Abstract
Purpose. Breast cancer and oncological treatment can result in significant acute and late localized and systemic negative effects on health-related physical fitness and physical function. The aim of this single-arm study was to examine the effects of a 12-week community-based multimodal exercise program on health-related physical fitness and physical function in breast cancer survivors. Methods. A total of 52 female breast cancer survivors (mean age = 59.7 ± 10.4 years) completed supervised exercise training consisting of (1) aerobic conditioning, (2) resistance training, and (3) balance and flexibility training, for 30 minutes each, totaling 90 minutes twice weekly for 12 weeks. Pretreatment and posttreatment outcome measures—mobility: (1) Timed Up and Go (TUG) and (2) 6-minute walk test (6MWT); muscular strength: (3) leg press strength and (4) chest press strength; upper-extremity flexibility: (5) back scratch test; and balance: (6) functional reach (FR) and (7) single-leg stance time—were assessed and compared. Results. Postintervention assessment measures given as percentage improvement and effect size (ES) for mobility, TUG (18%, 0.59), and 6MWT (14%, 0.74) were significantly (P < .001) improved. Outcome measures of muscular strength, leg press strength (32%, 0.58) and chest press strength (40%, 0.61), both significantly (P < .001) improved. Postintervention assessment measure of upper-extremity flexibility (42%, 0.41) showed significant (P < .001) improvements. Outcome measures for balance, FR (18%, 0.75) and single leg stance time (24%, 0.30), showed significant (P < .001) improvements. Conclusions. Outcome measures showed moderate to large ES improvements after participants completed the 12-week multimodal exercise program for breast cancer survivors.
Keywords: breast cancer survivors, exercise, physical fitness, physical function, community-based exercise
Introduction
Advances in medical diagnostics and treatment of cancer have progressed to the point where cancer may be considered a chronic disease. It is suggested that of all chronic diseases, cancer and its medical treatment may produce the most significant concern to those inflicted.1 Breast cancer and oncological treatment can result in significant acute and late localized and systemic effects on health-related physical fitness and physical function. Health-related physical fitness is generally recognized as having adequate cardiorespiratory fitness, muscular fitness, and flexibility—a bodily systems perspective. Physical function describes how individuals function when performing common daily activities. Health-related physical fitness parameters potentially impact physical function. When individual bodily systems related to health-related physical fitness are impaired, other bodily systems may compensate to sustain physical function, or physical function may be impaired.2 In breast cancer and oncological treatment, impairments in cardiorespiratory, musculoskeletal, and neurological systems have been identified.3 Impairments in these systems may affect breast cancer-related morbidity (eg, decreased functional mobility, muscular function, upper-extremity flexibility, and functional balance).
Whereas about one-third of health-related physical fitness is influenced by genetic factors,4 physical activity and physical exercise remain the predominant ways for people to increase their health-related physical fitness and physical function and, hence, the recommendations for cancer survivors to increase their physical activity and exercise participation.5
Research reveals that reductions in cardiorespiratory fitness increase risks for premature mortality in cancer survivor populations.6 Using a meta-analytic approach, researchers have identified a significant reduction in risk of total cancer mortality among individuals with high versus low cardiorespiratory fitness (RR = 0.55; 95% CI = 0.47-0.65).7 Additionally, research shows that reductions in muscular fitness are linked to increased morbidity8 and mortality in cancer survivor populations.9
Muscle tissue plays an important role in health and disease.10 Wolfe10 suggests that there is abundant evidence that muscle serves a vital role in many common pathological conditions and chronic diseases.10 High levels of muscular strength are associated with reduced cancer mortality in men.11 Reduced muscle mass (sarcopenia) is associated with an increased risk of overall mortality in breast cancer survivors and is reported to be associated with breast cancer–specific mortality.12 Additionally, it is reported that total mortality decreased 2% for every 1 kg increase in leg extension strength in community dwelling elderly.13 Decreased physical function is associated with decreased health-related physical fitness and increased breast cancer–related morbidity and mortality.14,15 Cancer survivors with higher levels of objective and patient-reported physical function are less likely to die prematurely than cancer survivors with lower levels of physical function.14,15
Physical function can be measured by objective performance-based outcome measures and by patient-reported outcome measures. Various performance-based outcome measures of physical function have been studied for associations to predict mortality.16,17 The Timed Up and Go (TUG) has been reported to predict 13.5-year mortality in elderly women.18 Additionally, the TUG has been reported as an objective prognostic indicator of early death for patients with cancer receiving chemotherapy.19 Another performance-based outcome measure, the 6-minute walk test (6MWT) is recognized as a surrogate indicator of physical functional capacity, mobility, and cardiorespiratory fitness in healthy20 and diseased populations, including those with cancer.21
Mobility is a common physical functional activity and requires adequate physical abilities to enable an individual to move about their environment safely for participation in society. In addition to adequate cardiorespiratory and musculoskeletal fitness, possessing sufficient balance is vital for safe ambulation. Impairments in body structure and function and functional mobility deficits have been linked to increased risk for falls. Furthermore, falls in older adults are a major public health concern.22 It is estimated that 1 in 3 adults older than 65 years will fall annually, and 50% of those will have repeated falls.23 Falls are more common in older adult cancer survivors than they are in the general population and are associated with risk factors unique to people with cancer.24 Patients who reported a fall in the month prior to receiving chemotherapy had increased risk of death compared with those who did not report a fall: hazard ratio (HR) = 3.2 (95% CI = 1.13-9.11).25 Therefore, it would seem reasonable that significant reductions in the aforementioned physiological requisites for mobility and balance would result in decreases in health-related physical fitness and physical function.
