Abstract
Background. Chinese herbal medicines reportedly increase efficacy and minimize toxicity of chemotherapy; however, little attention has been paid to how poor study quality can bias outcomes. Methods. We systematically searched MEDLINE, TCMLARS, EMBASE, and Cochrane Library for randomized controlled trials of Chinese herbal medicines combined with fluorouracil-based chemotherapy compared with the same chemotherapy alone. We screened for eligibility, extracted data, and pooled data with random-effects meta-analysis. Outcome measures were survival, toxicity, tumor response, performance status, quality of life, and Cochrane Risk of Bias (ROB) criteria to critically evaluate the quality of reporting in the randomized trials included in the meta-analysis. Results. We found 36 potentially eligible studies, with only 3 (those with low ROB) qualifying for meta-analysis. Two reported chemotherapy-related diarrhea reduced by 57% (relative risk [RR] = 0.43; 95% CI = 0.19-1.01; I2 test for variation in RR due to heterogeneity = 0.0%), with nonsignificant results. Two reported white blood cell toxicity reduced by 66% (RR = 0.34; 95% CI = 0.16-0.72; I2 test for variation in RR due to heterogeneity = 0.0%), with statistically significant results. Stratifying analysis by studies with high versus low ROB, we found substantial overestimation of benefit: Studies with high ROB overestimated by nearly 2-fold reduction of platelet toxicity by Chinese herbal medicines (RR = 0.35, 95% CI = 0.15-0.84 vs RR = 0.65, 95% CI = 0.11-3.92). Studies with high ROB overestimated by nearly 2-fold reduction of vomiting toxicity (RR = 0.45, 95% CI = 0.33-0.61 vs RR = 0.87, 95% CI = 0.48-1.58). And, studies with high ROB overestimated by 21% the reduction in diarrhea toxicity (RR = 0.34, 95% CI = 0.20-0.58 vs RR = 0.43, 95% CI = 0.19-1.01). Studies with high ROB also overestimated by 16% improvement in tumor response (RR = 1.39, 95% CI = 1.18-1.63 vs RR = 1.20; 95% CI = 0.81-1.79). Not accounting for ROB would have exaggerated evidence of benefit and failed to detect nonsignificance of results. Conclusions. In the present analysis, involving 36 studies, 2593 patients, 20 outcomes, 36 medical institutions, and 271 named research authors, 92% of the data points were from studies at high ROB. Given the poor quality of the data in studies identified, it cannot be concluded whether combining Chinese herbs with chemotherapy reduces toxicity of chemotherapy.
Keywords: meta-analysis, colorectal cancer, chemotherapy, Chinese herbal medicine, survival, performance status
Introduction
Colorectal cancer (CRC) is the third most commonly occurring cancer and cause of cancer death for both men and women in the United States population, and represents 9% of all cancer deaths for both men and women.1 In 2013, an estimated 102,480 new cases were diagnosed.1 Among colorectal cancer patients, approximately 90% of those diagnosed with localized disease will survive five years, but 5-year survival decreases to 68% if lymph nodes are involved, and to 10% if patients have evidence of metastatic spread at the time they are diagnosed.2 CRC patients with pre-existing diabetes mellitus have an increased risk of short and long-term mortality, post-operative complications, and 5-year cancer recurrence.3
First-line Therapies
Surgical resection is essential, and staging with sampling of at least 12 adjacent lymph nodes draining the tumor site provides important prognostic information.4 While adjuvant radiation therapy was in the past frequently included in treatment regimens, its use is now limited to treating patients who have advanced retroperitoneal tumors and for patients with rectal tumors.5
The earliest established adjuvant chemotherapy treatment was 5-fluorouracil (5-FU), has been in continuous use since 1957. Important improvements in the efficacy of 5-FU were the Mayo Clinic and Roswell Park protocols,6,7 which improved median survival in patients with metastatic disease to 11 months, compared with 5 months with supportive therapy.8 Efficacy of 5-FU/leucovorin has been significantly improved by combination with irinotecan and oxaliplatin for metastatic patients.9-12 Another significant new treatment development was the advent of FOLFOX regimens, which by adding oxaliplatin to 5-FU/leucovorin improved response rates and survival.13 Among metastatic patients, alternating FOLFOX and FOLFIRI protocols has helped achieve even greater survival outcomes when compared to 5-FU alone.14,15 However, more than 70% of patients receiving FOLFOX suffer from thrombocytopenia, and approximately 15% of patients receiving oxaliplatin exhibit a hypersensitivity reaction with 2% of patients having severe reactions. Serious complications of immune-mediated thrombocytopenia may occur if blood count and bleeding symptoms are not monitored after the appearance of oxaliplatin-induced hypersensitivity.16
Another important addition to treatment options has been the oral 5-FU pro-drug, capecitabine, which is frequently combined with irinotecan and oxaliplatin.17 Newly discovered genetic variations have helped to better understand variations in treatment success with irinotecan and 5-FU. Among patients treated with irinotecan and 5-FU, carriers of the ABCB1 haplotype not only responded to treatment less frequently, but also had a shorter survival time.18 Additionally, patients in whom there were genetic variations of the ABCB1 haplotype experienced earlier onset of toxicity and reduced effectiveness of irinotecan and 5-FU.18
Improved clinical outcomes have been found with the combined treatment of cetuximab, 5-FU/leucovorin and oxaliplatin (FOLFOX6) or irinotecan (FOLFIRI) for patients with the KRAS wild-type gene compared with KRAS mutated mCRC.19 Newly developed monoclonal antibodies include both bevacizumab, which specifically targets circulating vascular endothelial growth factor (VEGF) and cetuximab, which has an affinity for the epithelial growth factor receptor (EGFR)20,21. Adding the monoclonal antibody bevacizumab to chemotherapy has significantly increased progression-free survival by 17.1%, and overall survival by 8.6%.22 However, a limiting factor to this protocol is hypertension. Significant increase was shown in progression-free survival of 3.7 and 4.4 months, respectively, in a meta-analysis of bevacizumab in combination with 5-FU/FA, and bevacizumab in combination with irinotecan, fluorouracil and leucovorin (IFL), as first-line treatments of CRC.23
Herbal Therapies
Preliminary data have been published in cell culture, animal, and human trial studies suggesting Chinese herbal medicine may have an adjunctive role in colorectal cancer therapy. Mechanistic data have been published supporting the plausibility of Chinese herbal medicine having clinical benefit through either improving host defense or directly inhibiting tumor growth.
Inhibition of tumor cell growth and upregulation of apoptosis have been observed in colon cancer cell culture models using crude extracts from Chinese herbal medicines such as Radix Eleutherococcus senticosus,24 and Ganoderma lucidum.25 Inhibition of tumor cell growth has also been observed using well-characterized purified extracts, including curcumin (from turmeric root, Rhizoma Curcuma longa),26 allicin (from garlic, Bulbus Allium sativum),27,28 epigallocatechin gallate (EGCG, from green tea, Folium Thea sinensis),29,30 genistein (from the soybean, Semen Glycine max),31 and tanshinones (from Radix Salvia miltiorrhiza).32 Tumor inhibition has also been demonstrated in animal models: honokiol (from Cortex Magnolia officinalis),33 and triptolide (from Radix Trypterygium wilfordii).34
Suppression of colon cancer metastasis has been demonstrated with the herbal extract fucoidan (from the seaweeds Kun Bu and Hai Zao, Sargassum),35 and the herbal combination Pien Tze Huang.36
Anti-tumor effects of Chinese herbal medicines have also been identified as acting through multiple molecular pathways: inhibition of vascular endothelial growth factor (VEGF) and matrix metalloproteinases with Sargassum,35 Fas receptor upregulation, and caspase activation, and reduction of mitochondrial membrane potential (MMP, Psim) with Bax protein activation and cytochrome c release with Cortex Morus alba.37
Improvements in host defense mechanisms have also been observed, which may contribute to improved survival and quality of life. These data include reversal of muscle cell atrophy in cachexia induced by Rhizoma Anemarrhena asphodeloides and Cortex Phellodendron amurense,38 reduction of gastrointestinal dysfunction following colon surgery achieved by the Chinese herbal combination Da Jian Zhong Tang,39 reduction of FOLFOX6-related peripheral neuropathy with herbal combination Niu Che Sen Qi Wan,40 and reduction of post–colorectal surgical time to tolerance of regular diet with the combinations Da Jian Zhong Tang and Gui Zhi Fu Ling Tang.41 Chinese herbal medicines may derive their reported benefit for colon cancer patients because they are typically used in multi-ingredient combination formulas, thus taking advantage of multiple pathways of therapeutic action.
Prior meta-analysis of randomized trials of Chinese herbal medicine in colorectal cancer has shown a modest increase in 1-year survival (odds ratio [OR] 2.41, 95% CI 1.32-4.41) and 3-year survival (OR 2.40, 95% CI 1.49-3.87), reduction in cancer progression (OR 0.50, 95% CI 0.32-0.77), and improved quality of life (OR 3.43, 95% CI 2.35-5.02).42 However, the authors did not critically evaluate study quality. In the current article, we sought to critically examine the evidence for effectiveness of Chinese herbal medicines in colon cancer patients, with particular emphasis on study quality. We decided to identify for our systematic search and meta-analysis those published randomized trials using fluorouracil-based chemotherapy in both treatment and control groups because this therapy is a key component of most standard front-line treatment protocols for colorectal cancer.
Materials and Methods
Systematic Search
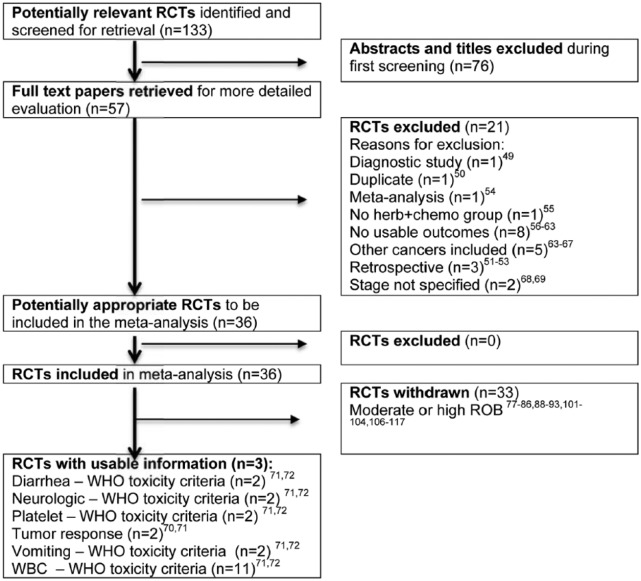
We conducted systematic searches of TCMLARS (1984 to 2014; www.cintcm.com), PubMed (1966 to 2014), Cochrane Library (1988 to 2014), and the Cochrane Central Register of Controlled Trials (1966 to 2014). We sought all randomized trials in any language, to reduce the risk of language bias seen in previous systematic reviews of Chinese herbal medicine.43 We sought studies reporting on the use of Chinese herbal medicine combined with fluorouracil-based chemotherapy for colorectal cancer patients compared to chemotherapy alone, and synonyms for each term. We also explored references from bibliographies of identified studies. We first screened titles and abstracts, ordered potentially relevant full-text articles, and subsequently screened those articles prior to data extraction (Figure 1).
Figure 1.
Flow diagram. RCT, randomized controlled trial.
