Abstract
Background. Resistance exercise is emerging as a potential adjunct therapy to aid in the management of breast cancer–related lymphedema (BCRL). However, the mechanisms underlying the relationships between the acute and long-term benefits of resistance exercise on BCRL are not well understood. Purpose. To examine the acute inflammatory response to upper-body resistance exercise in women with BCRL and to compare these effects between resistance exercises involving low, moderate, and high loads. The impact on lymphedema status and associated symptoms was also compared. Methods. A total of 21 women, 62 ± 10 years old, with BCRL participated in the study. Participants completed low-load (15-20 repetition maximum [RM]), moderate-load (10-12 RM), and high-load (6-8 RM) exercise sessions consisting of 3 sets of 6 upper-body resistance exercises. Sessions were completed in a randomized order separated by a 7- to 10-day wash-out period. Venous blood samples were obtained to assess markers of exercise-induced muscle damage and inflammation. Lymphedema status was assessed using bioimpedance spectroscopy and arm circumferences, and associated symptoms were assessed using Visual Analogue Scales for pain, heaviness, and tightness. Measurements were conducted before and 24 hours after the exercise sessions. Results. No significant changes in creatine kinase, C-reactive protein, interleukin-6, and tumor necrosis factor-α were observed following the 3 resistance exercise sessions. There were no significant changes in arm swelling or symptom severity scores across the 3 resistance exercise conditions. Conclusions. The magnitude of acute exercise-induced inflammation following upper-body resistance exercise in women with BCRL does not vary between resistance exercise loads.
Keywords: lymphedema, resistance exercise, weight training, inflammation, breast cancer
Introduction
Exercise is considered a safe, feasible, and effective adjuvant therapy for people with breast cancer.1 Exercise may reduce treatment-related side effects, the risk of breast cancer recurrence, and mortality and enhance health outcomes and quality of life.1,2 For breast cancer survivors who develop lymphedema, resistance exercise has been demonstrated to improve symptom severity, strength, endurance, and mobility of the affected limb, without exacerbating lymphedema.3-5 However, breast cancer survivors face a number of difficulties and barriers to exercise participation. These include treatment-related side effects such as fatigue, nausea, pain, weight gain, and depression6 as well as the presence of uncertainty and fear toward exercise, in that it may lead to worsening of lymphedema or its associated symptoms.7 Lymphedema risk reduction and management guidelines advise patients to avoid physical trauma to the affected limb to reduce the risk of infection and cellulitis occurring as a result of impaired lymphatic function.8 This includes avoiding needles, injections, blood draws and blood pressure cuffs, and cuts and scratches on the affected hand and arm.9 As a result, difficulties exist in undertaking invasive studies evaluating resistance exercise in women with breast cancer–related lymphedema (BCRL). Accordingly, the volume of previous research examining the physiological effect of resistance exercise in this cohort is limited. In addition, the mechanisms underlying the relationships between the acute and long-term benefits of resistance exercise and BCRL are not well understood.
Single bouts of resistance exercise elicit muscle tissue damage and an acute inflammatory response.10-12 The extent of this response is affected by the mode (eccentric and/or concentric muscular contractions), volume (total work of the session), load (weight lifted), and intensity (extent of neuromuscular fatigue) of resistance exercise.13-15 High-volume and eccentric resistance exercise causes a greater magnitude of exercise-induced muscle damage and inflammation.11,16-19 This response is characterized by a transient increase in creatine kinase (CK) and inflammatory biomarkers (eg, interleukin-6 [IL-6], tumor necrosis factor α [TNF-α], and C-reactive protein [CRP]) up to 72 hours postexercise.11,20-24 Whereas single bouts of resistance exercise cause increases in these biomarkers, chronic adaptation to regular exercise has an anti-inflammatory effect associated with downregulation of various inflammatory biomarkers.20,25-28 This adaptation is characterized by lower resting levels of inflammatory biomarkers. The acute inflammatory response to resistance exercise has been reported in healthy trained and untrained individuals14 but has yet to be examined in women with BCRL who have compromised lymphatic system function. There is a perception among health professionals and patients that if resistance exercise is conducted it should be prescribed at light loads. This perception is based on the assumption (yet to be empirically evaluated) that lighter loads elicit a lower inflammatory response in women with BCRL. However, it has been reported that at least in young healthy men, there are no differences to the response of CK, IL-6, or TNF-α 24 hours after upper-body resistance exercise at 4 different loads (ranging from 50% to 110% of maximal muscular strength; ie, 1 repetition maximum [RM]).29 At present, it is unknown if the impaired lymphatic system of women with BCRL prompts a different inflammatory response to varying loads of resistance exercise. The response may differ from that in healthy adults because the lymphatic system is implicated in cytokine production and removal. It is suggested that in addition to the blood, cytokines may also enter the lymph fluid, which can modify plasma cytokine levels.30 Furthermore, lymphatic fluid passes through lymph nodes, a site where immune cells are removed. As a result, cytokine production may be modulated when components of damaged muscle pass through lymph nodes.30 However, it is unknown whether the excision of lymph nodes and lymphatic fluid accumulation in BCRL may alter the inflammatory response to exercise.
Women who develop BCRL experience impairment within the lymphatic system, resulting in reduced or stagnant flow of lymph fluid.31,32 As a result of lymph stasis, fibrosis, fat accumulation, and inflammation occur within the affected limb.33 This impaired lymph flow results in the buildup of protein-rich fluid, which is associated with inflammation in the lymphadetamous limb.33-35 Ongoing and worsening inflammatory processes of the skin, subcutaneous tissue, and lymphatic system also occur.36 As a result of the pathological changes in the affected limb, it is plausible that the normal inflammatory response to a bout of upper-body resistance exercise is exacerbated or at least altered in patients with BCRL. This may have important implications in understanding the pathophysiology, upper-limb rehabilitation, and prescription of optimal resistance exercise loads for women with BCRL. Therefore, the aim of the present study was to examine the acute inflammatory responses to resistance exercise in women with BCRL. Markers of exercise-induced muscle damage and inflammation (CK, CRP, IL-6, and TNF-α) in women with unilateral BCRL were evaluated after performing acute bouts of low-, moderate-, and high-load upper-body resistance exercises. The impact on lymphedema status and associated symptoms (pain, heaviness, and tightness) were also assessed.
