Abstract
Background. Cognitive impairment is a common complaint among cancer survivors, significantly impacting working memory, attention, executive function, and information processing speed. This meta-analysis aims to evaluate the effect of neuropsychological interventions on the cognitive function of non–central nervous system (non-CNS) cancer survivors. Methods. Three databases (PubMed, PsycInfo, and CAJ Full-text Database) were searched from January 2010 to September 2015. Controlled clinical trials of neuropsychological interventions for the treatment of cognitive impairment in cancer survivors were considered for inclusion. Results. A total of 10 eligible trials were included in this meta-analysis. Three trials assessed the effects of cognitive rehabilitation (CR) interventions, and the weighted mean difference (WMD) for the overall intervention effect was −0.19 (95% confidence interval [CI] = −2.98 to 2.61). Two trials examined the effects of cognitive training (CT) interventions on the cognitive function of cancer survivors; the standardized mean difference (SMD) for the overall effect was 0.52 (95% CI = 0.06 to 0.98). The overall effect of CR interventions on neuropsychological status at postintervention was 5.66 (95% CI = 2.97 to 8.35). The SMD of CR and CT intervention for objective function by verbal learning tests was 0.50 (95% CI = 0.19 to 0.81) at postintervention, and 0.58 (95% CI = 0.19-0.98) at follow-up assessment within 6 months. Conclusion. Findings from this meta-analysis indicate that neuropsychological interventions can improve cognitive function in non-CNS cancer survivors, and support the need for future research. However, the conclusion from this meta-analysis was based on trials with small sample sizes. Future research should be conducted using a larger sample size. Relevant clinical implications were discussed accordingly.
Keywords: neuropsychological interventions, cognitive impairment, cancer survivors, meta-analysis
Introduction
Thanks to medical technology advances, coupled with earlier detection of cancer, survival rates for cancer patients have improved significantly. The 5-year relative survival rate from all cancer sites is 68%.1 Globally, 32.6 million people are cancer survivors.2 As cancer survival rates increase, cognitive impairment has emerged as a significant problem affecting survivors.3,4 The prevalence of cognitive impairment for cancer survivors was up to 75% both during and after treatment,5 particularly affecting attention, memory, executive function, and information processing speed.5-8
Increasing research evidence shows that cancer-related cognitive impairment was associated with having cancer, as well as with cancer treatment.9,10 There is an accumulating body of evidence suggesting that cancer patients could suffer cognitive impairment, even before systematic treatment begins.10,11 In addition, there are accumulating published studies showing that cancer treatments, particularly chemotherapy, could influence the cognitive function of cancer survivors upward of months, to even years.10,12 These cognitive impairments could exert a significant impact on social and occupational functioning, interfering with the ability to carry out normal daily activities, all of which in turn contributes to lower quality of life for cancer survivors.13-15
There are limited pharmacological treatment approaches for the management of cognitive impairment, and it is noted that pharmacological treatments often have side effects.8,16 Cognitive rehabilitation support and neuropsychological modulation strategies are an increasingly common approach to supporting cancer survivors.3,17 One review by Gehring and colleagues16 comprehensively examined a range of pharmacological and nonpharmacological interventions for cancer-related cognitive deficits. Hines et al18 conducted a systematic review focusing on the effectiveness of psychosocial interventions for chemotherapy-related cognitive dysfunction. However, both articles only reviewed relevant intervention studies published during or prior to 2011. According to King and Green,8 many studies related to psychological interventions for cognitive dysfunction among adult cancer patients following treatment were published after 2012. Therefore, this study’s aim was to quantitatively evaluate the most recent studies on the effects of neuropsychological interventions on cancer survivors’ cognitive function, and to identify implications for future research.
Methods
Data Sources and Searches
Three databases (PubMed, PsycInfo, and CAJ Full-text Database) were searched from January 2010 to September 2015, including articles published in both English and Chinese. The search terms included a combination of neuropsycholog*, cognit*, neurocognit*, neurobehavior*, intervention*, rehabilitation, trial, cancer, and cancer survivors. Searches were limited to adult human studies.
Study and Participant Types
Studies were eligible for inclusion if they were controlled clinical trials, including randomized controlled trials and clinical trials without randomization, which addressed the effects of neuropsychological interventions on the cognitive function of individuals with cancer. Inclusion criteria comprised (1) patients diagnosed with primary cancer during adulthood-onset (aged 18 years or older), because patient-reported cognitive function measures for childhood cancer survivors differ from adult measures19 and (2) with a non-brain or non–central nervous system (CNS) tumor, as a brain or CNS tumor can directly affect the brain, and thus the cognitive processes, of cancer survivors.20 Exclusion criteria included patients diagnosed with primary cancer during childhood-onset (aged 18 years or younger), and with a brain or CNS tumor, as there were existing reviews focused on brain tumors or other CNS tumor.20,21
Types of Interventions and Outcome Measures
Studies were included if they used any type of neuropsychological interventions aimed at the improvement of cognitive function in cancer survivors. The primary outcome was cognitive function by subjective and/or objective cognition outcome measures. Secondary outcomes included any adverse effects as a result of neuropsychological interventions.