Because of the inseparable relationship between health-related physical fitness and physical function, exercise interventions have been utilized as countermeasures for many chronic and lifestyle diseases, including cancer. Exercise interventions have shown promise as an adjuvant therapy in the management of cancer-related morbidity and mortality.26-28 A meta-analysis study on association between physical activity and mortality in breast cancer by Zhong et al26 concluded that both prediagnosis and postdiagnosis physical activity were associated with reduced breast cancer–specific and all-cause mortality. In a systematic review and meta-analysis on the effects of exercise on breast cancer survivors, McNeely et al29 report that despite heterogeneity and relative small samples in breast cancer cohort studies, exercise is an effective intervention to improve cardiorespiratory fitness and physical function in breast cancer survivors. However, the preponderance of published research related to exercise as an intervention for cancer-related morbidity and mortality has rightfully primarily focused on clinical trials, whereas cancer homogeneity and specific oncological treatment were foremost in the research design. We believe that there is a great need for translational research into the effects of community-based exercise programming for cancer survivors. Because of the overall impact breast cancer and oncological treatment can have on health-related physical fitness and physical function, we have examined the effects of a community-based multimodal exercise program in breast cancer survivors.
Purpose
The purpose of this study was to examine the effects of a community-based multimodal exercise program on health-related physical fitness and physical function—mobility: (1) TUG and (2) 6MWT; muscular strength: (3) leg press strength and (4) chest press strength; upper-extremity flexibility: (5) back scratch test; and balance: (6) functional reach (FR) and (7) single-leg stance time—in breast cancer survivors. We hypothesized that postintervention measures of health-related physical fitness and physical function would be improved as compared with respective preintervention outcome measures.
Methods
Study Design
This single-arm pre-post study enrolled breast cancer survivors from 6 separate local Central Savanah River Area (CSRA) Young Men’s Christian Association (YMCA) sites over a 17-month period.
Participant Population
This study was approved by the Georgia Regents University Institutional Review Board and was conducted through a community partnership with the CSRA YMCAs. Breast cancer survivors registered and enrolled in the CSRA LIVESTRONG at the YMCAs classes were approached by the researchers for voluntary participation in this study. Inclusion criteria for this study were the following: (1) consenting adult breast cancer survivors, (2) signed physician approval for participation, and (3) breast cancer survivors regardless of treatment/recovery phase as long as they had signed physician approval for participation. Minors (<18 years of age) were excluded from participation in this study. Written informed consent was obtained from all participants. Physician signed medical approval for participation was also obtained prior to participation in this study. The physician approval form did not include any information regarding the type or intensity of exercises. The researchers were not aware if there were any potential participants who were not able to obtain physician approval for participation. Prior to program initiation, participants completed a separate intake form, which included a review of health conditions, cancer history and treatment, general description of functional abilities, current medications, past and present exercise participation, participant goals, and any concerns for participation in the program. Additionally, all participants completed individualized face-to-face intake interviews prior to participation in the community-based multimodal exercise program. During the individualized interviews, the completed intake forms were reviewed and participants were provided opportunity to have any questions or concerns answered.
Description of the Multimodal Exercise Program
The multimodal exercise program was a free, voluntary supervised program that met twice weekly for 90-minute exercise classes for 12 weeks. Each exercise class was divided into three 30-minute components: (1) aerobic conditioning, (2) resistance exercise training (RET), and (3) balance and flexibility training. All sessions were limited to a maximum of 10 participants and were supervised by 2 LIVESTRONG Foundation certified instructors who were also trained YMCA fitness instructors. A licensed physical therapist and certified lymphedema therapist (MPF) with several years of oncology rehabilitation experience assisted in the program. The LIVESTRONG Foundation initially provided training and materials for the CSRA YMCAs for program development. However, the multimodal exercise program was specifically developed by the CSRA YMCA Community Program Director and the researchers. Health-related physical fitness and physical functional assessments were completed prior to beginning the supervised multimodal exercise training program and again on completion of the 12-week program by the certified fitness instructors and a licensed physical therapist who also supervised all the preintervention and postintervention assessments.
