Abstract
Objective. To evaluate the effectiveness of Jian Pi Li Qi (JPLQ) decoction in improving quality of life of patients with hepatocellular carcinoma (HCC) following transcatheter arterial chemoembolization (TACE). Methods. A randomized, double-blind, placebo-controlled trial was conducted. A total of 150 patients with HCC were randomly assigned into 3 groups. Groups were designed as follows: neither herbal medicine nor placebo administration (group A), placebo treatment (group B), and JPLQ decoction treatment (group C). The measurement methods of the observed outcomes include MD Anderson Symptom Inventory–Gastrointestinal module, armpit temperature, and laboratory tests. Results. Among the 140 patients studied, the 12 symptoms rated as most severe, which characterize postembolization syndrome (PES), were fever, pain, fatigue, nausea, disturbed sleep, distress, lack of appetite, drowsiness, dry mouth, vomiting, constipation, and feeling bloated. All these increased significantly (all P < .05) after TACE; 7 symptoms, including fever, pain, fatigue, lack of appetite, drowsiness, dry mouth, and constipation (all P < .05), were found to be relieved significantly by JPLQ. JPLQ also improved the liver function damage caused by TACE. Conclusion. JPLQ decoction may be an effective modality to relieve PES and protect liver function in patients with HCC after TACE.
Keywords: traditional Chinese medicine, postembolization syndrome, MDASI-GI, TACE, hepatocellular carcinoma
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the third most common cause of cancer-related deaths in the world.1 For patients with HCC in the early stage, surgical resection and liver transplantation are effective treatments. Unfortunately, most patients with HCC are diagnosed at an intermediate to advanced stage, with few effective therapies available at this point. Transcatheter arterial chemoembolization (TACE) is the recommended treatment modality for intermediate-stage HCC (Barcelona Clinic Liver Cancer stage B).2
Postembolization syndrome (PES), characterized by a series of symptoms, including fever, nausea, vomiting, loss of appetite, abdominal pain, and liver function impairment, is the affiliated complication of TACE. The incidence of PES after TACE ranges from 60% to 80%.3 PES usually has a manifestation and duration of action of approximately 1 to 2 weeks. It is widely considered as self-limited; however, it still remains the major reason for hospitalization and reduction in the quality of life (QOL) of patients.4 Although antipyretic analgesics, antiemetics, and cytoprotection agents may relieve these symptoms, the combination of too many drugs will exacerbate the metabolic load of the liver. Thus, a systematic approach to preventing and treating the PES is necessary.
Traditional Chinese medicine Jian Pi Li Qi (JPLQ) decoction is one of the most commonly used prescriptions in our hospital. A previous study has indicated that JPLQ decoction improved the liver function and immune function impairment in vivo caused by chemotherapy.5 A retrospective study also showed that JPLQ decoction could improve the efficacy and relieve the side effects of TACE for patients with unresectable primary liver cancer.6 Therefore, we designed a randomized, double-blind, placebo-controlled trial to confirm whether JPLQ decoction could prevent and treat the PES caused by TACE.
Methods
Patient Eligibility
Inclusion Criteria
Patients with histologically or cytologically documented or radiographically diagnosed unresectable primary HCC who were candidates for TACE were included. Radiographic diagnosis needed typical findings of HCC by a radiographic method—that is, on multidimensional dynamic CT, CT hepatic arteriography/CT arterial portography, or MRI.
Patients should not have received any herbal medicine and systemic treatment in the past 2 weeks.
The Eastern Cooperative Oncology Group Performance Status score had to be from 0 to 1.
Patients had to have compensated liver function (Child-Pugh class of A or B).
Patients had to have a life expectancy of at least 3 months.
Patients had to sign the informed consent form.
Exclusion Criteria
Those not meeting the inclusion criteria above were excluded.
Patients who received combinations of medications that could potentially affect the indices for observation of evaluation during the trial were also excluded.
According to the inclusion and exclusion criteria, patients were recruited between May 2010 and October 2012.