Data Extraction and Assessment of Bias Risk
For each study, data was independently extracted from the original article by one of the main researchers, then verified by the second researcher. Any disagreements on data extraction were resolved by discussion among the research team members. The Cochrane Risk of Bias Assessment Tool was used to evaluate the risk of bias of the included trials. This assessment tool consists of seven domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases.22 Each domain was carefully assessed as to whether it had low, high or unclear risk of bias in accordance with the judgment criteria.
Data Synthesis and Analysis
The data were synthesized and analyzed using the Cochrane Collaboration’s Review Manager (RevMan 5.3).23 The heterogeneity of included trials was assessed using chi-square and Ι2 statistics, and a chi-square of P value greater than 0.1 or an Ι2 value of less than 50% was considered to be indicative of statistical homogeneity.22 The random-effects model was used to combine statistically heterogeneous clinical trials, whereas the fixed effects model was used to combine statistically homogeneous trials.22 For the effects of intervention on cognitive function, weighted mean difference (WMD) was calculated when cognitive function outcomes were measured using the same scale, and the standardized mean difference (SMD) was used when different scales were used to measure cognitive functions among different trials, with corresponding 95% confidence intervals (CI).22 Data pooling in this meta-analysis was performed for the effects of neuropsychological interventions by subjective and objective outcome measures.
Results
Description of Included Trials
The flow diagram of the literature search process is given in Figure 1. A total of 10 trials3,8,10,14,24-29 were included in this meta-analysis. Table 1 summarizes the characteristics of these trials. Each trial was evaluated in terms of its risk of bias and the overall bias risk is shown in Figure 2. Of these 10 studies, 7 studies8,14,24-27,29 were randomized trials. Three studies3,10,28 were case-control designs, leading to a high risk of bias for random sequence generation and allocation concealment.
Figure 1.
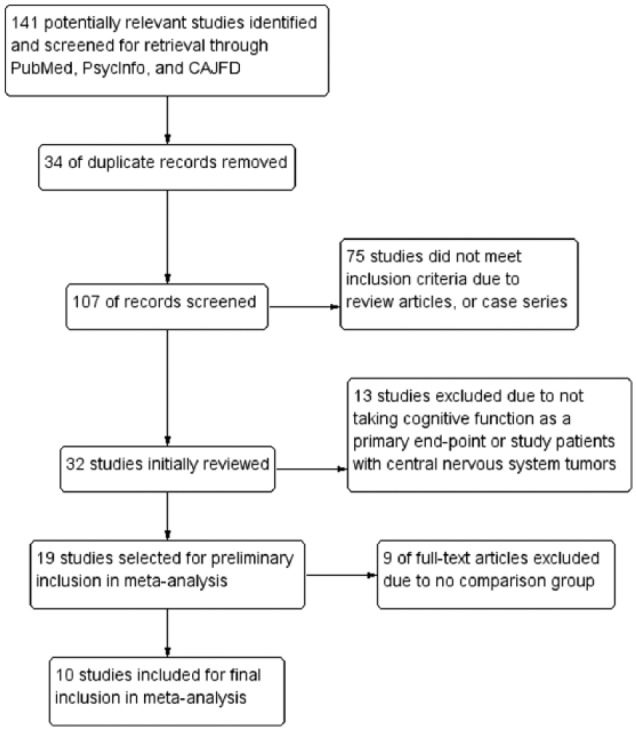
Study flow diagram.
Table 1.