Individualized exercise prescriptions were developed for each participant using American College of Sports Medicine exercise guidelines and position statements.5,30,31 Aerobic conditioning exercises initially (1-2 weeks) consisted of treadmill walking for 10 to 20 minutes at an intensity of 70% to 85% heart rate maximum and moderate to hard (3 to 5) rating of perceived exertion on the Borg Scale (0-10).32 The duration of aerobic conditioning exercises was progressed to 30 minutes for the remainder of the 12 weeks during which participants were encouraged to try other aerobic exercise machines with the instructors’ assistance (eg, cycle ergometer, elliptical trainer, and NuStep Recumbent Cross Trainer). The RET generally consisted of 1 to 2 sets of 8 to 12 repetitions at 60% to 70% of 1 repetition maximum (1 RM)30 for the major muscle groups. The RET was progressed by approximately 5% to 10% when participants were able to perform more than 12 repetitions for a given exercise.30 Balance and flexibility training mostly consisted of seated and standing static and dynamic balance exercises (eg, balance ball exercises, ball and balloon tosses, reaches, bends, dance exercises, and yoga poses) and stretching exercises. Deep diaphragmatic breathing techniques were also performed during the balance and flexibility training sessions. Participants began the program with the goal of 30 minutes of (1) aerobic conditioning, (2) 30 minutes of RET, and (3) 30 minutes of balance and flexibility exercises. However, not all participants were able to do this much exercise initially, and in such cases, they rested and exercised at lower intensities, usually building up to these levels over the first 2 weeks. Exercise prescription compliance was monitored by having a ratio of 5 participants to 1 certified exercise instructor. Participants were encouraged to follow their exercise prescriptions. In cases where the exercise intensity was not perceived as tolerable by the participant(s), they were given individualized attention and encouraged to work toward their intensity whenever possible. Participants were questioned and monitored throughout the exercise program for any signs and symptoms of exercise intolerance, and appropriate adjustments were made on an individualized basis.
Outcome Measures
Outcome Measures of Mobility
The TUG was performed according to the procedures originally described by Mathias et al.33 Test-retest reliability has been reported as high interclass correlation (ICC = 0.97) in elderly populations.34 The 6MWT was administered according to the original procedures described in the American Thoracic Society guidelines.35 High test-retest reliability (0.93) has been reported in patients with cancer.21
Outcome Measures for Muscular Strength
Lower- and upper-body strength were assessed by 1 RM using leg press and chest press machines. High test-retest reliability of 1 RM for leg press (0.99) and chest press (0.98) has been reported in untrained middle-aged individuals.36
Outcome Measure of Upper-Extremity Flexibility
The back scratch test was used to assess upper-extremity flexibility. The back scratch test was used rather than individual goniometric measurements for upper-extremity motions for several reasons: (1) participant convenience (less time compared with assessing a multitude of bilateral upper-extremity goniometric motions), (2) the certified fitness instructors were not trained in assessing goniometric measurements, and (3) the back scratch test is a functional test that encompasses upper-extremity motions that research37 indicates represent the greatest association with disability. The back scratch test was performed in the standing position by having participants reach over their head (arm external rotation and flexion) with their right hand and then reach downward (fingers extended and palm facing toward their back) along their back toward the left hand (fingers extended and facing away from their back while reaching upward toward the right hand), attempting to touch or overlap their fingers. The distance of overlap or space between the 2 middle fingers was measured as positive or negative, respectively. Participants performed this procedure twice, and the best (greatest overlap: +; or least gap: −) of the 2 measurements was utilized as right back scratch. Similarly, participants performed the back scratch again this time using the left hand for reaching over their head and the right hand reaching around their back. This test was identified as the left back scratch. Right- and left-upper extremity flexibility values were then averaged for respective pretraining and posttraining upper-extremity flexibility values. The intraclass correlation coefficient for the back scratch test in female patients with fibromyalgia has been reported to be 0.96.38
Outcome Measures of Balance
