Introduction
Pyoderma gangrenosum (PG) is a neutrophilic dermatitis, the prevalence of which is unknown. The only reported incidence in the literature estimates that there are 3 to 10 cases per million annually in the United Kingdom.1 The association with digestive tract and inflammatory rheumatic disease, neoplasia, and endocrinopathies is well known.2
New autoinflammatory syndromes with PG have been described: PAPA syndrome, combining PG with pyogenic sterile arthritis and cystic acne; PASH syndrome, combining PG with cystic acne and hidradenitis suppurativa (HS); and PAPASH syndrome, combining PG with pyogenic sterile arthritis, acne, and HS.3
PAPA and PAPASH syndromes arise from mutations in the coding region of the proline-serine-threonine-phosphatase interacting protein 1 gene (PSTPIP1) resulting in the loss of inhibitory effect on the NALP3 inflammasome with production of interleukin (IL)-1β. For PASH syndrome, the only known anomaly is an increase in the number of CCTG repetitions in the PSTPIP1 promoter, with no known functional impact.4
First-line treatment for PG is generally based on systemic corticosteroid therapy or antibiotics with an anti-inflammatory action (eg, dapsone and tetracyclines) or immunosuppressive drugs (eg, azathioprine, cyclosporine, and mycophenolate mofetil). Also introduced recently are anti–tumor necrosis factor-α agents, anti–IL-1 (anakinra), and finally anti–IL-12–IL-23 (ustekinumab) and anti–IL-17 (ixekizumab).