Characteristics of 10 Included Studies.
| Authors (Year) | Study Type | Study Sample | Study Interventions | Outcome Measures for Cognition | Main Findings and Conclusion |
|---|---|---|---|---|---|
| Alvarez et al (2013)3 | CCT | 23 female breast cancer survivors, aged 40 years or older, and with 6-60 months posttreatment | 10-week (20 sessions) whole brain EEG neurofeedback training regimen vs normative sample | Subjective measure: FACT-Cog | Study revealed strongly significant improvements on 4 domains of FACT-Cog (P < .001) |
| Cherrier et al (2013)24 | RCT | 28 female and male non-CNS cancer survivors, with a mean age of 60.5 years and with a median of 3 years posttreatment | 7-week cognitive rehabilitation intervention vs waitlist control | Subjective measure: FACT-Cog Objective measure: RAVLT for verbal memory; Stroop Trial for executive function; Digit Symbol and Digit Span for attention |
The treatment group demonstrated improvements in symptoms of PCI, PCA, and overall impact of quality of life related to cognitive symptoms (P < .01). This group also improved on objective measures of attention (P < .05) |
| Ercoli et al (2015)25 | RCT | 48 female breast cancer survivors with a mean age of 54.5 years and with 18 months to 5 years posttreatment | 5-week group-based interventions included psychoeducation and cognitive exercises vs waitlist control | Subjective measure: PAOFI; Objective measure: RAVLT for verbal memory | The cognitive rehabilitation group improved significantly on PAOFI total and memory score (both P = .01), and on RAVLT total trials (P = .02) and delayed recall scores (P < .01). On qEEG, this group also showed a decreased in delta “slow wave” power and alpha power (both P < .05) |
| Ferguson et al (2012)26 | RCT | 40 female breast cancer survivors, with a mean age of 50 years and after chemotherapy | 8-week CBT intervention focused on memory and attention adaptation training vs waitlist control | Subjective measure: MASQ; Objective measure: CVLT for verbal memory; Digit Symbol for attention; Trail Making Number-Letter trial for executive function |
The intervention group made significant improvements on verbal memory, but no statistical significance on self-reported cognitive complaints |
| Goedendorp et al (2014)27 | RCT | 98 female and male non-CNS cancer survivors, with a mean age of 44.6 years old, and with at least 1 year posttreatment | 6-month CBT intervention focused on memory and attention adaptation training vs waitlist control | Subjective measure: CIS-Concentration; Objective measure: Digit Symbol for attention; Reaction Time Task for speed of information processing | The CBT group reported significantly less cognitive disability. CBT also was associates with a clinically relevant reduction in concentration problems, but no significant differences in objective cognitive tests |
| Kesler et al (2013)14 | RCT | 41 female breast cancer survivors, with a mean age of 55 years and experiencing long-term cognitive deficits | 12-week online, home-based cognitive training program vs waitlist control | Subjective measure: BRIEF; Objective measure: HVLT-R for verbal memory; WCST for language; Digit Span for attention |
Cognitive training led to significant improvements in cognitive flexibility, verbal fluency and processing speed, and self-rating executive function skills |
| King and Green (2015)8 | RCT | 29 female and male non-CNS cancer survivors, with a mean age of 50.4 years and completed major treatment at least 6 months | 4-week cognitive rehabilitation program for adults recovering from cancer vs waitlist control vs normative sample | Subjective measure: FACT-Cog; Objective measure: RBANS for immediate and delayed memory; TMT for attention and executive function |
Participating in the intervention was associated with significantly faster performance on one objective cognitive task that measures processing speed and visual scanning. The intervention group also reported improvement on subjective measures of cognitive impairment and cognitive self-efficacy |
| McDougall et al (2011)28 | CCT | 22 female and male non-CNS older cancer survivors, with a mean age of 73.86 years and experienced treatment-induced memory impairments | Memory intervention vs health training intervention over a 2-year period | Subjective measure: MSEQ and MIA; Objective measure: HVLT-R for verbal memory; VMT-R for visual memory | The memory intervention group tended to improve more than the health training group in daily verbal memory performance scores, memory self-efficacy, strategy use and memory complaints |
| Schuurs and Green (2013)10 | CCT | 22 female and male non-CNS cancer survivors, with a mean age of 58.2 years and immediately completed cancer treatment | 4-week group-based cognitive rehabilitation treatment vs no intervention cancer survivors vs normal adults | Subjective measure: FACT-Cog and MASQ; Objective measure: RBANS for immediate and delayed memory; TMT for attention and executive function | The intervention was effective in improving overall cognitive function, visuospatial performance, immediate memory and delayed memory |
| Von Ah et al (2012)29 | RCT | 82 female breast cancer survivors, with a mean age of 56.5 years old, and at post–cancer treatment for at least 1 year | 8-week group-based memory training vs waitlist control | Subjective measure: FACT-Cog; Objective measure: RAVLT for verbal memory; UFOV for objective speed of process |
Memory training intervention improved memory performance at 2-month follow-up (P < .05); speed of processing training improved processing speed at postintervention and 2-month follow-up (both P < .05). Both interventions were associated with improvements in perceived cognitive functioning, symptom distress and quality of life |
Abbreviations: BRIEF, Behavioral Rating Inventory of Executive Function; BVMT-R, Brief Visuospatial Memory Test-Revised; CBT, Cognitive–Behavioral Therapy; CCT, Controlled Clinical Trial; CIS, Checklist Individual Strength; CNS, Central Nervous System; CVLT, California Verbal Learning Test; EEG, Electroencephalography; FACT-Cog, Functional Assessment of Cancer Therapy–Cognitive Function; HVLT-R, Hopkins Verbal Learning Test-Revised; MASQ, Multiple Ability Self-report Questionnaire; MSEQ, Memory Self-Efficacy Questionnaire; MIA, Meta-memory in Adulthood; PAOFI, Patient’s Assessment of Own Functioning Inventory; PCA, Perceived Cognitive Abilities; PCI, Perceived Cognitive Impairment; RAVLT, Rey Auditory Verbal Learning Test; RBANS, Repeatable Battery for Neuropsychological Status; RCT, Randomized Controlled Trial; TMT, Trail Making Test; UFOV, Useful Field of View; VMT-R, Visuospatial Memory Test–Revised.
