Abstract
Objectives
The prevalence of Srongyloides stercoralis infections is grossly underestimated because infections go mostly undetected, though they can persist for a lifetime due to the auto-infective cycle. In the Bolivian Chaco, the prevalence of soil-transmitted nematodes dropped dramatically in the past 25 years, but mebendazole used for preventive chemotherapy has no effect on S. stercoralis. Meanwhile, the prevalence of intestinal protozoan infections remains unchanged. The present study compared S. stercoralis seroprevalence in rural communities of the Bolivian Chaco from 1987 to 2013.
Methods
Sera collected during two previous sero-surveys, conducted in the Chaco region in 1987 and 2013, were tested for S. stercoralis using a commercial assay (Bordier-ELISA, Bordier Affinity Products, Switzerland).
Results
Overall, 355 sera were analyzed, 122 from the 1987 survey and 233 from the 2013 survey. Seropositivity for S. stercoralis was significantly more prevalent in 1987 (19/122, 16% in 1987 vs. 15/233, 6% in 2013, p=0.006), accounted for by a drop from 17% to 3% in the under-26. Multivariate analysis showed a significant association between seropositivity for S. stercoralis and age in the 2013 population (OR 1.03 for each one-year increase, 95%CI 1.00-1.05, p=0.04), but none in 1987.
Conclusions
The significant reduction in S. stercoralis seroprevalence in Bolivian Chaco cannot be explained by preventive chemotherapy or improved social-sanitary conditions. As the drop is seen in younger generations, it is consistent with little transmission occurring. However, the risk of transmission still exists, as prevalence is persistently high in older individuals, who represent a potential reservoir due to the lifelong nature of S. stercoralis infections.
Introduction
Strongyloides stercoralis is a soil-transmitted helminth widely diffused in tropical and subtropical regions of the world, including Southeast Asia, Latin America, sub-Saharan Africa, and parts of the south-eastern United States but also in some temperate areas at low endemicity [1]. There is an estimated 370 million individuals infected with S. stercoralis worldwide [2]. Most infections remain undetected because they are often asymptomatic, or cause mild, unspecific signs and symptoms such as pruritus, abdominal pain, diarrhea and respiratory disturbances; eosinophilia may be present. Due to a peculiar auto-infective cycle, the infection perpetuates in the human body indefinitely, if not treated. In the immunocompromised host, the number of larvae can highly increase and spread to any tissue of the body, leading to the hyperinfection syndrome and disseminated forms. These are severe life-threatening syndromes, which are often complicated by sepsis or meningitis due to intestinal bacteria carried into the bloodstream by the larvae. A number of severe syndromes has been reported due to concomitant use of immunosuppressant drugs, especially corticosteroids [3].
The global prevalence of the infection has long been underestimated, since data came from studies based on diagnostic technique with low accuracy [2]. Indeed, direct stool examination and Kato Katz method, the most common stool-based parasitological techniques used in surveys on helminths prevalence, have unsatisfactory sensitivity, due to the small numbers of S. stercoralis larvae in stool. More accurate parasitological techniques, such as specific concentration methods (e.g. the Baermann method), coproculture in agar plates and in-house molecular methods are not routinely performed, being available only in few reference laboratories. On the other hand, while serological techniques are more sensitive than stool-based methods, and also sufficiently specific above a given cut-off, there is a limited number of seroprevalence surveys for S. stercoralis [4].
A recent systematic review of the prevalence of strongyloidiasis in Latin America identified to Argentina, Ecuador, Venezuela, Peru and Brazil as high-prevalence countries (>20%), but reliable data were not available for most of the Latin America countries [5]. Moreover, studies were characterized by large heterogeneity in terms of population target and diagnostic methods used, with a small minority of serological-based surveys. Limited data on strongyloidiasis prevalence are available for Bolivia, all from surveys of soil-transmitted helminths (STH) prevalence, using stool-based methods [6–8]. Given the low sensitivity of these techniques, the reported prevalence of S. stercoralis, ranging from 0.9 to 1.8%, is likely to be grossly underestimated. The present study reports the S. stercoralis seroprevalence in rural communities of the Bolivian Chaco measured at two different time points, twenty-six years apart, in 1987 and 2013. In the period between the two surveys no large-scale control measures potentially affecting strongyloidiasis were implemented.