Study Design
A randomized, double-blind, placebo-controlled trial was performed in this study. Eligible patients enrolled by the investigators received a patient number and were randomly assigned to receive neither herbal medicine nor placebo (group A), placebo treatment (group B), or JPLQ decoction treatment (group C). Random assignment was generated by a statistician via a computer-generated random number (using SPSS 19.0 statistical software for Windows). The statistician made up a series of sealed envelopes with random numbers that specified group assignment. Every eligible participant obtained an envelope from the statistician before admission to the treatment groups. The envelope was then opened by a nurse. After that, the nurse telephoned the statistician for group assignment. The details of the assignment and administration were unknown to any of the investigators. All study personnel and participants were blinded to treatment assignment during the study. Only the study statistician could see unblinded data but could not have any contact with study participants. The study protocol was evaluated and approved by the Human Research and Ethics Committee for Clinical Studies of Fudan University Cancer Center.
Interventions
TACE Procedure
The TACE procedures, using the Seldinger technique, were performed by the same group of doctors in the Department of Integrative Medicine, University Cancer Center on all the patients. Guided by arterial angiography, the tip of a 4F catheter, or a microcatheter with 3F outer diameter in some cases, was inserted selectively into the left or right hepatic artery or the tumor-feeding artery if technically possible. Oxaliplatin (75 mg/m2) was then infused through the catheter followed by a mixture of iodized oil (Lipiodol Ultrafluide; Guerbet, France) and epirubicin (60 mg/m2). The dose of Lipiodol is calculated based on the maximum diameter of the tumor. Tumor embolization was terminated on appearance of the portal vein around the tumor. Occasionally, a gelatin sponge was used to enhance the embolic effect. If arterioportal shunts were detected on angiography, the gelatin foam particles or strips would be used to block the arteriovenous shunt.
Traditional Chinese Medicine Treatment
Patients in groups A, B, and C were medicated as follows: with no herbal medicine taken, 200 mL decoction of raw hawthorn taken twice daily as placebo; or 200 mL of JPLQ decoction with twice daily administration, respectively. The JPLQ decoction is composed of Poria cocos 20 g, Atractylodes macrocephala 10 g, Codonopsis pilosula 20 g, Fructus aurantii 10 g, raw hawthorn 20 g, five leaf akebia fruit 30 g, citrus chirocarpus 20 g. All herbal medicines were soaked for half an hour with clean cold water and simmered for half an hour with gentle heat after boiling. The herbal medicine was initially administered on the day of the performance of TACE and continued for 3 days.
Clinical Assessment
Evaluation of QOL was performed using MD Anderson Symptom Inventory–Gastrointestinal module (MDASI-GI), which was administered for 4 days, within 24 hours before TACE, and on the first, second, and third day after TACE. The MDASI-GI is a psychometrically validated 24-item questionnaire containing 13 core items representing important symptoms common to all cancer types, 5 GI items, and 6 questions for the measurement of how much the symptoms have interfered with daily activities. Every item is rated on a 0 to 10 numerical rating scale. The higher the score, the greater the severity of symptoms and more interference with the patient’s daily activities.7
Armpit temperature was taken daily. Grading for fever was as follows: grade 0 fever is body temperature ≤37.2°C grade I fever is body temperature 37.3°C to 38.0°C; grade II fever is body temperature of 38.1°C to 40.0°C; grade III fever is body temperature >40.0°C. Bacterial culture from blood and urine and chest X-rays were performed in patients who had body temperature ≥39°C after TACE to detect any possible infectious agents.
Liver function, including total bilirubin (TB) level, the ratio of glutamic oxalacetic transaminase (ALT) to glutamic pyruvic transaminase (AST), and alkaline phosphatase (AKP) level was performed before TACE and on the third day after TACE. The efficacy was assessed according to World Health Organization grading criteria on toxicities of antitumor drugs.
Statistical Analyses
Descriptive statistics were used to summarize patient and clinical characteristics and MDASI-GI scores. The overall mean symptom severity and the mean values for each MDASI-GI item over the whole treatment course were plotted for each treatment group. Continuous variables were expressed as mean ± standard deviation and compared using the Student t test, ANOVA, or Kruskal-Wallis test, as appropriate. Bonferroni correction was used for multiple comparisons. Categorical variables were described using percentages and compared using the χ2 test and Fisher’s exact test, as appropriate. P values ≤.05 were considered statistically significant for all tests. Analysis was conducted with SPSS 19.0 for Windows (SPSS Inc, Chicago, IL).