Methods
Participants
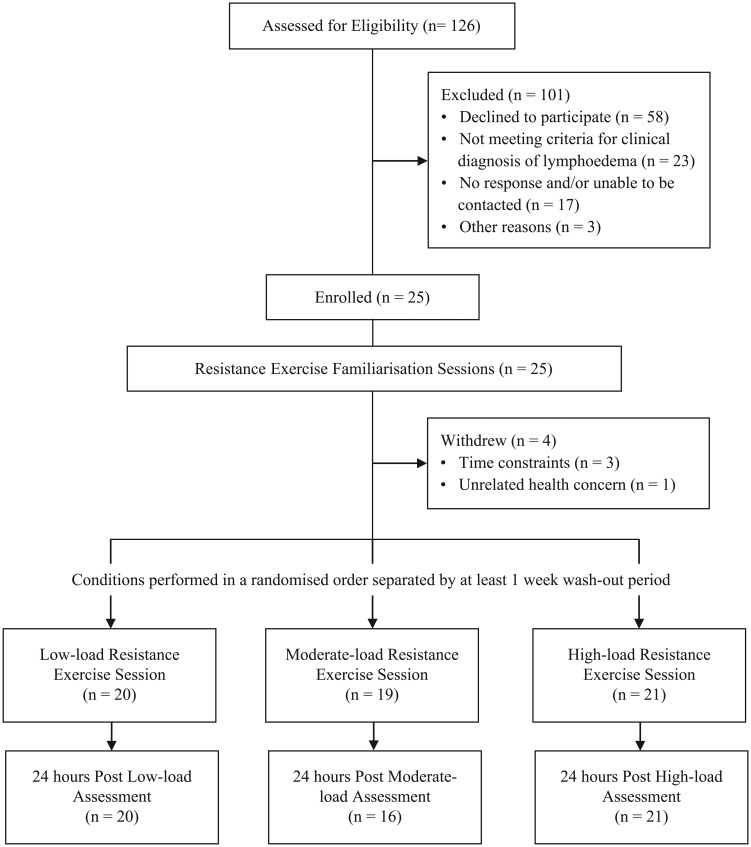
A total of 126 women with BCRL were screened for participation in this study between August 2011 and February 2012 (Figure 1). Potential participants were identified through referral from oncologists or physiotherapists and existing databases of the investigators. A total of 25 women were enrolled to participate. To be eligible, participants had to have had a histological diagnosis of breast cancer and a clinical diagnosis of unilateral BCRL defined as: (1) an impedance ratio of at least 3 standard deviations (SDs) greater than normative data;37,38 (2) a volume difference between affected and unaffected limbs of at least 5%;38,39 and (3) a difference in circumference between affected and unaffected limbs of at least 5%.5,38,39 Women were excluded from participation if they had unstable lymphedema, which was defined as receiving intensive therapy (ie, decongestive therapy or antibiotics for infection) within the previous 3 months. Any potential participants were also excluded if they had a musculoskeletal, cardiovascular, and/or neurological disorder that could inhibit them or place them at risk of harm from exercising, as determined by their general physician. This study was approved by the university’s human research ethics committee, and all participants provided written informed consent.
Figure 1.
Flow of participants throughout trial.
Experimental Design
A randomized, cross-over design was implemented in which participants completed 3 experimental conditions separated by a wash-out period of at least 1 week. Prior to any experimental conditions, all participants completed a series of 4 familiarization sessions implemented twice weekly for a fortnight. These sessions involved familiarizing participants with the resistance exercises and loads performed during the experimental conditions. The experimental conditions involved an upper-body resistance exercise session undertaken using: (1) low load; (2) moderate load; or (3) high load. Sessions were completed in a randomized order as determined by a random assignment computer program. A series of assessments were conducted immediately prior to and 24 hours after each of the experimental conditions. Instructions were provided to all participants to maintain their normal lymphedema management strategies throughout the duration of the study. Additionally, participants were instructed to maintain their standard diet and physical activity levels throughout the study period.
Resistance Exercise Sessions
Each of the experimental sessions involved a series of 6 standard resistance exercises targeting all the major muscle groups in the upper body. Specifically, the exercises included the chest press, lat pull-down, bicep curl, triceps extension, lateral raise, and wrist curl. Three sets of each exercise were performed in each session, whereas the load lifted and number of repetitions completed in each set differed between the 3 experimental conditions. The low-load trial involved sets of 15 to 20 RM (ie, load corresponding to the maximum weight that could be lifted 15-20 times). The moderate-load trial involved sets of 10 to 12 RM. The high-load trial involved sets of 6 to 8 RM. These RM ranges were estimated to represent 55% to 65%, 65% to 75%, and 80% to 85% of the 1 RM (ie, maximal strength), respectively.40 These loading protocols have been shown to be safe for women with BCRL3,4 and are consistent with common resistance exercise prescriptions used in clinical practice.41 The participant’s perception of exertion associated with each of the experimental conditions was assessed using the Rating of Perceived Exertion (RPE) Scale, which quantifies the difficulty of the exercise session on a scale from 6 (no exertion at all) to 20 (maximal exertion).42 Perceived tolerance of each exercise session was assessed using a 7-point Likert scale, ranging from 1 (strongly disagree) to 7 (strongly agree) in response to the statement, “I have found the exercise session to be tolerable.” All exercise sessions were supervised by an accredited exercise physiologist.