Note: In the RCTs Excluded box, 1 study had more than 1 reason for being excluded, therefore while the individual reasons total 22, the number of studies excluded is 21.
Study Eligibility
Eligible studies were randomized controlled trials recruiting patients with colon cancer, with allocation to either Chinese herbal medicines combined with fluorouracil-based combination chemotherapy or the same chemotherapy alone, reporting at least one outcome of interest (survival, toxicity, tumor response, performance status and quality of life), with enough detail to allow calculation of risk ratios. We followed a predefined protocol for our systematic review (protocol not registered), which included the PRISMA Statement guidelines (Supplemental Materials available at http://ict.sagepub.com/content/by/supplemental-data).44
Data Extraction
Three researchers fluent in both Chinese and English (M.M., H.L., and C.S.) extracted data on treatment details, patient characteristics, study quality and clinical outcomes. We grouped studies by outcome of interest for analysis. Only outcomes with 2 or more studies found were included in quantitative meta-analyses.
Study Quality
We used the Cochrane Collaboration’s Risk of Bias (ROB) criteria to critically evaluate the quality of reporting in the randomized trials included in the meta-analysis, for adequate random sequence allocation, group allocation concealment, participant blinding, completeness of outcome reporting, freedom from selective outcome reporting, and other potential sources of bias.45 Each of these 7 items on the ROB assessment tool was given a possible score by the assessor of 0 for low, 1 for medium and 2 for high ROB. The total is then combined to give an overall ROB score. Low ROB was assigned to studies with total score 0 to 4, medium ROB with total score 5 to 8, and high ROB with total score of 9 and higher, an approach similar to that used by previous researchers in applying the Cochrane ROB tool.46 The total possible score was 12, consisting of a range of 0, 1, or 2 for each scored quality item (Table 1).
Table 1.
Risk of Bias (ROB) Scores for Studies Identified: Randomized Trials of Chinese Herbal Medicine Combined With Fluorouracil-Based Chemotherapy, Compared With Chemotherapy Alone for Colon Cancer.a
| First Author (Year) | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Incomplete Outcomes Data | Selective Reporting | Other Bias | Total Score |
|---|---|---|---|---|---|---|---|
| Low ROB | |||||||
| Liu J (2005)70 | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| Zeng JY (2010)72 | 0 | 0 | 2 | 0 | 0 | 2 | 4 |
| Zhang Q (2010)71 | 0 | 0 | 2 | 0 | 0 | 0 | 2 |
| Moderate ROB | |||||||
| Huang ZF (2005) 82 | 0 | 0 | 2 | 2 | 1 | 2 | 7 |
| Wu GL (2010)80 | 2 | 2 | 2 | 0 | 0 | 0 | 6 |
| High ROB | |||||||
| Chen J (2010)101 | 0 | 2 | 2 | 2 | 2 | 2 | 10 |
| Chen L (2001)102 | 2 | 2 | 2 | 0 | 1 | 2 | 9 |
| Chen XD (2005)103 | 1 | 1 | 2 | 1 | 1 | 2 | 8 |
| Gao HD (2005)104 | 2 | 2 | 2 | 2 | 2 | 2 | 12 |
| Hu AM (2006)78 | 2 | 2 | 2 | 1 | 1 | 2 | 10 |
| Jia XQ (2000)105 | 2 | 2 | 2 | 2 | 2 | 2 | 12 |
| Li YJ (1999)91 | 2 | 2 | 2 | 2 | 2 | 2 | 12 |
| Liu H (2001)88 | 2 | 2 | 2 | 0 | 1 | 2 | 9 |
| Liu J (2005)77 | 2 | 1 | 2 | 1 | 1 | 2 | 9 |
| Liu JA (2000)106 | 2 | 2 | 2 | 0 | 1 | 2 | 9 |
| Luo L (2006)107 | 2 | 2 | 2 | 1 | 1 | 2 | 10 |
| Ma J (2005)93 | 2 | 2 | 2 | 1 | 1 | 2 | 10 |
| Niu CF (2003)89 | 2 | 2 | 2 | 1 | 1 | 2 | 10 |
| Pan MQ (2003)108 | 2 | 2 | 2 | 2 | 1 | 2 | 11 |
| Shu JH (2011)81 | 2 | 1 | 2 | 0 | 1 | 2 | 8 |
| Song CY (2012)109 | 2 | 2 | 2 | 2 | 2 | 2 | 12 |
| Tan XY (2006)110 | 2 | 2 | 2 | 1 | 1 | 2 | 10 |
| Wang HZ (2000)85 | 2 | 2 | 2 | 0 | 1 | 2 | 9 |
| Wang WP (2003)111 | 2 | 2 | 2 | 2 | 2 | 2 | 12 |
| Wu JY (2003)112 | 2 | 2 | 2 | 2 | 1 | 2 | 11 |
| Xiao H (2011)79 | 2 | 2 | 2 | 1 | 1 | 2 | 10 |
| Xiao ZQ (1998)113 | 2 | 2 | 2 | 2 | 1 | 2 | 11 |
| Xu YQ (2006)62 | 2 | 2 | 2 | 2 | 2 | 2 | 12 |
| Yang QR (2001)114 | 2 | 2 | 2 | 2 | 2 | 2 | 12 |
| Yang X (2006)115 | 2 | 1 | 2 | 1 | 1 | 2 | 9 |
| Yang ZY (2005)83 | 2 | 1 | 2 | 1 | 1 | 2 | 9 |
| Yu GY (2005)116 | 2 | 2 | 2 | 2 | 1 | 2 | 11 |
| Yu Y (2006)84 | 2 | 2 | 2 | 2 | 2 | 2 | 12 |
| Zhang JW (2004)86 | 2 | 2 | 2 | 0 | 0 | 2 | 8 |
| Zhao WS (2006)117 | 2 | 2 | 2 | 2 | 2 | 2 | 12 |
| Zhu WR (2005)90 | 2 | 2 | 2 | 1 | 1 | 2 | 10 |
“Other bias” refers to bias due to problems not covered elsewhere in the Cochrane ROB tool.
Analysis of Outcomes
Survival was defined as the number of patients who died at intervals of 1 year, 2 years, and 3 years following completion of chemotherapy. Probability of failure (death) was calculated by the number of patients in the Chinese herbal medicine plus chemotherapy group, divided by that same number in the chemotherapy-only group. Intention-to-treat analysis was used, treating in the analysis any non-reported patients at follow-up times as having failed. A relative risk of less than one would indicate the Chinese herbal medicine plus fluorouracil-based chemotherapy conferred a survival advantage, compared to the same chemotherapy alone.
Reduction of Chemotherapy Toxicity
Most studies identified in our search used the 5-point World Health Organization (WHO) scale to report severity of chemotherapy-related toxicity. We calculated toxicity reduction in each study as the number of patients reporting severe toxicity (WHO grades 2 or higher), divided by the total number in each group of treatment (WHO grades 0 + 1 + 2 + 3 + 4). The risk reduction was then calculated as toxicity reduction in the herbal medicine plus chemotherapy group, divided by that in the chemotherapy alone group. A relative risk of less than 1 would favor the herbal/chemotherapy combination therapy.
Objective Tumor Response
We calculated tumor response as the total number of patients experiencing complete as well as partial response divided by the total number in each treatment group (complete response, partial response, no change, and progressive disease). The relative risk of tumor response was calculated as the probability of tumor response in the Chinese medicine plus chemotherapy group, divided by the total in the chemotherapy-only group. A relative risk of more than 1 would favor the combination treatment regimen.
Performance Status
All studies reporting performance status used the Karnofsky Performance Scale; most used a 10-point change as a cutoff for worsening or improvement of performance status, a few used a 20-point change as a cutoff. We chose to calculate performance status as a proportion of improved or stable status: (greater than 10 point increase and no change) divided by a total status (no change plus greater than 10-point increase or greater than 10-point decrease). The relative risk of improved or stable status for this meta-analysis included the Chinese medicine plus fluorouracil-based chemotherapy in the numerator, divided by this proportion in the fluorouracil-based chemotherapy treatment group. A relative risk of greater than 1 would support the combination treatment regimen.
Meta-Analysis, Between-study Heterogeneity, and Publication Bias
We performed random-effects meta-analysis using the -metan- command in the Stata software package (Stata Corp, College Station, TX), and the I2 measure to evaluate between-study heterogeneity.47 The Harbord test was used to evaluate publication bias,48 and the I2 measure to evaluate between-study heterogeneity.47
Results
Studies Retrieved
Of the 133 potentially relevant abstracts found, we identified 57 full-text articles for further assessment. These were screened and 21 excluded because the study in question was a diagnostic study (n = 1),49 duplicate article (n = 1),50 retrospective study (n = 3),51-53 or meta-analysis (n = 1),54 or because the study design had no herb + chemo group (n = 1),55 there were no usable outcomes (n = 8),56-63 other cancers were included in the data reported (n = 5),63-67 or stage was not specified (n = 2).68,69 This yielded 36 studies for our meta-analysis (Figure 1).
In data extraction of these 36 studies, we found data potentially eligible for meta-analysis for reduction of chemotherapy toxicity (anemia, diarrhea, fatigue, kidney toxicity, liver toxicity, neurological toxicity, performance status, platelet toxicity, vomiting, white blood cell [WBC] toxicity) and recurrence, survival, and tumor response. However, of these 36 studies, we found only 3 that qualified for data analysis and reporting on the basis of our predefined eligibility criteria: those studies that (a) had low risk of bias (Cochrane ROB tool), (b) were free of publication bias based on the Harbord test,48 and (c) had low between-study heterogeneity (Table 1).70-72
These 3 studies provided sufficient data for meta-analysis for the following 6 outcomes: reduction of diarrhea toxicity,71,72 neurotoxicity,71,72 platelet toxicity,71,72 vomiting,71,72 WBC toxicity71,72 (all on WHO scale) and tumor response.70,71 Two of these 3 studies were based on the Chinese herbal medicine Astragalus membranaceus.70,72 Clinical outcomes are reported in Table 2, and each study’s treatment characteristics in Table 3.
Table 2.
