Looking for a model of spontaneous metastatic melanoma, we imported the Hgf-Cdk4R24C mouse line in which overexpression of hepatocyte growth factor (Hgf) promotes melanocyte proliferation and migration and the clinically relevant mutation in cyclin dependent kinase 4 (Cdk4R24C) prevents binding to the tumor suppressor protein p16 [1]. These mice have been reported to spontaneously develop a spectrum of primary melanomas, with 61% showing progressively growing melanomas at 1 year of age (74% nodular melanomas, 26% flat melanomas) [2]. The tumours appear mostly on the back (80%) and more rarely in other locations such as the ear, nose, belly, extremities, anus and tail (2-5% per site), with metastasis into the draining lymph nodes in all mice [2]. More recently, a study of the eyes of 11-month-old Hgf-Cdk4R24C mice found altered corneal stromal morphology but no evidence of spontaneous ocular melanoma [3].
In our Facility, Hgf-Cdk4R24C/R24C mice were generated by intercrossing Hgf-Cdk4R24C/R24C male mice with Cdk4R24C/R24C female mice (C57BL/6 background; the use and housing conditions of mice are detailed in Supplementary Data Table 1). Hgf-Cdk4R24C/R24C mice (12 females, 3 males)† were left to age with daily visual inspections, and typically were sacrificed due to hyper-proliferation of their anogenital areas at 341 ± 61 days of age (range: 178 – 429 days). At the time of necropsy we noticed a range of tissue sites undergoing hyper-proliferation with the presence of melanosis and the development of melanoma (and other tumours) in many cases (Table 1). All mice showed mixed anal mucosal melanoma/peri-anal skin malignant melanoma (Figure 1a-c) with concomitant vulval/penile mucosal melanoma (Figure 1d-e). Both flat and papillary skin melanomas (Figure 1f), were noted in all mice, with a few cases of nodular melanoma (Figure 1g). A high incidence of mucosal melanoma of the lips was also observed, as well as two cases of mucosal melanoma of the snout. Opacity and ulcerations of the eyes and/or discharge from the eyes was a recurrent observation; all eyes sampled showed retinal melanosis (Figure 1h) with increased melanin pigmentation of the retinal pigment epithelium (and usually the iris/uveal tract), some with a possible increase in numbers of retinal pigment epithelial cells, but no evidence of invasive melanoma of the retina or uveal tract (with one case showing a concomitant conjunctival melanoma). All Harderian glands sampled showed hyperplasia and adenoma formation (sometimes >1). All brains sampled showed meningeal melanosis with increased melanin pigmentation of the meninges, some with a probable increase in numbers of meningeal melanocytes, but no evidence of invasive melanoma infiltrating brain tissue (Figure 1i-j). A few livers showed hepatocellular carcinoma and most livers showed increased melanin pigment. Metastasis to the lymph nodes was noted in 10/15 mice, with the remaining 5/15 mice showing the presence of pigmented macrophages in the lymph nodes.
Table 1. Tissue distribution and frequency of melanosis and tumour development in Hgf-Cdk4R24C/R24C mice.
aOne mouse also developed a uterine/cervical melanoma. bFor one mouse, the eyes, harderian gland and brain were not collected for analysis. cOne mouse also developed conjunctival melanoma. d5/15 (33%) mice showed the presence of pigmented macrophages in lymph nodes, but no definite melanoma.
| Pathology | Frequency (%) |
|---|---|
| Peri-anal Skin Melanoma/Anal Canal Mucosal Melanoma | 15/15 (100%) |
| Vulval Mucosal Melanoma | 12/12 (100%)a |
| Penile Melanoma | 3/3 (100%) |
| Skin Nodular Melanoma | 3/15 (20%) |
| Skin Flat Melanoma | 15/15 (100%) |
| Skin Papillary Melanoma | 15/15 (100%) |
| Lip Mucosal Melanoma | 12/15 (80%) |
| Snout Mucosal Melanoma | 2/15 (13%) |
| Eye Harderian Gland Hyperplasia and Adenoma | 14/14 (100%)b |
| Eye Retinal Melanosis | 14/14 (100%)b,c |
| Brain Meningeal Melanosis | 14/14 (100%)b |
| Liver Hepatocellular Carcinoma | 2/15 (13%) |
| Lymph Node Metastatic Melanoma | 10/15 (66%)d |
Figure 1. Macroscopic and histological images of tissue melanosis and melanoma in Hgf-Cdk4R24C mice.