A FR was performed as an assessment of balance according to procedures outlined by Duncan et al.39 Excellent test-retest reliability has been reported for the FR in community-dwelling elderly (ICC = 0.92).39 Single-leg stance time was also used as a proxy measure of balance and was assessed by having each participant stand barefoot on a hard surface in a relaxed posture with their weight evenly distributed between their feet. Each participant then stood on their right leg, without using any assistance, for up to 60 s or until they placed their left foot back on the floor. Participants completed 2 trials, unless their first trial was 60 s. The best time of the 2 trials was identified as right single-leg stance time. Similarly, the single-leg stance was performed again standing on the left foot. Right and left single-leg stance times were then averaged for respective pretraining and posttraining single-leg stance times. Clinically, the single-leg stance shows good reproducibility and reliability (ICC = 0.95) in elderly populations.40
Data Analysis
Normality testing was performed on all the outcome measures using Kolmogrov-Smirnov with the Lillefors correction and the Shapiro-Wilk test at the P < .05 level. Bonferroni corrections were used for the outcome measures (7 total), resulting in P ≤.007 significance levels. All statistical analyses were performed using SPSS version 22.0 (Chicago, IL). All outcome measures were determined to be normally distributed; therefore, parametric testing using paired t tests for pre-post comparisons were performed. Null hypothesis testing was used for all outcome measures. Effect sizes (ESs) were calculated and interpreted according to procedures described by Cohen41: 0.2 to 0.50 = small to moderate; 0.51 to 0.80 = moderate to large; and >0.80 = large. Minimal clinically important differences (MCIDs) were calculated to procedures described by Albright et al.42 Minimal detectable changes at 90% CI (MDC90s) were calculated according to procedures described by Portney and Watkins.43
Results
Participant Characteristics
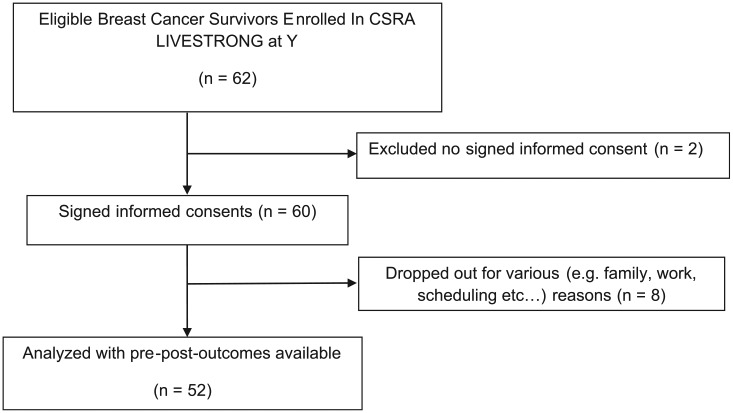
A diagram of study flow is provided in Figure 1. A total of 60 female breast cancer survivors signed informed consent for participation in this study, and 52 survivors completed the 12-week multimodal exercise program (86.7%). The most commonly reported reason for dropping was scheduling/employment conflicts and family conflicts. Average attendance for the participants over the 12-week program was 80.5%. Participant demographics and anthropometrics are presented in Table 1. All participants had breast cancer surgery; 85% received chemotherapy, 75% received radiation therapy, and 39.5% reported that they were taking hormonal deprivation therapy. Prior to participation, participants were asked about their current exercise activity. Participant exercise levels were later categorized as follows: 0 = no regular physical exercise; 1 = some exercise (<2 times per week); and 2 = exercise regularly (>3 times per week). Mean ± SD for exercise was 0.65 ± 0.82, and median was 0. Although weight loss was not an outcome measure for this study, mean and SD of weight loss were 1.47 ± 3.01 kg. We acknowledge that there may have been changes in lean muscle mass and fat mass and did not measure these. Participants were questioned and monitored throughout the exercise program for any signs and symptoms of exercise intolerance, and appropriate adjustments were made accordingly on an individualized basis. No significant adverse advents were reported.
Figure 1.
Study flow diagram.
Table 1.
Demographic and Anthropometric Measures of Breast Cancer Survivors.a
| Mean | SD | Range | n | |
|---|---|---|---|---|
| Age (years) | 59.7 | 10.4 | 46-82 | 52 |
| Weight (kg) | 81.0 | 2.5 | 76.7-127.1 | 44 |
| Height (cm) | 164 | 5 | 152-178 | 44 |
| BMI (kg/m2) | 30.11 | 0.93 | 28.5-47.2 | 44 |
| Resting heart rate (beats/min) | 76 | 9.4 | 60-100 | 44 |
| Resting systolic blood pressure (mm Hg) | 128 | 14.7 | 100-158 | 44 |
| Resting diastolic blood pressure (mm Hg) | 80 | 8.4 | 60-96 | 44 |
| Years since medical treatment | 4.96 | 6.3 | 0-24 | 52 |
Abbreviation: BMI, body mass index.
Medical treatment (surgery, chemotherapy, and or radiation therapy)
Health-Related Physical Fitness and Physical Function Outcome Measures
Results from pretraining and posttraining health-related physical fitness and physical function outcome measures are presented in Table 2. Both postintervention assessment measures of mobility, TUG (decrease 18%) and 6MWT (increase 14%), were significantly (P < .001) improved. Outcome measures of muscular strength, leg press (increase 32%) and chest press (increase 40%), both significantly (P < .001) improved. Postintervention assessment measure of upper-extremity flexibility (increase 42%) showed significant (P < .001) improvement. Outcome measures for balance, FR (increase 18%) and single-leg stance time (increase 24%), showed significant (P < .001) improvements. Moderate to large ESs were found for mobility and muscular strength (Table 3), and small to moderate ESs for upper-extremity flexibility were found. ESs for balance ranged from moderate to large for FR and small to moderate for single-leg stance. Improvement in TUG and muscular strength were greater than the respective MCIDs and MDC90s. The mean difference in upper-extremity flexibility was greater than the MCID. The ES improvement in FR was greater than the ES for single-leg stance, and the improvement in FR was greater than both the MCID and MDC90.
Table 2.
