Abstract
The relationship between lung function decline and dietary antioxidants over 10 years in adults from three European countries was investigated.
In 2002, adults from three participating countries of the European Community Respiratory Health Survey (ECRHS) answered a questionnaire and underwent spirometry (forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC)), which were repeated 10 years later. Dietary intake was estimated at baseline with food frequency questionnaires (FFQ). Associations between annual lung function decline (mL) and diet (tertiles) were examined with multivariable analyses. Simes’ procedure was applied to control for multiple testing.
A total of 680 individuals (baseline mean age 43.8±6.6 years) were included. A per-tertile increase in apple and banana intake was associated with a 3.59 mL·year−1 (95% CI 0.40, 7.68) and 3.69 mL·year−1 (95% CI 0.25, 7.14) slower decline in FEV1 and FVC, respectively. Tomato intake was also associated with a slower decline in FVC (4.5 mL·year−1; 95% CI 1.28, 8.02). Only the association with tomato intake remained statistically significant after the Simes’ procedure was performed. Subgroup analyses showed that apple, banana and tomato intake were all associated with a slower decline in FVC in ex-smokers.
Intake of fruits and tomatoes might delay lung function decline in adults, particularly in ex-smokers.
Short abstract
A higher intake of fruits and tomato is associated with a slower lung function decline, particularly in ex-smokers http://ow.ly/5LLv30gK9Bn
Introduction
Lung function is a predictor of mortality in the general population, as well as in patients with lung disease, even in those who have never smoked [1]. Maintaining lung function is an important goal in the prevention of chronic respiratory diseases and a major public health objective; yet, smoking cessation remains the main target to reduce the burden of these diseases [2].
The possible modulatory effect of diet on lung health has been investigated in several epidemiological studies, suggesting that dietary intake of various sources of antioxidants are associated with improved ventilatory function outcomes in adults [3]. Cross-sectional and longitudinal evidence has shown a positive association between forced vital capacity (FVC) and higher fruit and flavonoid intake in young [4], middle-aged [5] and elderly adults [6]. Similarly, in older adults, a ‘prudent’ dietary pattern, characterised by a higher intake of fruits and vegetables, has been associated with better lung function and a lower prevalence of chronic obstructive pulmonary disease (COPD) [7]. Longitudinal evidence is less consistent for other antioxidants. A 4-year follow-up study showed that having a higher intake of antioxidant nutrients was associated with an attenuated decline of forced expiratory volume in 1 s (FEV1) in ex- and current-smokers, compared to those who had a lower intake [8], whilst other studies have shown a positive association between serum vitamin E [9] and lung function decline, or no effect of vitamin E supplementation on lung function [10].
The European Community Respiratory Health Survey (ECRHS) is a three-phased, longitudinal, multi-centre, European cohort study that examines the role of environmental risk factors on respiratory health. In the present study, we sought to investigate whether a higher intake of dietary sources of antioxidants in middle-aged European adults could attenuate ageing-related lung function decline over 10 years.
Methods
Sample
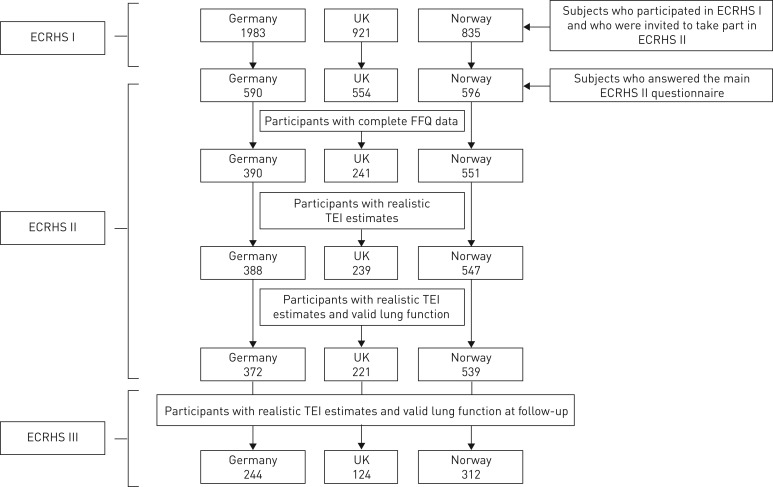
The ECRHS survey started in 1990, with outcomes and exposures studied at three time points: 1990–1995 (ECRHS I), 2002 (ECRHS II) and 2012 (ECRHS III). Details of the study design have been reported elsewhere [11, 12]. Briefly, in 29 participant centres in 1990, a random sample of at least 3000 adults, aged 20–44 years was selected, using a local sampling frame. From those who responded, a random sample of at least 600 adults was selected to undergo a detailed clinical examination (1991–1993). In ECRHS II (1998–2002), participants who had completed the extended questionnaire in ECRHS I, were reinvestigated to include spirometry. In ECRHS III, those who took part in the clinical stages of ECRHS I and II were again contacted, and responders were invited to a local testing centre, where measures of lung function were carried out once more. Figure 1 illustrates the flowchart of participants from ECRHS I to those included in the present analysis (ECRHS II and III).
FIGURE 1.