Results of Meta-Analyses of Randomized Trials, Chinese Herbal Medicine Combined With Fluorouracil-Based Chemotherapy, Compared With Chemotherapy Alone for Colon Cancer.a
| Endpoint | Cochrane Risk of Bias | No. of Studies | No. of Patients | RR | 95% CI | P | Test for Between-Study Heterogeneity (P)b | Test for no Publication Bias (P)c | Clinical Evidence of Benefit Found That Has Low ROB |
|---|---|---|---|---|---|---|---|---|---|
| Anemia (WHO Scale ≥2) | High ROB | 4 | 306 | 0.64 | (0.20, 2.03) | .44 | .03 | .03 | No |
| Low ROB | 1 | n/a | n/a | n/a | n/a | n/a | Nod | ||
| Chemotherapy completion | High ROB | 6 | 470 | 1.17 | (1.01, 1.37) | .04 | <.01 | .55 | No |
| Low ROB | None found | ||||||||
| Diarrhea (incidence) | High ROB | 3 | 268 | 0.33 | (0.21, 0.54) | <.01 | .54 | .21 | No |
| Low ROB | None found | ||||||||
| Diarrhea (WHO Scale ≥2) | High ROB | 5 | 316 | 0.34 | (0.20, 0.58) | <.01 | .84 | .65 | No |
| Low ROB | 2 | 224 | 0.43 | (0.19, 1.01) | .05 | .61 | Nondetectable | Yes | |
| Fatigue (incidence) | High ROB | 4 | 319 | 0.42 | (0.29, 0.61) | <.01 | .19 | .06 | No |
| Low ROB | None | ||||||||
| Karnofsky performance status | High ROB | 18 | 1.37 | (1.27, 1.48) | <.01 | .18 | <.01 | No | |
| Low ROB | 1 | n/a | n/a | n/a | n/a | n/a | Nod | ||
| Kidney toxicity (WHO Scale ≥2) | High ROB | 2 | 222 | 0.33 | (0.04, 2.46) | .28 | .66 | Nondetectable | No |
| Low ROB | None found | ||||||||
| Liver toxicity WHO Scale ≥2) | High ROB | 3 | 204 | 0.83 | (0.38, 1.80) | .64 | .87 | .83 | No |
| Low ROB | None found | ||||||||
| Neurotoxicity (WHO Scale ≥2) | High ROB | 5 | 309 | 0.63 | (0.37, 1.06) | .08 | .93 | .44 | No |
| Low ROB | 2 | 220 | 0.79 | (0.31, 1.31) | .27 | .77 | Nondetectable | No | |
| Platelet toxicity (WHO Scale ≥1) | High ROB | 4 | 265 | 0.35 | (0.15, 0.84) | .02 | .90 | .61 | No |
| Low ROB | 2 | 224 | 0.65 | (0.11, 3.92) | .64 | .74 | Nondetectable | Yes | |
| Recurrence, at 1 year | High ROB | 2 | 172 | 0.24 | (0.04, 1.53) | .13 | .58 | Nondetectable | No |
| Low ROB | None found | ||||||||
| Recurrence, at 3 years | High ROB | 3 | 237 | 0.43 | (0.25, 0.75) | <.01 | .85 | .08 | No |
| Low ROB | None found | ||||||||
| Survival, at 1 year | High ROB | 5 | 391 | 0.53 | (0.35, 0.78) | <.01 | .23 | .74 | No |
| Low ROB | None found | ||||||||
| Survival, at 2 year | High ROB | 2 | 216 | 0.50 | (0.35, 0.71) | <.01 | .59 | .85 | No |
| Low ROB | None found | ||||||||
| Survival, at 3 years | High ROB | 4 | 274 | 0.70 | (0.57, 0.88) | <.01 | .88 | .60 | No |
| Low ROB | None found | ||||||||
| Tumor response | High ROB | 15 | 967 | 1.39 | (1.18, 1.63) | <.01 | .93 | .68 | No |
| Low ROB | 2 | 198 | 1.20 | (0.81, 1.79) | .38 | .84 | Nondetectable | Yes | |
| Vomiting (incidence) | High ROB | 8 | 446 | 0.35 | (0.25, 0.48) | <.01 | .35 | .03 | No |
| Low ROB | None found | ||||||||
| Vomiting (WHO Scale ≥2) | High ROB | 8 | 481 | 0.45 | (0.33, 0.61) | <.01 | .75 | .03 | No |
| Low ROB | 2 | 224 | 0.87 | (0.48, 1.58) | .64 | .85 | Nondetectable | Yes | |
| WBC <4.0 (incidence) | High ROB | 3 | 293 | 0.24 | (0.14, 0.41) | <.01 | .98 | .78 | No |
| Low ROB | None found | ||||||||
| WBC toxicity (WHO Scale) | High ROB | 9 | 607 | 0.32 | (0.22, 0.47) | <.01 | .85 | .42 | No |
| Low ROB | 2 | 224 | 0.34 | (0.16, 0.72) | <.01 | .86 | Nondetectable | Yes |
Abbreviations: CI, confidence interval; n/a, not applicable; ROB, risk of bias; RR, relative risk; WBC, white blood cells; WHO, World Health Organization.
Significant therapeutic effects were allowed when meta-analysis results satisfied all 4 of these criteria: Within studies with low risk of bias, a significant finding for the pooled relative risk or clinical benefit, a significant finding of the test for no publication bias, and a nonsignificant finding of the test for between-study heterogeneity.
If P < .05: unbalanced effects between studies.
If P > .05: evidence of publication bias.
Only one study with low risk of bias found for this outcome, so no meta-analysis could be calculated.
Table 3.
Study Characteristics.
| First Author (Year) | Total No. of Patients | Stage | Chemotherapy Protocol | Herbs Fully Disclosed | Proprietary Formula | Herbal Formula Name (Manufacturer) | Herbal Ingredients | Risk of Bias |
|---|---|---|---|---|---|---|---|---|
| Chen J (2010)101 | 40 | Dukes’ B, Dukes’ C, Dukes’ D | FOLFOX 4 (L-OHP + Leucovorin + 5FU) | Yes | No | 健脾益气养血方 | Radix Codonopsis pilosula, Radix Actractylodis alba, Radix Rhemanniae preparatum, Radix Angelica sinensis, Radix Paeonia alba, Caulis Spatholobus suberectus, Rhizoma Pinellia ternata, Pericarpium Citrus riticulata, Endothelium Gallus gallus domesticus, Radix Polygonatum sibiricum, Semen Coix lachryma-jobi, Radix Dioscorea opposita, Sclerotium Poria cocos, Polyporus umbellatus, Fructus Cornus officinalis, Herba Agrimonia pilosa, Herba Hedyotiolis diffusa, Rhizoma Glycyrrhiza glabra | High |
| Chen L (2001)102 | 83 | Dukes’ B, Dukes’ C | 5FU + Mitomycin | No | Yes | 复方扶芳藤合剂 (广西中医学院制药厂) | Herba Euonymus fortuna, Radix Panax ginseng, Radix Astragalus membranaceus, etc. (ingredients not fully disclosed) | High |
| Chen XD (2005)103 | 93 | II, III, IV | LLF (L-OHP + Leucovorin + 5FU) | No | No | 复方丹参滴丸 | Radix Salvia miltiorrhiza, Radix et Rhizoma Panax notoginseng, Borneolum | High |
| Gao HD (2005)104 | 71 | Dukes B, C1, C2 | 5FU + Leucovorin | No | Yes | 贞 芪 扶 正 胶 囊 | Radix Astragalus membranaceus, Fructus Ligustricum lucidum | High |
| Hu AM (2006)78 | 50 | IV | FOLFOX (L-OHP + CF + 5FU) | No | No | 中药口服 | Radix Panax Ginseng, Rhizoma Atractylodis Macrocephala, Sclerotium Poria cocos, Radix Glycyrrhiza uralensis honey fried, Radix Rehmannia Glutinosa, Radix Paeonia Lactiflora, Radix Angelica Sinensis, Radix Ligusticum shuanxiong, Fructus Crataegus, fried Fructus Oryza Sativa germinatus, Fructus Hordeum germinatum, Fructus Cardamomum, with additions for symptoms | High |
| Huang ZF (2005)82 | 61 | Dukes’ B, Dukes’ C, Dukes’ D | 5FU + CF + Carmustine (MeCCNU) | Yes | Yes | 健脾消积汤 (自拟) | Rhizoma Atractylodis macrocephala, Sclerotium Poria cocos, Radix Glycyrrhiza uralensis, Pericarpium Citrus reticulata, Herba Hedyotidis diffusa, Semen Coix lachryma-jobi, Fructus Aurantium immaturis, Radix Astragalus membranaceus, Fructus Hordeum vulgaris | Moderate |
| Jia XQ (2000)105 | 56 | I, II, III | 5FU | Yes | No | 双养汤基本方 | Base prescription: Radix Astragalus membranaceus, Rhizoma Atractylodis macrocephala, Radix Angelica sinensis, Radix Paeonia lactiflora, Gelatinum Equus asinus, Pericarpium Citrus reticulata, Caulis Bambusa breviflora, Fructus Amomum villosum, with additions for symptoms | High |
| Li YJ (1999)91 | 96 | III, IV | 5FU + DXM + Mitomycin | Yes | No | 中药灌肠 | Herba Hedyotiolis diffusa, Radix Actinidia arguta,Fructus Mume, Herba Scutellaria barbatae, Caulis Sargentodoxa cuneata, Herba Solanum nigrum,Radix Pulsatillae chinensis, Radix Sophora flavescentis, Fructus Gleditsia sinensis, Radix Sanguisorba officinalis | High |
| Liu H (2001)88 | 67 | IV | 5FU + Leucovorin + Cisplatin | Yes | Yes | 抗瘤升白片 (湖南中医学院第一附属医院) | Radix Astragalus membranaceus, Radix Codonopsis pilosula, Radix Angelica sinensis, Rhizoma Atractylodis macrocephala, Gelatinum Equus asinus, Radix Panix notoginseng, Squama Manitis pentadactyla, Caulis Millettia Reticulata, Carapax Amyda sinensis, Herba Houttuynia cordata | High |
| Liu J (2005)70 | 78 | Not specified, post-surgical resection | Oxaliplatin + CF + FUDR | No | No | 健脾活血中药 | Radix Astragalus membranaceus, Radix Codonopsis pilosula, Rhizoma Atractylodis macrocephala, Sclerotium Poria cocos, Rhizoma Sparganum stoloniferum, Rhizoma Curcuma ezhu, Radix Ligusticum chuanxiong, Gecko, Lumbricus | Low |
| Liu J (2005)77 | 64 | IV | Oxaliplatin + Calcium folinate + Loxuridine | No | No | 健脾活血中药 | Radix Astragalus membranaceus, Radix Salvia miltiorrhiza, Rhizoma Atractylodis macrocephala, Sclerotium Poria cocos, Rhizoma Sparganum stoloniferum, Rhizoma Curcuma ezhu, Radix Liguticum chuanxiong, Gecko, Lumbricus | High |
| Liu JA (2000)106 | 154 | I, II, III | MFA (Mitomycin + 5FU + Doxorubicin) | Yes | No | 脾肾防 | Radix Astragalus membranaceus, Radix Codonopsis pilosula, Rhizoma Atractylodis macrocephala, Sclerotium Poria cocos, Pericarpium Citrus reticulata, Fructus Ligustrum lucidum, Fructus Lycium barbarum, Fructus Psoralea corylifolia, Semen Cuscuta chinensis, Radix Glycyrrhiza uralensis | High |
| Luo L (2006)107 | 101 | II, III | 5FU + CF or OXA + 5FU + CF | No | Yes | 扶正胶囊, 祛邪胶囊 (中国中医科学院西苑医院药剂科) |
Fu Zheng Capsule: Radix Panax ginseng, Rhizoma Atractylodis macrocephala, Sclerotium Poria cocos, Radix Glycyrrhiza uralensis, Semen Myristica fragrantis, Pericarpium Citrus reticulatae, Radix Aucklandia Lappa, fried Fructus Hordeum vulgaris, Endothelium Corneum Gallus gallus domesticus
Quxie capsule: Semen Croton tiglium, Fructus Evodia rutaecarpa, Rhizoma Zingiberis officinalis, Cortex Cinnamomum cassia, Radix Aconitum carmichaeli, Rhizoma Pinellia ternata, Pars Rubra picarpium, Fructus Citrus japonica |
High |
