Abstract
Objective. Both the Chinese herbal compound Songyou Yin (SYY) and swimming exercise have been shown to have protective effects against liver cancer in animal models. In this study, we investigated whether SYY and moderate swimming (MS) have enhanced effect on suppressing progression of liver cancer by immunomodulation. Methods. C57BL/6 mice were transplanted with Hepa1-6 murine liver cancer cell lines and received treatment with SYY alone or SYY combined with MS. The green fluorescent protein (GFP)-positive metastatic foci in lungs were imaged with a stereoscopic fluorescence microscope. Flow cytometry was used to test the proportion of CD4 +, CD8 + T cells in peripheral blood and the proportions of CD4 + CD25 + Foxp3 + Treg cells in peripheral blood, spleen, and tumor tissues. Cytokine transforming growth factor (TGF)-β1 level in serum was detected by ELISA. Results. SYY plus MS significantly suppressed the growth and lung metastasis of liver cancer and prolonged survival in tumor-burdened mice. SYY plus MS markedly raised the CD4 to CD8 ratio in peripheral blood and lowered the serum TGF-β1 level and the proportions of Treg cells in peripheral blood, spleen, and tumor tissue. The effects of the combined intervention were significantly superior to SYY or MS alone. Conclusion. The combined application of SYY and MS exerted an enhanced effect on suppressing growth and metastasis of liver cancer by strengthening immunity.
Keywords: herbal medicine, swimming, metastasis, liver cancer, immunity
Introduction
Primary liver cancer, mainly hepatocellular carcinoma (HCC), is the third leading cause of cancer deaths worldwide. The poor prognosis is attributed mainly to its high recurrence and metastasis rate,1 and the lungs are the most common metastatic site.2 Currently, the main strategies against HCC include surgery, regional therapy (transarterial chemoembolization, radiofrequency ablation, ethanol injection, etc), radiotherapy, and targeted therapy.3,4 However, the effects of these measures are limited in the treatment of HCC. It is, thus, necessary to explore complementary approaches to preventing and limiting the progression of HCC. Immunity plays an emerging important role in the initiation and progression of cancer.5 It is expected that immunotherapy will bring breakthroughs for future cancer treatment.6 Physical exercise has been recognized to reduce the incidence and recurrence of cancers and improve survival by enhancing immunocompetence.7-10 In animal models, it has been found that swimming training could inhibit the growth of hepatoma in rat and mouse models by improving metabolism and immunity.11-13 Chinese herbal traditional medicine shows effectiveness in the treatment of cancer.14-16 Songyou Yin (SYY), a composite formula composed of 5 agents, has been demonstrated to suppress the growth, metastasis, and recurrence of HCC as well as prolong survival in the mouse model system.17-21 Two components of SYY, Astragalus membranaceus and Lycium barbarum have been confirmed to have anticancer and immunomodulatory effects,22,23 but the impact of SYY on immune function has not been clarified. A previous study showed that SYY could inhibit the expression of transforming growth factor (TGF)-β1 by activated hepatic stellate cells.21 TGF-β1 is a potent inhibitor of immune function, which plays a key role in the proliferation and activation of regulatory T cells (Treg cells), a subgroup of T cells with immunosuppressive properties.22 A previous study showed that both swimming exercise and SYY may have potential effects on immune modulation and tumor suppression. We investigated whether the 2 strategies have an enhanced effect against liver cancer. C57BL/6 immunocompetent mice transplanted with liver cancer cell lines were treated with SYY alone and combined with swimming training. We found that SYY combined with moderate swimming (MS) markedly suppressed growth and lung metastasis of transplanted liver cancer by raising the CD4 to CD8 ratio in the peripheral blood and reducing serum TGF-β1 levels and proportions of CD4 + CD25 + Foxp3 + Treg cells in peripheral blood, spleen, and tumor tissue in tumor-bearing mice; furthermore, SYY plus MS had an enhanced effect in suppressing tumor growth and metastasis and enhancing immunity. The effects were superior to SYY or MS alone.
Materials and Methods
Cell Line and Culture
The murine liver cancer cell line Hepa1-6 was purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China) and was maintained in Dulbecco’s modified eagle medium (Genom, Hangzhou, China), supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA) and 1% penicillin-streptomycin (Genom, Hangzhou, China). For in vivo injections, cancer cells were trypsinized and centrifuged at 1000 rpm for 5 minutes at 4°C, washed twice, and reconstituted in phosphate buffered saline solution (PBS) (Genom, Hangzhou, China).
Animals and Hepatoma Model
A total of 48 male C57BL/6 mice (6 weeks old) were purchased from the Animal Center of the Chinese Academy of Science. The mice were habituated to vivarium conditions for 1 week before initiation of the intervention procedures. C57BL/6 mice were injected with Hepa1-6 cells subcutaneously (Hepa1-6, 5 × 106 cells) and through the tail vein (Hepa1-6–green fluorescent protein [GFP], 3 × 106 cells) to establish subcutaneous tumor and experimental lung metastasis models, respectively.23 All experiments were approved by the Institutional Animal Care and Use Committee, Fudan University.