Inflammatory Markers
Venous blood samples were collected from the nonaffected arm by a qualified phlebotomist immediately prior to and 24 hours following the exercise sessions. Because of risk reduction guidelines among this cohort (ie, guidelines to minimize injections to the hand and arm), we aimed to minimize patient burden by allowing 24 hours between blood withdrawals. This sampling time point (24 hours) also allowed us to determine whether a robust and sustained inflammatory response was induced by exercise. Samples were assessed for CK, CRP, IL-6, and TNF-α using standard techniques. CK was assessed by a commercial pathology laboratory using the ADVIA Chemistry System (Western Diagnostics, Perth, Western Australia). The assay sensitivity was <1 U/L, and the precision (coefficient of variation) was 1.5%. CRP, IL-6, and TNF-α were assessed using multiplex bead-based immunoassays on the Luminex platform (Luminex, Austin, TX). Assays were conducted using magnetic multiplex kits in accordance with manufacturer’s protocol (Merck Millipore, St Charles, MO). The sensitivity was 451 ng/mL for CRP, 0.13 pg/mL for IL-6, and 0.23 pg/mL for TNF-α. The precision was 8.8% for CRP and IL-6 and 6.6% for TNF-α.
Lymphedema Status and Associated Symptoms
The extent of swelling was determined by 2 independent techniques: bioimpedance spectroscopy (BIS) and arm circumferences. In accordance with the manufacturer’s guidelines for the BIS device (ImpediMed IMPTM DF50; ImpediMed, San Diego, CA), the impedance of the extracellular fluid in the affected and nonaffected arms was assessed using a range of frequencies and compared to produce a L-Dex score.37 Regional arm circumferences of the affected and nonaffected arms were assessed according to established protocols.38,43 The participant was seated with the arm abducted at 90°; a constant tension tape was used to measure circumferences immediately distal to the metacarpal-phalangeal joint and at 5-cm intervals to the base of the axilla. Arm circumference swelling was reported as the percentage difference in total circumference between the affected and nonaffected arms. The severity of lymphedema symptoms was assessed using Visual Analogue Scales (VAS) for pain, heaviness, and tightness.44 The scales ranged from 0 (no discomfort) to 10 (worst imaginable), and participants rated both the affected and nonaffected arms.
Statistical Analyses
Summary descriptive statistics for participants’ characteristics included counts (and percentages) for categorical variables and means ± SDs or medians, minimum, and maximum for continuous variables. Generalized estimating equations (GEEs) were used to model continuous primary outcomes (CK, CRP, TNF-α, and IL-6) to determine time (pre-exercise and 24-hours postexercise) and trial (low-, moderate-, and high-load) effects and Time × Trial interactions. GEEs were considered the most appropriate multivariate modeling technique. In comparison to conventional repeated-measure techniques, GEEs incorporate all available data (including from participants with missing data at various time points). Secondary outcomes (lymphedema status and associated symptoms) were analyzed using repeated-measures analysis of variance. All tests were 2-tailed, with an α level of P ≤ .05 as the criterion for statistical significance. No imputation of data was generated. Data were analyzed using SPSS Statistics for Windows, Version 21 (IBM SPSS, Chicago, IL).
Results
Participant Characteristics
Participants were on average 61.5 years old and had had lymphedema for an average of 9 years (Table 1). Most participants were either overweight or obese (91%) as determined by body mass index and had undergone previous surgery (96%), with an average of 17 lymph nodes removed. Between 60% and 80% of participants had had previous radiotherapy and/or chemotherapy or were currently and/or previously taking hormone therapy. According to the American Physical Therapy Association lymphedema criteria,39 41%, 18%, and 41% of participants had mild, moderate, and severe lymphedema, respectively. Most participants (86%) self-reported being physically active prior to study participation. All participants maintained their usual lymphedema self-care management, physical activity, and diet behaviors for the duration of the study. One participant completed only the low-load trial and withdrew from the study as a result of a change in work commitments, whereas another participant did not complete the moderate-load trial (because of unrelated health concerns). Three participants did not complete the 24-hour assessment after moderate-load resistance exercise. It was also not possible to perform the BIS assessments on 1 participant.
Table 1.
Participant Characteristics (n = 21).
| Characteristics | Mean ± SD or n (%) |
|---|---|
| Age (years) | 61.5 ± 10.1 |
| Body mass (kg) | 82.2 ± 16.2 |
| Body mass index (kg/m²) | |
| Normal (18.5-24.9) | 2 (9.1%) |
| Overweight (25-29.9) | 8 (36.4%) |
| Obese (≥30) | 12 (54.5%) |
| Presence of comorbiditiesa | |
| 0 | 7 (31.8%) |
| 1 | 4 (18.2%) |
| 2 | 7 (31.8%) |
| 3+ | 4 (18.2%) |
| Time since cancer diagnosis (years) | 9.4 ± 8.9 |
| Cancer stage | |
| I | 4 (18.2%) |
| II | 5 (22.7%) |
| III | 4 (18.2%) |
| IV | 2 (9.1%) |
| Missing | 7 (31.8%) |
| Adjuvant treatment (yes) | |
| Radiotherapy | 17 (77.3%) |
| Chemotherapy | 16 (72.7%) |
| Hormone therapy (currently and/or previously) | 13 (59.1%) |
| Surgery (yes) | 21 (95.5%) |
| Full axillary clearance (yes) | 16 (72.7%) |
| Number of lymph nodes dissected | 16.6 ± 7.7 |
| Years since lymphedema diagnosis | 9.4 ± 8.9 |
| Lymphedema treatment in previous 3 months (yes) | 8 (36.4%) |
| Lymphedema severityb | |
| Mild | 9 (40.9%) |
| Moderate | 4 (18.2%) |
| Severe | 9 (40.9%) |
| Currently physically activec (yes) | 19 (86.4%) |
Abbreviation: SD, standard deviation.