| Ma J (2005)93 | 53 | Dukes’ B, Dukes’ C | CF + 5FU, CF + 5FU + L-OHP | Yes | Yes | 健脾消瘤方(自拟) | Radix Codonopsis pilosula, Radix Astragalus membranaceus, Rhizoma Atractylodis macrocephala, Fructus Akebia, Sclerotium Poria cocos, Semen Coix lachryma-jobi, Rhizoma Smilax chinensis, Rhizoma Curcuma ezhu, Tuber Curcuma longa, Rhizoma Smilax glabra, Caulis Vitis vinifera (wild grapevine), Scolopendra subspinipes, Gecko, Concha Arca, Radix Semiaquilegia, Rhizoma Polygonatum, Fructus Cornus officinalis, Herba Epimedium grandiflorum, Semen Cuscuta chinensis | High |
| Niu CF (2003)89 | 65 | Not specified, post-surgical resection | MeF(V) (5FU + Semustine + Oncovin) | No | No | 扶正祛邪汤 | Semen Coix lachryma-jobi, Radix Panax quinquefolium, Ganoderma lucidum, Radix et Rhizoma Panax notoginseng, Radix Astragalus membranaceus, Rhizoma Atractylodes alba, Rhizoma Polygonum tataricis, Fructus Ficus carica, Sclerotium Polyporus umbellatum, Rhizoma Iphigenia indica, Rhizoma Menispermus dauricus, Radix Salvia miltiorrhiza, Herba cum Radix Patrinia scabiosaefolia, with additions for symptoms | High |
| Pan MQ (2003)108 | 83 | I, II, III | 5FU + Leucovorin | No | No | 益气调腑汤 | Radix Panax quinqfolium, Radix Astragalus membranaceus, Rhizoma Atractylodis macrocephala, Sclerotium Poria cocos, Fructus Aurantium immaturis, Rhizoma Cyperus rotundus, Radix Aucklandia lappa, Fructus Amomum, Fructus Crataegus, Radix et Rhizoma Rheum palmatum, Herba Salvia chinensis, Herba cum Radix Patrinia, Radix Glycyrrhiza uralensis | High |
| Shu JH (2011)81 | 90 | IV | CapeOX (L-OHP + Capecitabine) | Yes | No | 益气解毒汤 | Radix Panax ginseng, Radix Astragalus membranaceus, Radix Atractylodes alba, Sclerotium Poria cocos, Rhizoma Pinellia ternata, Pericarpium Citrus reticulata, Herba Agrimonia eupatorium, Spica Prunella vulgaris, Herba Hedyotis diffusa, Rhizoma Lyratum septemlobus, Herba Scutellaria barbata, Cortex Moutan radicis, Herba Duchesne indica, Rhizoma Glycyrrhiza glabra | High |
| Song CY (2012)109 | 58 | Dukes’ C | FOLFOX (L-OHP + Leucovorin + 5FU) | Yes | No | 四君子汤 | Radix Codonopsis pilosula, Radix Actrylodes alba, Sclerotium Poria cocos, Semen Coix lachryma-jobi, Radix Dioscorea opposita, Endothelium Gallus Gallus domesticus, Fructus Zizyphus jujuba | High |
| Tan XY (2006)110 | 68 | IV | L-OHP + CF + 5FU | No | Yes | 康赛迪胶囊aka复方斑蝥胶囊(贵州益佰制药股份有限公司) | Mylabris, Spina Acanthopanax senticosus, Rhizoma Sparganium stoloniferum, Rhizoma Curcuma ezhu, Herba Scutellaria barbata, Radix Panax ginseng, Radix Astragalus membranaceus, Vesica Fellea ursi, Fructus Ligustricum lucidum, Fructus Cornus officinalis, Radix Glycyrrhiza uralensis | High |
| Wang HZ (2000)85 | 98 | III, IV | 5FU + Leucovorin | Yes | Yes | Mutouhui Glycoside Pill(河南省人民医) | Radix Patrinia Heterophylla seu Scabra | High |
| Wang WP (2003)111 | 80 | Dukes’ B, Dukes’ C | CF + 5FU, MF (MMC + 5FU), or FUL (5FU + levamisole) | No | No | 复方肠安泰 | Radix Actinidia chinensis, Caulis Millettia reticulatae, Squama Mantis pentadactyla | High |
| Wu GL (2010)80 | 58 | Dukes’ A, Dukes’ B, Dukes’ C, Dukes’ D | FOLFOX 4 (L-OHP + Leucovorin + 5FU) | No | Yes | 扶脾益胃方 (浙江大学医学院附属第一医院中药制剂室) | Herba Dendrobium nobile, Rhizoma Atractylodes chinensis, Semen Coix lachryma-jobi, Rhizoma Pinellia ternata, Radix Dioscorea opposita, Sclerotium Poria cocos, Semen Alpinia katsumadai, Herba Gynostemma pentaphyllum, Radix Paeonia alba, Herba Agastaches rugosus | Moderate |
| Wu JY (2003)112 | 216 | III, IV | FAM | No | No | 岩舒注射液 | Radix Sophora Flavescentus | High |
| Xiao H (2011)79 | 45 | I – IV | FOLFOX 4 | Yes | Yes | 加味四君子汤 (无锡中天天然药物有限公司) | Radix Codonopsis pilosula, Radix Atractylodes alba, Sclerotium Poria cocos, Pericarpium Citrus reticulata, Rhizoma Pinellia ternata, Radix Astragalus membranaceus, Semen Coix lachryma-jobi, Caulis Sargentodoxa cuneatae, Herba Patrinia scabiosaefolia, Herba Hedyotis diffusa, Rhizoma Glycyrrhiza glabra, Fructus Crataegus pinnatifida, Massa Fermanta preparatum, Fructus Setaria germinatus, Fructus Hordeum germinatus | High |
| Xiao ZQ (1998)113 | 75 | III, IV | 5FU + MMC | No | Yes | 复元口服液 (广西中医学院第二附属医院制剂室) | Radix Astragalus membranaceus, Radix Pseudostellaria heterophylla, Rhizoma Polygonatum sibiricum, Radix Rehmannia glutinosa, Placenta Hominis, Radix et Caulis Jixueteng, Radix Paeonia rubra, Rhizoma Paris polyphylla, Herba Hedyotis diffusa, Pericarpium Citrus reticulata, Endothelium Corneum Gallus gallus domesticus, Sclerotium Poria cocos | High |
| Xu YQ (2006)92 | 52 | Dukes’ D | 5FU + L-OHP, or HCPT + 5FU + L-OHP | Yes | No | 扶正化瘀解毒散 | Radix Astragalus membranaceus, Rhizoma Atractylodis macrocephala, Semen Coix lachryma-jobi, Semen Sinapis alba, Radix Patrinia heterophyllae seu scabra, Rhizoma Curcuma ezhu, Radix et Caulis Jixueteng, Herba Agrimonia pilosae, Herba hedyotis diffusa, Radix Pueraria lobata, with additions for symptoms. | High |
| Yang QR (2001)114 | 72 | Dukes’ B, Dukes’ C, Dukes’ D | LDLV/FP (5FU + Leucovorin + Adriamycin) | No | Yes | 扶固液 (厦门市中医院) | Herba Anoectochilus formosanus, Radix Panax ginseng, Radix Astragalus membranaceus, Sclerotium Poria cocos, Rhizoma Atractylodis macrocephala, Fructus Cornus officinalis, Fructus Lycium barbaratum, Fructus Ligustricum lucidum, Squama Manitis pentadactyla, Herba Epimedium grandiflorum, Radix Dioscorea oppositae, etc. (ingredients not fully disclosed) | High |
| Yang X (2006)115 | 43 | III, IV | L-OHP + CF/LV + 5FU | No | No | 中药煎剂灌肠 | Enema: Caulis Sargentodoxa cuneata, Radix et Rhizoma Panax notoginseng, Sclerotium Poria cocos, Herba hedyotidis diffusa, Radix Paeonia lactiflora | High |
| Yang ZY (2005)83 | 60 | III | 5FU + Leucovorin + Oxaliplatin | Yes | Yes | 血塞通注射液(昆明制药集团股份有限公司), 黄芪注射液(成都地奥九泓制药厂), 参麦注射液(雅安三九药业有限公司), 口服中药 | Radix Codonopsis pilosula, Sclerotium Poria cocos, Rhizoma Atractylodis macrocephala, Radix Glycyrrhiza uralensis, Radix Dioscorea opposita, Fructus Amomum villosum, Radix Aucklandia lappa, Cortex Magnolia officinalis, Semen Coix lachryma-jobi, Fried Fructus Hordeum vulgaris, Fried Fructus Oryza sativa germinatus, Massa Fermentata, Endothelium Corneum Gallus gallus domesticus, Fructus Crataegus pinnatifida | High |
| Yu GY (2005)116 | 58 | IIII, IV | OLF (5FU + HCPT + Oxaliplatin) | No | Yes | 扶脾益胃饮煎剂 (浙江大学医学院附属第一医院中药制剂室) | Herba Dendrobium nobile, Rhizoma Atractylodis alba, Semen Coix lachryma-jobi, Rhizoma Pinellia ternatae, Sclerotium Poria cocos, Radix Dioscorea opposita, Semen Myristica fragrantis, Herba Gynostemma pentaphyllum | High |
| Yu Y (2006)84 | 54 | Dukes’ B, C1, C2 | 5FU + Leucovorin + Cisplatin | Yes | No | 扶正固本汤 | Radix Codonopsis Pilulosa, Radix Astragalus membranaceous, Sclerotium Poria cocos, Radix Atractylodes alba, Herba Epimedium grandiflorum, Gelatinum Cornu Cervi, Radix Salvia miltiorrhiza, Caulis Millettia reticulata, Pericarpium Citrus reticulata, Rhizoma Pinellia ternata, Rhizoma Zingiberis officinalis, Radix Rehmannia glutinosae, Fructus Ligustrum lucidum, Plastrum testudinis | High |
| Zeng JY (2010)72 | 104 | II, III | 5FU + Cisplatin | Yes | No | 消瘤汤 |
Radix Codonopsis pilosulae, Radix Astragalus membranaceus, Placenta Hominis, Radix et Rhizoma Panax notoginseng, Caulis et Folium Euonymus fortuneum, Bulbus Tulipa, Herba Scutellaria barbata, Semen Coix lachryma-jobi, Rhizoma Glycyrrhiza glabra |
Low |
| Zhang JW (2004)86 | 103 | Dukes’ B,Dukes’ C | HLF (calcium folinate + 5FU + H-CPT) | No | Yes | 艾迪注射液(贵州益佰制药有限责任公司) | Radix Panax ginseng, Radix Astragalus membranaceus, Radix Eleutherococcus senticosus, Mylabiris cichoi | High |
| Zhang Q (2010)71 | 120 | III, IV | FOLFOX 4 (L-OHP + CV + 5FU) | No | Yes | 固本消瘤胶囊 (首都医科大学附属北京中医医院院内制剂) | Cordyceps sinensis, Ganoderma lucidum, Herba Epimedium grandiflorum, Semen Coix lachryma-jobi, Hirudo, Scorpio, Buthus martensii, Herba Solanum nigrum, Bulbus Fritillaria thunbergii | Low |
| Zhao WS (2006)117 | 80 | III, IV | L-OHP + CF + 5FU | Yes | No | 加味升血汤 | Fresh Radix Astragalus membranaceus, Radix Pseudostellaria heterophylla, Radix et Caulis Jixueteng, Rhizoma Atractylodis macrocephala, Sclerotium Poria cocos, Fructus Lycium barbarum, Fructus Ligustrum lucidum, Semen Cuscuta chinensis, Fructus Psoralea corylifolia, Radix Paeonia rubra, Hirudo seu Whitmania | High |
| Zhu WR (2005)90 | 58 | Dukes’ B2, C | Intraperitoneal 5FU + cisplatin | Yes | Yes | 参脉注射液 | Radix Panax ginseng, Tuber Ophiopogonis japonicus | High |
For an additional 2 outcomes, there was only 1 study identified per outcome, which made formal meta-analysis not possible: reduction in anemia toxicity (WHO scale),71 and improvement in Karnofsky Performance Status.71
Reduction of Diarrhea Toxicity (WHO Scale ≥2)
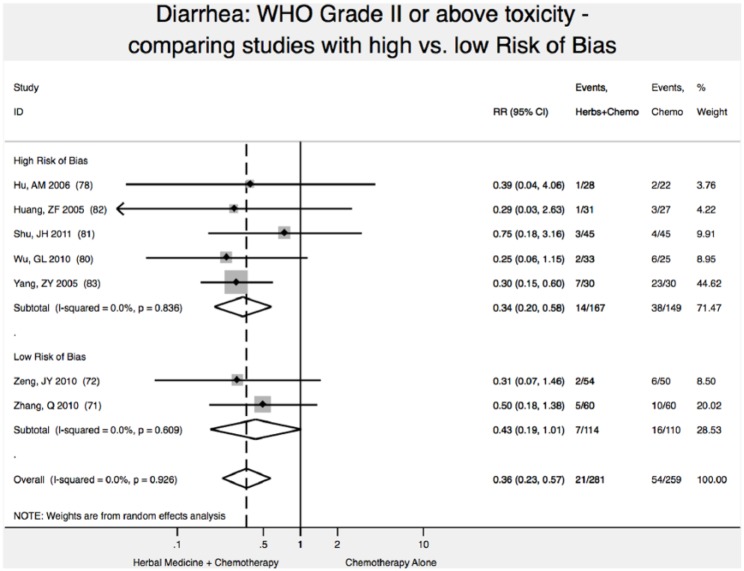
In meta-analysis, we found 2 high-quality (low risk of bias) studies reporting that addition of Chinese herbal medicine to fluorouracil-based chemotherapy reduced the relative risk of severity of diarrhea toxicity (WHO Scale ≥2) by 57% (relative risk [RR] 0.43; 95% CI 0.19-1.01); however, results were not statistically significant (P = .05; Figure 2),71,72 with P < .05 defined as the upper bound of statistical significance.