Characterization and Preparation of Herbal Extracts
The Chinese herbal medicine formula SYY, a dietary component, is authorized by the Chinese State Food and Drug Administration (Grant No. G20070160). The mixture includes 5 Chinese medicinal herbal extracts with a fingerprint in the following proportions (w/w): Salvia miltiorrhiza Bge, 14.3%; A membranaceus Bge, 14.3%; L barbarum L, 23.8%; Crataegus pinnatifida Bge, 23.8%, and Trionyx sinensis Wiegmann, 23.8% (all from China). The fingerprint and protocol of preparation have previously been reported.17 SYY used in vitro with the same batch number (#20120901) was produced by Shanghai Fang Xin Pharmaceutical Technology Co, Ltd. (Shanghai, China).
Mice Grouping and Treatment Schema
The mice were divided into 4 groups (12 mice in each group): (1) control group, (2) SYY group, (3) MS group, and (4) SYY-MS group. Each mouse in the SYY group received 0.2 mL of SYY (4 g/kg/d per mouse) via the oral gavage method after inoculation. The MS group mice were subjected to swimming training in water (28°C-30°C) 5 days per week. For adaptation to swimming, all trained mice swam 5 minutes in the first week, 6 min/d in the second week, and 8 min/d in the third week. The mice were injected with liver cancer lines at the end of the third week. This level of swimming (8 min/d) was maintained after inoculation. Based on our pilot study, voluntary swimming for 8 min/d was defined as moderate swimming. The SYY-SM group combined application of SYY and MS. At day 42 after inoculation, 6 mice of each group were killed humanely by cervical dislocation under anesthesia, and peripheral blood, subcutaneous tumor, spleen, and lung were retrieved for examination; the remaining mice of each group were maintained for survival observation. Subcutaneous tumors were completely stripped from mice and then weighed using a precision electronic scale.
Lung Metastasis Evaluation
The fluorescent protein–positive (GFP) metastatic foci in lungs were imaged with a stereoscopic fluorescence microscope (Leica M205FA, Image Source: Leica DFC500, Germany); then, Image-Pro Plus software (Media Cybernetics, Bethesda, MD) was used to calculate the ratio of total size of metastatic foci to the size of a whole lung. This ratio was called the lung metastases ratio.
Flow Cytometry for CD4+, CD8+ T lymphocyte, CD4+ CD25+ Foxp3+ Treg Cell Detection
The peripheral blood, spleen, and tumor tissue were acquired from mice under anesthesia using aseptic procedures. The procedures of isolation of peripheral blood monocyte (PBMC), spleen lymphocyte, and tumor-infiltrating lymphocyte (TIL) conform to those in the previous report.24,25 Isolated lymphocytes were washed with PBS supplemented with 2% fetal bovine serum. The washed cells were incubated with antimouse CD3-FITC, CD4-PC, and CD8-PE (BD Pharmingen, USA) at 4°C for 15 minutes. Tregs were detected using a mouse Treg detection kit (anti-CD4-FITC/anti-CD25-APC/anti-FoxP3-PE) in accordance with the manufacturer’s instructions (Miltenyi Biotec, Germany). Then, fluorescent antibody incubated cells were analyzed with flow cytometry using a FACSCalibur (BD Pharmingen, USA).
Determination of TGF-β1 Concentration in the Serum of Mice
TGF-β1 levels were detected in the serum of mice using a mouse TGF-β1 ELISA kit (Boster, Wuhan, China) in accordance with the manufacturer’s instructions.
Statistical Analysis
All statistical analyses were performed using SPSS v17. Continuous variables were compared with the Student’s t test (between 2 groups) or analysis of variance (ANOVA, among all groups) if normally distributed. Kaplan-Meier survival analysis with the log-rank test was used to evaluate the relationship between various interventions and survival. For the analysis, a P value <.05 in a 2-tailed test was considered statistically significant. Charts and graphs were drawn with GraphPad Prism 5.0.