Methods
Study area and population
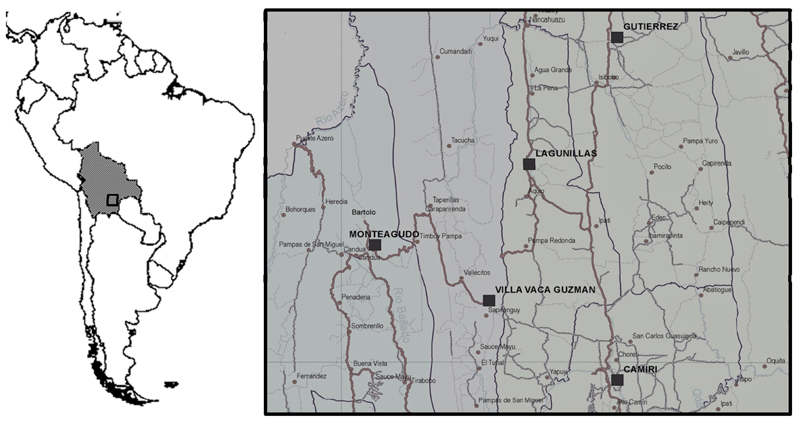
The study populations consisted of people living in the Chaco region, south-eastern Bolivia between longitude 64°30’ and 58°50’ west of the Greenwich meridian and at latitude 17°58’ and 22°20’ south. The former survey dates back 1987 and it was conducted in Camiri (Cordillera Province, Department of Santa Cruz, 20°06’ south; 63°32’ west), a municipality of about 30000 inhabitants situated in the foothills of the Andes, and Javillo, a very small community, isolated in the jungle north-east of Camiri, at an altitude of about 1500 m. The latter survey was conducted in 2013 in Bartolo (municipality of Monteagudo, Hernando Siles Province, Department of Chuquisaca: 16°30’ south; 59°88’ west) and Ivamirapinta (municipality of Gutierrez, Cordillera Province, Department of Santa Cruz: 19°45' south; 63° 30' west). A map of the study area is provided in Figure 1.
Figure 1. Study area map, within the Bolivian Chaco Region.
In 1987 the study was conducted in the municipality of Camiri (Cordillera Province, Department of Santa Cruz) and the community of Javillo (municipality of Gutierrez, Cordillera Province, Department of Santa Cruz); in 2013 in the communities of Bartolo (municipality of Monteagudo, Hernando Siles Province, Department of Chuquisaca) and Ivamirapinta (municipality of Gutierrez, Cordillera Province, Department of Santa Cruz).
Serologic assay
Sera, obtained from a sample of 5 ml venous blood, were stored at -20 °C in Bolivia, transported within 2-4 weeks to Italy in dry ice and stored at -70 °C until tested. In July 2016, all the samples were tested with a commercial enzyme-linked immunosorbent assay (ELISA) test, (Bordier Affinity Products, Switzerland) using Strongyloides ratti L3 larvae as antigen, according to the instructions of the manufacturers. A cut-off of 1.1 was used to define positive tests; this threshold showed a good balance between sensitivity (around 90%) and specificity (around 97%) [9].
Statistical analysis
Data were entered into Microsoft Excel 2010 software (Microsoft, Redmond, WA, USA). Statistical analysis of the data was performed with STATA 11.0 (StataCorp, College Statio, TX, USA). Frequencies and percentages with 95% confidence intervals (CI) for categorical variables, medians and interquantile ranges (IQR) for continuous variables were calculated. Two age categories were created: <26 and ≥26 years of age (the time difference between the two surveys, so as to have a group of 2013 subjects who were not yet born when the first survey was conducted).
Difference in sex and age was estimated by proportion test and Mann-Whitney test, respectively. The Cochran-Mantel-Haenszel stratified test was used to compare seroprevalence. To account for differences in the sample size between the two surveys, frequency ratios were calculated overall and by age category and the seropositivity rates recalculated accounting for these differences; a sensitivity analysis was conducted on this recalculated sample.
Multivariate analysis was performed by logistic regression to establish the association of positive ELISA test with sex and age. Results were considered significant when the p-value ≤ 0.05.