Figure 2.
Reduction in diarrhea toxicity.
Note: Vertical dashed line indicates the effect size in this analysis.
Reduction of Neurological Toxicity (WHO Scale ≥2)
We found 2 high-quality (low risk of bias) studies reporting that addition of Chinese herbal medicine to fluorouracil-based chemotherapy reduced the relative risk of severity of neurological toxicity (WHO Scale ≥2) by 21% (RR 0.79; 95% CI 0.31-1.31); however, results were not statistically significant (P = .27).71,72
Reduction of Platelet Toxicity (WHO Scale ≥2)
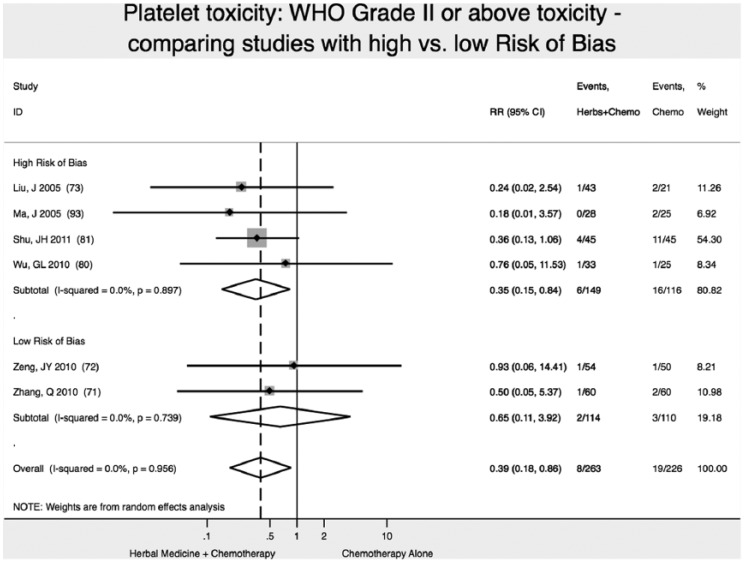
We found 2 high-quality (low risk of bias) studies reporting that addition of Chinese herbal medicine to fluorouracil-based chemotherapy reduced the relative risk of severity of platelet toxicity (WHO Scale ≥2) by 35% (RR 0.65; 95% CI 0.11-3.92), although results were not statistically significant (P = .64; Figure 3).71,72
Figure 3.
Reduction in platelet toxicity.
Note: Vertical dashed line indicates the effect size in this analysis.
Reduction of Vomiting Toxicity (WHO Scale ≥2)
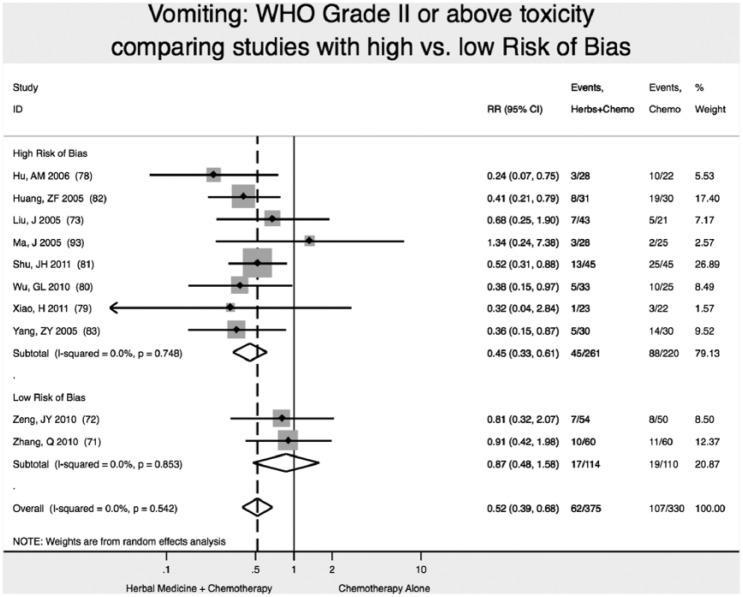
We found 2 high-quality (low risk of bias) studies reporting that addition of Chinese herbal medicine to fluorouracil-based chemotherapy reduced the relative risk of severity of vomiting toxicity (WHO Scale ≥2) by 13% (RR 0.87; 95% CI 0.48-1.58), although results were not statistically significant (P = .64; Figure 4).71,72
Figure 4.
Reduction in vomiting toxicity.
Note: Vertical dashed line indicates the effect size in this analysis.
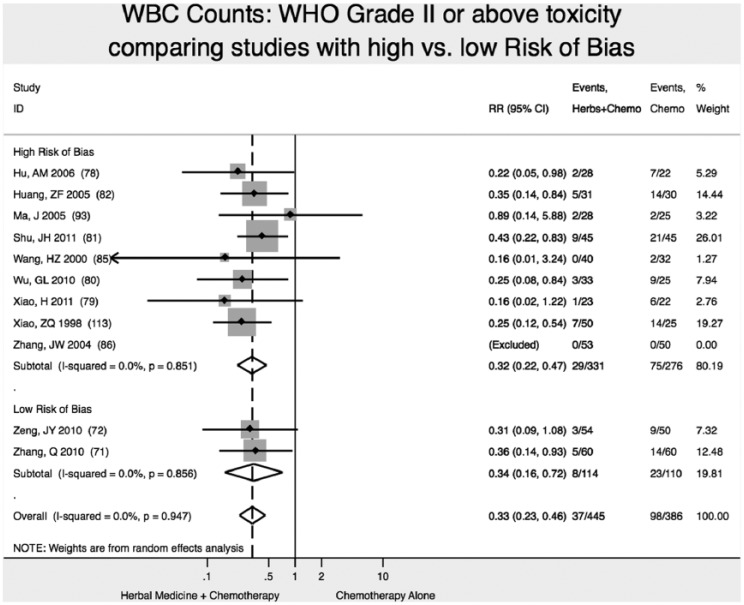
Reduction of WBC Toxicity (WHO Scale ≥2)
We found 2 high-quality (low risk of bias) studies reporting that addition of Chinese herbal medicine to fluorouracil-based chemotherapy reduced the relative risk of severity of WBC toxicity (WHO Scale ≥2) by 66% (RR 0.34; 95% CI 0.16-0.72), with statistically significant results (P < .01; Figure 5).71,72
Figure 5.
Reduction in white blood cell (WBC) toxicity.
Note: Vertical dashed line indicates the effect size in this analysis.
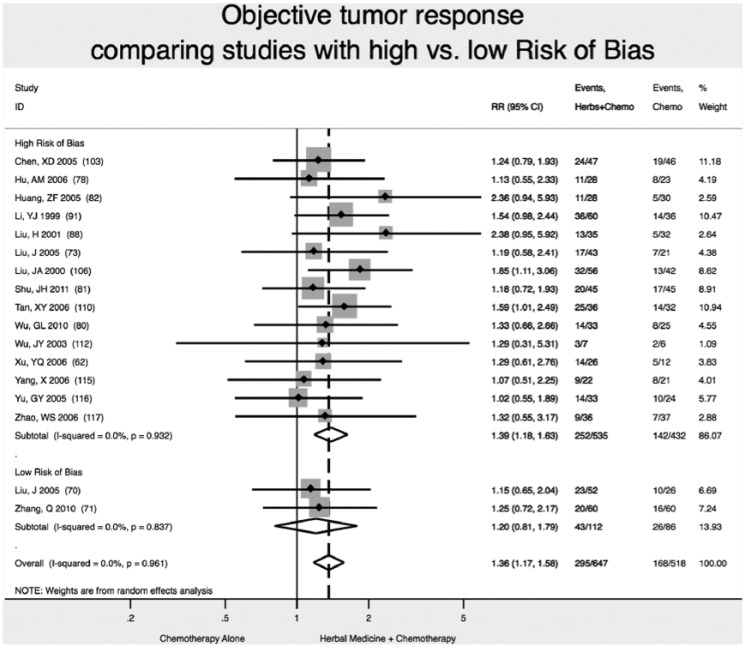
Improvement of Tumor Response (Partial Response + Complete Response)
We found 2 high-quality (low risk of bias) studies reporting that addition of Chinese herbal medicine to fluorouracil-based chemotherapy increase the likelihood of tumor response (partial response or complete response) by 20% (RR 1.20; 95% CI 0.81-1.79), although the results were not statistically significant (P = .38; Figure 6).70,71
Figure 6.
Increase in objective tumor response.
Note: Vertical dashed line indicates the effect size in this analysis.
Comparing Results of Meta-Analysis of Studies With High Versus Low Risk of Bias
Studies with high ROB overestimated by 21% the reduction in diarrhea toxicity (WHO grade II or higher): RR = 0.34 (95% CI 0.20-0.58) versus RR = 0.43 (95% CI 0.19-1.01). In this analysis, not accounting for study ROB would have failed to detect the nonsignificant result of meta-analysis for reduction of diarrhea toxicity (Figure 2).