Results
SYY Combined With MS Significantly Suppressed Growth and Lung Metastasis of Transplanted Liver Cancer and Prolonged Survival in a Mouse Model
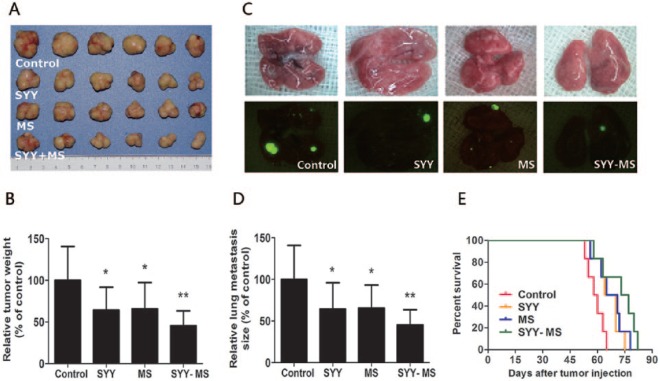
On day 42 after inoculation of liver cancer cell lines, compared with the control group, the mean weight of the subcutaneous tumors was reduced by 22.9% in the SYY group (control 3.19 ± 1.12 g vs SYY 2.46 ± 0.77 g, P < .05), 19.4% in the MS group (control 3.19 ± 1.12 g vs MS 2.57 ± 0.68 g, P < .05), and 56.4% in the SYY-MS groups (control 3.19 ± 1.12 g vs SYY-MS 1.39 ± 0.52 g, P < .01; Figures 1A and 1B). Compared with the control group, the mean lung metastases ratio was reduced by 35.6% in the SYY group (control 10.16% ± 4.13% vs SYY 6.54% ± 3.21%, P < .05), 34.3% in the MS group (control 10.16% ± 4.13% vs MS 6.68% ± 2.79%, P < .05), and 54.5% in the SYY-MS groups (control 10.16% ± 4.13% vs SYY-MS 4.62% ± 1.83%, P < .01; Figures 1C and 1D). In the log-rank survival analysis, the median survival time, compared with the control group, was prolonged by 8 days in the SYY group (control 59 days vs SYY 67 days, P = .041), by 9 days in the MS group (control 59 days vs MS 68 days, P = .041), and by 16 days in the MS-SYY group (control 59 days vs SYY-MS 75 days, P = .016; Figure 1E).
Figure 1.
SYY combined with MS suppressed growth and lung metastases of transplanted liver cancer in a mouse model. (A) Subcutaneous Hepa1-6 hepatomas were dissected 42 days after inoculation. Hepatomas were analyzed from each of the 4 groups (control, SYY, MS, and SYY-MS; n = 6 in each group). The tumor weights (mean ± SD) were quantified and plotted in (B). (C) Four representative foci from lung metastases from Hepa1-6-GFP-inoculated animals (n = 6 in each group) 42 days after injection through the tail vein. The relative lung metastases sizes (mean ± SD) are quantified and plotted in (D). (E) Log-rank survival analysis of the 4 groups. For all panels, *P < .05 and **P <.01.
Abbreviations: SYY, Songyou Yin; MS, moderate swimming; GFP, green fluorescent protein.
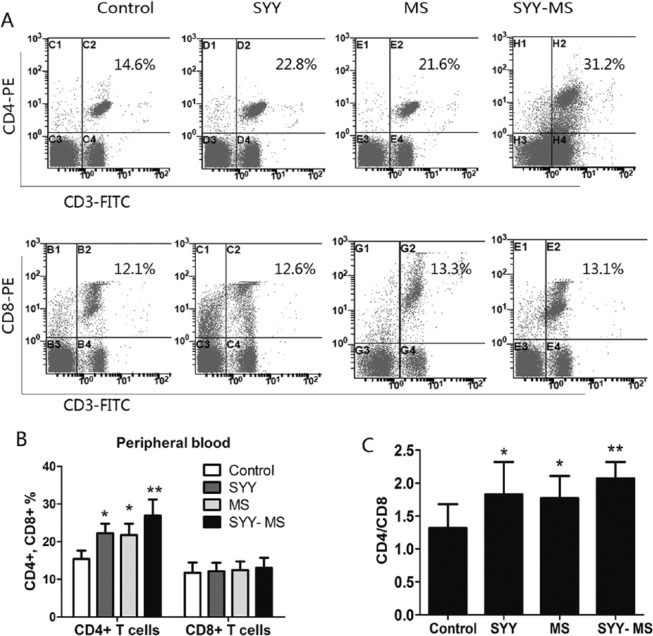
SYY Combined With MS Markedly Increased CD4/CD8 Ratio in Peripheral Blood
Compared with the control group, CD4 + T lymphocyte proportion in PBMC was increased in the SYY group (P < .05) and MS group (P < .05) and dramatically in the SYY-MS group (P < .01). There was no significant difference in CD8 + T lymphocyte proportions in PBMC among the 4 groups (Table 1, Figures 2A and 2B). Compared with the control group, the CD4 to CD8 ratio was increased in the SYY group (P < .05) and MS group (P < .05) and dramatically in the MS-SYY group (P < .01, Table 1, Figure 2C).
Table 1.