Comorbidities include hypertension/high blood pressure, high cholesterol, cardiovascular disease or heart disease, diabetes, and osteoporosis.
According to the American Physical Therapy Association lymphedema criteria.15
Physically active defined as meeting the Australian national physical activity guidelines.53
Resistance Exercise Sessions
Mean ratings of perceived exertion for the 3 exercise conditions were similar. The participants rated the exercise sessions between “light” and “somewhat hard” (Table 2). Participants rated the 3 exercise conditions as equally tolerable (Table 2). The load lifted across all 6 exercises differed significantly between the 3 exercise conditions (low load = 31.7 ± 18.5 kg; moderate load = 40.7 ± 21.2 kg; high load = 46.9 ± 26.8 kg; P < .001 [Table 2]). Total volume-load (load [kg] × repetitions × sets) was 1300 ± 1091 kg in the low-load trial, 840 ± 514 kg in the moderate-load trial, and 1011 ± 650 kg in the high-load trial. There were no adverse events during participation in the resistance exercise sessions, including no exacerbations of lymphedema or symptom severity.
Table 2.
Mean Perceived Exertion and Tolerability, as Well as the Loads Lifted, for the Low-, Moderate-, and High-Load Conditions.
| Low Load,a Mean ± SD | Moderate Load,a Mean ± SD | High Load,a Mean ± SD | |
|---|---|---|---|
| Perceived exertion (RPE scale)b | 12.7 ± 1.9 | 12.6 ± 0.9 | 13.1 ± 1.8 |
| Tolerability (7-point Likert scale)c | 6.4 ± 0.8 | 5.9 ± 1.5 | 6.4 ± 0.7 |
| Resistance exercise loads | |||
| Chest press (kg) | 10.8 ± 6.4 | 12.8 ± 7.4 | 14.7 ± 8.3 |
| Lat-pulldown (kg) | 13.2 ± 8.0 | 16.2 ± 9.5 | 19.0 ± 10.9 |
| Biceps curl (kg) | 2.2 ± 0.4 | 2.8 ± 0.6 | 3.5 ± 0.7 |
| Lateral raise (kg) | 1.7 ± 0.4 | 2.1 ± 0.5 | 2.6 ± 0.6 |
| Triceps extension (kg) | 3.4 ± 3.6 | 5.1 ± 5.5 | 6.5 ± 6.7 |
| Wrist curl (kg) | 1.9 ± 0.5 | 4.3 ± 3.5 | 3.2 ± 0.9 |
Abbreviation: RPE, Rating of Perceived Exertion.
The load lifted differed significantly between the 3 exercise conditions.
Perceived exertion assessed using a 6 (no exertion at all) to 20 (maximal exertion) scale.
Tolerability assessed using a 1 (strongly disagree) to 7 (strongly agree) scale in response to the statement, “I have found the exercise session to be tolerable.”
Inflammatory Markers
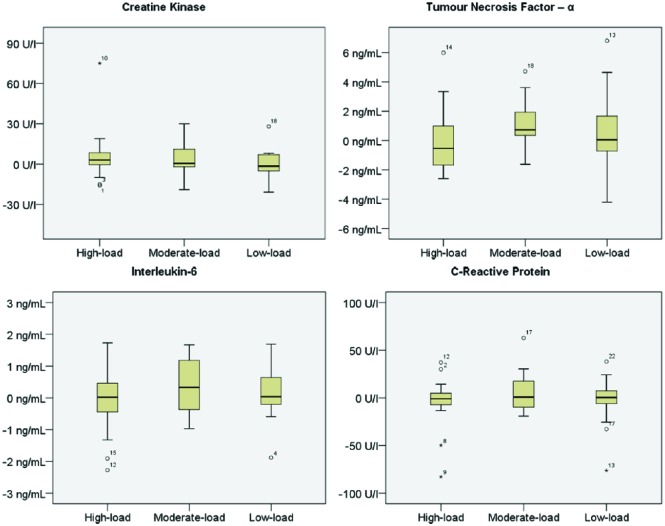
There was no significant interaction effect between time and trial for CK, CRP, IL-6, and TNF-α (Table 3, Supplementary Table [available at http://ict.sagepub.com/supplemental]). There was a trend for CK to increase (nonsignificantly) 24 hours after each of the 3 exercise conditions, with no significant differences between the low-, moderate-, and high-load conditions. There were no clear trends in terms of the impact of resistance exercise load on CRP, IL-6, or TNF-α from pre-exercise to 24 hours postexercise in any of the 3 conditions. Individual responses varied markedly from no change to increases and/or reductions in CK, CRP, TNF-α, and IL-6 (Figure 2).
Table 3.
Lymphedema Status and Associated Symptom Severity Before and 24 Hours After Low-, Moderate-, and High-Load Conditions (Mean ± SD).