Studies investigating PASH syndrome found that cyclosporine and dapsone and biotherapy using anti–tumor necrosis factor-α5 and anti–IL-1 (anakinra)4 are effective. The clinical course is, however, marked by the risk of repeated relapses and resistance to conventional treatments for PG. Furthermore, any proposed therapeutic strategy should be effective against all 3 entities (ie, PG, HS, and cystic acne).
Case report
We report the case of a 59-year-old man with PASH syndrome without a PSTPIP1 mutation. The patient had cystic acne since adolescence and HS since the age of 22. In 2013, he noted the appearance of an ulcer on the lateral aspect of his right leg that developed rapidly, progressively, and spontaneously. Initially, the lesion was papulopustular, rapidly becoming a deep painful ulcer with a raised, irregular, and violet-colored border (Fig 1, A). Vascular Doppler ultrasound scan failed to provide any evidence suggestive of a vascular ulcer, and the clinical appearance was not in favor of hypertensive leg ulcers.
Fig 1.
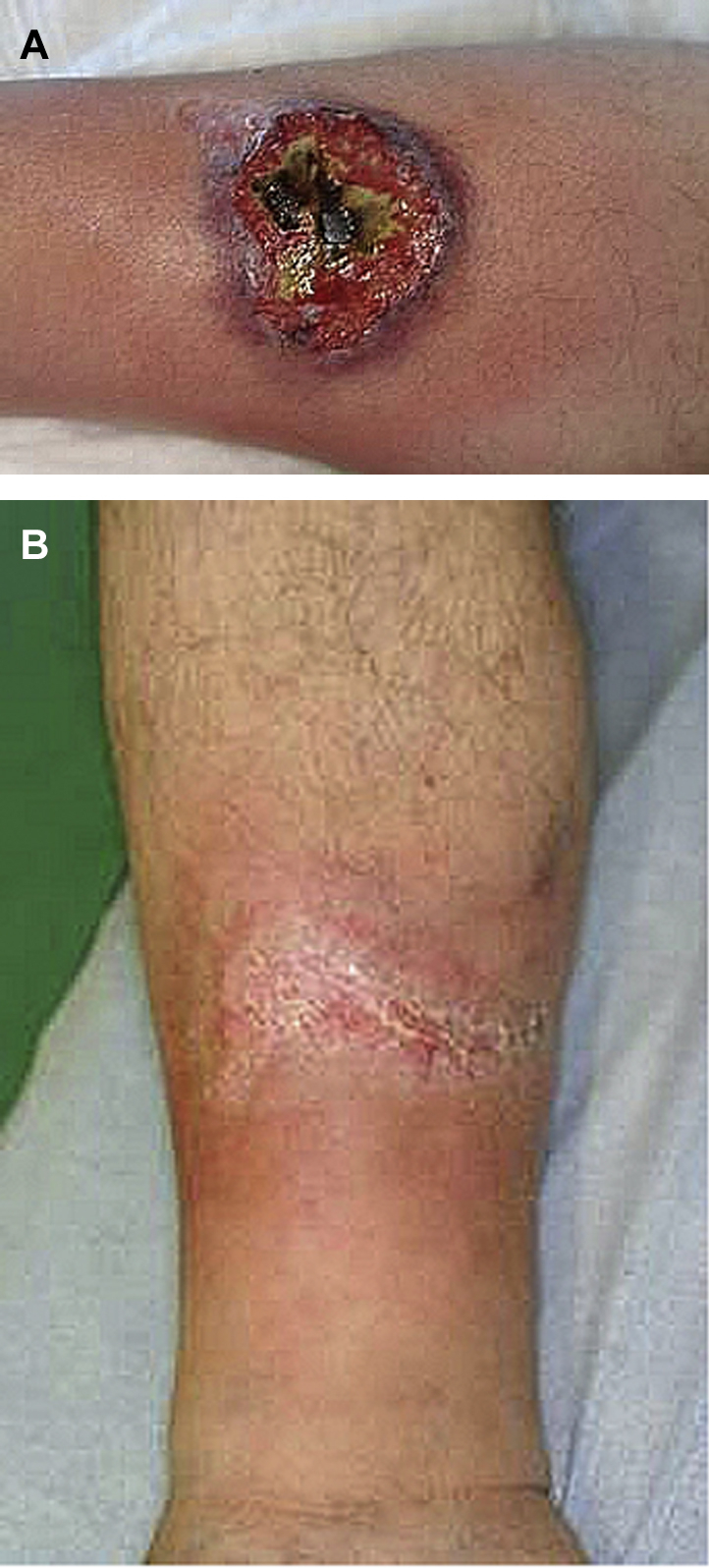
A, PG of the right leg. B, PG has completely healed after antibiotic therapy.
Histopathology reported neutrophilic infiltration affecting the dermis and to a lesser extent the epidermis with no vasculitis or thrombosis. No pathogens were identified. These characteristics were compatible with the diagnosis of PG.
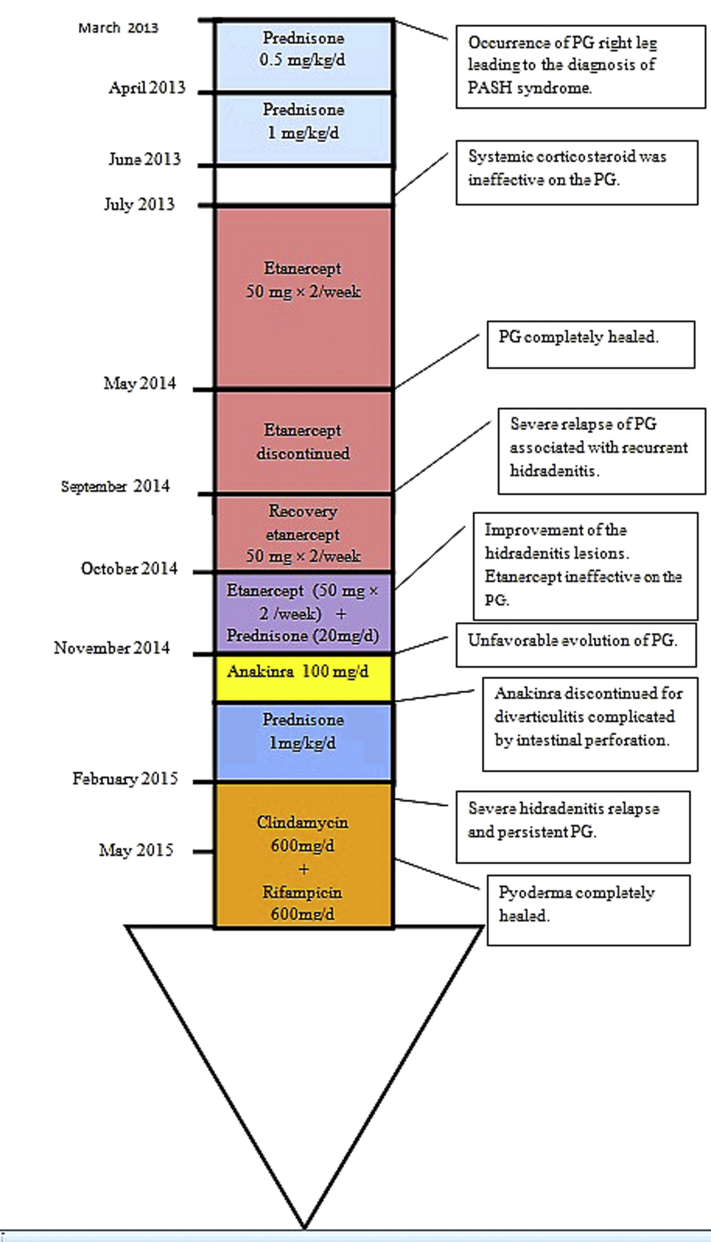