Peripheral Blood CD4+ and CD8+ T Cell Proportion and CD4 to CD8 Ratio in the 4 Groups.a
| Control | SYY | MS | SYY-MS | |
|---|---|---|---|---|
| CD4+ T cell (%) | 15.38 ± 2.24 | 22.26 ± 2.51* | 21.96 ± 3.01* | 26.88 ± 4.32** |
| CD8+ T cell (%) | 11.68 ± 2.76 | 12.15 ± 2.14* | 12.43 ± 2.27* | 13.02 ± 2.68** |
| CD4/CD8 ratio | 1.32 ± 0.36 | 1.83 ± 0.49* | 1.77 ± 0.34* | 2.07 ± 0.25** |
Abbreviations: SYY, Songyou Yin; MS, moderate swimming.
Data are presented as the mean ± SD. *P < .05, **P < .01 (indicates a significant difference with respect to the control group).
Figure 2.
SYY combined with MS increased the CD4 to CD8 ratio in peripheral blood. (A) Flow cytometric analysis of CD4+ and CD8+ T cell proportions in PBMC from tumor-burdened C57 mice treated with SYY, MS, and SYY-MS. The proportion of CD4+ and CD8+ T cells in PBMC were quantified and plotted in (B). (C) CD4+/CD8+ T cells ratio of PBMC from C57BL/6 mice of the 4 groups. For all panels, n = 6 in each group. For all panels, *P < .05 and **P < .01.
Abbreviations: SYY, Songyou Yin; MS, moderate swimming; PBMC, peripheral blood monocyte.
SYY Combined With MS Markedly Reduced Serum TGF-β1 Levels
Serum TGF-β1 levels were detected in mice of 4 groups. Compared with the control group, TGF-β1 was significantly decreased (Figure 3) in the SYY group (control 50.16 ± 16.35 pg/mL vs SYY 39.17 ± 13.26 pg/mL, P < .05), MS group (control 50.16 ± 16.35 pg/mL vs MS 36.53 ± 12. 46 pg/mL, P < .05), and SYY-MS group (control 50.16 ± 16.35 pg/mL vs SYY-MS 31.64 ± 10.19 pg/mL, P < .01).
Figure 3.
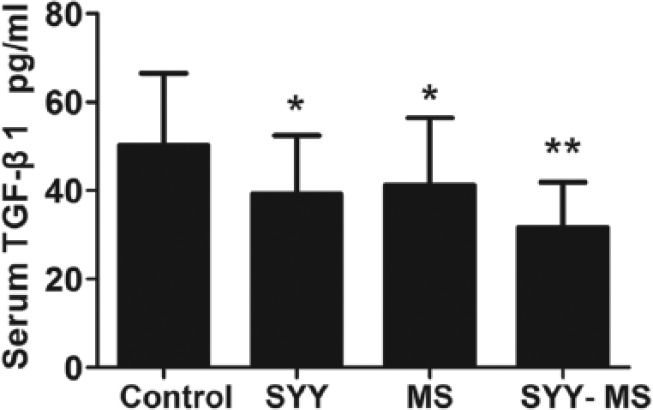
TGF-β1 levels in the serum of tumor-burdened mice exposed to SYY, MS, and SYY-MS; n = 6 in each group. *P < .05 and **P < .01.
Abbreviations: SYY, Songyou Yin; MS, moderate swimming; TGF, transforming growth factor.
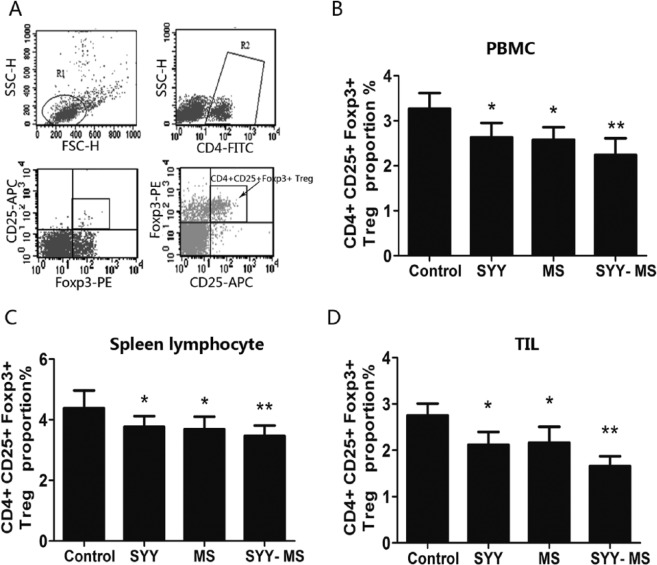
SYY Combined With MS Markedly Decreased CD4+ CD25+ Foxp3+ Treg Proportion in Peripheral Blood, Spleen, and Tumor Tissue
Tregs comprise a subgroup of T lymphocytes with immune suppressive properties. Compared with controls, the proportion of CD4+ CD25+ Foxp3+ Tregs in PBMC, spleen lymphocytes, and TIL were significantly decreased in the SYY (P < .05) and MS groups (P < .05) and dramatically in the SYY-MS group (P < .01, Table 2 and Figures 4A-4D).