Health-Related Physical Fitness and Physical Function Outcome Measures.
| Mean | SD | 95% CI | Range | n | P Value (2-tailed) | |
|---|---|---|---|---|---|---|
| Mobility | ||||||
| Preintervention TUG (s) | 8.05 | 2.39 | 7.4 to 8.7 | 4.7 to 15.7 | 49 | .001,a t = 6.53 |
| Postintervention TUG (s) | 6.64 | 2.08 | 6.0 to 7.2 | 3.9 to 13.5 | 49 | |
| Preintervention 6MWT (m) | 416.7 | 81.11 | 392.3 to 441.1 | 392.3 to 441.0 | 45 | .001,a t = 34.46 |
| Postintervention 6MWT (m) | 476.7 | 97.39 | 447.4 to 505.9 | 447.4 to 505.9 | 45 | |
| Muscular strength | ||||||
| Preintervention leg press (kg) | 60.3 | 33.5 | 50.5 to 70.2 | 6.8 to 139.5 | 47 | .001,a t = −7.53 |
| Postintervention leg press (kg) | 79.6 | 34.7 | 69.5 to 89.8 | 15.8 to 144.0 | 47 | |
| Preintervention chest press (kg) | 18.8 | 12.4 | 15.1 to 22.4 | 0.0 to 45.0 | 47 | .001,a t = −6.63 |
| Postintervention chest press (kg) | 26.4 | 12.4 | 22.8 to 30.1 | 2.2 to 47.2 | 47 | |
| Upper-extremity flexibility | ||||||
| Preintervention back scratch (cm) | −11.4 | 11.7 | −14.9 to −8.0 | −36.8 to 16.5 | 47 | .001,a t = −3.54 |
| Postintervention back scratch (cm) | −6.6 | 11.4 | −10.0 to −3.2 | −31.75 to 22.9 | 47 | |
| Balance | ||||||
| Preintervention FR (cm) | 29.7 | 7.4 | 27.2 to 32.1 | 12.7 to 50.8 | 38 | .001,a t = 4.07 |
| Postintervention FR (cm) | 35.2 | 7.6 | 32.7 to 37.7 | 20.3 to 50.8 | 38 | |
| Preintervention single-leg stance (seconds) | 26.8 | 20.9 | 20.7 to 32.0 | 2.0 to 60 | 47 | .001,a t = −3.58 |
| Postintervention single-leg stance (seconds) | 33.1 | 21.3 | 26.8 to 39.3 | 1.75 to 60 | 47 | |
Abbreviations: TUG, Timed Up and Go; 6MWT, 6-minute walk test; FR, functional reach.
P ≤ .001.
Table 3.
Clinimetric Data for Health Related Physical Fitness and Physical Function Outcome Measures.
| Mean Difference | 95% CI | Effect Size | MCID | MDC90 | |
|---|---|---|---|---|---|
| Mobility | |||||
| TUG (s) | 1.41 | 1.0-1.8 | 0.59 | 1.21 | 0.96a |
| 6MWT (m) | 60.0 | 41.2-78.8 | 0.74 | 62.5 | 49.06b |
| Muscular strength | |||||
| Leg press (kg) | 19.3 | 14.17-24.5 | 0.58 | 9.12 | 7.86c |
| Chest press (kg) | 7.7 | 5.3-9.9 | 0.61 | 2.84 | 4.07d |
| Upper-extremity flexibility | |||||
| Average back scratch (cm) | 4.8 | 0.83-3.0 | 0.41 | 0.68 | 1.52e |
| Balance | |||||
| Functional reach (cm) | 5.58 | 2.8-8.4 | 0.75 | 4.45 | 2.44f |
| Single-leg stance (s) | 6.3 | 2.7-9.8 | 0.30 | 4.02 | 7.59g |
Discussion
The main finding in this study is that outcome measures of health-related physical fitness and physical function (mobility, muscular strength, upper-extremity flexibility, and balance) related to breast cancer morbidity were significantly (P < .001) improved after participants completed the 12-week community-based multimodal exercise program. Direct comparison of the present study with other published research on the effects of exercise training on patients with cancer is somewhat difficult because of variations in cancer oncotypes, oncological treatments, timing of exercise, and the specifics of the exercise regimes. Therefore, our discussion primarily focuses on comparisons of health-related physical fitness and physical function outcome with the published age-gender-related normative data, with emphasis on breast cancer morbidity and mortality.
In a recently published systematic review44 examining physical function for women diagnosed with breast cancer, it was reported that the TUG was used to evaluate mobility in 2 included studies. However, it is noted that the 2 included studies utilized distances for the TUG—8 feet and 3 m. Nevertheless, the reported TUG times were slower for the breast cancer groups as compared with age-gender normative values.44 In relating the present outcome measures of health-related physical fitness and physical function with mortality, research18 has shown that TUG performance and FR reach predicted 13.5-year mortality in elderly women. Of the 300 randomly selected community-dwelling women (mean age = 81 years at baseline), 71% died. Idland et al18 reported significant (P < .001) differences in mean (±SD) TUG scores for those who survived (6.6 ± 1.3 s) as compared with those who died (8.3 ± 3.3 s). In the present study, preintervention and postintervention TUG scores were 8.0 ± 2.4 s and postintervention to 6.6 ± 2.1 seconds, respectively. We believe that comparison of the present TUG scores with those reported by Idland et al18 could be suggestive that it is possible for these patients to achieve profiles more similar to those survivors than to those of individuals who die. Likewise, comparison of FR scores reported by Idland et al18 in the group that survived (28.9 ± 6.0 cm) and the group that died (24.9 ± 6.9 cm) with the present findings—preintervention FR (29.7 ± 7.4 cm) and postintervention FR (35.2 ± 7.6 cm)—showed a similar trend.