| Extent of Swelling |
Symptom Severity |
||||
|---|---|---|---|---|---|
| BIS (L-Dex) | Circumference Differencea (%) | Pain (mm) | Heaviness (mm) | Tightness (mm) | |
| Low-load resistance exercise | |||||
| Pre-exercise (n = 20) | 15.19 ± 18.97 | 6.73 ± 5.79 | 0.35 ± 0.75 | 0.67 ± 1.18 | 0.75 ± 1.19 |
| 24 Hours postexercise (n = 20) | 12.87 ± 16.55 | 5.96 ± 6.11b | 0.40 ± 0.88 | 0.68 ± 1.13 | 0.87 ± 1.31 |
| Mean change from pre-exercise to 24 hours postexercise (95% CI) | −2.51 (−7.22, 2.19) | −0.77 (−1.41, −0.13) | +0.04 (−0.07, 0.17) | +0.01 (−0.33, 0.33) | +0.12 (−0.17, 0.42) |
| Moderate-load resistance exercise | |||||
| Pre-exercise (n = 16) | 16.38 ± 23.00 | 8.13 ± 6.76 | 0.11 ± 0.14 | 0.78 ± 1.67 | 0.67 ± 1.23 |
| 24 Hours postexercise (n = 16) | 13.86 ± 19.25 | 6.93 ± 6.06 | 0.50 ± 1.58 | 1.22 ± 1.93 | 1.28 ± 2.35 |
| Mean change from pre-exercise to 24 hours postexercise (95% CI) | −2.51 (−7.22, 2.19) | −1.20 (–3.32, 0.92) | +0.38 (−0.55, 1.32) | +0.43 (−0.38, 1.25) | +0.60 (−0.34, 1.55) |
| High-load resistance exercise | |||||
| Pre-exercise (n = 21) | 16.71 ± 18.89 | 5.59 ± 6.24 | 0.40 ± 0.87 | 0.98 ± 1.60 | 1.18 ± 1.86 |
| 24 Hours postexercise (n = 21) | 14.16 ± 16.85 | 5.49 ± 5.26 | 0.26 ± 0.52 | 0.73 ± 1.49 | 0.65 ± 1.44c |
| Mean change from pre-exercise to 24 hours postexercise (95% CI) | −2.54 (−5.23, 0.14) | −0.10 (−1.48, 1.28) | −0.13 (−0.33, 0.61) | −0.25 (−0.55, 0.03) | −0.52 (−0.93, −0.11) |
Abbreviations: SD, standard deviation; BIS, bioimpedance spectroscopy; CI, confidence interval.
Interlimb circumference difference.
Significantly different from pre-exercise (P = .02).
Significantly different from pre-exercise (P = .015).
Figure 2.
Individual response in markers of muscle damage and inflammation presented as the mean change from pre-exercise to 24 hours postexercise for low-, moderate-, and high-load conditions.a
a*Denotes extreme outliers (≥3 × interquartile range); ○ denotes mild outliers (≥1.5 × interquartile range).
Lymphedema Status and Associated Symptoms
No significant increases were observed in BIS or interlimb circumference difference at any time point during the 3 exercise conditions (Table 4). BIS scores and interlimb circumference differences generally tended to decrease at 24 hours after exercise in all 3 conditions. Circumference difference decreased significantly 24 hours after low-load resistance exercise (P = .02). There was no worsening of symptom severity of the affected arm at any time throughout the study. Tightness ratings of the affected arm decreased significantly 24 hours after the high-load trial (P = .015).
Table 4.
Inflammatory Marker Response, Including CK, CRP, TNF-α, and IL-6, to the Resistance Exercise Conditions From Pre-exercise to 24 Hours Postexercise.
| Low Load, Mean ± SD | Moderate Load, Mean ± SD | High Load, Mean ± SD | |
|---|---|---|---|
| CK (U/L) | |||
| Pre-exercise | 85.80 ± 45.95 | 98.07 ± 76.15 | 83.52 ± 54.06 |
| Change from pre-exercise to 24 hours postexercise (95% CI) | 0.70 (−9.29, 10.69) | 1.07 (−5.73, 7.87) | 5.89 (−3.29, 15.08) |
| CRP (ng/mL) | |||
| Pre-exercise | 7.56 ± 5.36 | 5.97 ± 5.63 | 6.69 ± 5.79 |
| Change from pre-exercise to 24 hours postexercise (95% CI) | −0.30 (−1.39, 0.78) | 0.66 (−0.57, 1.90) | −0.40 (−1.65, 0.84) |
| TNF-α (pg/mL) | |||
| Pre-exercise | 10.13 ± 6.81 | 8.65 ± 8.37 | 10.06 ± 6.97 |
| Change from pre-exercise to 24 hours postexercise (95% CI) | 0.67 (−0.50, 1.84) | 0.04 (−2.73, 2.83) | −0.36 (−1.66, 0.92) |
| IL-6 (ng/mL) | |||
| Pre-exercise | 2.23 ± 4.29 | 2.58 ± 5.59 | 2.65 ± 5.42 |
| Change from pre-exercise to 24 hours postexercise (95% CI) | 0.58 (−0.48, 1.65) | 0.64 (−0.27, 1.56) | −0.05 (−0.51, 0.39) |
Abbreviations: CK, creatine kinase; CRP, C-reactive protein; TNF, tumor necrosis factor; IL-6, interleukin-6; SD, standard deviation; CI, confidence interval.
Discussion
The main findings of this study were the following: (1) no significant changes in indicators of muscle damage or inflammation occurred 24 hours after resistance exercise in women with BCRL; (2) the acute inflammatory responses in women with BCRL were similar across low-, moderate-, and high-load resistance exercise conditions; (3) resistance exercise did not acutely exacerbate BCRL, regardless of the resistance exercise load; and (4) participants reported no differences in tolerability or perceived exertion between the 3 different loading conditions. However, the changes observed following all 3 conditions in the present study were modest and not clinically relevant (all changes represented small effects: d < 0.2).
Resistance exercise may elicit muscle damage and associated inflammation characterized by increases in CK and various inflammatory biomarkers.45 Inflammatory cytokines are intracellular signaling molecules involved in initiating the inflammatory responses to exercise-induced muscle damage and adaptation.14,30 Clinicians have been hesitant to recommend resistance training (and in particular moderate to high loads) for women with BCRL. There is a perception that lighter-load resistance training will produce less muscle damage and inflammation compared with higher-load resistance training (eg, large increases in CK have been observed after strenuous exercise46), and there are concerns that the inflammation would exacerbate BCRL as a result of impaired lymph flow. Furthermore, there are concerns that resistance exercise with heavy loads may exacerbate lymphedema symptoms, and women with BCRL are typically apprehensive about lifting weights, especially at heavier loads.7 However, participation in an acute high-load resistance exercise session has recently been demonstrated to not acutely increase swelling or symptom severity of the affected limb in women with BCRL.3 Importantly, the current study extends these previous observations to indicate that there may be no difference in the inflammatory response 24 hours following participation in acute bouts of resistance exercise involving low, moderate, or high loads. Because inflammatory markers returned to normal within 24 hours, there is little evidence that any clinically important inflammation occurred. However, it is important to note that these are preliminary observations based on a small sample of patients who were relatively active. Higher-load resistance exercise is known to stimulate greater morphological and neural adaptations compared with lower loads.41,47,49 As such, these preliminary findings suggest that women with BCRL can safely participate in moderate- to high-load resistance exercise.