Table 2.
The Proportion of CD4+ CD25+ Foxp3+ Treg Cells in Peripheral Blood, Spleen, and Tumor Tissues.a
| Treg Cells Proportion | Control | SYY | MS | SYY-MS |
|---|---|---|---|---|
| In peripheral blood (%) | 3.27 ± 0.35 | 2.63 ± 0.32* | 2.58 ± 0.28* | 2.24 ± 0.37** |
| In spleen (%) | 4.38 ± 0.52 | 3.77 ± 0.33* | 3.69 ± 0.41* | 3.46 ± 0.29** |
| In tumor tissue (%) | 2.75 ± 0.26 | 2.12 ± 0.28* | 2.16 ± 0.35* | 1.66 ± 0.21** |
Abbreviations: SYY, Songyou Yin; MS, moderate swimming.
Data are presented as the mean ± SD. *P < .05, **P < .01 (indicates a significant difference with respect to the control group).
Figure 4.
SYY combined with MS reduced CD4+CD25+Foxp3+ Treg cell proportions in PBMC, spleen, and TIL from tumor-burdened mice. A. Flow cytometric selection and analysis for CD4+ CD25+ Foxp3+ Treg cell. B. Treg cell proportion in PBMC from C57BL/6 mice. C. Treg cell proportion in spleen lymphocytes. D. Treg cell proportion in TIL. For all panels, n = 6 in each group. For all panels, *P < .05 and **P < .01.
Abbreviations: SYY, Songyou Yin; MS, moderate swimming; PBMC, peripheral blood monocyte; TIL, tumor-infiltrating lymphocytes.
Discussion
With a history of more than 2000 years, traditional Chinese Medicine (TCM) has formed a unique theoretical system and had a practical clinical effect. Chinese herbal medicine is the most common modality of TCM.15,26 TCM was used against cancer for centuries, and one of its primary treatment principles is Fuzheng—namely, enhancing immunocompetence.27 As is well known, physical exercise has been observed to reduce the morbidity and mortality associated with malignant tumors, mainly by enhancement of immunity.9,10
An immunosuppressive status facilitates growth and metastasis of malignant tumors, and immunotherapy for cancer has been specifically emphasized recently.6 This study showed that the Chinese herbal formula SYY combined with moderate swimming training markedly suppressed growth and experimental lung metastasis of liver cancer and prolonged survival in a mouse model by enhancing immune function. SYY plus MS markedly raised the CD4 to CD8 ratio and reduced the serum TGF-β1 levels and proportion of Tregs in peripheral blood, spleen, and tumor tissue in tumor-burdened mice. The effects of the combined intervention were significantly superior to SYY or MS alone.
There are many studies of drug combinations (including combinations of Western medicines or Western medicine and herbal medicine) in the therapy of cancers, and most of them exhibit enhanced or synergistic efficacy and reduced toxicity.28-32 To our knowledge, there is no related report on the combined application of physical exercise and herb medicine in cancers. But there are studies on combined exercise and diet. A previous study showed that both short-term moderate exercise training and consumption of the soluble oat β-glucan can decrease the metastatic spread of injected B16 melanoma cells by increasing macrophage cytotoxicity; the 2 therapies, however, had no additive effects.11 It has also been shown that swimming training may attenuate liver carcinogenesis under an adequate dietary regimen with lowered fat intake.13
CD4+ T cells play a central role in initiating and maintaining anticancer immune responses.33 Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with HCC.34 Clinically, the CD4 to CD8 ratio is a key criterion to evaluate the immune status, and a reverse CD4 to CD8 ratio usually indicates tumor progression and poor prognosis.29,31 The cytokine TGF-β plays a role in immunosuppression, and tumors that produce high levels of TGF-β may be shielded from immune surveillance.35 Treg cells, a subgroup of T cells with immunosuppressive property have been confirmed to play an important role in HCC.22,32 Liver cancer cells can recruit Tregs or induce differentiation of CD4+ T cells to Tregs by secreting TGF-β1, as a result creating an immunosuppressive microenvironment that promotes tumor growth and metastasis.22,36
The effect of SYY in the treatment of liver cancer has been studied for nearly 10 years. This series of studies showed that SYY can inhibit growth and metastasis of liver cancer by downregulating vascular endothelial growth factor (VEGF) and matrix metalloproteinase 2 expression,17 enhancing the antitumor capacity of interferon-α,18 reversing epithelial to mesenchymal transition (EMT) induced by chemotherapy,19 reducing the proportion of cancer stem cells in liver cancer tissue cells,20 and inhibiting the secretion of cytokines: hepatocyte growth factor, interleukin 6, TGF-β, and VEGF by hepatic stellate cells.21 These experimental results indicate that the tumor-suppressing effect of SYY is complex. The present study revealed that enhancing immunity is the novel mechanism underlying SYY suppression of liver cancer. In this study, SYY suppressed experimental growth and lung metastasis of liver cancer in mice by raising CD4+ T lymphocytes in peripheral blood and reducing serum TGF-β1 level and CD4+ CD25+ Foxp3+ Treg percentage in blood, spleen, and tumor tissue. TGF-β potently suppresses CD4+ T cell differentiation and effector functions of cytotoxic CD8+ T cells37 and promotes proliferation and activation of Treg cells.22 Previous studies showed that SYY inhibits the secretion of TGF-β by hepatic stellate cells21; thus, reduction of serum TGF-β1 may be an initiating factor causing the reduction of Treg proportions and rise of CD4 to CD8 ratio in the study.