Flowchart of participants in European Community Respiratory Health Survey (ECRHS) II and ECRHS III surveys included in the present study. FFQ: food frequency questionnaires; TEI: total energy intake
Assessment of diet
Dietary assessments were included in ECRHS II at five centres (three countries), although the method and protocol differed among countries. For the current analyses, we present the results from these centres. The same centres also participated in ECRHS III (Hamburg and Erfurt in Germany; Ipswich and Norwich in the UK; and Bergen in Norway).
Food frequency questionnaires (FFQ)
The German FFQ was developed for use in the German part of the European Prospective Investigation into Cancer and Nutrition (EPIC-Heidelberg) [13]. It recorded the consumption of 158 different foods over the previous 12 months as frequencies (zero to five or more portions per day). Portion size was selected from multiple-choice questions, sometimes with reference photos. Supplementary questions covered aspects of diet, such as the preparation and fat content of foods. The FFQ was distributed after the clinical and questionnaire assessments, and the participants were asked to return the completed FFQ by mail. Further details on the characteristics of the FFQs, exclusion of dietary data and the validity and repeatability of the FFQs are provided in the supplementary material.
Exclusion of dietary data
On the FFQs, respondents sometimes left individual items blank. This was assumed to denote zero intake of these foods; however, if 20% of the items were blank, the FFQ was considered incomplete, and the subject was excluded from further analyses. Participants were also excluded if they had extreme values of total energy intake, which might suggest an unrealistic response. We calculated the expected basal metabolic rate (BMR) with the given age, weight and sex [4], and excluded subjects with a ratio of energy intake to expected BMR that was either below the 0.5th sample centile or above the 99.5th sample centile for their country [5]. This is what we referred to as having “unrealistic total energy intake (TEI)”.
Nutrient intakes
Nutrient intakes were calculated for each country from the FFQ data and supplementary questions, using local food tables [14–16]. The intake of nutrients with antioxidant properties was measured by whole food intake (total fruit and total vegetable intakes) and micronutrient intake, calculated from all dietary sources of antioxidant vitamin intake (vitamins C and E, and β-carotene). In addition to total fruit and vegetable intake, we selected 17 individual fruits (n=5), vegetables (n=5), or other foods or beverages (n=7) for their high content of β-carotene and vitamin C [15], and because they generally have the highest estimates of total flavonoids, or for their reported potential antioxidant effects on lung health [5].
Lung function measurements
During ECRHS II and ECRHS III, lung function measurements of the participants were taken. During ECRHS II, participants had at least five, and up to nine attempts to provide two technically satisfactory forced expiratory manoeuvres. In 2002 (ECRHS II), different spirometers were used at each centre (Biomedin in the UK, Sensor Medics in Norway and Jaeger Pneumolab in Germany); however, during ECRHS III, lung function was tested at all centres using the NDD spirometer (ndd Medical Technologies, Zurich, Switzerland). During ECRHS II, participants had at least five, and up to eight attempts to provide reproducible (150 mLs) FEV1 and FVC. The maximum FEV1 and FVC reproducible to 150 mL, possibly coming from different expiratory manoeuvres [17], were used as the outcome. Decline in FEV1 and FVC was expressed per year of follow-up (ECRHS III value minus ECRHS II value, a negative value represented a decline). All measures were assessed pre-bronchodilator.
Potential confounders
Body mass index (BMI) was based on the measured weight and height. Subjects were categorised as never-, ex- or current smokers based on questionnaire responses. Pack-years of smoking were calculated, based on questions about ever-smoking; and among smokers, additional questions about age at which smoking commenced, current smoking, reducing or quitting smoking were posed. Educational level was estimated as the age at which full-time education was completed, and three categories were created (completed education before 18 years of age, between 18–21 years, or >21 years). Socio-economic status was based on the reported occupation group of the International Standard Classification of Occupations-88 codes [18]. Physical activity was based on the reported frequency of physical exercise (the question “How often do you usually exercise so much that you get out of breath or sweat?” was posed), and categorised as follows: never; less than once a week; one to three times a week; or more than three times a week.
Statistical analyses
The total intake in grams of fruits and vegetables, as well as the fifteen individual food items analysed, was considered as tertiles. The decline in FEV1 and FVC in mL per year between ECRHS II and ECRHS III per tertile of each food consumed was assessed using multivariate linear regression, controlling for two sets of confounders; a baseline model (Model 1) included the covariates age, height and country (results presented in the supplementary material). Model 2 added the covariates sex, BMI, socio-economic status, physical activity, years of education and TEI. Data from centres within the same country were merged and treated as a single sample in the analyses. The Simes’ procedure was used to adjust for multiple testing [19].
All analyses were repeated and stratified by country, and the effect estimates were combined using random effects meta-analysis [20]. Heterogeneity was summarised using the I2 statistic [21]. Further analyses were carried out after stratifying by smoking status, using the same models of adjustment as those used with the dietary exposures that showed a statistically significant association in the analyses of the whole sample. All analyses were conducted using Stata 14 (Stata Corporation, College Station, TX, USA).
Results
Figure 1 shows the number of participants per country. Clinical and questionnaire assessments were available for 1740 subjects from ECRHS II. Of the 1182 subjects with complete FFQ data, i.e. subjects with more than 80% of the FFQ completed, eight subjects were further excluded for having an “unrealistic TEI” (n=1174). Then, 42 subjects were also excluded for not having valid lung function measures, leaving 1132 subjects with realistic TEI and valid lung function data in the ECRHS II. Of this sample, at the time of follow-up (ECRHS III), 680 individuals had valid lung function measured at this second time point, and comprised the final sample for analysis. The general characteristics of responders (ECRHS III) and non-responders were similar between both groups, in relation to age, sex, socio-economic status, BMI and physical activity (Supplementary table S1).