Resistance training–induced muscle damage and subsequent inflammatory responses are mediated by a range of individual and training program variables.14,15 In the current study, increasing the load of resistance exercise did not elicit a significantly greater inflammatory response. Similarly, previous findings suggest that the load of upper-body resistance exercise may not affect the magnitude of change in blood markers of muscle damage and inflammation. Uchida et al29 compared 4 different loads of upper-body resistance exercise (ranging from 50% to 110% of 1 RM) on various inflammatory biomarkers, including CK, IL-6, and TNF-α in physically trained men. Regardless of the loading protocol, no changes in IL-6 or TNF-α were observed 24 hours after exercise. Uchida et al29 reported significant increases in CK 24 hours after each exercise trial; however, no difference was observed between the different loading protocols in the magnitude of CK change.29 Although there were clear differences in the participants sampled, exercise protocols, blood sampling, and measurement techniques between the previous and current studies, the findings were consistent. These initial findings suggest that BCRL and its associated pathology do not alter acute inflammatory response in the 24 hours following low-, moderate-, and high-load resistance exercise.
Although more than half of the participants had chronic lymphedema (present for on average 9 years), our findings suggest that symptom severity may still acutely improve with appropriately prescribed resistance exercise. Consistent with previous findings,50 a significant reduction in tightness of the affected arm was observed 24 hours after a high-load resistance exercise session. The possible mechanisms of such benefit are unknown, but it could be speculated that more effective hydrostatic pressure drove lymph return with more forceful muscle actions. In addition, more forceful muscle actions may result in favorable hormone and cytokine production, affecting the tissue locally and systemically. The observation that high-load resistance exercise did not induce significantly greater exercise-induced muscle damage or inflammation in the present study is supported by the subjective improvements in symptom severity of the affected limb 24 hours after exercise. It is also important to consider that although many of the participants in the study were not accustomed to performing regular resistance exercise, participants were generally physically active and had undergone a 2-week familiarization protocol. Regular exercise may cause a cumulative anti-inflammatory effect in response to repeated bouts of exercise.25,51 Participants were likely accustomed to physical activity (albeit not resistance exercise in particular); therefore, these findings may not represent the acute inflammatory response of sedentary or less physically active women with BCRL. However, previous research in healthy individuals indicates that even a single bout of eccentric exercise results in considerable protection from muscle damage in subsequent training sessions.30
This is the first study to our knowledge that has compared whether resistance exercise load influences the exercise-induced inflammatory response and the potential implications this may have for women with BCRL. We examined various markers of exercise-induced muscle damage and inflammation in response to varying exercise loads. Importantly, all levels of loading (low, moderate, and high) were equally tolerable in this sample of women with BCRL. Nevertheless, our study was subject to several limitations that are worthy of comment. First, our study was limited by a relatively small sample size, which may limit our ability to detect statistically significant differences. Second, blood samples were only assessed 24 hours postexercise. As a result, we cannot discount that the lack of significant increase or difference among the 3 exercise bouts may be a result of the clearance of cytokines prior to blood withdrawal. Although more frequent sampling would have been preferable to demonstrate changes in cytokines following resistance exercise (eg, Izquierdo et al19), 24 hours between blood samples was selected to alleviate patient concerns and burden among patients with BCRL. Additionally, systemic biomarkers were assessed, and it is acknowledged that local responses within skeletal muscle may differ.
Findings from the present study add to the existing body of knowledge by establishing that acute bouts of moderate- and high-load resistance exercise may not induce a greater level of muscle damage or inflammation compared with low-load resistance exercise in breast cancer survivors who have established lymphedema. This extends previous research demonstrating that high-load resistance exercise does not exacerbate lymphedema or associated symptoms of the affected limb.3,4 Data from this present study may inform future exercise intervention studies, and given the greater time efficiency and strength adaptation resulting from moderate- to high-load resistance training,41 the efficacy of low-load resistance exercise appears limited.41 It is well established that a strong dose-response relationship exists with resistance exercise load.41 Greater improvements in muscle strength and hypertrophy, physical function, and health-related quality of life are experienced with higher load resistance exercise compared with low loads.47,48,52 Participants reported the same tolerability and RPE regardless of load, further supporting the potential appropriateness of moderate to high loads for this population. Because regular exercise has been shown to reduce resting levels of inflammatory cytokines in breast cancer survivors without lymphedema,51 future studies should aim to examine changes in chronic low-grade inflammation with longer-term (eg, ≥12 weeks) exercise in women with BCRL.
Conclusions
As research continues to emerge demonstrating the safety and efficacy of resistance exercise in women with BCRL, there remains a limited amount of research investigating the physiological responses underlying these benefits. Findings from the current exploratory study demonstrate that the magnitude of exercise-induced muscle damage and inflammation following upper-body resistance exercise in women with BCRL may not be dependent on the load of resistance exercise and that low-, moderate-, and high-load upper-body resistance exercise may not result in a greater 24-hour inflammatory response in individuals with impaired lymph flow in the upper limb. Lymphedema status and symptom severity as well as perceived tolerability and exertion of resistance exercise were also not affected by the load lifted. Given the extensive evidence that moderate to high loads in resistance training produce greater strength and morphological adaptations as well as physical function and health-related quality of life compared with low loads,47,48,52 this study lends further support for the prescription of moderate- to high-load exercise for women with BCRL.