The World Health Organization recommends regular physical activity to reduce the risk of cancers,38 and physical activity has even been recommended as a standard cancer treatment.10 The mechanism underlying the ability of physical exercise to suppress tumor progression is quite complicated. Postulated mechanisms underlying the potential effects of exercise on cancer progression include modulation of metabolic (eg, glucose-insulin homeostasis) and sex-steroid (eg, estrogens) hormone levels, improvements in immune surveillance, and reduced systemic inflammation and oxidative damage.39 Among these, exercise-induced immunomodulation has received a great deal of attention in the exercise oncology field.40-43 Furthermore, physical activity can have different influences on carcinogenesis, depending on intensity, duration, and frequency of activity.44 Moderate regular exercise exhibits cancer-preventive potential, whereas exhaustive exercise may increase the risk of development of some cancers.45 Wang et al41 showed that moderate-intensity exercise lowered the risk of cancer and infectious illness through enhancing proinflammatory responses, whereas high-intensity exercise might increase the risk of common infections because of an upregulation of CD4+CD25+ Treg cells and anti-inflammatory responses. Demarzo et al46 reported that exhaustive physical exercise increased the susceptibility of rats to colon cancer via increased free radical DNA oxidative damage and depressed immune function. Murphy et al,11 however, reported that moderate exercise can decrease lung metastases of B16 melanoma via increased macrophage antitumor cytotoxicity. Consistent with previous studies, our results showed that MS exercise suppressed growth and lung metastasis of liver cancer in mice via enhancement of immunity represented by a rise in CD4 to CD8 ratio in peripheral blood and reduction of serum TGF-β1 and CD4+ CD25+ Foxp3+ Treg proportion in PBMC, spleen lymphocytes, and TIL.
Conclusion
The combined intervention of SYY and MS exerted enhanced effects on suppressing growth and lung metastasis of liver cancer via dramatic enhancement of immunocompetence, and the effect is superior to SYY or MS alone. Although the herbal compound SYY and swimming exercise are 2 quite different types of intervention, they had similar effects on strengthening immunity and suppressing liver cancer progression. Whether this model would be beneficial for patients suffering from malignant tumors would need further clinical study in the future.
Footnotes
Author’s Note: Quan-Bao Zhang and Xiang-Ting Meng contributed equally to the work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This project was supported by the National Natural Science Foundation of China (Grant Number: 81372314, 81173391, 81272565), and the National Key Project for Infectious Diseases of China (2012ZX10002012-004).
References
- 1. Tang ZY, Ye SL, Liu YK, et al. A decade’s studies on metastasis of hepatocellular carcinoma. J Cancer Res Clin Oncol. 2004;130:187-196. [DOI] [PubMed] [Google Scholar]
- 2. Abbas A, Medvedev S, Shores N, et al. Epidemiology of metastatic hepatocellular carcinoma, a nationwide perspective. Dig Dis Sci. 2014;59:2813-2820. [DOI] [PubMed] [Google Scholar]
- 3. Verslype C, Van Cutsem E, Dicato M, et al. The management of hepatocellular carcinoma: current expert opinion and recommendations derived from the 10th World Congress on Gastrointestinal Cancer, Barcelona, 2008. Ann Oncol. 2009;20(suppl 7):vii1-vii6. [DOI] [PubMed] [Google Scholar]
- 4. Kim KW, Lee JM, Choi BI. Assessment of the treatment response of HCC. Abdom Imaging. 2011;36:300-314. [DOI] [PubMed] [Google Scholar]
- 5. Heger M. Cancer immunotherapy shows promise in multiple tumor types. Nat Med. 2012;18:993. [DOI] [PubMed] [Google Scholar]
- 6. Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432-1433. [DOI] [PubMed] [Google Scholar]
- 7. Heinen MM, Verhage BA, Goldbohm RA, Lumey LH, van den Brandt PA. Physical activity, energy restriction, and the risk of pancreatic cancer: a prospective study in the Netherlands. Am J Clin Nutr. 2011;94:1314-1323. [DOI] [PubMed] [Google Scholar]