Table 1 summarises the main general characteristics and dietary intake of participants with FFQ at baseline and lung function at baseline and during ECRHS III (n=680). The average age at baseline was 43.8±6.6 years. Over 40% of the participants had smoked at some point before ECRHS III, and 16% were current smokers. The mean decline in FEV1 and FVC in the present study sample over the 10-year period was 445 mL and 389 mL, respectively. The median total intake of fruits and vegetables was 278 g·day−1 and 114 g·day−1, respectively, with the highest consumption in Norway.
TABLE 1.
General characteristics of participants in ECRHS II and ECRHS III with FFQ and lung function measures
| Variables (measured in 2002) | Countries | |||
| Germany | UK | Norway | Overall | |
| Subjects n | 244 | 124 | 312 | 680 |
| Mean±sd years of follow-up ECRHS II and III | 10.3±0.4 | 12.6±0.5 | 9.3±0.2 | 10.3±1.2 |
| Mean±sd age years | 44.2±7.0 | 43.3±6.2 | 43.6±6.5 | 43.8±6.6 |
| Males n (%) | 115 (47.1) | 54 (43.6) | 167 (53.5) | 336 (49.4) |
| Mean±sd height m | 1.71±0.1 | 1.68±0.1 | 1.73±0.1 | 1.71±0.1 |
| Mean±sd BMI kg·m−2 | 25.7±4.5 | 26.10±4.4 | 25.6±4.0 | 25.7±4.2 |
| Smoking# n (%) | ||||
| Never-smokers | 82 (33.8) | 74 (59.7) | 126 (41.6) | 282 (42.2) |
| Ex-smokers gave up before ECRHS II | 94 (38.9) | 36 (29.0) | 74 (24.4) | 204 (30.5) |
| Ex-smokers gave up after ECRHS II | 21 (8.7) | 7 (5.7) | 42 (13.9) | 70 (10.5) |
| Current smokers | 45 (18.6) | 7 (5.7) | 61 (20.1) | 113 (16.9) |
| Mean number of pack-years smoked up to ECRHS III¶ | 21.7 | 14.7 | 20.1 | 20.1 |
| Age when completed education years+ n (%) | ||||
| Before 18 | 112 (17.1) | |||
| 18–20 | 213 (32.7) | |||
| ≥21 | 327 (50.2) | |||
| FEV1 at baseline (mean z-score GLI) | 0.25 | 0.15 | −0.39 | −0.06 |
| FVC at baseline (mean z-score GLI) | 0.14 | 0.31 | −0.12 | 0.05 |
| Whole sample 10-year mean±sd change in FEV1 mL§ | −445.6±290.0 | |||
| Whole sample 10-year mean±sd change in FVC mL§ | −381.1±347.9 | |||
| Median (IQR) total fruit intake g·day−1 | 205.9 (116.4–347.0) | 289.8 (175.4–442.7) | 321.5 (191.7–502.6) | 278.5 (156.5–443.9) |
| Median (IQR) total vegetable intake g·day−1 | 69.6 (51.8–95.9) | 155.8 (107.9–225.4) | 180.4 (97.5–319.8) | 114.3 (69.7–213.3) |
| Median (IQR) vitamin A IU | 872.6 (570–1381) | 545.9 (375–844) | 482.4 (291–872) | 633.6 (376–113) |
| Median (IQR) vitamin C mg | 104.1 (76.5–145.5) | 225.2 (151.2–348.9) | 230.0 (148.6–340.8) | 170.8 (105.8–273.1) |
| Median (IQR) vitamin D µg | 3.4 ((2.3–4.8) | 2.7 (1.9–3.5) | 4.5 (3.3–6.4) | 3.7 (2.5–5.4) |
| Median (IQR) vitamin E µg | 8.7 (6.7–10.4) | 8.7 (6.3–11.0) | 13.6 (10.1–18.1) | 10.1 (7.4–14.4) |
| Median (IQR) total energy intake kcal | 2251 (1470–3069) | 2587 (1788–3386) | 2819 (1710–3918) | 2573 (1748–3421) |
ECRHS: European Community Respiratory Health Survey; FFQ: food frequency questionnaires; BMI: body mass index; FEV1: forced expiratory volume in 1 s; GLI: Global Lung Function Initiative; FVC: forced vital capacity; IQR: interquartile range. #: Smoking status was based on self-report in 2000 and 2013. Current smokers were defined as those who smoked in 2000 and 2013. Ex-smokers were split into two groups: those who had quit before baseline and those who quit between baseline and follow-up. Never-smokers reported no smoking at both instances. ¶: Estimated for ever-smokers. +: From 652 subjects with information on number of years of education who had lung function data in ECRHS II and III. §: Standardised to a 10-year interval period.