The 6MWT is sometimes used as a surrogate indicator of aerobic fitness. In the previously cited systematic review,44 women diagnosed with breast cancer have reduced aerobic fitness. Comparison of the present 6MWT and 6MWT for community-residing older adults45 revealed that the present breast cancer survivors’ (mean age = 59.7 years) preintervention 6MWT (416.7 ± 81.1 m) was closest to the 6MWT (422.3 ± 107 m) of 80- to 84-year-old women.45 Additional comparison of the present 6MWT with age-gender-related scores published in another study34 showed similar results, in that the present breast cancer survivors’ preintervention 6MWT was closest to that of 80- to 90-year-old women. Additionally, the postintervention 6MWT (476.7 ± 97.4 m) in this study was closest to the scores in the 70- to 80-year-old range (471 ± 75 m) reported by Steffen et al,34 exemplifying what might be construed as a 10-year age-related improvement.
In an 85-year-old community-dwelling population, decreased leg extension strength was associated with a significantly (P = .015) increased mortality hazard ratio (HR = 0.962; CI = 0.929-1.001).13 To compare the preintervention and postintervention leg strength values with published46 age and gender values, we converted the leg press values to ratios of weight pushed divided by body weight. The preintervention leg press strength ratio was 0.74, which corresponded to the well-below average category (10th percentile value = 0.78). The present postintervention leg press strength ratio was 1.00, which corresponded to average (50th percentile value = 1.05). In the present postintervention assessment, lower-extremity muscle strength was significantly increased (P < .001) and showed moderate to large ES.
Research37 reveals that breast cancer survivors have reduced upper-extremity function (eg, patient-reported upper-extremity function and decreased upper-extremity range of motion and strength). Harrington et al37 reported that deficits in active range of motion and shoulder strength represented the greatest percentage of shoulder disability after breast cancer treatments. Notably, Harrington et al37 report that flexion and external rotation have the greatest relationships to shoulder disability. Although, in the present study, we did not specifically measure upper-extremity range of motion with goniometry, we assessed upper extremity flexibility by administering a bilateral back scratch test, which requires shoulder external rotation and flexion in the ipsilateral upper extremity reaching over the back and internal rotation and extension in the contralateral upper extremity. Comparison of the present preintervention back scratch scores (−11.5 ± 12.6 cm) with those published by Rikli47 revealed that these breast cancer survivors had comparable back scratch scores between the female age ranges of 75 to 79 years (−12.7 to 1.27 cm) and 70 to 74 years (−10.16 to 2.54 cm). Comparison of the present postintervention back scratch scores (−6.6 ± 11.4 cm) with the youngest available range (60-64 years of age = −7.62 to 3.81 cm) by Rikli47 shows age normalization of the present postintervention back scratch scores. The preintervention and postintervention chest press strength values were divided by postintervention body weight to convert to chest press by body weight ratio for comparison with published46 age-gender values. The present preintervention chest press ratio was 0.23, which corresponded to the very poor category (<first percentile). The postintervention chest press ratio was 0.33 and corresponded to the very poor category (approximately seventh percentile). Although there was significant improvement in postintervention chest press strength, the aforementioned comparison with upper-body strength ratio to age- and gender-related upper-body strength ratios suggests that these breast cancer survivors still show very poor upper-body strength.
It is estimated that approximately one-third of community-dwelling elderly adults fall each year.48 In a recent systematic review of falls in older adults,24 it was reported that falls in older adults with cancer are 16% to 17% higher than in older adults in the general population. The etiology of falls is multifactorial; however, balance likely plays a key role in fall risk. In the current study, postintervention assessments of balance (FR and single-leg stance time) were significantly (P < .001) improved. We compared the preintervention FR scores (29.7 cm) with published39 age- and gender-related normative values and found that the preintervention FR score was between the 41 to 69 years age range (35.1 cm) and the 70 to 87 years age range (26.6 cm). Therefore, for comparative purposes, we calculated 95% CIs for the age- and gender-related FR scores39 for both the 41 to 69 (33.0-37.1 cm) and 70 to 87 (21.9-31.3 cm) years range. In the present study, the 95% CI for preintervention FR (27.2-32.1) was closest to the 70 to 87 years range. This suggests that the preintervention FR scores for the present breast cancer survivors (mean age = 59.7 years) may indicate decreased age-related balance. The present postintervention FR (32.7-37.7) was closest to the 41 to 69 year range, suggestive of more appropriate age-related balance.
Study Strengths
We believe that this pilot study provides relevant translational findings for supporting community-based multimodal exercise training, including aerobic conditioning, RET, and balance and flexibility training for breast cancer survivors. Additionally, we also believe that the outcome measures utilized in this study provide a basis for assessment of health-related physical fitness and physical function that are relevant to breast cancer morbidity. Finally, we think that providing ESs, MCIDs, and MDCs for the outcome measures is also a strength that provides other researchers the opportunity to calculate sample size and perform power analyses.