Figure 2.
Overall risk of bias assessment using the Cochrane tool.
From Table 1, there are subjective cognitive measures and formal neurocognitive tests. The most common subjective cognitive measures include FACT-Cog (Functional Assessment of Cancer Therapy–Cognitive Function) and MASQ (Multiple Ability Self-Report Questionnaire). Common objective measures include brain imaging via quantitative electroencephalography (qEEG) and formal neurocognitive tests, such as verbal learning tests by RBANS (Repeatable Battery for Neuropsychological Status), RAVLT (Rey Auditory Verbal Learning Test), CVLT (California Verbal Learning Test), or HVLT-R (Hopkins Verbal Learning Test–Revised), Trial Making Test, Digit Symbol, and Digit Span. These neurocognitive tests were applied to measure participants’ attention, verbal and visual memory, executive function and information processing speed. In terms of interventions, one study3 used a neuromodulation intervention by EEG neurofeedback for breast cancer survivors. Three studies14,28,29 made use of cognitive training interventions. Six studies8,14,24-27 used cognitive rehabilitation interventions, mainly delivering interventions in a group format. Intervention duration ranged from 4 weeks to 6 months (Table 1).
Effects of Neuropsychological Interventions on Subjective Cognitive Function in Cancer Survivors
Three trials with a total of 86 subjects measured improved FACT-Cog subscales of perceived cognitive impairment (PCI), perceived cognitive abilities (PCA), and impact of perceived cognitive impairments on quality of life (IPCIQL). Figure 3.1 shows the WMD for the overall effect of cognitive rehabilitation (CR) interventions was −0.19 (95% CI = −2.98 to 2.61). The WMDs for the three subscales of PCI, PCA, and IPCIQL were −0.76 (95% CI = −18.90 to 17.38), 0.28 (95% CI = −4.29 to 4.85), and −1.50 (95% CI = −4.59 to 1.60), respectively. Although the improvement of subjective cognitive function was in favor of CR interventions, there is no statistically significant difference (Z score = 0.13, P = .90). Figure 3.2 shows the SMD for the effect of cognitive training (CT) interventions was 0.52 (95% CI = 0.06-0.98). By follow-up assessment of the effect of CT interventions for the subjective cognitive function, Figure 3.3 also shows its positive effects and the SMD was 0.54 (95% CI = 0.08-1.00; Z score = 2.29, P = .02), indicating that CT interventions had positive effects on improving the subjective cognitive function of cancer survivors in the follow-up evaluation.
Figure 3.
(3.1) Subjective cognitive function (FACT-Cog) at postintervention. (3.2) Subjective cognitive function at postintervention. (3.3) Subjective cognitive function at follow-up (≤6 months).