Results
Enrollment and Patient Characteristics
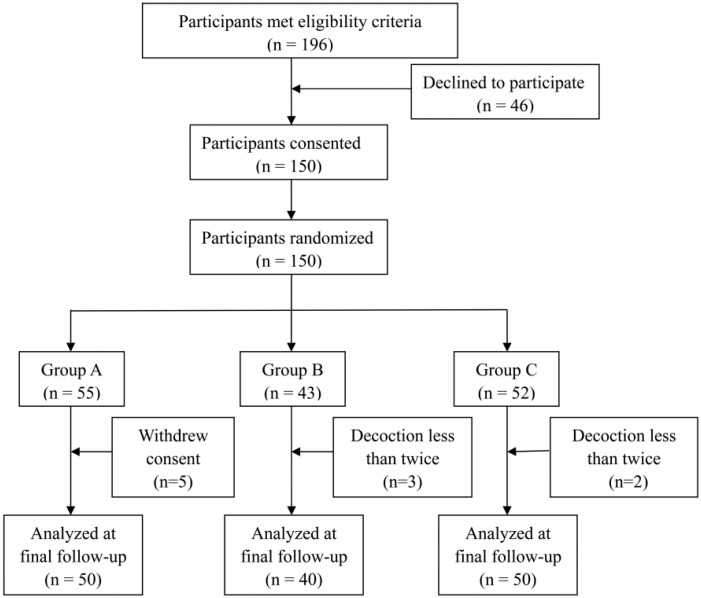
Between May 2010 and October 2012, a total number of 150 patients receiving their first TACE therapy were enrolled in this study, with 55, 43, and 52 patients being randomly allocated to group A, group B, and group C, respectively (Figure 1). After randomization, 5 patients in group A withdrew their consent, and 3 patients in group B and 2 patients in group C took the allocated decoction less than twice because they could not tolerate the experimental and placebo formulas. All these 10 patients were not included in data analysis. Patient baseline characteristics are shown in Table 1. All analyzed patients were in the hospital for all 4 days and did not return home during the therapy.
Figure 1.
Patients’ progress through the trial.
Table 1.
Demographics of HCC patients receiving TACE.
| Group A |
Group B |
Group C |
Total |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristics | n | Percentage | n | Percentage | n | Percentage | n | Percentage | P |
| Age (years) | |||||||||
| Mean | 54.7 | 55.3 | 55.8 | 55.2 | .904 | ||||
| SD | 10.4 | 11.6 | 9.2 | 10.2 | |||||
| Gender | |||||||||
| Male | 45 | 90.0 | 34 | 85.0 | 44 | 88.0 | 123 | 87.9 | .770 |
| Female | 5 | 10.0 | 6 | 15.0 | 6 | 12.0 | 17 | 12.1 | |
| HBV infection | 38 | 76.0 | 31 | 77.5 | 41 | 82.0 | 110 | 78.6 | .751 |
| HCV infection | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | 1.000 |
| Liver cirrhosis | 23 | 46.0 | 21 | 52.5 | 24 | 48.0 | 68 | 48.6 | .824 |
| Child-Pugh | |||||||||
| A | 49 | 98.0 | 40 | 100.0 | 49 | 98.0 | 138 | 98.6 | .666 |
| B | 1 | 2.0 | 0 | 0.0 | 1 | 2.0 | |||
| Eastern Cooperative Oncology Group | |||||||||
| 0 | 49 | 98.0 | 39 | 97.5 | 50 | 100.0 | 138 | 98.6 | .558 |
| 1 | 1 | 2.0 | 1 | 2.5 | 0 | 0.0 | |||
| Barcelona Clinic Liver Cancer stage | |||||||||
| A | 0 | 0.0 | 1 | 2.5 | 1 | 2.0 | 2 | 1.4 | .115 |
| B | 38 | 76.0 | 20 | 50.0 | 34 | 68.0 | 92 | 65.7 | |
| C | 12 | 24.0 | 19 | 47.5 | 15 | 30.0 | 46 | 32.9 | |
Abbreviations: HCC, hepatocellular carcinoma; TACE, transcatheter arterial chemoembolization; SD, standard deviation; HBV, hepatitis B virus; HCV, hepatitis C virus.