The patient was given prednisone, 0.5 mg/kg/d, in March and April 2013 with no effect, even with an increased dose, 1 mg/kg/d, from April to June. The patient was then switched to etanercept, 50 mg, with 2 subcutaneous injections per week from July 2013 to March 2014. The PG healed within 10 months, allowing complete treatment withdrawal in May 2014. Six months later, the patient presented with a severe relapse of PG associated with recurrent HS lesions. Etanercept was reintroduced at the same dose. The HS skin lesions improved at 1 month, but there was no effect on the PG. An alternative strategy using anakinra (100 mg/d) was tried but had to be discontinued 15 days after introduction because of sigmoid diverticulitis and a benign exanthematous drug reaction. Prednisone, 1 mg/kg/d was introduced again but quickly discontinued because of a gastric ulcer with hemorrhagic shock. An oral antibiotic regimen with rifampicin, 600 mg/d and clindamycin, 600 mg/d was started after a severe relapse of HS. This treatment was followed by marked improvement in his HS and PG. Wound care with hydrocellular dressings (Biatain Silicone, Coloplast Laboratory, Rosny sous Bois, France) and local corticosteroids (Diprosone, MSD France, Courbevoie, France) was also given to partially improve the pain. Complete healing of the PG was achieved in less than 3 months (Fig 1, B) without recurrence at 22 months. His HS healed 11 months after antibiotic treatment onset (Fig 2). Use of rifampicin and clindamycin was discontinued in June 2016 (after 12 months of treatment; Fig 3).
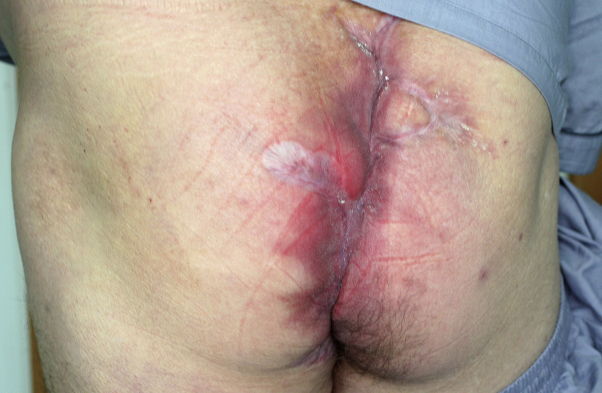
Fig 2.
Completely healed HS lesions.
Fig 3.
Timeline of interventions and outcomes.