Study Limitations
In this single-arm study, no control group was utilized for comparison, resulting in time being the independent variable. Therefore, definitive conclusions cannot be stated as to whether the improvements in health-related physical fitness and physical function made in this study were a direct result of the community-based multimodal exercise program or if time was a factor. A randomized controlled design would help elucidate this limitation. However, we believe that despite this limitation, the breast cancer survivors showed decreases in breast cancer–related dysfunction. Power size calculations were not performed because there was no known ES given the type of study conducted and that the sample was based in a community survivor exercise program.
Conclusions
Outcome measures of health-related physical fitness and physical function showed moderate to large ES improvements after participants completed the 12-week community-based multimodal exercise program. We hope that this study will help provide additional support for community-based multimodal exercise programming for breast cancer survivors as well as suggesting relevant readily available outcome measures for measuring program effectiveness.
Footnotes
Authors’ Note: All authors disclose no potential conflicts of interest. This study was approved by an institutional review board in accordance to ethical treatment of human subjects as laid down in the 1964 Helsinki Declaration. All participants signed written informed consents.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Garland SN, Tamagawa R, Todd SC, Speca M, Carlson LE. Increased mindfulness is related to improved stress and mood following participation in a mindfulness-based stress reduction program in individuals with cancer. Integr Cancer Ther. 2013;12:31-40. [DOI] [PubMed] [Google Scholar]
- 2. Ferrucci L, Bandinelli S, Benvenuti E, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618-1625. [DOI] [PubMed] [Google Scholar]
- 3. Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10:598-605. [DOI] [PubMed] [Google Scholar]
- 4. Wei M, Kampert JB, Barlow CE, et al. Relationship between low cardiorespiratory fitness and mortality in normal-weight, overweight, and obese men. JAMA. 1999;282:1547-1553. [DOI] [PubMed] [Google Scholar]
- 5. Schmitz KH, Courneya KS, Matthews C, et al. American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409-1426. [DOI] [PubMed] [Google Scholar]
- 6. Sanchis-Gomar F, Lucia A, Yvert T, et al. Physical inactivity and low fitness deserve more attention to alter cancer risk and prognosis. Cancer Prev Res (Phila). 2015;8:105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schmid D, Leitzmann MF. Television viewing and time spent sedentary in relation to cancer risk: a meta-analysis. J Natl Cancer Inst. 2014;106(7). [DOI] [PubMed] [Google Scholar]
- 8. Winters-Stone KM, Dobek JC, Bennett JA, et al. Resistance training reduces disability in prostate cancer survivors on androgen deprivation therapy: evidence from a randomized controlled trial. Arch Phys Med Rehabil. 2015;96:7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christensen JF, Jones LW, Andersen JL, Daugaard G, Rorth M, Hojman P. Muscle dysfunction in cancer patients. Ann Oncol. 2014;25:947-958. [DOI] [PubMed] [Google Scholar]
- 10. Wolfe RR. The underappreciated role of muscle in health and disease. Am J Clin Nutr. 2006;84:475-482. [DOI] [PubMed] [Google Scholar]
- 11. Ruiz JR, Sui X, Lobelo F, et al. Muscular strength and adiposity as predictors of adulthood cancer mortality in men. Cancer Epidemiol Biomarkers Prev. 2009;18:1468-1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villasenor A, Ballard-Barbash R, Baumgartner K, et al. Prevalence and prognostic effect of sarcopenia in breast cancer survivors: the HEAL Study. J Cancer Surviv. 2012;6:398-406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Takata Y, Ansai T, Soh I, et al. Physical fitness and 6.5-year mortality in an 85-year-old community-dwelling population. Arch Gerontol Geriatr. 2012;54:28-33. [DOI] [PubMed] [Google Scholar]
- 14. Braithwaite D, Satariano WA, Sternfeld B, et al. Long-term prognostic role of functional limitations among women with breast cancer. J Natl Cancer Inst. 2010;102:1468-1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brown JC, Harhay MO, Harhay MN. Physical function as a prognostic biomarker among cancer survivors. Br J Cancer. 2015;112:194-198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cesari M, Onder G, Zamboni V, et al. Physical function and self-rated health status as predictors of mortality: results from longitudinal analysis in the ilSIRENTE study. BMC Geriatr. 2008;8:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cooper R, Kuh D, Hardy R. Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ. 2010;341:c4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Idland G, Engedal K, Bergland A. Physical performance and 13.5-year mortality in elderly women. Scand J Public Health. 2013;41:102-108. [DOI] [PubMed] [Google Scholar]
- 19. Soubeyran P, Fonck M, Blanc-Bisson C, et al. Predictors of early death risk in older patients treated with first-line chemotherapy for cancer. J Clin Oncol. 2012;30:1829-1834. [DOI] [PubMed] [Google Scholar]