Table 2 shows the adjusted associations between annual FEV1 and FVC decline, and dietary intake of the relevant foods and antioxidant vitamins over the 10-year follow-up. In the fully adjusted model, total intake of fruits was positively associated with a 3.5 mL·year−1 slower decline in FEV1 (95% CI 0.04, 6.92); however, the association was of borderline statistical significance. Similarly, per-tertile increases in the intake of apples, bananas, tomatoes, herbal tea and vitamin C were all significantly associated with a slower decline in FVC. Among these, only the association between FVC and tomato intake survived the Simes’ procedure (4.74 mL·year−1 slower decline in FVC; 95% CI 1.35, 8.13).
TABLE 2.
Adjusted associations of FEV1 and FVC decline with dietary intake measured at baseline (2001)
| Dietary intake (per-tertile increase) |
FEV1
decline mL·year−1
(continuous) regression coefficient (95% CI) |
FVC decline mL·year−1
(continuous) regression coefficient (95% CI) |
||
| Fully adjusted model# | p-value | Fully adjusted model¶ | p-value | |
| Foods g | ||||
| Total fruit | 2.99 (0.37, 5.61) | 0.025 | 3.48 (0.04, 6.92) | 0.048 |
| Apple | 2.53 (0.10, 4.94) | 0.04 | 3.96 (0.76, 7.15) | 0.01 |
| Banana | 2.57 (−0.02, 5.17) | 0.05 | 3.97 (0.51, 7.43) | 0.03 |
| Orange | 2.40 (−0.07, 4.87) | 0.06 | 2.94 (−0.33, 6.21) | 0.08 |
| Pear | 2.19 (−0.80, 5.17) | 0.15 | 1.28 (−2.66, 5.22) | 0.52 |
| Berries | 0.52 (−2.31, 3.35) | 0.72 | 1.59 (−2.11, 5.29) | 0.40 |
| Total vegetables | −0.29 (−3.47, 2.89) | 0.86 | −0.01 (−4.26, 4.25) | 0.96 |
| Potato (boiled/mashed/baked) | −1.83 (−4.47, 0.82) | 0.18 | −3.27 (−6.78, 0.24) | 0.07 |
| Broccoli, cabbage and cauliflower | −1.26 (−4.44, 1.93) | 0.44 | −2.94 (−7.21, 1.32) | 0.18 |
| Carrots | 0.72 (−2.09, 3.53) | 0.61 | −0.86 (−4.59, 2.87) | 0.65 |
| Garlic | −0.45 (−3.26, 2.35) | 0.75 | −0.02 (−3.66, 3.70) | 0.99 |
| Tomato | 2.80 (0.23, 5.38) | 0.03 | 4.74 (1.35, 8.13) | 0.006+ |
| Flavonoid-rich foods/beverages mL or g | ||||
| Chocolate | −0.35 (−3.10, 2.39) | 0.80 | −2.26 (−5.89, 1.36) | 0.22 |
| Nut | −0.82 (−4.14, 6.77) | 0.63 | −1.72 (−3.67, 10.70) | 0.34 |
| Lentils, dahl, mixed beans | 1.31 (−4.28, 6.60) | 0.68 | −3.51 (−6.6, 0.23) | 0.07 |
| Tea (black and green) | −1.43 (−4.01, 1.15) | 0.28 | −2.59 (−6.01, 0.83) | 0.14 |
| Herbal tea | 4.64 (−0.23, 9.53) | 0.06 | 7.75 (1.28, 14.2) | 0.02 |
| Coffee | 1.05 (−1.51, 3.60) | 0.42 | 1.35 (−2.01, 4.70) | 0.43 |
| Wine | 0.59 (−1.96, 3.14) | 0.65 | 3.62 (−3.56, 10.8) | 0.32 |
| Antioxidant vitamins | ||||
| Vitamin A (retinol) UI | 0.72 (−3.10, 2.73) | 0.90 | 0.17 (−3.66, 4.00) | 0.77 |
| Vitamin A (β-carotenoid) UI | −0.19 (−0.59, 2.11) | 0.61 | 0.02 (−3.82, 3.87) | 0.99 |
| Vitamin C (ascorbic acid) mg | 2.64 (−0.91, 5.86) | 0.11 | 4.54 (0.28, 8.82) | 0.037 |
| Vitamin D µg | −2.66 (−5.60, 0.29) | 0.08 | −1.17 (−5.07, 2.73) | 0.56 |
| Vitamin E µg | 0.17 (−3.40, 3.75) | 0.93 | 0.58 (−4.17, 5.34) | 0.81 |
Fully adjusted model (adjusted for height, age, country, sex, smoking status, socio-economic class, body mass index, total energy intake, physical activity and years of education). Bold font indicates a statistically significant p-value (<0.05). FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity. #: n=680; ¶: n=654; +: Only dietary exposure to survive Simes’ procedure (p=0.03).
As IgE-sensitisation to airborne allergens, such as birch pollen could affect the intake of some fruits through cross-reactivity, we further investigated whether IgE sensitisation could be a confounder in the associations investigated. The effect sizes for the associations between total fruit intake (per-tertile increase) and FEV1 was 2.74 mL (95% CI 0.08, 5.4), p=0.04; and FVC, 3.21 mL (95% CI −0.02, 6.72), p=0.055 (data not shown).