Studies with high ROB overestimated by nearly 2-fold the reduction in platelet toxicity (WHO grade II or higher): RR = 0.35 (95% CI 0.15-0.84) versus RR = 0.65 (95% CI 0.11-3.92). In this analysis, not accounting for study ROB would have failed to detect the nonsignificant result of meta-analysis for reduction of platelet toxicity (Figure 3).
Studies with high ROB overestimated by nearly 2-fold the reduction in vomiting toxicity (WHO grade II or higher): RR = 0.45 (95% CI 0.33-0.61), versus RR = 0.87 (95% CI 0.48-1.58). In this analysis, not accounting for study ROB would have failed to detect the nonsignificant result of meta-analysis for reduction of vomiting toxicity (Figure 4).
Studies with high ROB overestimated by 16% the improvement in objective tumor response: RR = 1.39 (95% CI 1.18-1.63) versus RR = 1.20 (95% CI 0.81-1.79). In this analysis, not accounting for study ROB would have failed to detect the nonsignificant result of meta-analysis for improvement in objective tumor response (Figure 5). Statistically significant results on reduction of neurological toxicity were not found in meta-analysis of studies at either high or low ROB.
We found no difference in results of meta-analysis comparing studies with high ROB versus low ROB for reduction of WBC toxicity (Figure 6).
Discussion
Our meta-analysis found very limited published evidence supporting the efficacy of Chinese herbal medicines when used in combination with fluorouracil-based chemotherapy for patients with colon cancer. We found qualified reportable results (those published in articles with low ROB) for only 20% (n = 4) of the 20 outcomes identified: diarrhea, neurological, platelet, vomiting, and WBC toxicity (all on WHO Scale), and objective tumor response. In only 5% (n = 1) of all outcomes analyzed were the results of the analysis both at low ROB and also statistically significant: WBC toxicity.
Study Quality in Articles Assessed
Over the past decade, our team has conducted meta-analyses of Chinese herbal medicines in patients with chronic hepatitis B,73 advanced non–small cell lung cancer,74 and hepatocellular carcinoma,75 initially using the Jadad quality scale.76 The availability in 2011 of the Cochrane Risk of Bias tool has provided meta-analysts with a clearly-defined set of metrics with which to efficiently evaluate the quality of underlying studies.45 This tool asks analysts to assign to each study being assessed a 0 to 2 score, that in their judgment the study’s reporting indicates a low ROB, unclear, or high risk. This is assigned in 6 domains: adequate random sequence allocation, group allocation concealment, participant blinding, completeness of outcome reporting, freedom from selective outcome reporting, and other potential sources of bias. This score when totaled is used to assign a low risk (score 0-4), medium (5-8), or high (9-12) risk that the study’s design or outcomes are subject to bias. In effect, it is an indicator of apparent trustworthiness of the reported data.
However, 80% of the evidence (16/20 outcomes) we found has virtually no clinical usefulness, because the articles in which they were published were at high ROB using the Cochrane Risk of Bias tool.45 Most studies used the outdated clinical outcome for chemotherapy toxicity—the WHO Scale71,72,77-86—a method that is more prone to bias than the 12-year-old CTCAE scale.87 All studies reporting patient survival and time to recurrence reported these outcomes at fixed time points (1, 2, or 3 years),88-93 a method prone to substantial analytic bias compared to the more modern hazard ratio method.94 To help understand the broader scientific context within which our results are found, we informally compared our results with the findings of other recently published meta-analyses of Chinese herbal medicines, and found similar issues with study quality and risk of bias.42,95,96
Limitations
Heavy metal contamination in Chinese herbal medicines has been reported.97,98 It is additionally known that heavy metal accumulation can contribute to cancer development, in part due to increased activation of tumor initiation and promotion signaling pathway such as epidermal growth factor receptor (EGFR), phosphatidyl inositol 3-kinase (PI3K), AKT, and mammalian target of rapamycin (mTOR) in carcinogenesis and cancer progression.99 Therefore, unmeasured confounding may exist in our results due to unknown interaction of heavy metal residues with chemotherapy efficacy.
Most symptomatic outcomes were measured on the WHO Scale, which we have specified in the manuscript. However, several (incidence of diarrhea, fatigue, and vomiting) were not measured using this scale, and in the underlying studies analyzed, it is not clear in the reporting how they were measured, further diminishing the clinical value of our results.
Conclusions
Although these 36 studies involved 2,593 patients, 20 outcomes, 36 medical institutions, and 271 named research authors, unfortunately most of the data points suggesting clinical benefit are of virtually no clinical value due to very poor methodological quality of the studies. Because stratifying our analysis by the articles’ level of risk of bias showed no statistically significant difference in effect size, we suggest that virtually all of these studies to some extent suffer from such risk of bias. Additionally, no studies reported any adverse effects monitoring associated with the use of Chinese herbal medicines, a shortcoming common to many other such RCTs published in China.42,95,96,100 In short, the high frequency of low-quality and/or biased studies of Chinese herbal medicine undermines confidence in the results of published meta-analyses of these trials. This is unfortunate given the wealth of information on combination therapies available from the tradition of Chinese medicine. The solutions to these problems may be found in improved researcher methodology and ethics training, comprehensive clinical trial oversight, and reformed medical journal peer-review and editorial practices.
Supplementary Material
Acknowledgments
We appreciate the support of Michelle Ching, Daniel Eng, Raleigh Harrell, Andrew Liszt, Nina Ng, Anita Pietrofita, Heather Voborsky, and Carolyn Yeh in conducting background research.
Footnotes
Authors’ Note: Data are available to readers on request.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [DOI] [PubMed] [Google Scholar]
- 2. Ellenhorn JD, Cullinane CA, Coia LR, Alberts SR. Colon, rectal and anal cancers. In: Pazdur R, Coia LR, Hoskins WJ, Wagman LD, eds. Cancer Management: A Multidisciplinary Approach. 10th ed. Lawrence, KS: CMP Medica; 2007:343-376. [Google Scholar]
- 3. Stein KB, Snyder CF, Barone BB, et al. Colorectal cancer outcomes, recurrence, and complications in persons with and without diabetes mellitus: a systematic review and meta-analysis. Dig Dis Sci. 2010;55:1839-1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swanson RS, Compton CC, Stewart AK, Bland KI. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol. 2003;10:65-71. [DOI] [PubMed] [Google Scholar]
- 5. Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [DOI] [PubMed] [Google Scholar]
- 6. Petrelli N, Douglass HO, Jr, Herrera L, et al. The modulation of fluorouracil with leucovorin in metastatic colorectal carcinoma: a prospective randomized phase III trial. Gastrointestinal Tumor Study Group. J Clin Oncol. 1989;7:1419-1426. Erratum in: J Clin Oncol. 1990;8:185. [DOI] [PubMed] [Google Scholar]
- 7. Poon MA, O’Connell MJ, Wieand HS, et al. Biochemical modulation of fluorouracil with leucovorin: confirmatory evidence of improved therapeutic efficacy in advanced colorectal cancer. J Clin Oncol. 1991;9:1967-1972. [DOI] [PubMed] [Google Scholar]
- 8. Scheithauer W, Rosen H, Kornek GV, Sebesta C, Depisch D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ. 1993;306:752-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. de Gramont A, Figer A, Seymour M, et al. Leucovorin and fluorouracil with or without oxaliplatin as first-line treatment in advanced colorectal cancer. J Clin Oncol. 2000;18:2938-2947. [DOI] [PubMed] [Google Scholar]
- 10. Douillard JY, Cunningham D, Roth AD, et al. Irinotecan combined with fluorouracil compared with fluorouracil alone as first-line treatment for metastatic colorectal cancer: a multicentre randomised trial. Lancet. 2000;355:1041-1047. [DOI] [PubMed] [Google Scholar]
- 11. Giacchetti S, Perpoint B, Zidani R, et al. Phase III multicenter randomized trial of oxaliplatin added to chronomodulated fluorouracil-leucovorin as first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2000;18:136-147. [DOI] [PubMed] [Google Scholar]
- 12. Saltz LB, Cox JV, Blanke C, et al. : Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med. 2000;343:905-914. [DOI] [PubMed] [Google Scholar]
- 13. Andre T, Boni C, Mounedji-Boudiaf L, et al. : Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343-2351. [DOI] [PubMed] [Google Scholar]
- 14. Goldberg RM, Sargent DJ, Morton RF, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer. J Clin Oncol. 2004;22:23-30. [DOI] [PubMed] [Google Scholar]
- 15. Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229-237. [DOI] [PubMed] [Google Scholar]
- 16. Bautista MA, Stevens WT, Chen CS, Curtis BR, Aster RH, Hsueh CT. Hypersensitivity reaction and acute immune-mediated thrombocytopenia from oxaliplatin: two case reports and a review of the literature. J Hematol Oncol. 2010;3:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N Engl J Med. 2005;352:2696-2704. [DOI] [PubMed] [Google Scholar]
- 18. Glimelius B, Garmo H, Berglund A, et al. Prediction of irinotecan and 5-fluorouracil toxicity and response in patients with advanced colorectal cancer. Pharmacogenomics J. 2011;11:61-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Licar A, Cerkovnik P, Ocvirk J, Novakovic S. KRAS mutations in Slovene patients with colorectal cancer: frequency, distribution and correlation with the response to treatment. Int J Oncol. 2010;36:1137-1144. [DOI] [PubMed] [Google Scholar]
- 20. Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337-345. [DOI] [PubMed] [Google Scholar]
- 21. Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [DOI] [PubMed] [Google Scholar]