Ethics Consideration
Sera used in the present study were collected during two previous serological survey, focused on other infectious diseases, conducted in the years 1987 and 2013. Sera were completely anonymized. The previous and present studies were programmed and conducted in agreement with the Ministry of Health of the Plurinational State of Bolivia (Convenio Ministerio de Salud y Deportes, Estado Plurinacional de Bolivia/Cátedra de Enfermedades Infecciosas, Universidad de Florencia, Italia) and with the support of the Guaraní political organization (Asamblea del Pueblo Guaraní). Ethical approval for the study was obtained from both these above-mentioned institutions.
Results
We analysed a total of 355 sera from subjects aged 1-70 years-old, of whom 122 from the 1987 survey in Camiri and Javillo and 233 from the 2013 survey in Bartolo and Ivamirapinta. Demographic characteristics are presented and compared both within and between the two surveys in Table 1. Within each survey (Camiri versus Javillo, Ivamirapinta versus Bartolo) the study populations were homogenous, except a higher female frequency in Camiri than in Javillo in 1987.
Table 1. Demographic data of the human population surveyed in 1987 and 2013 in the Bolivian Chaco.
| 1987 Survey | 2013 Survey | |||||
|---|---|---|---|---|---|---|
| Camiri | Javillo | p-value | Ivamirapinta | Bartolo | p-value | |
|
Sex Female N (%,95%CI) |
48 (85.7%, 76.5-95.0) | 35 (53.0%, 41.0 – 65.1) | <0.01 | 72 (53.3%, 44.9-61.7) | 45 (45.9%, 36.1-55.8) | 0.26 |
| 83 (68.0%, 59.8-76.3) | 117 (50.2%, 43.8 – 56.6) | <0.01 | ||||
| Median age (IQR) | 16 (14-17) | 15.5 (10-30) | 0.95 | 23 (12-44) | 27 (14-43) | 0.40 |
| 16 (11-18) | 26 (12– 43) | <0.01 | ||||
| Mean age (95% CI) | 15.6 (13.9-17.3) | 19.7 (16.1-23.1) | 28.7 (25.3-32.0) | 30.2 (26.4-33.9) | ||
| 17.8 (15.8-19.8) | 29.3 ( 26.8-31.8) | |||||
N: number; IQR: interquartile range; CI: confidence intervals.
When comparing the two populations screened, significant differences were: (i) a higher female frequency among 1987 participants (68.0% vs. 50.2% in 2013); (ii) the 2013 population from Bartolo and Ivamirapinta was significantly older than that enrolled in 1987 (mean age 29.3 years versus 17.8).
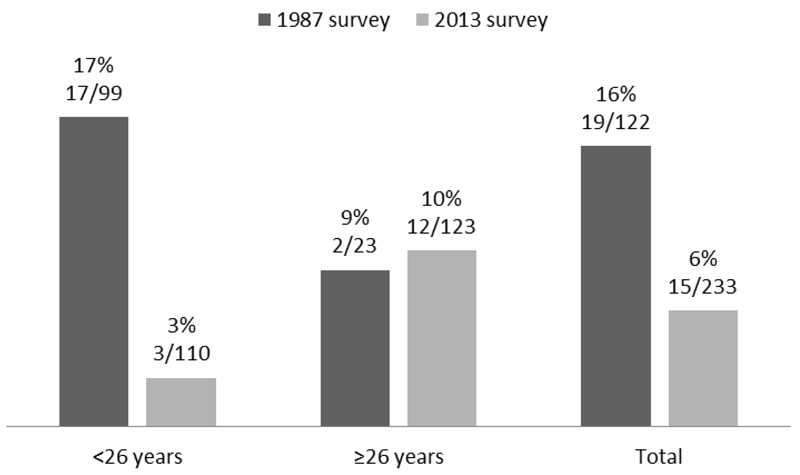
The S. stercoralis seroprevalence was statistically significantly higher in 1987 than in 2013 (19/ 122, 16% in 1987 vs. 15/233, 6% in 2013, Cochran-Matel-Haenszel test age-stratified p=0.006 on uncorrected data; 0.028 on sensitivity analysis) The overall and age-stratified prevalences are presented in Figure 2. By multivariate analysis, we found a significant association between seropositivity for S. stercoralis and age in the 2013 population (OR 1.03 for each one-year increase, 95%CI 1.00-1.05, p=0.04), but none in 1987.