The hypothesis that smoking modifies the effects of dietary antioxidants was investigated within categories of cigarette smokers (current, ex- (prior to ECRHS III) and never-smokers). Tables 3 and 4 show the adjusted stratified analyses according to smoking habit for the dietary exposures that showed a statistically significant association in the analyses presented in table 2. Total dietary intake of fruits, apples, tomatoes and herbal tea were all associated with a slower decline in FEV1 and FVC in ex-smokers (p<0.05); however, no effect was observed in never- or current smokers (table 3). There was evidence that smoking modified the effect of fruit intake on FEV1 and FVC decline (p for interaction=0.03, and p for interaction=0.04, respectively); however, smoking did not modify the effect of tomatoes, herbal tea, or vitamin C (all p for interaction >0.05). Direction and magnitude of effects seen in ex- and current smokers were only slightly altered by further adjustment for pack-years of smoking (table 4).
TABLE 3.
Adjusted associations# between FEV1 or FVC decline and dietary intake in adults stratified by smoking status with dietary data collected in 2001
| Dietary intake (per-tertile increase) |
Average decline in lung function mL·year−1
(continuous) regression coefficient (95% CI) |
||||||
| Never-smoker | p-value | Quit before ECRHS III | p-value | Smoker | p-value | p-value for interaction | |
| Subjects n | 270 | 255 | 109 | ||||
| FEV1 | |||||||
| Total fruit g | 0.51 (−3.62, 4.65) | 0.81 | 6.41 (2.29, 10.5) | 0.002 | 3.83 (−2.93, 10.60) | 0.26 | 0.03 |
| Apple g | 0.16 (−3.51, 3.82) | 0.93 | 4.79 (0.87, 8.72) | 0.017 | 0.62 (−6.22, 7.46) | 0.86 | 0.09 |
| Banana g | 2.63 (−1.11, 6.37) | 0.17 | 2.92 (−1.52, 7.35) | 0.20 | −0.82 (−7.70, 6.06) | 0.81 | 0.25 |
| Tomato g | 0.52 (−3.36, 4.40) | 0.79 | 5.15 (0.87, 9.44) | 0.019 | 5.71 (−1.21, 12.63) | 0.11 | 0.06 |
| Herbal tea mL | −3.89 (−11.5, 3.71) | 0.32 | 12.8 (5.13, 20.54) | 0.001 | 1.97 (−13.36, 17.3) | 0.80 | 0.21 |
| Vitamin C mg | 1.66 (−3.36, 6.69) | 0.52 | 3.99 (−1.45, 9.44) | 0.15 | 3.19 (−5.59, 11.97) | 0.47 | 0.05 |
| FVC | |||||||
| Total fruit g | 0.13 (−4.79, 5.06) | 0.96 | 8.13 (2.22, 14.01) | 0.007 | 4.15 (−5.41, 13.7) | 0.39 | 0.04 |
| Apple g | 1.45 (−2.91, 5.80) | 0.51 | 6.75 (1.14, 12.34) | 0.018 | 0.78 (−9.13, 10.69) | 0.88 | 0.29 |
| Banana g | 4.07 (−0.54, 8.67) | 0.08 | 6.23 (0.01, 12.5) | 0.05 | −3.79 (−13.6, 5.99) | 0.44 | 0.04 |
| Tomato g | 1.02 (−3.66, 5.70) | 0.67 | 9.09 (3.04, 15.14) | 0.003 | 7.16 (−3.05, 17.37) | 0.17 | 0.11 |
| Herbal tea mL | −2.52 (−11.7, 6.67) | 0.59 | 14.4 (3.16, 25.69) | 0.01 | 12.34 (−9.47, 34.15) | 0.26 | 0.11 |
| Vitamin C mg | 3.17 (−2.83, 9.16) | 0.30 | 4.65 (−3.08, 12.37) | 0.24 | 10.58 (−1.96, 23.11) | 0.10 | 0.30 |
Bold font indicates a statistically significant p-value (<0.05). FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity; ECRHS: European Community Respiratory Health Survey. #: Adjusted for height, age, country, sex, socio-economic status, body mass index, total energy intake, years of education and physical activity.
TABLE 4.
Adjusted associations of FEV1 or FVC decline with dietary intake as reported in 2001 (controlling for lifetime pack-years of smoking at follow-up)#
| Dietary exposures (tertiles) |
Decline in lung function mL·year−1
(continuous) regression coefficient (95% CI) |
|||
| Quit before ECRHS III | p-value | Smoker | p-value | |
| Subjects n | 227 | 95 | ||
| FEV1 | ||||
| Total fruit g | 5.45 (1.12, 9.78) | 0.01 | 1.21 (−5.92, 8.33) | 0.74 |
| Apple g | 4.97 (0.94, 9.01) | 0.016 | 6.95 (−0.84, 14.73) | 0.08 |
| Banana g | 1.41 (−3.21, 6.04) | 0.55 | −3.22 (−10.28, 3.83) | 0.37 |
| Tomato g | 5.44 (0.98, 9.90) | 0.017 | 3.63 (−3.60, 10.86) | 0.32 |
| Herbal tea mL | 12.03 (4.09, 19.98) | 0.003 | 2.64 (−14.66, 19.94) | 0.76 |
| Vitamin C mg | 2.83 (−2.90, 8.57) | 0.33 | 0.93 (−8.07, 9.92) | 0.84 |
| FVC | ||||
| Total fruit g | 7.38 (1.17, 13.6) | 0.02 | 1.47 (−8.91, 11.9) | 0.78 |
| Apple g | 7.28 (1.59, 13.0) | 0.01 | 6.41 (−5.38, 18.2) | 0.28 |
| Banana g | 4.67 (−1.88, 11.2) | 0.16 | −7.44 (−17.4, 2.48) | 0.14 |
| Tomato g | 8.45 (2.16, 14.7) | 0.009 | 5.12 (−4.93, 15.2) | 0.31 |
| Herbal tea mL | 11.57 (−0.01, 23. 15) | 0.05 | 14.93 (−8.90, 38.76) | 0.22 |
| Vitamin C mg | 2.80 (−5.24, 10.8) | 0.49 | 6.90 (−6.43, 20.23) | 0.31 |
Adjusted for height, age, country, sex, social class, body mass index, total energy intake, years of education and physical activity. Bold font indicates a statistically significant p-value (<0.05). FEV1: forced expiratory volume in 1 s; FVC: forced vital capacity. #: Only ex- or current smokers included in this analysis).