Effects of Neuropsychological Interventions on Objective Cognitive Function in Cancer Survivors
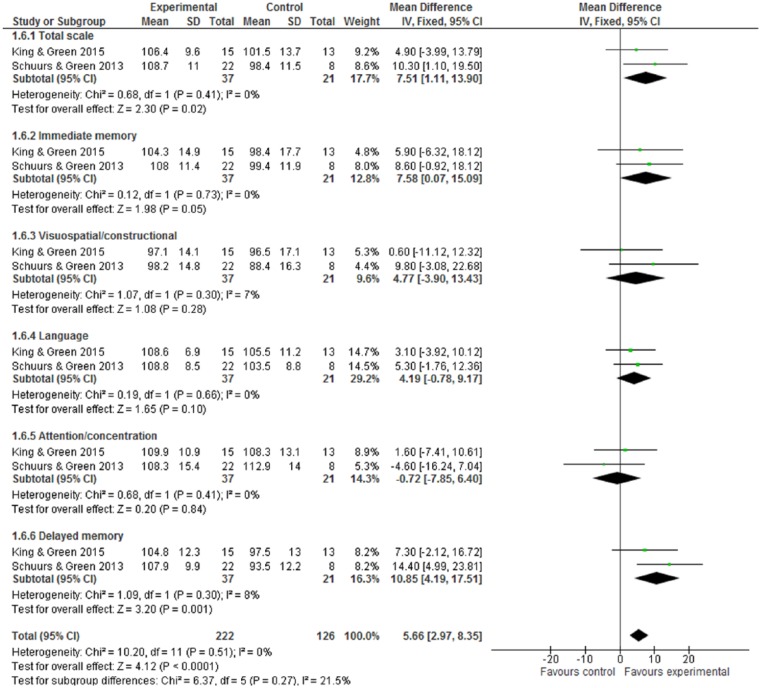
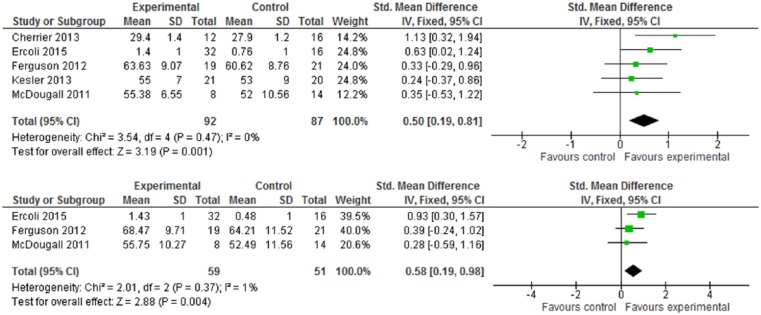
One trial3 used brain imaging assessment via qEEG, and reported that the intervention group showed positive effects in terms of cognitive function improvement: a decrease in alpha power and delta “slow wave” power (both P values <.05). By formal neurocognitive tests, Figure 4 shows the improvement of neuropsychological status in favor of intervention (WMD 5.66, 95% CI = 2.97-8.35) and with statistical significance (Z score = 4.12, P < .0001). Within the RBANS test, there were 5 subscales, but only 2 subscales—immediate memory and delayed memory—with statistical significance: The WMDs were 7.58 (95% CI = 0.07-15.09) and 10.85 (95% CI = 4.19-17.51). For the verbal learning tests by RAVLT, CVLT, or HVLT-R, Figure 5.1 indicates the intervention group experienced an improvement in verbal learning function, with the SMD at 0.50 (95% CI = 0.19-0.81). Within a 6-month follow-up, Figure 5.2 also shows the intervention had statistically significant effects on improved verbal learning function among cancer survivors. The SMD was 0.58 (95% CI = 0.19-0.98; Z score = 2.88, P = .004).
Figure 4.
Repeatable Battery for Neuropsychological Status (RBANS) test at postintervention.
Figure 5.
(5.1) Verbal Learning Test (VLT) at postintervention. (5.2) Verbal Learning Test (VLT) at follow-up (≤6 months).
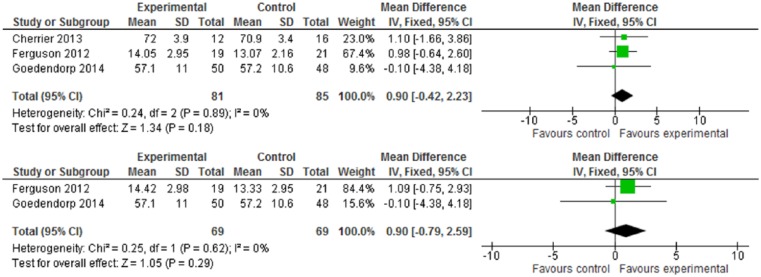
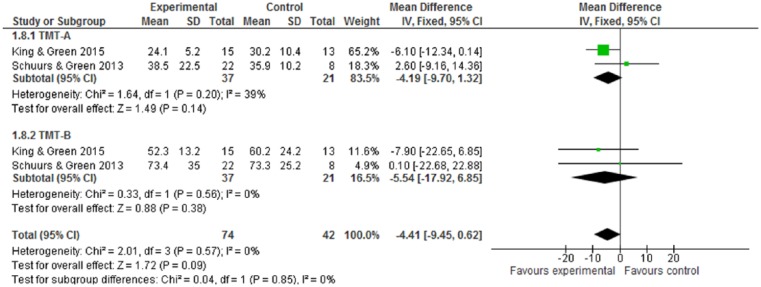
For cognitive performance, as measured by Digit Symbol, Digit Span, and TMT, none of these cognitive tests have statistical significance (Figures 6-8). While CR interventions showed trends in the direction of improving attention, processing speed, and working memory by Digit Symbol at postintervention and follow-up within 6 months (Figure 6.1 and 6.2), the intervention effect sizes’ CI crossed zero (both WMDs = 0.90, 95% CI = −0.42 to 2.23; −0.79 to 2.59, respectively). Cognitive performance, measured by Digit Span and TMT—including functions of attention, spatial organization, executive function, and mental flexibility—was also in favor of intervention, but found no statistical significance (Figures 7 and 8). Of 10 included trials, no study reported adverse effects related to neuropsychological interventions.