Figure 2. Seroprevalence for S. stercoralis antibodies in the human population surveyed in 1987 and 2013 in the Bolivian Chaco, both overall and age-stratified.
Cochran-Mantel-Haenszel p value 0.006
Discussion
We report a significant drop in S. stercoralis infections over a 26-year period in rural Bolivia (from 16% to 6%) in the absence of disease-specific interventions.
To the best of our knowledge, this is the first study of this kind. Few serological surveys for S. stercoralis have been conducted in Latin America, with disparate results. In a rural community of the Peruvian Amazon region, a study published in 2006 reported a seroprevalence of 72% among 609 sera by ELISA, whereas S. stercoralis was identified in only 7.8% of stool samples evaluated by three methods (direct examination, Baermann method and simple sedimentation) [10]. In a Brazilian review, which considered data from 1990-2009, the mean seroprevalence for S. stercoralis from epidemiological studies in the general population was 21.7% and 29.2%, using immunofluorescence antibody test (IFAT) and ELISA, respectively [11]. In Chile, 0.25% of apparently healthy individuals were infected with S. stercoralis by ELISA; infections were found in few areas of the country and in high-risk groups, such as the mentally ill hospitalized patients (12.1% out of 675 patients of two psychiatric hospitals) [12]. Indirect information about S. stercoralis seroprevalence in the Bolivian population can be inferred from few community studies on Bolivian immigrants in European countries. A serological survey carried out in Bergamo, Italy, between 2009 and 2011, involving 739 Bolivian migrants (mean age 34.7 years) tested with an in-house IFAT, reported a global prevalence of 9.7% [13]. In 2015, among 486 Bolivian migrants in Geneva, Switzerland (mean age 36.5 years), the prevalence was 13.4%, using the Bordier-ELISA test [14]. In that study, which included also migrants from others Latin American countries (such as Brazil, Ecuador, Colombia, Peru), Bolivian nationality, as well as the provenience from rural area, were associated with a higher risk to be infected with S. stercoralis.
Importantly, the reduction in S. stercoralis seroprevalence was accounted for by a drop from 17% to 3% in the under-26, while the prevalence in older subjects remained stable (9% and 10%, respectively). This latter group is likely to be the same generation who were infected 26 years earlier as children and young adults, while the low prevalence in the under 26 in 2013 reflects the cumulative risk of infections in the last quarter of a century.
The association found here in 2013 between age and risk of S. stercoralis infection is known, but was not present in 1987, possibly because it was comparatively younger; there was no difference between the two surveys in male representation, another risk factor for S. stercoralis infection [10, 14]. Of note, in 2013 we also found 3/8 subjects over 70 to be positive for S. stercoralis (data not shown). Considering that S. stercoralis infection, when acquired, can potentially last for life because of its auto-infective cycle, older adults will likely represent a reservoir in the community; however, they do not seem to be an efficient source of transmission, as infections among the younger generations were now far less common.
The overall reduction of S. stercoralis seroprevalence is consistent with the dramatic decrease of STHs evidenced by parasitological surveys conducted in the same areas of the Bolivian Chaco, during this lapse of time: in 1987, the prevalence of hookworm and T. trichiura in a rural community near Camiri were 64.7% and 25.6%, respectively. In 2013 no STH infections were found among 111 people lìving in Ivamirapinta, except 1 case of S. stercoralis infection, while only 3 A. lumbricoides infections (3%) and 2 hookworm infections (2%) were documented among 112 inhabitants of Bartolo [6, 15]. This result may be largely ascribed to the implementation since 1986 of a preventive chemotherapy program based on the delivery of single-dose mebendazole to 2 to 9-year-old children, approximately every 6 months. However, given that mebendazole is not likely to affect S. stercoralis, other factors must have contributed to the trend. Theoretically, a lower S. stercoralis seroprevalence could be attributed to the STH decrease because of serologic cross-reactivity, but this phenomenon was shown to be of limited importance [9, 10]. Another reason could be improved sanitary conditions; however, the persistent high prevalence of intestinal of infections caused by waterborne protozoa such as Entamoeba coli, Giardia intestinalis and Blastocystis hominis, documented in the same parasitological studies cited above, suggests that general hygienic and sanitary conditions continues to be inadequate in these area [6, 7, 15]. Other factors, such as climatic change and less risky behaviours, e.g. walking barefoot or indiscriminate defecation, are worthy to be further investigated to better understand the trend of S. stercoralis infection over the time. Moreover, cross-reactivity of antifilarial antibodies should be considered in areas of endemicity. In 1997, a cross-sectional survey carried out in the Bolivian Chaco showed that 26% of the rural population near Camiri harbored Mansonella ozzardi microfilariae [16]; meanwhile, no specific interventions were implemented to reduce M. ozzardi prevalence.