- 22. Loupakis F, Bria E, Vaccaro V, et al. Magnitude of benefit of the addition of bevacizumab to first-line chemotherapy for metastatic colorectal cancer: meta-analysis of randomized clinical trials. J Exp Clin Cancer Res. 2010;29:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tappenden P, Jones R, Paisley S, Carroll C. Systematic review and economic evaluation of bevacizumab and cetuximab for the treatment of metastatic colorectal cancer. Health Technol Assess. 2007;11:1-128, iii-iv. [DOI] [PubMed] [Google Scholar]
- 24. Cichello SA, Yao Q, Dowell A, Leury B, He XQ. Proliferative and inhibitory activity of Siberian ginseng (Eleutherococcus senticosus) extract on cancer cell lines; A-549, XWLC-05, HCT-116, CNE and Beas-2b. Asian Pac J Cancer Prev. 2015;16:4781-4786. [DOI] [PubMed] [Google Scholar]
- 25. Liang ZE, Yi YJ, Guo YT, Wang RC, Hu QL, Xiong XY. Inhibition of migration and induction of apoptosis in LoVo human colon cancer cells by polysaccharides from Ganoderma lucidum. Mol Med Rep. 2015;12:7629-7636. [DOI] [PubMed] [Google Scholar]
- 26. Dimas K, Tsimplouli C, Houchen C, et al. : An ethanol extract of Hawaiian turmeric: extensive in vitro anticancer activity against human colon cancer cells. Altern Ther Health Med. 2015;21(suppl 2):46-54. [PubMed] [Google Scholar]
- 27. Robert V, Mouillé B, Mayeur C, Michaud M, Blachier F. Effects of the garlic compound diallyl disulfide on the metabolism, adherence and cell cycle of HT-29 colon carcinoma cells: evidence of sensitive and resistant sub-populations. Carcinogenesis. 2001;22:1155-1161. [DOI] [PubMed] [Google Scholar]
- 28. Matsuura N, Miyamae Y, Yamane K, et al. Aged garlic extract inhibits angiogenesis and proliferation of colorectal carcinoma cells. J Nutr. 2006;136(3 suppl):842S-846S. [DOI] [PubMed] [Google Scholar]
- 29. Mueller-Klieser W, Schreiber-Klais S, Walenta S, Kreuter MH. Bioactivity of well-defined green tea extracts in multicellular tumor spheroids. Int J Oncol. 2002;21:1307-1315. [DOI] [PubMed] [Google Scholar]
- 30. Norwood AA, Tan M, May M, Tucci M, Benghuzzi H. Comparison of potential chemotherapeutic agents, 5-fluoruracil, green tea, and thymoquinone on colon cancer cells. Biomed Sci Instrum. 2006;42:350-356. [PubMed] [Google Scholar]
- 31. Zhu Q, Meisinger J, Van Thiel DH, Zhang Y, Mobarhan S. Effects of soybean extract on morphology and survival of Caco-2, SW620, and HT-29 cells. Nutr Cancer. 2002;42:131-140. [DOI] [PubMed] [Google Scholar]
- 32. Hu T, Wang L, Zhang L, et al. Sensitivity of apoptosis-resistant colon cancer cells to tanshinones is mediated by autophagic cell death and p53-independent cytotoxicity. Phytomedicine. 2015;22:536-544. [DOI] [PubMed] [Google Scholar]
- 33. Chen F, Wang T, Wu YF, et al. Honokiol: a potent chemotherapy candidate for human colorectal carcinoma. World J Gastroenterol. 2004;10:3459-3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tang XY, Zhu YQ, Tao WH, Wei B, Lin XL. Synergistic effect of triptolide combined with 5-fluorouracil on colon carcinoma. Postgrad Med J. 2007;83:338-343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Huang TH, Chiu YH, Chan YL, et al. Prophylactic administration of fucoidan represses cancer metastasis by inhibiting vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs) in Lewis tumor-bearing mice. Mar Drugs. 2015;13:1882-1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin W, Zhuang Q, Zheng L, et al. Pien Tze Huang inhibits liver metastasis by targeting TGF-β signaling in an orthotopic model of colorectal cancer. Oncol Rep. 2015;33:1922-1928. [DOI] [PubMed] [Google Scholar]
- 37. Hyun HB, Lee WS, Go SI, et al. The flavonoid morin from Moraceae induces apoptosis by modulation of Bcl-2 family members and Fas receptor in HCT 116 cells. Int J Oncol. 2015;46:2670-2678. [DOI] [PubMed] [Google Scholar]
- 38. Zhuang P, Zhang J, Wang Y, et al. Reversal of muscle atrophy by Zhimu and Huangbai herb pair via activation of IGF-1/Akt and autophagy signal in cancer cachexia. Support Care Cancer. 2016;24:1189-1198. [DOI] [PubMed] [Google Scholar]
- 39. Katsuno H, Maeda K, Kaiho T, et al. Clinical efficacy of Daikenchuto for gastrointestinal dysfunction following colon surgery: a randomized, double-blind, multicenter, placebo-controlled study (JFMC39-0902). Jpn J Clin Oncol. 2015;45:650-656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mamiya N, Kono T, Mamiya K, Satomi M, Chisato N, Ebisawa Y. A case of neurotoxicity reduced with goshajinkigan in modified FOLFOX6 chemotherapy for advanced colon cancer [in Japanese]. Gan To Kagaku Ryoho. 2007;34:1295-1297. [PubMed] [Google Scholar]
- 41. Suehiro T, Matsumata T, Shikada Y, Sugimachi K. The effect of the herbal medicines dai-kenchu-to and keishi-bukuryo-gan on bowel movement after colorectal surgery. Hepatogastroenterology. 2005;52:97-100. [PubMed] [Google Scholar]
- 42. Zhong LL, Chen HY, Cho WC, Meng XM, Tong Y. The efficacy of Chinese herbal medicine as an adjunctive therapy for colorectal cancer: a systematic review and meta-analysis. Complement Ther Med. 2012;20:240-252. [DOI] [PubMed] [Google Scholar]
- 43. Wu XY, Tang JL, Mao C, Yuan JQ, Qin Y, Chung VC. Systematic reviews and meta-analyses of traditional chinese medicine must search chinese databases to reduce language bias. Evid Based Complement Alternat Med. 2013;2013:812179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6:e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wider B, Pittler MH, Thompson-Coon J, Ernst E. Artichoke leaf extract for treating hypercholesterolaemia. Cochrane Database Syst Rev 2013;3:CD003335. [DOI] [PubMed] [Google Scholar]
- 47. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Harbord RM, Egger M, Sterne JA. A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med. 2006;25:3443-3457. [DOI] [PubMed] [Google Scholar]
- 49. Zhao GX, Zheng J, Gu Y, et al. Colorectal cancer syndrome: analysis of the literature. Liaoning Zhongyiyao Daxue Xuebao. 2009;11:72-74. [Google Scholar]
- 50. Liu JA, Zhang YH. Clinical observation of Chinese and Western integrative treatment of 154 cases of postoperative colon cancer. Zhongcaoyao. 2000;31:367-368. [Google Scholar]
- 51. Lin SY, Shen MH, Shu JN, et al. Retrospectively analysis of the characteristics of the TCM syndromes of colorectal cancer. Zhejiang Zhongyiyao Daxue Xuebao. 2011;35:322-324. [Google Scholar]
- 52. Li M. Observation of short-term efficacy of Chinese medicine in treatment of late stage colorectal cancer. Hebei Zhongyiyao Xuebao. 2011;26:21-23. [Google Scholar]
- 53. Li M. Clinical research on treating advanced colorectal cancer patients with the methods of invigorating spleen and resolving mass. Xibu Zhongyiyao. 2011;24:70-72. [Google Scholar]
- 54. Liu J, Zhu Q. The reduction of adverse drug reaction incidences of colorectal caner patients receiving Jianpi herbs combined with chemotherapy: a systematic review. Zhongguo Xunzheng Yixue Zazhi. 2009;9:802-808. [Google Scholar]
- 55. Li ZY, Deng XJ. Clinical observation of treatment of late-stage colon cancer with Curcuma oil and other Chinese medicinals via implanted regional infusion pump. Zhongguo Zhongxiyijiehe Zazhi. 2003;23:72. [Google Scholar]
- 56. Jiang YL, Pan B, Qiu XZ. Summary of 40 cases of the treatment of postoperative colon cancer with a combination of Strengthen the Spleen and Eliminate Cancer Beverage and chemotherapy. Hunan Zhongyi Zazhi. 2001;17:9-10. [Google Scholar]
- 57. Huang GD, Huang CR, He YH. Clinical observation of the treatment of late-stage colon cancer with Gastrodia injection. Hunan Zhongyi Xueyuan Xuebao. 2002;22:50-51. [Google Scholar]
- 58. Jiang T, Zhu YK. Effect of Aidi Injection on immune function of colon cancer patients. Changchun Zhongyiyao Daxue Xuebao. 2007;23:67. [Google Scholar]
- 59. Yang YF, Xu Y, Wu Y. Clinical randomized double-blinded controlled study on Quxie Capsule in reducing post-operational relapse and metastasis of colorectal cancer [in Chinese]. Zhongguo Zhongxiyijiehe Zazhi. 2007;27:879-882. [PubMed] [Google Scholar]
- 60. Zhou H, Shen KP. Clinical study on Weichang An in treating postoperative colorectal carcinoma of spleen deficiency. Shanghai Zhongyiyao Zazhi. 2009;43:36-38. [Google Scholar]
- 61. Fan HJ, Yao RM, Xu CY, et al. A study on imbilicus moxibustion therapy and intraabdominal cavity administration of Chinese medicine in the treatment of malignant peritoneal effusion. Zhongguo Zhongyiyao. 2012;10:34-35. [Google Scholar]
- 62. Xu CY, Li LN, Pan DM, et al. Retrospective analysis of TCM syndrome types associated with adverse reactions after chemotherapy who were advanced large intestine cancer. Liaoning Zhongyiyao Daxue Xuebao. 2011;13:104-106. [Google Scholar]
- 63. Mok TS, Yeo W, Johnson PJ, et al. A double-blind placebo-controlled randomized study of Chinese herbal medicine as complementary therapy for reduction of chemotherapy-induced toxicity. Ann Oncol. 2007;18:768-774. [DOI] [PubMed] [Google Scholar]
- 64. Zheng ZX, Zhang LJ, Liu ZY, et al. Integrative medical treatment of 42 cases of advanced stomach and colon cancers. Chin J Surg Integr Tradit Western Med. 2007;13:244-245. [Google Scholar]
- 65. Sun H, Li ZD, Wang W, et al. A comparative study of Renshen Yangrong Tang in reducing cancer-related fatigue of chemotherapy patients. Zhongguo Zhongyi Jichu Yixue Zazhi. 2010;16:155-157. [Google Scholar]
- 66. Lu M. Effect of Jianpi Yiqi treatment on life quality and immune function of advanced stomach and colon cancer patients. Shanxi Zhongyi. 2008;29:1130-1131. [Google Scholar]
- 67. Li Y. An analysis of Huachan Su in the treatment of 19 cases of late stage cancers. Shandong Yiyao. 2011;51:97-98. [Google Scholar]
- 68. Xu YQ, Xue HN, Zou J. Observation of the short-term efficacy, toxicity and side effects of the treatment of malignant tumors with a combination of Sophora Flavescens Formula injection and chemotherapy. Anhui Zhongyi Linchuang Zazhi. 2001;13:160-162. [Google Scholar]
- 69. Jin J, Zhang MX. Observation of the efficacy of Great Peace Pill to reduce the gastrointestinal side effects of postoperative chemotherapy in patients with colon cancer. Zhongguo Zhongyiyao Xinxi Zazhi. 2004;11:823-824. [Google Scholar]
- 70. Liu J, Wang WP, Zhou YY, et al. Effects of treatment with Chinese medicinals to strengthen the Spleen and invigorate blood combined with chemotherapy on postoperative immunity and hemorheology in patients with colon cancer. Jiangsu Zhongyiyao. 2005;26:13-14. [Google Scholar]
- 71. Zhang Q, Wang XM, Yang GW. Guben Xiaoliao Capsule combined with FOLFOX4 chemotherapy in the treatment of late-stage colon cancer. Beijing Zhongyiyao. 2010;29:255-257. [Google Scholar]