Pattern of Postembolization Syndrome Burden During Therapy
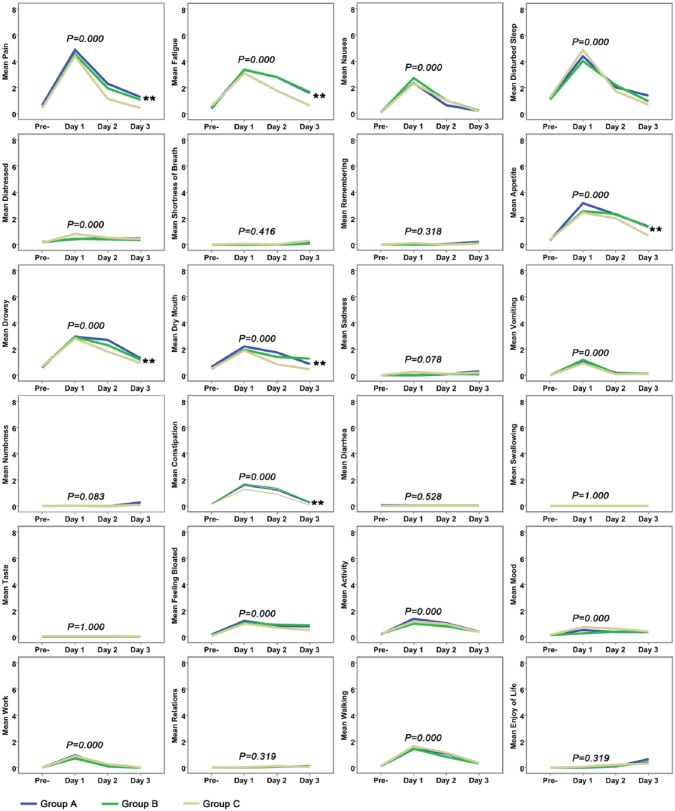
MDASI-GI was used to assess the postembolization syndrome during therapy. The mean MDASI-GI item ratings are shown in Figure 2. On the whole, the symptoms shifted during the treatment course in terms of symptom severity in each group. On the first day after TACE, the symptoms in core items and GI items in all 3 groups that were most severe were pain, fatigue, nausea, disturbed sleep, distress, lack of appetite, drowsiness, dry mouth, vomiting, constipation, and feeling bloated, which interfered with the patients’ life items: activity, mood, work, and walking (P < .05, shown in Figure 2). Fortunately, in the remaining 2 days after TACE, all symptoms were gradually relieved. Combining results for Groups A, B, and C, we compared the percentages of patients experiencing severe levels of the 10 most prominent symptoms 1 day before TACE to 3 days after TACE, except for distress, for which all values were below 5. We found that the percentages of each prominent symptom in the first day after TACE were higher than that for the other days (P = .000, shown in Figure 3). Therefore, postembolization syndrome was most serious on the first day after TACE and was mainly displayed by the above-mentioned symptoms as measured by the MDASI-GI.
Figure 2.
Mean severity of the University of Texas MD Anderson Cancer Center Symptom Inventory–Gastrointestinal Module individual symptom items and symptom interferences are shown across the treatment period among the 3 treatment groups.a
a **The symptom was found to be significantly relieved in group C compared with the other 2 groups.
Figure 3.
Percentages of patients experiencing severe levels (≥7 on a 0-10 scale) of the 10 symptoms rated as most severe after TACE.
Abbreviation: TACE, transcatheter arterial chemoembolization.
We wanted to determine whether JPLQ decoction could relieve the PES after TACE; as shown in Figure 2, 6 symptoms, including pain (P = .000), fatigue (P = .000), lack of appetite (P = .000), drowsiness (P = .002), dry mouth (P = .005), and constipation (P = .000), were found to be significantly relieved in group C compared with the other groups beginning on the second day after TACE. There were no statistical differences on the first day after TACE. There were no differences among the 3 groups on the question of interference of symptoms with daily life. The percentages of patients experiencing severe levels of the 10 most prominent symptoms except for distress in each treatment group during the treatment period are shown in Figure 4. On the first day after TACE, the proportion of patients with severe symptoms, such as drowsiness (P = .046) and dry mouth (P = .016), was obviously lower in group C than in group A and group B. Although the proportions of severe pain and fatigue are lower in group C than in others, there were no statistical differences among the 3 groups. On the second day and third day after TACE, there were no statistical differences in the severity of each symptom. These results indicate that although JPLQ decoction could provide overall relief for the 6 symptoms (such as pain, fatigue, lack of appetite, drowsiness, dry mouth, and constipation) after TACE, it could not reduce severe pain, fatigue, lack of appetite, or constipation.
Figure 4.