- 8. Ballard-Barbash R, Friedenreich CM, Courneya KS, Siddiqi SM, McTiernan A, Alfano CM. Physical activity, biomarkers, and disease outcomes in cancer survivors: a systematic review. J Natl Cancer Inst. 2012;104:815-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Abdalla DR, Murta EF, Michelin MA. The influence of physical activity on the profile of immune response cells and cytokine synthesis in mice with experimental breast tumors induced by 7,12-dimethylbenzanthracene. Eur J Cancer Prev. 2013;22:251-258. [DOI] [PubMed] [Google Scholar]
- 10. Giovannucci EL. Physical activity as a standard cancer treatment. J Natl Cancer Inst. 2012;104:797-799. [DOI] [PubMed] [Google Scholar]
- 11. Murphy EA, Davis JM, Brown AS, Carmichael MD, Mayer EP, Ghaffar A. Effects of moderate exercise and oat beta-glucan on lung tumor metastases and macrophage antitumor cytotoxicity. J Appl Physiol (1985). 2004;97(3):955-959. [DOI] [PubMed] [Google Scholar]
- 12. Almeida PW, Gomes-Filho A, Ferreira AJ, et al. Swim training suppresses tumor growth in mice. J Appl Physiol (1985). 2009;107:261-265. [DOI] [PubMed] [Google Scholar]
- 13. Aguiar e, Silva MA, Vechetti-Junior IJ, Nascimento AF, et al. Effects of swim training on liver carcinogenesis in male Wistar rats fed a low-fat or high-fat diet. Appl Physiol Nutr Metab. 2012;37:1101-1109. [DOI] [PubMed] [Google Scholar]
- 14. Wong BY, Nguyen DL, Lin T, et al. Chinese medicinal herb Scutellaria barbata modulates apoptosis and cell survival in murine and human prostate cancer cells and tumor development in TRAMP mice. Eur J Cancer Prev. 2009;18:331-341. [DOI] [PubMed] [Google Scholar]
- 15. Liu TG, Xiong SQ, Yan Y, Zhu H, Yi C. Use of Chinese herb medicine in cancer patients: a survey in southwestern China. Evid Based Complement Alternat Med. 2012;2012:769042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang N, Feng Y, Cheung F, Wang X, Zhang Z, Feng Y. A Chinese medicine formula Gegen Qinlian decoction suppresses expansion of human renal carcinoma with inhibition of matrix metalloproteinase-2. Integr Cancer Ther. 2015;14:75-85. [DOI] [PubMed] [Google Scholar]
- 17. Huang XY, Wang L, Huang ZL, Zheng Q, Li QS, Tang ZY. Herbal extract “Songyou Yin” inhibits tumor growth and prolongs survival in nude mice bearing human hepatocellular carcinoma xenograft with high metastatic potential. J Cancer Res Clin Oncol. 2009;135:1245-1255. [DOI] [PubMed] [Google Scholar]
- 18. Huang XY, Huang ZL, Wang L, et al. Herbal compound “Songyou Yin” reinforced the ability of interferon-alfa to inhibit the enhanced metastatic potential induced by palliative resection of hepatocellular carcinoma in nude mice. BMC Cancer. 2010;10:580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xiong W, Ren ZG, Qiu SJ, et al. Residual hepatocellular carcinoma after oxaliplatin treatment has increased metastatic potential in a nude mouse model and is attenuated by Songyou Yin. BMC Cancer. 2010;10:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jia QA, Ren ZG, Bu Y, et al. Herbal compound “Songyou Yin” renders hepatocellular carcinoma sensitive to oxaliplatin through inhibition of stemness. Evid Based Complement Alternat Med. 2012;2012:908601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jia QA, Wang ZM, Ren ZG, et al. Herbal compound “Songyou Yin” attenuates hepatoma cell invasiveness and metastasis through downregulation of cytokines secreted by activated hepatic stellate cells. BMC Complement Altern Med. 2013;13:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yang P, Li QJ, Feng Y, et al. TGF-beta-miR-34a-CCL22 signaling-induced Treg cell recruitment promotes venous metastases of HBV-positive hepatocellular carcinoma. Cancer Cell. 2012;22:291-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li Y, Tang Y, Ye L, et al. Establishment of a hepatocellular carcinoma cell line with unique metastatic characteristics through in vivo selection and screening for metastasis-related genes through cDNA microarray. J Cancer Res Clin Oncol. 2003;129:43-51. [DOI] [PubMed] [Google Scholar]
- 24. Yoon HL, Singh KP, Ratner S, Reiners JJ., Jr. Phorbol ester effects on splenic lymphocyte composition and cytotoxic T cell activities of SSIN mice: a strain deficient in CD8+ T cells. Carcinogenesis. 1996;17:2617-2624. [DOI] [PubMed] [Google Scholar]