- 20. Casanova C, Celli BR, Barria P, et al. The 6-min walk distance in healthy subjects: reference standards from seven countries. Eur Respir J. 2011;37:150-156. [DOI] [PubMed] [Google Scholar]
- 21. Schmidt K, Vogt L, Thiel C, Jager E, Banzer W. Validity of the six-minute walk test in cancer patients. Int J Sports Med. 2013;34:631-636. [DOI] [PubMed] [Google Scholar]
- 22. Avin KG, Hanke TA, Kirk-Sanchez N, et al. Management of falls in community-dwelling older adults: a clinical guidance statement from the Academy of Geriatric Physical Therapy of the American Physical Therapy Association. Phys Ther. 2015;95:815-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moreland J, Richardson J, Chan D, et al. Evidence-based guidelines for the secondary prevention of falls in older adults. Gerontology. 2003;49:93-116. [DOI] [PubMed] [Google Scholar]
- 24. Wildes TM, Dua P, Fowler SA, et al. Systematic review of falls in older adults with cancer. J Geriatr Oncol. 2015;6:70-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wildes TM, Ruwe AP, Fournier C, et al. Geriatric assessment is associated with completion of chemotherapy, toxicity, and survival in older adults with cancer. J Geriatr Oncol. 2013;4:227-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhong S, Jiang T, Ma T, et al. Association between physical activity and mortality in breast cancer: a meta-analysis of cohort studies. Eur J Epidemiol. 2014;29:391-404. [DOI] [PubMed] [Google Scholar]
- 27. Santa Mina D, Alibhai SM, Matthew AG, et al. Exercise in clinical cancer care: a call to action and program development description. Curr Oncol. 2012;19:e136-e144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clague J, Bernstein L. Physical activity and cancer. Curr Oncol Rep. 2012;14:550-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McNeely ML, Campbell KL, Rowe BH, Klassen TP, Mackey JR, Courneya KS. Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. CMAJ. 2006;175:34-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kraemer WJ, Adams K, Cafarelli E, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34:364-380. [DOI] [PubMed] [Google Scholar]
- 31. Garber CE, Blissmer B, Deschenes MR, et al. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: guidance for prescribing exercise. Med Sci Sports Exerc. 2011;43:1334-1359. [DOI] [PubMed] [Google Scholar]
- 32. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377-381. [PubMed] [Google Scholar]
- 33. Mathias S, Nayak US, Isaacs B. Balance in elderly patients: the “get-up and go” test. Arch Phys Med Rehabil. 1986;67:387-389. [PubMed] [Google Scholar]
- 34. Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: Six-Minute Walk Test, Berg Balance Scale, Timed Up & Go Test, and gait speeds. Phys Ther. 2002;82:128-137. [DOI] [PubMed] [Google Scholar]
- 35. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111-117. [DOI] [PubMed] [Google Scholar]
- 36. Levinger I, Goodman C, Hare DL, Jerums G, Toia D, Selig S. The reliability of the 1RM strength test for untrained middle-aged individuals. J Sci Med Sport. 2009;12:310-316. [DOI] [PubMed] [Google Scholar]
- 37. Harrington S, Padua D, Battaglini C, Michener LA. Upper extremity strength and range of motion and their relationship to function in breast cancer survivors. Physiother Theory Pract. 2013;29:513-520. [DOI] [PubMed] [Google Scholar]
- 38. Carbonell-Baeza A, Alvarez-Gallardo IC, Segura-Jimenez V, et al. Reliability and feasibility of physical fitness tests in female fibromyalgia patients. Int J Sports Med. 2015;36:157-162. [DOI] [PubMed] [Google Scholar]
- 39. Duncan PW, Weiner DK, Chandler J, Studenski S. Functional reach: a new clinical measure of balance. J Gerontol. 1990;45:M192-M197. [DOI] [PubMed] [Google Scholar]
- 40. Takacs J, Garland SJ, Carpenter MG, Hunt MA. Validity and reliability of the community balance and mobility scale in individuals with knee osteoarthritis. Phys Ther. 2014;94:866-874.24557649 [Google Scholar]
- 41. Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum; 1988. [Google Scholar]
- 42. Albright J, Allman R, Bonfiglio RP, et al. Philadelphia Panel evidence-based clinical practice guidelines on selected rehabilitation interventions: overview and methodology. Phys Ther. 2001;81:1629-1640. [PubMed] [Google Scholar]
- 43. Portney LG, Watkins MP. Foundations of Clinical Research: Applications to Practice. 3rd ed. Upper Saddle River, NJ: Pearson Prentice Hall; 2009. [Google Scholar]
- 44. Neil-Sztramko SE, Kirkham AA, Hung SH, Niksirat N, Nishikawa K, Campbell KL. Aerobic capacity and upper limb strength are reduced in women diagnosed with breast cancer: a systematic review. J Physiother. 2014;60:189-200. [DOI] [PubMed] [Google Scholar]
- 45. Rikli R, Jones CJ. Development and validation of a functional fitness test for community-residing older adults. J Aging Phys Act. 1999;7:129-161. [Google Scholar]
- 46. Thompson PD, Arena R, Riebe D, Pescatello LS. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr Sports Med Rep. 2013;12:215-217. [DOI] [PubMed] [Google Scholar]
- 47. Rikli RJJ. Functional fitness normative scores for community-residing older adults, ages 60-94. J Aging Phys Act. 1999;7:162-181. [Google Scholar]
- 48. Rubenstein LZ, Josephson KR. The epidemiology of falls and syncope. Clin Geriatr Med. 2002;18:141-158. [DOI] [PubMed] [Google Scholar]