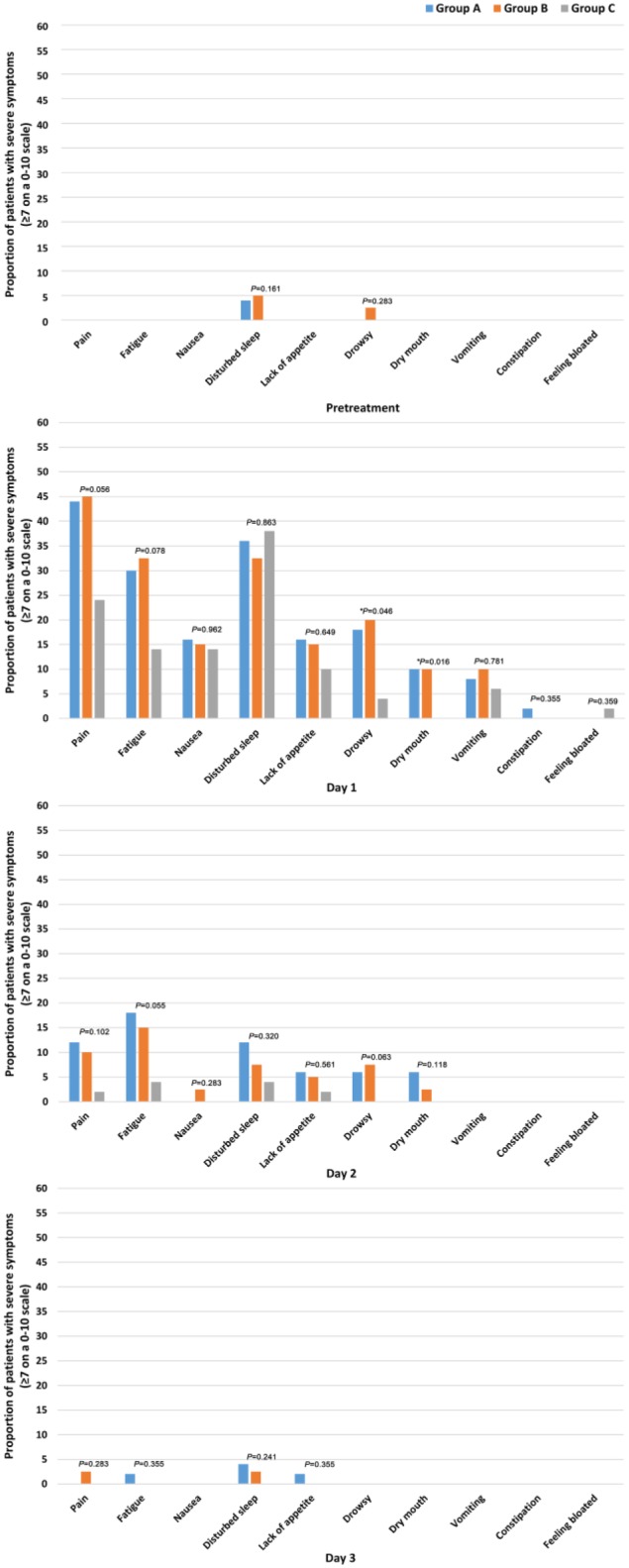
Percentages of patients experiencing severe levels of the 10 most prominent symptoms in each treatment group during the treatment (P: χ2 test; *P: Fisher’s exact test).
Fever
As shown in Figure 5A, the patients’ temperatures shifted during the treatment period. The post-TACE fever peaked on the first day and second day after TACE and was gradually relieved on the third day after TACE (P = .000) when results for the 3 groups were combined. All biological fluid cultures (including blood cultures) and radiological examinations were negative with body temperatures ≥39.0°C after TACE. With regard to differences among the 3 treatment groups during the therapy (shown in Figure 5B and Table 2), the incidence of fever (grade I-IV) was lower in group C than in group A and group B (P = .019) on the first day after TACE. On the second day and the third day after TACE, among the 3 groups, the fever incidence was lowest in group C, but the difference was not statistically significant, which may be related to the use of indomethacin suppositories when the body temperature was higher than 37.2°C.
Figure 5.