- 25. Hara T, Nishimura H, Hasegawa Y, Yoshikai Y. Thymus-dependent modulation of Ly49 inhibitory receptor expression on NK1.1+gamma/delta T cells. Immunology. 2001;102:24-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang L, Wu C, Zhang Y, et al. Comparison of efficacy and toxicity of traditional Chinese medicine (TCM) herbal mixture LQ and conventional chemotherapy on lung cancer metastasis and survival in mouse models. PLoS One. 2014;9:e109814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li J, Wang JC, Ma B, et al. Shenqi Fuzheng Injection for advanced gastric cancer: a systematic review of randomized controlled trials. Chin J Integr Med. 2015;21:71-79. [DOI] [PubMed] [Google Scholar]
- 28. Jia QA, Ren ZG, Bu Y, et al. Herbal compound “Songyou Yin” renders hepatocellular carcinoma sensitive to oxaliplatin through inhibition of stemness. Evid Based Complement Alternat Med. 2012;2012:908601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lin Y, Peng N, Li J, Zhuang H, Hua ZC. Herbal compound triptolide synergistically enhanced antitumor activity of amino-terminal fragment of urokinase. Mol Cancer. 2013;12:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hau DM, Chen TH, Cheng JF, You JS. Combined therapy of mitomycin C and Chinese medical herbs on experimental liver cancer. Am J Chin Med. 1993;21:51-58. [DOI] [PubMed] [Google Scholar]
- 31. Jiang ZY, Qin SK, Yin XJ, Chen YL, Zhu L. Synergistic effects of Endostar combined with beta-elemene on malignant ascites in a mouse model. Exp Ther Med. 2012;4:277-284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dong J, Su SY, Wang MY, Zhan Z. Shenqi fuzheng, an injection concocted from Chinese medicinal herbs, combined with platinum-based chemotherapy for advanced non-small cell lung cancer: a systematic review. J Exp Clin Cancer Res. 2010;29:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shah W, Yan X, Jing L, Zhou Y, Chen H, Wang Y. A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes and a high percentage of CD4(+)FOXP3(+) regulatory T cells are significantly associated with clinical outcome in squamous cell carcinoma of the cervix. Cell Mol Immunol. 2011;8:59-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fu J, Zhang Z, Zhou L, et al. Impairment of CD4+ cytotoxic T cells predicts poor survival and high recurrence rates in patients with hepatocellular carcinoma. Hepatology. 2013;58:139-149. [DOI] [PubMed] [Google Scholar]
- 35. Massague J. TGFbeta in cancer. Cell. 2008;134:215-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tosolini M, Kirilovsky A, Mlecnik B, et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263-1271. [DOI] [PubMed] [Google Scholar]
- 37. Travis MA, Sheppard D. TGF-beta activation and function in immunity. Annu Rev Immunol. 2014;32:51-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. World Health Organization. Global Recommendations on Physical Activity for Health. Geneva, Switzerland: WHO; 2010:10. [PubMed] [Google Scholar]
- 39. Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun. 2013;30:S75-S87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Abdalla DR, Murta EFC, Michelin MA. The influence of physical activity on the profile of immune response cells and cytokine synthesis in mice with experimental breast tumors induced by 7,12-dimethylbenzanthracene. Eur J Cancer Prev. 2013;22:251-258. [DOI] [PubMed] [Google Scholar]
- 41. Wang J, Song H, Tang X, et al. Effect of exercise training intensity on murine T-regulatory cells and vaccination response. Scand J Med Sci Sports. 2012;22:643-652. [DOI] [PubMed] [Google Scholar]
- 42. Walsh NP, Gleeson M, Shephard RJ, et al. Position statement part one: immune function and exercise. Exerc Immunol Rev. 2011;17:6-63. [PubMed] [Google Scholar]
- 43. Kruijsen-Jaarsma M, Revesz D, Bierings MB, Buffart LM, Takken T. Effects of exercise on immune function in patients with cancer: a systematic review. Exerc Immunol Rev. 2013;19:120-143. [PubMed] [Google Scholar]
- 44. Thompson HJ. Effect of exercise intensity and duration on the induction of mammary carcinogenesis. Cancer Res. 1994;54(7, suppl):1960s-1963s. [PubMed] [Google Scholar]
- 45. Na HK, Oliynyk S. Effects of physical activity on cancer prevention. Ann N Y Acad Sci. 2011;1229:176-183. [DOI] [PubMed] [Google Scholar]
- 46. Demarzo MM, Garcia SB. Exhaustive physical exercise increases the number of colonic preneoplastic lesions in untrained rats treated with a chemical carcinogen. Cancer Lett. 2004;216:31-34. [DOI] [PubMed] [Google Scholar]