Discussion
This case illustrates an excellent response of this neutrophilic dermatosis to a rifampicin-clindamycin combination despite the failure of 2 biotherapy regimens. Recently, Join-Lambert et al6 reported a case series of PG remissions in 4 patients with PASH syndrome who were treated by prolonged antibiotic therapy targeting the microbiota found in hidradenitis skin lesions. This protocol was effective on the PG skin lesions in 3 of 4 patients.6 Very few cases of successful antibiotic combinations for refractory PG have been reported, especially in the context of PASH.6, 7
Recently, a mutation of the gene NCSTN was described in PASH syndrome.8 This mutation is common with HS. It can lead to a loss of γ-secretase function,9 which might be the cause of the widespread follicle obstruction. Follicular occlusion, in the presence of a specific skin microbiota, might be the cause of chronic inflammation.9
Several clinical studies have shown the effectiveness of antibiotic therapy using the rifampicin-clindamycin combination in HS.10 Compared with regimens for acute infectious diseases, both antibiotics were given at a relatively low dose for a long period. Clindamycin and rifampicin both have antibacterial and anti-inflammatory effects. The exact mechanism of the beneficial effect of clindamycin and rifampicin is not well known. HS can be considered an inflammatory disease often complicated by bacterial infections, which would explain the effectiveness of antibiotics via their antibacterial and anti-inflammatory effects.11
Rifampicin is a broad-spectrum antibiotic highly effective and bactericidal against Staphylococcus aureus and negative-coagulase Staphylococcus but also against other gram-positive bacteria such as Streptococcus and gram-negative bacteria, especially anaerobes, Haemophilus influenzae, Neisseria gonorrhoeae, Neisseria meningitidis, and some mycobacterium species. Clindamycin is active against gram-negative bacilli and anaerobic bacteria. It is used in combination to prevent the emergence of bacterial resistance against rifampicin.10 Furthermore, rifampicin could have an anti-inflammatory effect inhibiting neutrophil function and the synthesis of chemokines and cytokines by CD4+ T cells.12
The study by Join-Lambert et al13 suggests that there is a significant pharmacokinetic interaction between rifampicin and clindamycin. Rifampicin is a major inducer of cytochrome P450 3A4, the main metabolic pathway of clindamycin, causing a decrease in its plasma concentration. There is a risk that bacterial resistance could emerge when using the rifampicin-clindamycin combination. If continued over a long period, antibiotic blood levels must be monitored.
The hypothesis proposed to explain the effectiveness of antibiotics on PG is the same as that for HS. There would be a dysregulation of the host microbiota, with replacement of commensal bacteria by pathogenic bacteria, inducing an inappropriate inflammatory reaction responsible for the symptoms.6, 11 The effectiveness of a prolonged and targeted antibiotic therapy would therefore have an action on the skin microbiota and on the inappropriate inflammatory response. The other argument put forward to explain the effectiveness of antibiotic therapy by rifampicin-clindamycin in this case of neutrophilic dermatosis is the anti-inflammatory effect of rifampicin, potentially targeting polymorphonuclear neutrophils.12 Despite better tolerance compared with immunosuppressants and biotherapy, antibiotics do have their digestive, cutaneous, and hematologic side effects. In addition, the risk of bacterial resistance implies precautious use. Antibiotics can be unsuccessful in some cases of PG. Our hypothesis is that there are several subtypes of PG for which treatment effectiveness would depend on the underlying condition. In the case of PASH syndrome, the occurrence of PG might be caused by dysfunctional skin microbiota triggering an inflammatory reaction seen as HS and acne, explaining the effectiveness of the rifampicin-clindamycin regimen.
Prospective studies examining the effectiveness of this combination regimen on PG depending on the context (PG as part of versus independent of PASH syndrome) would be needed to establish the expected therapeutic response by origin of the PG.
Footnotes
Funding sources: None.
Conflicts of interest: None declared.