In a post-hoc analysis, we examined the effect of total intake of fruits, apples, and tomatoes on FEV1 and FVC per country, and pooled the effect estimates. A trend towards an association between higher intake of these foods and a slower decline of both spirometric measures, with little or no heterogeneity among countries was observed, although the overall estimate did not reach significance (Supplementary figures S1–S4).
Discussion
Our findings suggest that a higher total intake of fruits, and of apples in middle-aged adults in Europe, was associated with a slower FEV1 decline; whilst the intake of apples, bananas, tomatoes, herbal tea and vitamin C was associated with a slower FVC decline. These associations remained robust even after adjustment for relevant potential confounders, and our results suggest that these protective effects are likely of greater impact in those who have quit smoking. The intake of fruits and vegetables in the adult participants of the present study averaged just over 400 g (four fruits), with Germany reporting the lowest intake (265 g) and Norway reporting the highest (445 g).
Ageing and smoking are established risk factors for a steeper lung function decline in adults [22]; the role of diet, however, is less clear [23]. Evidence from randomised controlled trials (RCTs) is limited to very few studies, which have shown no effect of β-carotene or α-tocopherol [24, 25] on lung function. These studies have used very specific adult populations affected by serious comorbidities, and are not representative of the general population. More recently, an intervention using vitamin E and selenium showed no effect on lung function in healthy adults and smokers [10]. Observational evidence suggests that the intake of foods that are rich in antioxidants (e.g. fruits and vegetables), as well as specific antioxidants, is associated with a slower decline in lung function in adults [26].
We found associations between tomato intake and a slower decline in both FEV1 and FVC in the whole sample, and particularly in ex-smokers. Tomatoes are the richest dietary sources of lycopene [27], a carotenoid with no vitamin A activity. This might explain why we observed beneficial associations with this food, but not with vitamin A or other sources of this antioxidant. A RCT in asthmatic adults showed that a diet low in antioxidants reduced FEV1 and FVC % predicted values [28], and that intake of a tomato extract and tomato juice for 10 days led to reduced airway inflammation in the intervention group [29]. The potential benefits of lycopene in lung health might extend to reduction of the risk of mortality in individuals with COPD. A longitudinal analysis based on the National Health and Nutrition Examination Survey (NHANES) III study showed that serum levels of lycopene and vitamin C were the only antioxidants to be negatively associated with the risk of all-cause mortality in fully adjusted regression models [30]. Serum lycopene and pro-vitamin carotenoids were also negatively associated with FEV1 and FVC decline over 14 years in young adults [31].
Given our a priori hypothesis that antioxidant intake might contribute to the preservation of lung function and attenuation of decline, we also investigated the association between specific food items and a high content of flavonoids. Flavonoids are widely distributed in plant foods and are found in particularly high amounts in fruits, vegetables and herbal teas [32]. Intervention studies in adults have demonstrated their antioxidant properties [33], and extensive experimental evidence demonstrates their anti-inflammatory effects [34], a mechanism through which they might exert beneficial effects on lung health. The scant evidence from population-based studies so far, suggests that catechins are positively associated with higher ventilatory function in adults [4, 5], and pro-anthocyanidins are prospectively associated with a slower lung function decline in older adults [6]. Albeit we found no evidence of an association between these foods and lung function decline, our observation that total fruit intake is associated with a slower decline in FEV1 and FVC might be partly explained by the flavonoid contents in this food group. Our observation that herbal tea intake is associated with a slower lung function decline further supports the notion that regular consumption of foods rich in polyphenolic compounds is beneficial for lung health [35, 36].
Our results show that the effects observed for apples, tomato and herbal tea were particularly strong in ex-smokers, regardless of the number of pack-years smoked between the two surveys. The Health Aging and Body Composition (Health ABC) study, a population-based survey in older adults, also showed that a higher intake of antioxidant nutrients was associated with a slower lung function decline (only FEV1 measured) [8]. Similarly, earlier studies on dietary antioxidant intake and lung function decline have reported similar effects to those found in our study. A study in middle-aged Welshmen (45–59 years old) followed for 5 years, reported a 10–15 mL·year−1 slower decline in FEV1 in those with the highest versus those with the lowest intakes of apples; however, no effect was observed for the antioxidant vitamins C or E [37]. In the Health ABC study, a higher intake of fruits or vegetables was associated with an 18 mL and 24 mL·year−1 slower decline in FEV1, respectively, in older adults [8]. In our study, the effect size was smaller, but there was a longer follow-up time.