Figure 6.
(6.1) Digit Symbol test at postintervention. (6.2) Digit Symbol test at follow-up (≤6 months).
Figure 7.
Digit Span test at postintervention.
Figure 8.
Trial Making Tests (TMT) at postintervention.
Discussion
Based on the most recent research literature, most common neuropsychological interventions could alleviate cognitive impairment in cancer survivors, including cognitive rehabilitation interventions by behavioral therapy approaches, mainly in a group format, and with cognitive brain training delivered mainly in an individual format. Findings from this meta-analysis indicated that cognitive training interventions have positive effects on improving subjective and objective cognitive function in cancer survivors, although the effect sizes have been modest (SMDs ranging from 0.50 to 0.58). For example, CT interventions by an online and home-based program significantly improved multiple executive function skills, as reported by objective and self-report measures.14 CR interventions have positive effects in formal neurocognitive tests, such as the domains of immediate and delayed memory by RBANS, and several verbal learning tests. In the study by Ferguson et al,26 interventions by cognitive behavioral treatment were effective at improving memory and attention problems. Cognitive neuromodulation strategies offer new and noninvasive approaches for ameliorating cognitive dysfunction.25 One study, which used neurofeedback, found positive effects in self-reported cognitive measures and objective cognitive functions in breast cancer survivors.3
Most trials included in this meta-analysis only assessed the immediate effects at postintervention or short-term follow-up (6 months or less), as long-term follow-up assessment can monitor the sustainability of intervention effects. Hence, future research should be conducted in a longer term follow-up to establish whether neuropsychological interventions have long-term effects on the improvement of cognitive function in cancer survivors. More than half of the trials included in this meta-analysis focused on a study population of breast cancer survivors, and the remaining trials, with mixed types of cancer survivors, also included a study sample of breast cancer survivors. While breast cancer is the most common type of cancer globally, with a relatively good 5-year survival rate, many other cancer patients may also experience similar survivorship issues, as the 5-year relative survival rate for all cancer populations is now up to 68%.1
In addition, studies of other cancer populations could help researchers understand whether different types of cancers have specific risk factors and different underlying mechanisms leading to cognitive impairment.6 In terms of outcomes, most trials included in this review used a combination of self-reported cognitive measures and formal neurocognitive tests. Self-reported measures may ask about cancer survivors’ cognitive problems over a period of time, but neurocognitive tests can only detect their cognitive function at a certain point in time.6 Hence, future research should also utilize subjective and objective cognitive function measures, in order to better capture cancer-related cognitive impairment in cancer survivors. Furthermore, this meta-analysis found that various neuropsychological tests have been used, which may contribute to error variance and type II error. A task force has recommended that a core set of neuropsychological tests be used across studies to facilitate interpretation of study findings.30
While the process of meta-analysis could obtain a weighted average effect size across a number of different trials,31 it is important to note that an important result found in one study could be washed out by the null results of other studies.32 Ideally, the methodological limitations of meta-analysis could be resolved by a presentation of integrative data analysis, which is also expected to increase statistical power and generalizability of results by combining raw data.33 Similar to a meta-analysis, raw data from multiple samples (eg, different types of cancer survivors) could be combined into a single data analysis, despite the fact that all cognitive outcomes may not be measured using the same instruments.33 Hence, the integrative data analysis method analyzes the combined original data, and may overcome the limitations of the synthesis of summary statistics drawn from multiple studies, by calculating secondary data as meta-analysis. Another limitation of this meta-analysis was the conclusion drawn in a number of trials with small sample sizes. Findings of this meta-analysis should be confirmed in future randomized trials with larger sample sizes.