The main limitation of the study is that the two surveys took place in different communities. However, given that the Bolivian Chaco is considered as a homogeneous ecological zone and rural population share the same hygienic and sanitary conditions, no substantial differences in S. stercoralis distribution is expected throughout the region.
In conclusion, despite the observed reduction over the last decades, S. stercoralis continues to be endemic within the rural areas of the Bolivian Chaco, with a persistently transmission potential, thus requiring local health care providers to be alerted about the risk and to implement all the appropriate preventive interventions against S. stercoralis.
WHO and its partners consider strongyloidiasis as one of the STHs that are particularly neglected. There are theoretical indications and practical experiences showing that large-scale administration of ivermectin can drastically reduce the prevalence and the morbidity due to strongyloidiasis [17–19]. In order to implement this control strategy at global level, we need more detailed knowledge of the distribution patterns of the infection and sufficient supplies of good-quality and low-cost ivermectin, which is presently missing. WHO, in order to find a solution for the limited drug availability, is now inviting the submission of dossiers by generic manufacturers for the prequalification of their product [20].
Acknowledgements
We are grateful to Father Tarcisio Ciabatti, Sister Maria Bettinsoli and Francesco Cosmi for their support in carrying out this study, and to Freddy Zuñiga, Jorge Changaray, Coralí Jimenez, Carlos Daza, Yuni Lara, Claudia Padilla, Petrona Rocha, the students of the Escuela de Salud del Chaco, Tekove Katu, Gutierrez, Reinaldo Chuve and Adan Siquevi, for their valuable assistance during the fieldwork. We would also like to thank the inhabitants of the communities for participating in the study.
References
- [1].Schar F, Trostdorf U, Giardina F, Khieu V, Muth S, Marti H, et al. Strongyloides stercoralis: Global Distribution and Risk Factors. PLoS Negl Trop Dis. 2013;7(7):e2288. doi: 10.1371/journal.pntd.0002288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bisoffi Z, Buonfrate D, Montresor A, Requena-Méndez A, Muñoz J, Krolewiecki AJ, et al. Strongyloides stercoralis: A Plea for Action. PLoS Negl Trop Dis. 2013;7(5):e2214. doi: 10.1371/journal.pntd.0002214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Buonfrate D, Requena-Mendez A, Angheben A, Muñoz J, Gobbi F, Van Den Ende J, et al. Severe strongyloidiasis: a systematic review of case reports. BMC Infect Dis. 2013;8:13–78. doi: 10.1186/1471-2334-13-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Requena-Méndez A, Chiodini P, Bisoffi Z, Buonfrate D, Gotuzzo E, Muñoz J. The laboratory diagnosis and follow up of strongyloidiasis: a systematic review. PLoS Negl Trop Dis. 2013;7(1):e2002. doi: 10.1371/journal.pntd.0002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Buonfrate D, Mena MA, Angheben A, Requena-Mendez A, Muñoz J, Gobbi F, et al. Prevalence of strongyloidiasis in Latin America: a systematic review of the literature. Epidemiol Infect. 2015;143(3):452–60. doi: 10.1017/S0950268814001563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Cancrini G, Bartoloni A, Paradisi F, Nunez LE. Parasitological observations on three Bolivian localities including rural communities, cities and institutions. Ann of Trop Med Parasitol. 1989;83:591–594. doi: 10.1080/00034983.1989.11812392. [DOI] [PubMed] [Google Scholar]