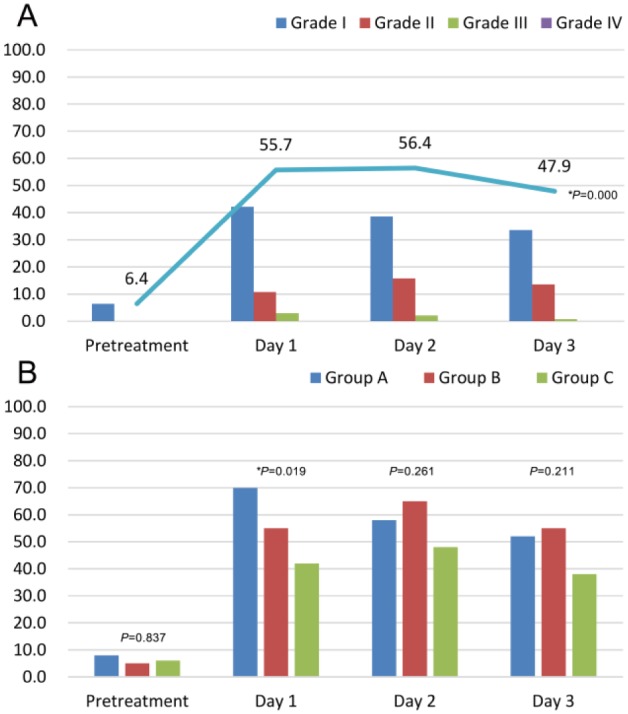
Changes in fever in different groups during the treatment: A. Overall changes in the percentages of fever grades across the treatment. B. The percentages of fever (>37.2°C) among the 3 groups in different periods (P: χ2 test; *P: Fisher’s exact test).
Table 2.
Percentages of Patients With Grade I to IV Fever Among Group A, Group B, and Group C During the Treatment Period.
| Pretreatment |
Day 1 |
Day 2 |
Day 3 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group A | Group B | Group C | Group A | Group B | Group C | Group A | Group B | Group C | Group A | Group B | Group C | |
| Grade I | 8 | 5 | 6 | 46 | 47.5 | 34 | 40 | 47.5 | 30 | 36 | 42.5 | 24 |
| Grade II | 0 | 0 | 0 | 18 | 7.5 | 6 | 14 | 17.5 | 16 | 14 | 12.5 | 14 |
| Grade III | 0 | 0 | 0 | 6 | 0 | 2 | 4 | 0 | 2 | 2 | 0 | 0 |
| Grade IV | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Liver and Renal Function Before and After TACE
After TACE, TB level (P = .000) and AST/ALT ratio (P = .000) with combined results for the 3 groups was obviously increased. There were no differences in AKP (P = .369), blood urea nitrogen (BUN; P = .246), and serum creatinine (CR; P = 1.000) after TACE (Table 3). We analyzed whether JPLQ decoction could protect liver function after TACE. We compared the average value of TB after TACE among the 3 groups and found that the average of TB in group C was lower than in group A and group B (P = .047) after TACE. The average value of AST/ALT ratio in group C after TACE, which was similar to that in group B (P = .296), was lower than that in group A (P = .037). There were no significant differences in AKP, BUN, and CR after TACE among the 3 groups.
Table 3.
Comparison of Liver and Renal Function Before and After TACE in Each Group.a
| TB (%) |
AST/ALT (%) |
AKP (%) |
BUN (%) |
CR (%) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Day | 0 | I | II | III | 0 | I | II | III | 0 | I | II | III | 0 | I | II | III | 0 | I | II | III |
| Group A | Pretreatment | 88 | 12 | 0 | 0 | 36 | 46 | 18 | 0 | 62 | 26 | 12 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| After TACE | 62 | 32 | 4 | 2 | 6 | 68 | 22 | 4 | 54 | 42 | 2 | 2 | 94 | 6 | 0 | 0 | 100 | 0 | 0 | 0 | |
| Group B | Pretreatment | 80 | 20 | 0 | 0 | 47.5 | 37.5 | 15 | 0 | 72.5 | 22.5 | 5 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| After TACE | 55 | 37.5 | 5 | 2.5 | 17.5 | 50 | 30 | 2.5 | 70 | 25 | 5 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | |
| Group C | Pretreatment | 92 | 8 | 0 | 0 | 34 | 48 | 14 | 4 | 78 | 16 | 6 | 0 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 |
| After TACE | 72 | 24 | 2 | 2 | 16 | 50 | 30 | 4 | 74 | 18 | 6 | 2 | 100 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | |
Abbreviations: TACE, transcatheter arterial chemoembolization; TB, total bilirubin; AST, glutamic-pyruvic transaminase; ALT, glutamic-oxalacetic transaminase; AKP, alkaline phosphatase; BUN, blood urea nitrogen; CR, serum creatinine.