This meta-analysis found that neuropsychological interventions had positive effects, improving cognitive function in cancer survivors. Further research should be conducted to explore relevant risk factors for identifying patients at increased risk for cancer-related cognitive impairment, and to explore the possible underlying mechanisms of cognitive impairment in cancer survivors by using neuroimaging studies.5,13,15 Although breast cancer survivors have received relatively more attention in published literature, many other types of cancer survivors experience similar survivorship issues.5 Thus, further research should be conducted on different types of cancer survivors to identify disease-specific risk factors in cognitive impairment. Moreover, the trials in this meta-analysis show moderate to high risk of bias. Future trial design should be randomized and the outcome assessors blinded, in order to minimize potential methodological bias.
From the review by Hines et al,18 patients treated on psychosocial interventions for cancer related cognitive dysfunction was limited, as current therapies only indicated short-term effects (<6 months) on their symptoms. This review of most recent intervention studies also indicated that the neuropsychological interventions did not show any long-term effects on cognitive function outcomes. The review by Gehring et al19 included pharmacological and nonpharmacological interventions and found that “of the pharmacological agents studied and reviewed, off-label modafinil has the strongest evidence base for beneficial effects on cognitive function in patients with cancer.” This review also indicated that neuropsychological interventions may improve aspects of objective cognitive function and subjective cognitive function. But this review concluded that overall subjective cognitive effects are larger than objective cognitive effects. In contrast, this meta-analysis found effect size by objective neurocognitive tests (up to 5.66) are large than subjective cognitive measures (0.52-0.54).
Conclusion
Findings from this meta-analysis indicate that neuropsychological interventions can improve cognitive function in cancer survivors, and support the need for future research. However, since the conclusion from this meta-analysis was drawn based on trials using small sample sizes, future research should be conducted on a larger sample size.
Implications for Practice
Clinical staff should recognize that non-CNS cancer per se may also be involved in cognitive impairment that patients find distressing.33 This meta-analysis found 3 types of neuropsychological interventions, which were used to manage cognitive impairment in non-CNS cancer survivors. CT interventions demonstrated benefits in subjective and objective cognitive function, especially in the domain of executive function. CR interventions produced significant effects in objective cognitive function, mainly in the domain of memory and verbal learning. While neuromodulation strategies indicated positive effects in the improvement of subjective and objective cognitive function, these intervention strategies are largely anecdotal based on theorized causes, as the causes of cognitive impairment in cancer survivors are still unknown.34 Because of this, it is difficult to determine which intervention strategies will be better than others for patients experiencing cognitive impairment. Thus, quality research is required in order to determine the exact mechanism and cause of cognitive impairment, which will allow clinical staff to design better intervention strategies to ameliorate this distress symptom in non-CNS cancer survivors.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5-29. [DOI] [PubMed] [Google Scholar]
- 2. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [DOI] [PubMed] [Google Scholar]
- 3. Alvarez J, Meyer FL, Granoff DL, Lundy A. The effect of EEG biofeedback on reducing postcancer cognitive impairment. Integr Cancer Ther. 2013;12:475-487. [DOI] [PubMed] [Google Scholar]
- 4. Denlinger CS, Ligibel JA, Are M, et al. Survivorship: cognitive function, version 1.2014: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2014;12:976-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wefel JS, Schagen SB. Chemotherapy related cognitive dysfunction. Curr Neurol Neurosci Rep. 2012;12:267-275. [DOI] [PubMed] [Google Scholar]
- 6. Janelsins MC, Kesler SR, Ahles TA, Morrow GR. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int Rev Psychiatry. 2014;26:102-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Treanor C, Donnelly M. Late effects of cancer and cancer treatment—a rapid review. J Community Support Oncol. 2014;12:137-148. [DOI] [PubMed] [Google Scholar]
- 8. King S, Green HJ. Psychological intervention for improving cognitive function in cancer survivors: a literature review and randomized controlled trial. Front Oncol. 2015;5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cimprich B, Reuter-Lorenz P, Nelson J, et al. Prechemotherapy alterations in brain function in women with breast cancer. J Clin Exp Neuropsychol. 2010;32:324-331. [DOI] [PubMed] [Google Scholar]