One strength of this study is the use of a random community-based sample of adults from three European countries, which affords some confidence in generalising the results to wider populations. Lung function was measured with standardised protocols at baseline and at follow-up, with a quality control programme in place. At follow-up, visual quality control of forced expiratory curves was conducted. Given the various dietary exposures being tested and the fact that we individually investigated the possible association between single nutrients and outcomes, we used the Simes’ procedure as a more rigorous way to control for multiple comparisons, to reduce the possibility of false discovery. The FFQ used in this study was previously validated in a similar population of adults from the participating countries, showing a good level of reproducibility and validity [38]. One limitation of the study is that we only had dietary assessments at baseline on one occasion for the three countries. We had to assume that diet remained relatively constant in adult life, and evidence for this exists [39]. Investigations of the association between single nutrients and disease might not accurately reflect a specific dietary habit or dietary behaviour, as neither foods, nor nutrients are consumed in isolation. Information on nutrient supplementation was not collected, and we cannot rule out whether adjustment for this might have altered estimates. However, studies on nutrient supplementation and lung function have shown little evidence of an association [10].
In conclusion, our study suggests that dietary factors might play a role in preserving ventilatory function in adults, by slowing down a decline in lung function. In particular, dietary antioxidants possibly contribute to restoration, following damage caused by exposure to smoking, among adults who have quit.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary Material ERJ-02286-2016_SupplementaryMaterial (212.1KB, pdf)
Acknowledgements
We are indebted to the participants of ECRHS II and III for their willingness to help with the collection of data.
Footnotes
This article has supplementary material available from erj.ersjournals.com
Support statement: The current study is part of the Ageing for Lungs in European Cohorts (ALEC) study. The ALEC Study is funded by the European Union's Horizon 2020 Research and Innovation programme under grant agreement no. 633212. The coordination of the ECRHS II was supported by the European Commission, as part of their Quality of Life programme. The following bodies funded the local studies in ECRHS II included in this paper. Bergen: Norwegian Research Council, Norwegian Asthma and Allergy Association (NAAF), Glaxo Wellcome AS, Norway Research Fund. Erfurt: GSF-National Research Centre for Environment and Health, Deutsche Forschungsgemeinschaft (DFG) (grant code FR 1526/1-1). Hamburg: GSF-National Research Centre for Environment and Health, Deutsche Forschungsgemeinschaft (DFG) (grant code MA 711/4-1). Ipswich and Norwich: Asthma UK (formerly known as National Asthma Campaign (UK)). ECRHS III was funded by the Medical Research Council (Grant Number 92091). Funding information for this article has been deposited with the Crossref Funder Registry.
Conflict of interest: None declared.
References
- 1.Strachan DP. Ventilatory function, height, and mortality among lifelong non-smokers. J Epidemiol Community Health 1992; 46: 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young RP, Hopkins RJ. Primary and secondary prevention of chronic obstructive pulmonary disease: where to next? Am J Respir Crit Care Med. 2014; 190: 839–840. [DOI] [PubMed] [Google Scholar]
- 3.Hanson C, Rutten EP, Wouters EF, et al. Diet and vitamin D as risk factors for lung impairment and COPD. Transl Res 2013; 162: 219–236. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Larsen V, Amigo H, Bustos P, et al. Ventilatory function in young adults and dietary antioxidant intake. Nutrients 2015; 7: 2879–2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tabak C, Smit HA, Heederik D, et al. Diet and chronic obstructive pulmonary disease: independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study). Clin Exp Allergy 2001; 31: 747–755. [DOI] [PubMed] [Google Scholar]
- 6.Mehta AJ, Cassidy A, Litonjua AA, et al. Dietary anthocyanin intake and age-related decline in lung function: longitudinal findings from the VA Normative Aging Study. Am J Clin Nutr 2016; 103: 542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shaheen SO, Jameson KA, Syddall HE, et al. The relationship of dietary patterns with adult lung function and COPD. Eur Respir J 2010; 36: 277–284. [DOI] [PubMed] [Google Scholar]
- 8.Bentley AR, Kritchevsky SB, Harris TB, et al. Dietary antioxidants and forced expiratory volume in 1 s decline: the Health, Aging and Body Composition study. Eur Respir J 2012; 39: 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanson C, Lyden E, Furtado J, et al. Serum tocopherol levels and vitamin E intake are associated with lung function in the normative aging study. Clin Nutr 2016; 35: 169–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cassano PA, Guertin KA, Kristal AR, et al. A randomized controlled trial of vitamin E and selenium on rate of decline in lung function. Respir Res 2015; 16: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burney PG, Luczynska C, Chinn S, et al. The European Community Respiratory Health Survey. Eur Respir J 1994; 7: 954–960. [DOI] [PubMed] [Google Scholar]
- 12.The European Community Respiratory Health Survey II. Eur Respir J 2002; 20: 1071–1079. [DOI] [PubMed] [Google Scholar]
- 13.Bohlscheid-Thomas S, Hoting I, Boeing H, et al. Reproducibility and relative validity of energy and macronutrient intake of a food frequency questionnaire developed for the German part of the EPIC project. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol 1997; 26: Suppl. 1, S71–S81. [DOI] [PubMed] [Google Scholar]
- 14.(BgVV) FIfHPoC. Der Bundeslebensmittelschlussel (BLS II.3). [The German Food Code and Nutrient Database]. Berlin, BgVV, 1999. [Google Scholar]
- 15.McCance R. McCance and Widdowson's the Composition of Foods. 6th Summary Edn Cambridge, Royal Society of Chemistry, 2002. [Google Scholar]
- 16.Norwegian Food Safety Authority DfHaSA, and University of Oslo http://matportalen.no/matvaretabellenn 2006 Date last accessed: October 17, 2016. Date last updated: 2017.