- 72. Zeng JY, He J, Wang QJ, et al. Effect of Xiaoliutang combined with heated intraperitoneal chemotherapy on prevention of recurrence in invasive colon cancer. Guangxi Yixue. 2010;32:645-648. [Google Scholar]
- 73. McCulloch M, Broffman M, Gao J, Colford JM., Jr. Chinese herbal medicine and interferon in the treatment of chronic hepatitis B: a meta-analysis of randomized, controlled trials. Am J Public Health. 2002;92:1619-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. McCulloch M, See C, Shu XJ, et al. Astragalus-based Chinese herbs and platinum-based chemotherapy for advanced non-small-cell lung cancer: meta-analysis of randomized trials. J Clin Oncol. 2006;24:419-430. [DOI] [PubMed] [Google Scholar]
- 75. Shu X, McCulloch M, Xiao H, Broffman M, Gao J. Chinese herbal medicine and chemotherapy in the treatment of hepatocellular carcinoma: a meta-analysis of randomized controlled trials. Integr Cancer Ther. 2005;4:219-229. [DOI] [PubMed] [Google Scholar]
- 76. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [DOI] [PubMed] [Google Scholar]
- 77. Liu J, Wang WP, Zhou YY, et al. Observation of the efficacy of postoperative treatment of patients with colon cancer with Chinese medicinals to strengthen the Spleen and invigorate blood combined with chemotherapy. Zhongguo Zhongxiyijiehe Zazhi. 2005;25:207-209. [PubMed] [Google Scholar]
- 78. Hu AM, Xie CY, Li ZP. Observation of the efficacy of Chinese medicinals combined with chemotherapy in the treatment of late-stage colon cancer. Shiyong Aizheng Zazhi. 2006;21:74-76. [Google Scholar]
- 79. Xiao H, Yang J. Immune-augmentive effect of Jia Wei Si Junzi Tang in colon cancer patients receiving chemotherapy Zhongguo Zhongxiyijiehe Zazhi. 2011;31:164-167. [Google Scholar]
- 80. Wu GL, Yu GY, Li JP, et al. Short term therapeutic effect on treatment of postoperational large intestine carcinoma by Fupiyiwei decoction combined with chemotherapy and its effect on immune fuction. Zhongguo Zhongyao Zazhi. 2010;35:782-785. [PubMed] [Google Scholar]
- 81. Shu JH, Zhou RY, Zhong Y, et al. Qi-enhancing Detoxifying Decoction combined with CapeOX chemotherapy in the treatment of 45 cases of late stage colon cancer. Shanghai Zhongyiyao Zazhi. 2011;45:33-35. [Google Scholar]
- 82. Huang ZF, Li HZ, Liu JB, et al. Observation of the efficacy of Strengthen the Spleen and Eliminate Accumulations Decoction in combination with chemotherapy in the treatment of late-stage colon cancer. Xiandai Zhongxiyijiehe Zazhi. 2005;14:1281-1282. [Google Scholar]
- 83. Yang ZY, Liu C, Luo J, et al. A clinical study on postoperative treatment of patients with Stage III colon cancer using a combination of Chinese medicinals and chemotherapy. Zhongguo Zhongliu Linchuang Yu Kangfu. 2005;12:190-192. [Google Scholar]
- 84. Yu Y, Zhang B, Cai QR, et al. 22 cases of post-surgical colon cancer patients treated with chemotherapy combinedwith Fuzhenggubentang. Zhongguo Zhongyiyao Keji. 2006;13:269-270. [Google Scholar]
- 85. Wang HZ, Wang SZ, Wang YH, et al. Clinical observation of the treatment of colon cancer with Patrinia heterophylla total glycoside tablets. Shanghai Zhongyiyao Zazhi. 2000;34:16-18. [Google Scholar]
- 86. Zhang JW, Wang JS. Clinical observation of Aidi Injection in combination with postoperative adjuvant chemotherapy in patients with colon cancer. Zhongguo Zonghe Linchuang. 2004;20:999-1000. [Google Scholar]
- 87. Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176-181. [DOI] [PubMed] [Google Scholar]
- 88. Liu H, Luo WH. Observation of the efficacy of treatment of 35 cases of late-stage colon cancer with a combination of chemotherapy and Resist Tumor and Raise White Blood Cell Count Tablets. Hunan Zhongyi Zazhi. 2001;17:13. [Google Scholar]
- 89. Niu CF, Wang XL, Wang BH. Observation of the efficacy of treatment of 34 cases of postoperative colon cancer with Support the Normal and Dispel the Pathogenic Decoction combined with chemotherapy. Zhongguo Laonianxue Zazhi. 2003;23:865-866. [Google Scholar]
- 90. Zhu WR, Zheng L, Guo YB, et al. Clinical observation of the treatment of progressive-stage colon cancer with Panax ginseng and Ophipogon japonicus injection in combination with intra-abdominal chemotherapy. Zhongxiyijiehe Xuebao 2005;3:265-269. [Google Scholar]
- 91. Li YJ, Li QS. 60 cases of progressive-stage colon cancer treated with a combination of Chinese medicinal enema and intra-abdominal chemotherapy. Jiangsu Zhongyi. 199;20:30. [Google Scholar]
- 92. Xu YQ, Xiao Y, Luo Y. Clinical observation of 32 cases of late-stage colon cancer treated using the strategies of supporting the normal, transforming stasis, resolving toxicity and dispersing aggregation in combination with intravenous chemotherapy. Jiangsu Zhongyiyao. 2006;27:41-42. [Google Scholar]
- 93. Ma J, Wang GH, Cai DF, et al. Clinical observation of the prevention of postoperative metastasis and recurrence in patients with colon cancer using prescriptions that strengthen the spleen and eliminate tumors. Shanghai Zhongyiyao Zazhi. 2005;39:24-25, 2005 [Google Scholar]
- 94. Cox DR. Regression models and life tables (with discussion). J R Stat Soc Ser B. 1972;34:187-220. [Google Scholar]
- 95. Nie XL, Shen H, Xie YM, Hu J, Zhang YL, Li YY. Meta-analysis of Dengzhanxixin injection treatment for unstable angina pectoris [in Chinese]. Zhongguo Zhong Yao Za Zhi. 2012;37:2768-2773. [PubMed] [Google Scholar]
- 96. See CJ, McCulloch M, Smikle C, Gao J. Chinese herbal medicine and clomiphene citrate for anovulation: a meta-analysis of randomized controlled trials. J Altern Complement Med. 2011;17:397-405. [DOI] [PubMed] [Google Scholar]
- 97. Ting A, Chow Y, Tan W. Microbial and heavy metal contamination in commonly consumed traditional Chinese herbal medicines. J Tradit Chin Med. 2013;33:119-124. [DOI] [PubMed] [Google Scholar]
- 98. Harris ES, Cao S, Littlefield BA, et al. Heavy metal and pesticide content in commonly prescribed individual raw Chinese herbal medicines. Sci Total Environ. 2011;409:4297-4305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Carpenter RL, Jiang BH. Roles of EGFR, PI3K, AKT, and mTOR in heavy metal–induced cancer. Curr Cancer Drug Targets. 2013;13:252-266. [DOI] [PubMed] [Google Scholar]
- 100. Cheng CW, Bian ZX, Li YP, et al. Transparently reporting adverse effects of traditional Chinese medicine interventions in randomized controlled trials. Zhong Xi Yi Jie He Xue Bao. 2008;6:881-886. [DOI] [PubMed] [Google Scholar]
- 101. Chen J, Xu L, Yang XH, et al. Effect of Jianpi Yiqi Yangxue Fang in combination with chemotherapy on immune function of postoperative colon cancer patients. Shanxi Zhongyi. 2-1;31:1107-1109. [Google Scholar]
- 102. Chen L. Observation of the efficacy of Euonymus Fortunei Formula Mixture to resist chemotherapy-induced leukopenia in postoperative colon cancer patients. Guangxi Zhongyiyao. 2001;24:49-50. [Google Scholar]
- 103. Chen XD, Liang QL, Li XY, et al. Treatment of 47 cases of colon cancer with a combination of Danshenform and chemotherapy. Zhongguo Zhongxiyijiehe Waike Zazhi. 2005;11:300-302. [Google Scholar]
- 104. Gao HD, Liu ZL, Chen HS. The use of Ligustrum and Astragalus Support the Normal Capsules with postoperative chemotherapy in patients with colon cancer. Yishi Jinxiu Zazhi: Waike Ban. 2005;28:39-40. [Google Scholar]
- 105. Jia XQ, Zhang LJ. Clinical observation of Chinese and Western integrative prevention and treatment of toxicity and side effects of postoperative chemotherapy in patients with colon cancer. Zhonghua Shiyong Zhongxiyi Zazhi. 2000;13:800-801. [Google Scholar]
- 106. Liu JA, Zhang YH. Summary of 96 cases of Spleen and Kidney prescriptions treating side effects of postoperative chemotherapy in patients with colon cancer. Hunan Zhongyi Zazhi. 2000;16:9-10. [Google Scholar]
- 107. Luo L, Yang YF, Li PH, et al. A cohort study on the reduction of recurrence and metastasis in postoperative Stage II and III colon cancer using the Chinese medicinals Support the Normal Capsules and Dispel the Pathogenic Capsules. Zhongguo Zhongxiyijiehe Zazhi. 2006;26:677-680. [Google Scholar]
- 108. Pan MQ, Pan B, Li YH. Clinical observation of 43 cases of colon cancer treated by Benefit Qi and Harmonize Bowels Decoction in combination with chemotherapy. Hunan Zhongyiyao Daobao. 2003;9:12-14. [Google Scholar]
- 109. Song CY, Wang CY, Shen FM. Observation of Sijunzi Tang in combination with chemotherapy on 29 cases of advanced colorectal cancer. Guiyang Zhongyi Xueyuan Xuebao. 2012;34:57-58. [Google Scholar]
- 110. Tan XY, Gong HW. 36 cases of treatment of hepatic metastasis from postoperative colon cancer using a combination of Kangsaidi capsules and chemotherapy. Zhongxiyijiehe Ganbing Zazhi. 2006;16:248-249. [Google Scholar]
- 111. Wang WP, Wang CJ, Liu F, et al. Clinical observation of the prevention of postoperative metastasis in 80 cases of colon cancer using the Chinese medicine Safe Intestines Formula. Beijing Zhongyiyao Daxue Xuebao. 2003;26:60-62. [Google Scholar]
- 112. Wu JY, Su SY. Clinical observation of treatment of middle- and late-stage malignant tumors using a combination of matrine and chemotherapy. Guangxi Yixue. 2003;25:1662-1664. [Google Scholar]
- 113. Xiao ZQ, Luo CD. Observation of the efficacy of Return the Origin Oral Liquid in 50 cases of colon cancer with postoperative chemotherapy, compared with 25 cases of chemotherapy alone. Liaoning Zhongyi Zazhi. 1998;25:516-517. [Google Scholar]
- 114. Yang QR, Chen XL, Lu TK. Clinical observation of the use of Supporting and Stabilizing Liquid to prevent post-chemotherapy leukopenia. Zhongyiyao Xuebao. 2001;29:6-7. [Google Scholar]
- 115. Yang X. Clinical observation of the treatment of late-stage colon cancer using enemas containing chemotherapy and Chinese medicinals. Zhongguo Gangchangbing Zazhi. 2006;26:13-15. [Google Scholar]
- 116. Yu GY, Wu GL, Li JP. Clinical observation of the use of Support the Spleen and Benefit the Stomach Beverage for prevention and treatment of toxicity and side effects of postoperative chemotherapy in patients with colon cancer. Zhonghua Shiyong Zhongxiyi Zazhi. 2005;18:276-277. [Google Scholar]
- 117. Zhao WS, Zhang Q, Yu J, et al. A clinical study on the treatment of middle- and late-stage colon cancer using a combination of Raise Blood Counts Decoction with Added Ingredients and chemotherapy. Guoji Zhongyi Zhongyao Zazhi. 2006;28:306-308. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.