- 10. Schuurs A, Green HJ. A feasibility study of group cognitive rehabilitation for cancer survivors: enhancing cognitive function and quality of life. Psychooncology. 2013;22:1043-1049. [DOI] [PubMed] [Google Scholar]
- 11. Mandelblatt JS, Stern RA, Luta G, et al. Cognitive impairment in older patients with breast cancer before systemic therapy: is there an interaction between cancer and comorbidity? J Clin Oncol. 2014;32:1909-1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wefel JS, Saleeba AK, Buzdar AU, Meyers CA. Acute and late onset cognitive dysfunction associated with chemotherapy in women with breast cancer. Cancer. 2010;116:3348-3356. [DOI] [PubMed] [Google Scholar]
- 13. Craig CD, Monk BJ, Farley JH, Chase DM. Cognitive impairment in gynecologic cancers: a systematic review of current approaches to diagnosis and treatment. Support Care Cancer. 2014;22:279-287. [DOI] [PubMed] [Google Scholar]
- 14. Kesler S, Hosseini SMH, Heckler C, et al. Cognitive training for improving executive function in chemotherapy-treated breast cancer survivors. Clin Breast Cancer. 2013;13:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wefel JS, Kesler SR, Noll KR, Schagen SB. Clinical characteristics, pathophysiology, and management of noncentral nervous system cancer-related cognitive impairment in adults. CA Cancer J Clin. 2015;65:123-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gehring K, Roukema JA, Sitskoorn MM. Review of recent studies on interventions for cognitive deficits in patients with cancer. Expert Rev Anticancer Ther. 2012;12:255-269. [DOI] [PubMed] [Google Scholar]
- 17. Hofmann W, Schmeichel BJ, Baddeley AD. Executive functions and self-regulation. Trends Cogn Sci. 2012;16:174-180. [DOI] [PubMed] [Google Scholar]
- 18. Hines S, Ramis MA, Pike S, Chang AM. The effectiveness of psychosocial interventions for cognitive dysfunction in cancer patients who have received chemotherapy: a systematic review. Worldviews Evid Based Nurs. 2014;11:187-193. [DOI] [PubMed] [Google Scholar]
- 19. Gehring K, Sitskoorn MM, Aaronson NK, Taphaorn MJ. Interventions for cognitive deficits in adults with brain tumours. Lancet Neurol. 2008;7:548-560. [DOI] [PubMed] [Google Scholar]
- 20. Gehring K, Aaronson NK, Taphoorn MJ, Sitskoorn MM. Interventions for cognitive deficits in patients with a brain tumor: an update. Expert Rev Anticancer Ther. 2010;10:1779-1795. [DOI] [PubMed] [Google Scholar]
- 21. Gross-King M, Booth-Jones M, Couluris M. Neurocognitive impairment in children treated for cancer: how do we measure cognitive outcomes? J Pediatric Oncol Nurs. 2008;25:227-232. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JPT, Green S. eds. Cochrane Handbook for Systematic Reviews of Interventions. The Cochrane Collaboration; 2011. [Google Scholar]
- 23. Review Manager (RevMan). 5.3. Copenhagen, Denmark: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- 24. Cherrier MM, Anderson K, David D, et al. A randomized trial of cognitive rehabilitation in cancer survivors. Life Sci. 2013;93:617-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ercoli LM, Petersen L, Hunter AM, et al. Cognitive rehabilitation group intervention for breast cancer survivors: results of a randomized clinical trial. Psychooncology. 2015;24:1360-1367. [DOI] [PubMed] [Google Scholar]
- 26. Ferguson RJ, McDonald BC, Rocque MA, et al. Development of CBT for chemotherapy-related cognitive change: results of a waitlist control trial. Psychooncology. 2012;21:176-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Goedendorp MM, Knoop H, Gielissen MF, Verhagen CA, Bleijenberg G. The effects of cognitive behavioral therapy for postcancer fatigue on perceived cognitive disabilities and neuropsychological test performance. J Pain Symptom Manage. 2014;47:35-44. [DOI] [PubMed] [Google Scholar]
- 28. McDougall GJ, Becker H, Acee TW, Vaughan PW, Delville CL. Symptom management of affective and cognitive disturbance with a group of cancer survivors. Arch Psychiatr Nurs. 2011;25:24-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Von Ah D, Carpenter JS, Saykin A, et al. Advanced cognitive training for breast cancer survivors: a randomized controlled trial. Breast Cancer Res Treat. 2012;135:799-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wefel JS, Vardy J, Ahles T, Schagen SB. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011;12:703-708. [DOI] [PubMed] [Google Scholar]
- 31. Jim HS, Phillips KM, Chait S, et al. Meta-analysis of cognitive functioning in breast cancer survivors previously treated with standard-dose chemotherapy. J Clin Oncol. 2012;30:3578-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treat Rev. 2013;39:297-304. [DOI] [PubMed] [Google Scholar]
- 33. Joly F, Giffard B, Rigal O, et al. Impact of cancer and its treatment on cognitive function: advances in research from the Paris International Cognition and Cancer Task Force Symposium and Update since 2012. J Pain Symptom Manage. 2015;50:830-841. [DOI] [PubMed] [Google Scholar]
- 34. McHenry AJ. Management of chemotherapy induced cognitive impairment. http://www.oncolink.org/resources/article.cfm?id=1057. Accessed December 29, 2015.