- 17.American Thoracic Society. Standardization of Spirometry, 1994 Update. Am J Respir Crit Care Med 1995; 152: 1107–1136. [DOI] [PubMed] [Google Scholar]
- 18.International Labour Organisation. International Standard Classification of Occupations (ISCO-88). Geneva, International Labour Organisation, 1991. [Google Scholar]
- 19.Simes RJ. An improved Bonferroni procedure for multiple tests of significance. Biometrika 1986; 73: 4. [Google Scholar]
- 20.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986; 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee PN, Fry JS. Systematic review of the evidence relating FEV1 decline to giving up smoking. BMC Med 2010; 8: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eisner MD, Anthonisen N, Coultas D, et al. An official American Thoracic Society public policy statement: novel risk factors and the global burden of chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2010; 182: 693–718. [DOI] [PubMed] [Google Scholar]
- 24.Rautalahti M, Virtamo J, Haukka J, et al. The effect of alpha-tocopherol and beta-carotene supplementation on COPD symptoms. Am J Respir Crit Care Med 1997; 156: 1447–1452. [DOI] [PubMed] [Google Scholar]
- 25.Agler AH, Kurth T, Gaziano JM, et al. Randomised vitamin E supplementation and risk of chronic lung disease in the Women's Health Study. Thorax 2011; 66: 320–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carey IM, Strachan DP, Cook DG. Effects of changes in fresh fruit consumption on ventilatory function in healthy British adults. Am J Respir Crit Care Med 1998; 158: 728–733. [DOI] [PubMed] [Google Scholar]
- 27.Agarwal S, Rao AV. Tomato lycopene and its role in human health and chronic diseases. CMAJ 2000; 163: 739–744. [PMC free article] [PubMed] [Google Scholar]
- 28.Wood LG, Garg ML, Smart JM, et al. Manipulating antioxidant intake in asthma: a randomized controlled trial. Am J Clin Nutr 2012; 96: 534–543. [DOI] [PubMed] [Google Scholar]
- 29.Wood LG, Garg ML, Powell H, et al. Lycopene-rich treatments modify noneosinophilic airway inflammation in asthma: proof of concept. Free Radic Res 2008; 42: 94–102. [DOI] [PubMed] [Google Scholar]
- 30.Ford ES, Li C, Cunningham TJ, et al. Associations between antioxidants and all-cause mortality among US adults with obstructive lung function. Br J Nutr 2014; 112: 1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thyagarajan B, A Meyer K, Smith LJ, et al. Serum carotenoid concentrations predict lung function evolution in young adults: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Clin Nutr 2011; 94: 1211–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manach C, Scalbert A, Morand C, et al. Polyphenols: food sources and bioavailability. Am J Clin Nutr 2004; 79: 727–747. [DOI] [PubMed] [Google Scholar]
- 33.Riso P, Klimis-Zacas D, Del Bo C, et al. Effect of a wild blueberry (Vaccinium angustifolium) drink intervention on markers of oxidative stress, inflammation and endothelial function in humans with cardiovascular risk factors. Eur J Nutr 2013; 52: 949–961. [DOI] [PubMed] [Google Scholar]
- 34.Leyva-Lopez N, Gutierrez-Grijalva EP, Ambriz-Perez DL, et al. Flavonoids as cytokine modulators: a possible therapy for inflammation-related diseases. Int J Mol Sci 2016; 17: 921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghasemi Pirbalouti A, Siahpoosh A, Setayesh M, et al. Antioxidant activity, total phenolic and flavonoid contents of some medicinal and aromatic plants used as herbal teas and condiments in Iran. J Med Food 2014; 17: 1151–1157. [DOI] [PubMed] [Google Scholar]
- 36.Yin DD, Yuan RY, Wu Q, et al. Assessment of flavonoids and volatile compounds in tea infusions of water lily flowers and their antioxidant activities. Food Chem 2015; 187: 20–28. [DOI] [PubMed] [Google Scholar]
- 37.Butland BK, Fehily AM, Elwood PC. Diet, lung function, and lung function decline in a cohort of 2512 middle aged men. Thorax 2000; 55: 102–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hooper R, Heinrich J, Omenaas E, et al. Dietary patterns and risk of asthma: results from three countries in European Community Respiratory Health Survey-II. Br J Nutr 2010; 103: 1354–1365. [DOI] [PubMed] [Google Scholar]
- 39.Westerterp KR. Metabolic adaptations to over--and underfeeding--still a matter of debate? Eur J Clin Nutr 2013; 67: 443–445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary Material ERJ-02286-2016_SupplementaryMaterial (212.1KB, pdf)