References
- 1.Langan S.M., Groves R.W., Card T.R., Gulliford M.C. Incidence, mortality, and disease associations of pyoderma gangrenosum in the United Kingdom: a retrospective cohort study. J Invest Dermatol. 2012;132(9):2166–2170. doi: 10.1038/jid.2012.130. [DOI] [PubMed] [Google Scholar]
- 2.Modiano P. Developments in pyoderma gangrenosum therapy in 2015. Ann Dermatol Venereol. 2015;142(8-9):502–505. doi: 10.1016/j.annder.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Staub J., Pfannschmidt N., Strohal R. Successful treatment of PASH syndrome with infliximab, cyclosporine and dapsone. J Eur Acad Dermatol Venereol JEADV. 2015;29(11):2243–2247. doi: 10.1111/jdv.12765. [DOI] [PubMed] [Google Scholar]
- 4.Braun-Falco M., Kovnerystyy O., Lohse P., Ruzicka T. Pyoderma gangrenosum, acne, and suppurative hidradenitis (PASH)–a new autoinflammatory syndrome distinct from PAPA syndrome. J Am Acad Dermatol. 2012;66(3):409–415. doi: 10.1016/j.jaad.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 5.Campanati A., Brisigotti V., Ganzetti G. Finally, recurrent pyoderma gangrenosum treated with Adalimumab: case report and review of the literature. J Eur Acad Dermatol Venereol JEADV. 2015;29(6):1245–1247. doi: 10.1111/jdv.12703. [DOI] [PubMed] [Google Scholar]
- 6.Join-Lambert O., Duchatelet S., Delage M. Remission of refractory pyoderma gangrenosum, severe acne, and hidradenitis suppurativa (PASH) syndrome using targeted antibiotic therapy in 4 patients. J Am Acad Dermatol. 2015;73(5 Suppl 1):S66–S69. doi: 10.1016/j.jaad.2015.07.040. [DOI] [PubMed] [Google Scholar]
- 7.Goldberg N.D., Vadlamudi A., Parrish N. Treatment of refractory Crohn's disease and pyoderma gangrenosum with a combination regimen of rifaximin, gentamicin and metronidazole. Case Rep Gastroenterol. 2015;9(1):25–28. doi: 10.1159/000369965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duchatelet S., Miskinyte S., Join-Lambert O. First nicastrin mutation in PASH (pyoderma gangrenosum, acne and suppurative hidradenitis) syndrome. Br J Dermatol. 2015;173(2):610–612. doi: 10.1111/bjd.13668. [DOI] [PubMed] [Google Scholar]
- 9.Pink A.E., Simpson M.A., Desai N., Trembath R.C., Barker J.N.W. γ-Secretase mutations in hidradenitis suppurativa: new insights into disease pathogenesis. J Invest Dermatol. 2013;133(3):601–607. doi: 10.1038/jid.2012.372. [DOI] [PubMed] [Google Scholar]
- 10.Gener G., Canoui-Poitrine F., Revuz J.E. Combination therapy with clindamycin and rifampicin for hidradenitis suppurativa: a series of 116 consecutive patients. Dermatol Basel Switz. 2009;219(2):148–154. doi: 10.1159/000228334. [DOI] [PubMed] [Google Scholar]
- 11.Kathju S., Lasko L.-A., Stoodley P. Considering hidradenitis suppurativa as a bacterial biofilm disease. FEMS Immunol Med Microbiol. 2012;65(2):385–389. doi: 10.1111/j.1574-695X.2012.00946.x. [DOI] [PubMed] [Google Scholar]
- 12.Spisani S., Traniello S., Martuccio C., Rizzuti O., Cellai L. Rifamycins inhibit human neutrophil functions: new derivatives with potential antiinflammatory activity. Inflamm. 1997;21(4):391–400. doi: 10.1023/a:1027314419843. [DOI] [PubMed] [Google Scholar]
- 13.Join-Lambert O., Ribadeau-Dumas F., Jullien V. Dramatic reduction of clindamycin plasma concentration in hidradenitis suppurativa patients treated with the rifampin-clindamycin combination. Eur J Dermatol. 2014;24(1):94–95. doi: 10.1684/ejd.2013.2213. [DOI] [PubMed] [Google Scholar]