TB, AST/ALT, AKP, BUN, CR: 0 degree, ≤1.25N; I degree, 1.26N to 2.5N; II degree, 2.6N to 5N; III degree, 5.1N to 10N, where N is the upper limit of the normal.
Discussion
TACE has been widely used as first-line therapy for patients without surgical indications or applied to prevent the recurrence of liver cancer after surgery. As a palliative modality, TACE prolongs overall survival.8-10 However, TACE procedures are associated with an increased risk of TACE-related side effects that may adversely affect a patient’s prognosis. The high incidence of PES is a major cause of reduced QOL and prolonged hospitalization.11,12 Therefore, it is necessary to seek an approach to improve the syndrome systematically.
Traditional Chinese medicine, used in China for a long time, has a unique theoretical system and practical approach to the treatment of diseases. Previous clinical studies have demonstrated that traditional Chinese medicine can relieve the PES after TACE. Chen et al13 found that Chinese herbs (Jian Pi Bu Qi) improve prevention and reduction of PES syndrome (vomiting, nausea, fever, and pain).13 Qingre Jiedu Decoction combined with Western medicine has a positive effect in preventing and treating syndromes after TACE, such as fever and pain in HCC. This combination can also improve the QOL and preserve the liver function.14 However, although these herbs relieve the PES systematically, these trials do not describe the randomization sequence generation and allocation concealment. In addition, these trials are not double blind or single blind. Therefore, it was necessary to design a randomized, double-blind, placebo-controlled trial to demonstrate the effect of traditional Chinese medicine in preventing PES of TACE.
Traditional Chinese medicine JPLQ decoction has been used in clinical practice for a long time. JPLQ decoction comprises Poria cocos, Atractylodes macrocephala, Codonopsis pilosula, Fructus aurantii, raw hawthorn, five leaf akebia fruit, and citrus chirocarpus. In our previous study, JPLQ decoction was found to improve the efficacy of chemotherapy and relieve its side effects.5 JPLQ can improve liver function and relieve the side effects caused by TACE, especially for the moderate to advanced stage HCC patients who accepted TACE.6 This randomized, double-blind and placebo-controlled trial also demonstrated that JPLQ decoction could improve QOL and relieve the PES after TACE.
PES after TACE mainly includes fever, nausea, vomiting, loss of appetite, abdominal pain, and fatigue as well as other symptoms. Our study has shown that the 12 symptoms, including fever, pain, fatigue, nausea, disturbed sleep, distress, lack of appetite, drowsiness, dry mouth, vomiting, constipation, and feeling bloated, rose in severity on the first day after TACE in 140 patients. There was no statistical difference among the 3 groups except for fever at that time, which was lower in group C, the patients assigned to receive the JPLQ formula. However, in the following days, 6 of the symptoms, including pain, fatigue, lack of appetite, drowsiness, dry mouth, and constipation, were found to be relieved more significantly in patients receiving JPLQ decoction than in the other 2 groups. However, on the second day and the third day after TACE, there were no statistical differences in fever among the 3 groups, possibly because of the use of indomethacin suppository, even though fever incidence was lower in group C than in groups A and B. On the second and third day after TACE, the percentages of patients experiencing severe levels of the 7 symptoms (including fever, pain, fatigue, lack of appetite, drowsiness, dry mouth, and constipation) were lower in the JPLQ group than in others. However, there were no statistical differences in severe levels of the 7 symptoms among the 3 groups during the 3 days after TACE except for drowsiness and dry mouth on the first day after TACE. Nausea, vomiting, disturbed sleep, distressed, and feeling bloated did not differ among the 3 groups. The reasons for this may be as follows: (1) before TACE, we usually used antiemetics such as tropisetron hydrochloride to prevent the reaction of nausea and vomiting; (2) disturbed sleep may be a psychological reaction to TACE; (3) during TACE, chemotherapeutic agents and Lipiodol may enter the gastric supplying arteries, causing gastric bloating, which needs more time to subside; (4) distress could be a psychological problem, which can be self-healing.