- [7].Macchioni F, Segundo H, Gabrielli S, Totino V, Rojas Gonzales P, Salazar E, et al. Dramatic decrease in prevalence of soil-transmitted helminthiasis and new insight into intestinal protozoa in children living in the Chaco region, Bolivia. Am J Trop Med Hyg. 2015;92(Suppl4):794–796. doi: 10.4269/ajtmh.14-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Muñoz Ortiz V, Lizarazu Chacón P, Limache G, Condori Matias D. Blastocistosis and other intestinal parasitosis in elderly residents from San Ramón home, La Paz, Bolivia. Biofarbo. 2008;16:9–15. [Google Scholar]
- [9].Bisoffi Z, Buonfrate D, Sequi M, Mejia R, Cimino RO, Krolewiecki AJ, et al. Diagnostic accuracy of five serologic tests for Strongyloides stercoralis infection. PLoS Negl Trop Dis. 2014;8:e2640. doi: 10.1371/journal.pntd.0002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yori PP, Kosek M, Gilman RH, Cordova J, Bern C, Chavez CB, et al. Seroepidemiology of strongyloidiasis in the Peruvian Amazon. Am J Trop Med Hyg. 2006;74(1):97–102. [PMC free article] [PubMed] [Google Scholar]
- [11].Paula FM, Costa-Cruz JM. Epidemiological aspects of strongyloidiasis in Brazil. Parasitology. 2011;138(11):1331–40. doi: 10.1017/S003118201100120X. [DOI] [PubMed] [Google Scholar]
- [12].Mercado R, Jercic MI, Alcayaga S, De paula FM, Ueta MT, Costa-cruz JM. Seroepidemiological aspects of human Strongyloides stercoralis infections in Chile. Rev Inst Med trop S Paulo. 49(4):247–249. doi: 10.1590/s0036-46652007000400010. [DOI] [PubMed] [Google Scholar]
- [13].Angheben A, Anselmi M, Andreoni F, Talamo M, Postiglione C, Gobbi F, et al. Strongyloidosis and Chagas disease sero-prevalence in a Bolivian community, Italy. Trop Med Int Health. 2011;16(S1):94. [Google Scholar]
- [14].Jackson Y, Santos L, Arm-Vernez I, Mauris A, Wolff H, Chappuis F, et al. Prevalence of chronic infections and susceptibility to measles and varicella-zoster virus in Latin American immigrants. Infect Dis Poverty. 2016;5(1):41. doi: 10.1186/s40249-016-0136-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Macchioni F, Segundo H, Totino V, Gabrielli S, Rojas P, Roselli M, et al. Intestinal parasitic infections and associated epidemiological drivers in two rural communities of the Bolivian Chaco. J Infect Dev Ctries. 2016;10(9):1012–1019. doi: 10.3855/jidc.7657. [DOI] [PubMed] [Google Scholar]
- [16].Bartoloni A, Cancrini G, Bartalesi F, Marcolin D, Roselli M, Caceres C, et al. Mansonella ozzardi infection in Bolivia: prevalence and clinical associations in the Chaco region. Am J Trop Med Hyg. 1999;61(5):830–833. doi: 10.4269/ajtmh.1999.61.830. [DOI] [PubMed] [Google Scholar]
- [17].Gabrielli A, Montresor A, Engels D, Savioli L. Preventive Chemotherapy in Human Helminthiasis: Theoretical and operational aspects. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2011;105:683–693. doi: 10.1016/j.trstmh.2011.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Anselmi M, Buonfrate D, Guevara Espinoza A, Prandi R, Gobbo M, Montresor A, et al. Mass administration of ivermectin for the elimination of onchocerciasis significantly reduced and maintained low the prevalence of Strongyloides stercoralis in Esmeraldas, Ecuador. PloS NTD PLoS Negl Trop Dis. 2015;9(11):e0004150. doi: 10.1371/journal.pntd.0004150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Barda B, Albonico M, Buonfrate D, Ame SM, Ali S, Speich B, Keiser J. Side benefits of mass drug administration for lymphatic filariasis on Strongyloides stercoralis prevalence on Pemba Island, Tanzania. Am J Trop Med Hyg. 2017;97(3):681–683. doi: 10.4269/ajtmh.17-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].WHO. Essential Medicines and Health Products: Prequalification of medicines. Available from: https://extranet.who.int/prequal/news/4th-invitation-expression-interest-eoi-treatment-neglected-tropical-diseases-ntds-published.