Supplementary Material
Acknowledgments
We would like to thank exercise physiologists Courtney Ishiguchi, Mark Trevaskis, Melissa Newton, and Jena Buchan.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the Cancer Council Western Australia and Cancer Council Queensland Project Grants. SH is supported by a Cancer Council Queensland Fellowship. DAG is supported by a Cancer Council Western Australia Fellowship.
References
- 1. Casla S, Hojman P, Márquez-Rodas I, et al. Running away from side effects: physical exercise as a complementary intervention for breast cancer patients. Clin Transl Oncol. 2015;17:180-196. [DOI] [PubMed] [Google Scholar]
- 2. Baumann FT. Physical exercise programs following cancer treatment. Eur Rev Aging Phys Act. 2013;10:57-59. [Google Scholar]
- 3. Cormie P, Galvão DA, Spry N, Newton RU. Neither heavy nor light load resistance exercise acutely exacerbates lymphedema in breast cancer survivor. Integr Cancer Ther. 2013;12:423-432. [DOI] [PubMed] [Google Scholar]
- 4. Cormie P, Pumpa K, Galvão DA, et al. Is it safe and efficacious for women with lymphedema secondary to breast cancer to lift heavy weights during exercise: a randomised controlled trial. J Cancer Surviv. 2013;7:413-424. [DOI] [PubMed] [Google Scholar]
- 5. Schmitz KH, Ahmed RL, Troxel A, et al. Weight lifting in women with breast-cancer–related lymphedema. N Engl J Med. 2009;361:664-673. [DOI] [PubMed] [Google Scholar]
- 6. Shapiro CL, Recht A. Side effects of adjuvant treatment of breast cancer. N Engl J Med. 2001;344:1997-2008. [DOI] [PubMed] [Google Scholar]
- 7. Meiklejohn J, Heesch K, Janda M, Hayes SC. Physical activity in the lives of those living with lymphoedema following cancer treatment. Lymphology. 2013;44(suppl):131-137. [Google Scholar]
- 8. Moseley A, Piller N. The assessment and care of the patient with secondary limb lymphoedema. Aust Nurs J. 2002;10(2):1-4. [PubMed] [Google Scholar]
- 9. Cemal Y, Pusic A, Mehrara BJ. Preventative measures for lymphedema: separating fact from fiction. J Am Coll Surg. 2011;213:543-551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murton AJ, Billeter R, Stephens FB, et al. Transient transcriptional events in human skeletal muscle at the outset of concentric resistance exercise training. J Appl Physiol (1985). 2014;116:113-125. [DOI] [PubMed] [Google Scholar]
- 11. Clarkson PM, Nosaka K, Braun B. Muscle function after exercise-induced muscle damage and rapid adaptation. Med Sci Sports Exerc. 1992;24:512-520. [PubMed] [Google Scholar]
- 12. McKune AJ, Semple SJ, Peters-Futre EM. Acute exercise-induced muscle injury. Biol Sport. 2012;29:3-10. [Google Scholar]
- 13. Hortobágyi T, Denahan T. Variability in creatine kinase: methodological, exercise and clinically related factors. Int J Sports Med. 1989;10:69-80. [DOI] [PubMed] [Google Scholar]
- 14. Calle MC, Fernandez ML. Effects of resistance training on the inflammatory response. Nutr Res Pract. 2010;4:259-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Clarkson PM, Hubal MJ. Exercise-induced muscle damage in humans. Am J Phys Med Rehabil. 2002;81(11, suppl):52-69. [DOI] [PubMed] [Google Scholar]
- 16. Fridén J, Lieber RL. Eccentric exercise-induced injuries to contractile and cytoskeletal muscle fibre components. Acta Physiol Scand. 2001;171:321-326. [DOI] [PubMed] [Google Scholar]
- 17. Heavens KR, Szivak TK, Hooper DR, et al. The effects of high intensity short rest resistance exercise on muscle damage markers in men and women. J Strength Cond Res. 2014;28:1041-1049. [DOI] [PubMed] [Google Scholar]
- 18. Howatson G, van Someren KA. The prevention and treatment of exercise-induced muscle damage. Sports Med. 2008;38:483-503. [DOI] [PubMed] [Google Scholar]
- 19. Izquierdo M, Ibañez J, Calbet JAL, et al. Cytokine and hormone responses to resistance training. Eur J Appl Physiol. 2009;107:397-409. [DOI] [PubMed] [Google Scholar]
- 20. Salles BFd, Simão R, Fleck SJ, Dias I, Kraemer-Aguiar LG, Bouskela E. Effects of resistance training on cytokines. Int J Sports Med. 2010;31:441-450. [DOI] [PubMed] [Google Scholar]
- 21. Buford TW, Cooke MB, Willoughby DS. Resistance exercise-induced changes of inflammatory gene expression within human skeletal muscle. Eur J Appl Physiol. 2009;107:463-471. [DOI] [PubMed] [Google Scholar]
- 22. Clarkson PM, Kearns AK, Rouzier P, Rubin R, Thompson PD. Serum creatine kinase levels and renal function measures in exertional muscle damage. Med Sci Sports Exerc. 2006;38:623-627. [DOI] [PubMed] [Google Scholar]
- 23. Pedersen BK, Febbraio MA. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379-1406. [DOI] [PubMed] [Google Scholar]
- 24. Serrão FV, Foerster B, Spada S, et al. Functional changes of human quadriceps muscle injured by eccentric exercise. Braz J Med Biol Res. 2003;36:781-786. [DOI] [PubMed] [Google Scholar]
- 25. Anne Marie WP, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol (1985). 2005;98:1154-1162. [DOI] [PubMed] [Google Scholar]
- 26. Mastana SS, Gleeson M, Lindley MR, Stensel DJ, Bishop NC, Nimmo MA. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat Rev Immunol. 2011;11:607-615. [DOI] [PubMed] [Google Scholar]
