing chemotherapy: randomised controlled trial. BMJ. 2009;339:b3410. doi: 10.1136/bmj.b3410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mishra SI, Scherer RW, Snyder C, Geigle PM, Berlanstein DR, Topaloglu O. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012;(8):CD008465. doi: 10.1002/14651858.CD008465.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rami-Porta R, Crowley JJ, Goldstraw P. The revised TNM staging system for lung cancer. Ann Thorac Cardiovasc Surg. 2009;15:4-9. [PubMed] [Google Scholar]
- 21. Sørensen JB, Klee M, Palshof T, Hansen HH. Performance status assessment in cancer patients. An inter-observer variability study. Br J Cancer. 1993;67:773-775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kreider ME, Grippi MA. Impact of the new ATS/ERS pulmonary function test interpretation guidelines. Respir Med. 2007;101:2336-2342. doi: 10.1016/j.rmed.2007.06.019. [DOI] [PubMed] [Google Scholar]
- 23. Boutron I, Moher D, Altman DG, Schulz KF, Ravaud P; CONSORT Group. Extending the CONSORT statement to randomized trials of nonpharmacologic treatment: explanation and elaboration. Ann Intern Med. 2008;148:295-309. [DOI] [PubMed] [Google Scholar]
- 24. Buchanan DR, Miller FG. A public health perspective on research ethics. J Med Ethics. 2006;32:729-733. doi: 10.1136/jme.2006.015891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. World Health Organization. International Classification of Functioning, Disability and Health (ICF). http://www.who.int/classifications/icf/icf_more/en/. Accessed February 16, 2016.
- 26. Bandura A. Self-efficacy: toward a unifying theory of behavioral change. Psychol Rev. 1977;84:191-215. [DOI] [PubMed] [Google Scholar]
- 27. Miller R. Motivational interviewing: research, practice, and puzzles. Addict Behav. 1996;21:835-842. [DOI] [PubMed] [Google Scholar]
- 28. Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334-1359. doi: 10.1249/MSS.0b013e318213fefb. [DOI] [PubMed] [Google Scholar]
- 29. Sommer MS, Trier K, Vibe-Petersen J, et al. Perioperative rehabilitation in operation for lung cancer (PROLUCA): rationale and design. BMC Cancer. 2014;14:404. doi: 10.1186/1471-2407-14-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Conraads VM, Deaton C, Piotrowicz E, et al. Adherence of heart failure patients to exercise: barriers and possible solutions. Eur J Heart Fail. 2012;14:451-458. doi: 10.1093/eurjhf/hfs048. [DOI] [PubMed] [Google Scholar]
- 31. Coats V, Maltais F, Simard S, et al. Feasibility and effectiveness of a home-based exercise training program before lung resection surgery. Can Respir J. 2013;20(2):10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Neubeck L, Freedman SB, Clark AM, Briffa T, Bauman A, Redfern J. Participating in cardiac rehabilitation: a systematic review and meta-synthesis of qualitative data. Eur J Prev Cardiol. 2012;19:494-503. doi: 10.1177/1741826711409326. [DOI] [PubMed] [Google Scholar]
- 33. Dalal HM, Zawada A, Jolly K, Moxham T, Taylor RS. Home based versus centre based cardiac rehabilitation: Cochrane systematic review and meta-analysis. BMJ. 2010;340:b5631. doi: 10.1136/bmj.b5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Silver JK, Baima J. Cancer prehabilitation: an opportunity to decrease treatment-related morbidity, increase cancer treatment options, and improve physical and psychological health outcomes. Am J Phys Med Rehabil. 2013;92:715-727. doi: 10.1097/PHM.0b013e31829b4afe. [DOI] [PubMed] [Google Scholar]
- 35. Cesario A, Ferri L, Galetta D, et al. Post-operative respiratory rehabilitation after lung resection for non-small cell lung cancer. Lung Cancer. 2007;57:175-180. doi: 10.1016/j.lungcan.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 36. Peddle-McIntyre CJ, Bell G, Fenton D, McCargar L, Courneya KS. Lung cancer feasibility and preliminary efficacy of progressive resistance exercise training in lung cancer survivors. Lung Cancer. 2012;75:126-132. doi: 10.1016/j.lungcan.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 37. Hoffman AJ, Brintnall RA, von Eye A, et al. Home-based exercise: promising rehabilitation for symptom relief, improved functional status and quality of life for post-surgical lung cancer patients. J Thorac Dis. 2014;6:632-640. doi: 10.3978/j.issn.2072-1439.2014.06.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Arbane G, Douiri A, Hart N, et al. Effect of postoperative physical training on activity after curative surgery for non-small cell lung cancer: a multicentre randomised controlled trial. Physiotherapy. 2014;100:100-107. doi: 10.1016/j.physio.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 39. Hoffman AJ, Brintnall RA, von Eye A, et al. A rehabilitation program for lung cancer patients during postthoracotomy chemotherapy. Onco Targets Ther. 2014;7:415-423. doi: 10.2147/OTT.S57262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boffa DJ, Allen MS, Grab JD, Gaissert HA, Harpole DH, Wright CD. Data from the Society of Thoracic Surgeons General Thoracic Surgery database: the surgical management of primary lung tumors. J Thorac Cardiovasc Surg. 2008;135:247-254. doi: 10.1016/j.jtcvs.2007.07.060. [DOI] [PubMed] [Google Scholar]
- 41. Edvardsen E, Skjonsberg OH, Holme I, Nordsletten L, Borchsenius F, Anderssen SA. High-intensity training following lung cancer surgery: a randomised controlled trial. Thorax. 2014;70:244-250. doi: 10.1136/thoraxjnl-2014-205944. [DOI] [PubMed] [Google Scholar]
- 42. Temel J, Greer J, Goldberg S, et al. A structured exercise program for patients with advanced non-small cell cancer. J Thorac Oncol. 2009;4:595-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kuehr L, Wiskemann J, Abel U, Ulrich CM, Hummler S, Thomas M. Exercise in patients with non-small cell lung cancer. Med Sci Sports Exerc. 2014;46:656-663. doi: 10.1249/MSS.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 44. Jones LW, Eves ND, Peterson BL, et al. Safety and feasibility of aerobic training on cardiopulmonary function and quality of life in postsurgical nonsmall cell lung cancer patients: a pilot study. Cancer. 2008;113:3430-3439. doi: 10.1002/cncr.23967. [DOI] [PubMed] [Google Scholar]
- 45. Ruiz JR, Sui X, Lobelo F, et al. Association between muscular strength and mortality in men: prospective cohort study. BMJ. 2008;337:a439. doi: 10.1136/bmj.a439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Little AG, Rusch VW, Bonner JA, et al. Patterns of surgical care of lung cancer patients. Ann Thorac Surg. 2005;80:2051-2056. doi: 10.1016/j.athoracsur.2005.06.071. [DOI] [PubMed] [Google Scholar]