Liver function damage and renal injury were also related to the TACE procedure. The main reasons are as follows: (1) Lipiodol-induced embolization may result in ischemia, hypoxia, and necrosis in some normal hepatic cells; (2) chemotherapeutic drugs have toxicities in the liver and kidney; (3) the procedure itself can lead to considerable releasing of inflammatory factors. In our study, we found that TB level and AST/ALT ratio increased in the 3 groups after TACE, whereas there were no significant changes in AKP and renal function. After TACE, TB level and ALT/AST ratio in the herbal medicine group were markedly lower than in the control group, indicating that JPLQ decoction can relieve the liver function damage caused by TACE.
However, there are some study limitations in our trial. First of all, in our study, we did not use stratified randomization, which may be the better choice in this trial. Second, the decoction may not be the best pharmaceutical dosage form in our trial; the capsule may be more reasonable. Last but not least, the results of our study may have been more accurate and generalizable if we had conducted this trial in multiple centers with a larger population.
Conclusion
In summary, JPLQ decoction may be used to relieve PES, improve the QOL of patients after TACE, and repair liver function damage, based on this randomized controlled clinical trial.
Footnotes
Authors’ Note: Litao Xu and Shiying Wang are first authors. Both Hongbin Wu and Zhiqiang Meng are the co-corresponding authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by a grant from Shanghai Municipal Science and Technology Commission, No. 09dZ16500.
References
- 1. Jemal A, Murray T, Ward E, et al. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10-30. [DOI] [PubMed] [Google Scholar]
- 2. Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marelli L, Stigliano R, Triantos C, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007;30:6-25. [DOI] [PubMed] [Google Scholar]
- 4. Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12:321-326. [DOI] [PubMed] [Google Scholar]
- 5. You J, Song MZ, Huang H, Yu EX, Huang WX, Lin YL. Effect of JianpiLiqi prescription combined with γIL-2 on immune suppression caused by chemotherapy. J Shanghai Med Univ. 1999;2:37-39. [Google Scholar]
- 6. Meng ZQ, Liu LM, Ma X, et al. Prognosis risk factors and therapeutic effect analysis in primary hepatic carcinoma: 394 cases report. China Oncol. 2007;08:628-632. [Google Scholar]
- 7. Wang XS, Williams LA, Eng C, et al. Validation and application of a module of the M. D. Anderson Symptom Inventory for measuring multiple symptoms in patients with gastrointestinal cancer (the MDASI-GI). Cancer. 2010;116:2053-2063. [DOI] [PubMed] [Google Scholar]
- 8. Lu W, Li Y, He X, Chen Y. Transcatheter arterial chemoembolization for hepatocellular carcinoma in patients with cirrhosis: evaluation of two kinds of dosages of anticancer drugs and analysis of prognostic factors. Hepatogastroenterology. 2003;50:2079-2083. [PubMed] [Google Scholar]
- 9. Saccheri S, Lovaria A, Sangiovanni A, et al. Segmental transcatheter arterial chemoembolization treatment in patients with cirrhosis and inoperable hepatocellular carcinomas. J Vasc Interv Radiol. 2002;13:995-999. [DOI] [PubMed] [Google Scholar]
- 10. Ikeda K, Kumada H, Saitoh S, Arase Y, Chayama K. Effect of repeated transcatheter arterial embolization on the survival time in patients with hepatocellular carcinoma: an analysis by the Cox proportional hazard model. Cancer. 1991;68:2150-2154. [DOI] [PubMed] [Google Scholar]
- 11. Leung DA, Goin JE, Sickles C, Raskay BJ, Soulen MC. Determinants of postembolization syndrome after hepatic chemoembolization. J Vasc Interv Radiol. 2001;12:321-326. [DOI] [PubMed] [Google Scholar]
- 12. Ruszniewski P, Malka D. Hepatic arterial chemoembolization in the management of advanced digestive endocrine tumors. Digestion. 2000;62(suppl 1):79-83. [DOI] [PubMed] [Google Scholar]
- 13. Chen X, Zhang P, Hu P, Huang G, Xu B. The alleviation of TACE syndrome in liver cancer by Chinese herbs: a clinical research. China Oncol. 2001;3:54-55. [Google Scholar]
- 14. Qian Z, Xie G, Guo X, et al. “Qingre Jiedu Decoction” combined western medicine in preventing and treating syndromes after transcatheter arterial chemoembolization in middle-late primary liver cancer. Shanghai J Tradit Chin Med. 2012;2:35-37. [Google Scholar]