- 27. Olson TP, Dengel DR, Leon AS, Schmitz KH. Changes in inflammatory biomarkers following one-year of moderate resistance training in overweight women. Int J Obes. 2007;31:996-1003. [DOI] [PubMed] [Google Scholar]
- 28. Petersen AMW, Pedersen BK. The role of IL-6 in mediating the anti-inflammatory effects of exercise. J Physiol Pharmacol. 2006;57(10):43-51. [PubMed] [Google Scholar]
- 29. Uchida MC, Nosaka K, Ugrinowitsch C, et al. Effect of bench press exercise intensity on muscle soreness and inflammatory mediators. J Sports Sci. 2009;27:499-507. [DOI] [PubMed] [Google Scholar]
- 30. Hirose L, Nosaka K, Newton M, et al. Changes in inflammatory mediators following eccentric exercise of the elbow flexors. Exerc Immunol Rev. 2004;10:75-90. [PubMed] [Google Scholar]
- 31. Mortimer PS, Levick JR. Chronic peripheral oedema: the critical role of the lymphatic system. Clin Med. 2004;4:448-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ridner SH. Pathophysiology of lymphedema. Semin Oncol Nurs. 2013;29:4-11. [DOI] [PubMed] [Google Scholar]
- 33. Rockson SG. The lymphatics and the inflammatory response: lessons learned from human lymphedema. Lymphat Res Biol. 2013;11:117-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dylke ES, Ward LC, Meerkin JD, Nery L, Kilbreath SL. Tissue composition changes and secondary lymphedema. Lymphat Res Biol. 2013;11:211-218. [DOI] [PubMed] [Google Scholar]
- 35. Wiig H, Swartz MA. Interstitial fluid and lymph formation and transport: physiological regulation and roles in inflammation and cancer. Physiol Rev. 2012;92:1005-1060. [DOI] [PubMed] [Google Scholar]
- 36. Olszewski WL. Pathophysiological aspects of lymphedema of human limbs: I. Lymph protein composition. Lymphat Res Biol. 2003;1:235-243. [DOI] [PubMed] [Google Scholar]
- 37. Ward LC, Bunce IH, Cornish BH, Mirolo BR, Thomas BJ, Jones LC. Multi-frequency bioelectrical impedance augments the diagnosis and management of lymphoedema in post-mastectomy patients. Eur J Clin Invest. 1992;22:751-754. [DOI] [PubMed] [Google Scholar]
- 38. Hayes SC, Speck RM, Reimet E, Stark A, Schmitz KH. Does the effect of weight lifting on lymphedema following breast cancer differ by diagnostic method: results from a randomized controlled trial. Breast Cancer Res Treat. 2011;130:227-234. [DOI] [PubMed] [Google Scholar]
- 39. Cheville AL, McGarvey CL, Petrek JA, Russo SA, Thiadens SR, Taylor ME. The grading of lymphedema in oncology clinical trials. Semin Radiat Oncol. 2003;13:214-225. [DOI] [PubMed] [Google Scholar]
- 40. Baechle TR, Earle RW, Wathen D. Resistance training. In: Baechle TR, Earle RW, eds. Essentials of Strength Training and Conditioning. Champaign, IL: Human Kinetics; 2008:381-412. [Google Scholar]
- 41. American College of Sports Medicine. American College of Sports Medicine position stand: progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009;41:687-708. [DOI] [PubMed] [Google Scholar]
- 42. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377-381. [PubMed] [Google Scholar]
- 43. Schmitz KH, Troxel AB, Cheville A, et al. Physical activity and lymphedema (the PAL trial): assessing the safety of progressive strength training in breast cancer survivors. Contemp Clin Trials. 2009;30:233-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45-56. [DOI] [PubMed] [Google Scholar]
- 45. Tee JC, Bosch AN, Lambert MI. Metabolic consequences of exercise-induced muscle damage. Sports Med. 2007;37:827-836. [DOI] [PubMed] [Google Scholar]
- 46. Koch AJ, Pereira R, Machado M. The creatine kinase response to resistance exercise. J Musculoskelet Neuronal Interact. 2014;14:68-77. [PubMed] [Google Scholar]
- 47. Seynnes O, Fiatarone Singh MA, Hue O, Pras P, Legros P, Bernard PL. Physiological and functional responses to low-moderate versus high-intensity progressive resistance training in frail elders. J Gerontol A Biol Sci Med Sci. 2004;59:503-509. [DOI] [PubMed] [Google Scholar]
- 48. Rhea MR, Alvar BA, Burkett LN, Ball SD. A meta-analysis to determine the dose response for strength development. Med Sci Sports Exerc. 2003;35:456-464. [DOI] [PubMed] [Google Scholar]
- 49. Fry AC. The role of resistance exercise intensity on muscle fibre adaptations. Sports Med. 2004;34:663-679. [DOI] [PubMed] [Google Scholar]
- 50. Johansson K, Piller N. Weight-bearing exercise and its impact on arm lymphoedema. J Lymphoedema. 2007;2(1):15-22. [Google Scholar]
- 51. Gómez AM, Martínez C, Fiuza-Luces C, et al. Exercise training and cytokines in breast cancer survivors. Int J Sports Med. 2011;32:461-467. [DOI] [PubMed] [Google Scholar]
- 52. Singh NA, Stavrinos TM, Scarbek Y, Galambos G, Liber C, Fiatarone Singh MA. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. J Gerontol A Biol Sci Med Sci. 2005;60:768-776. [DOI] [PubMed] [Google Scholar]
- 53. Australian Government. Australia’s Physical Activity and Sedentary Behaviour Guidelines. Canberra, Australia: Department of Health; 2012:2. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.