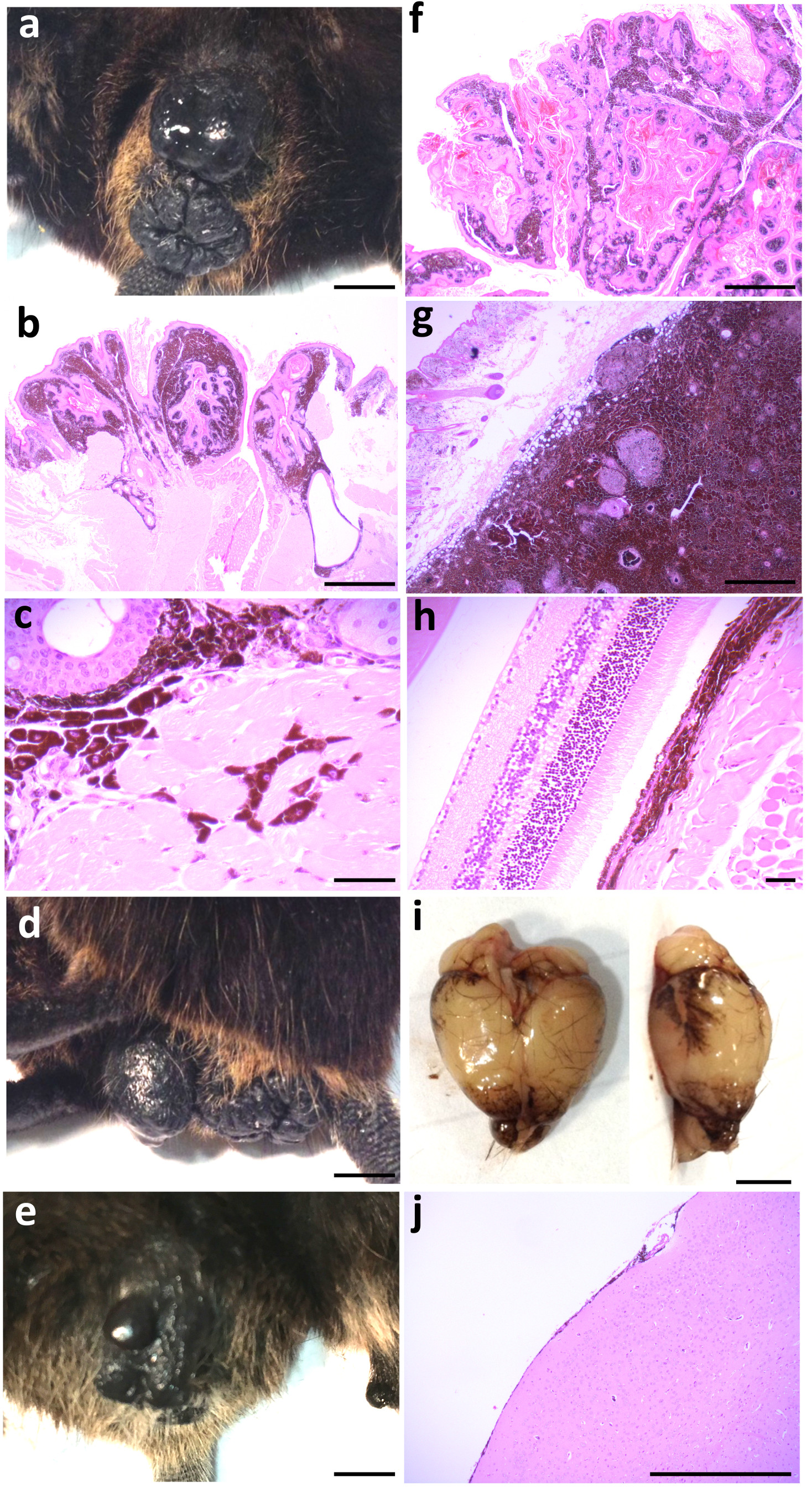
(a) Macroscopic image of a peri-anal (and vulval) melanoma (scale bar is 0.4 cm). (b-c) Haematoxylin and eosin (H&E)-stained section of a peri-anal melanoma, showing a papillary melanoma with both junctional and dermal melanoma components with the melanoma extending up around the anal canal near to the anorectal junction (scale bar is 1 mm, b), and showing invasion of atypical, malignant, heavily-pigmented melanoma cells into muscle (scale bar is 50 μm, c). (d) Macroscopic image of a vulval (and anal) melanoma (scale bar is 0.4 cm). (e) Macroscopic image of a penile melanoma (scale bar is 0.4 cm). (f) H&E-stained section of a skin papillary melanoma, showing junctional and dermal melanomatous components admixed with squamous epithelium and keratinous material displaying a papillomatous architecture (scale bar is 1 mm). (g) H&E-stained section of skin showing nodular melanoma in the sub-epithelial tissue, mostly heavily pigmented with a few small oval foci of loss of pigmentation (scale bar is 1 mm). (h) H&E-stained section of retinal melanosis, showing thickened and prominent pigmented layer under the retina due to over-activity of the retinal pigment epithelium with pigment extending into the underlying muscle and other tissue (scale bar is 50 μm). (i) Macroscopic image of a brain showing meningeal melanosis (scale bar is 0.4 cm). (j) H&E-stained section of meningeal melanosis, showing variably increased pigment within the thin meningeal layer on the surface of the brain (scale bar is 1 mm). All images are representative of those seen across the cohort (as detailed in Table 1).
Thus, in our Facility we had an increased incidence of mucosal melanoma, retinal melanosis and brain meningeal melanosis in preference to cutaneous melanoma, which is in contrast to previous reports [2], although spontaneous melanocytic hyperplasia of the mucosa, particularly of the perianal and genital mucosa, has been previously observed but not reported (Thomas Tuting, unpublished observations). Also, the cutaneous melanomas we observed were of the flat and/or papillary types, less commonly the nodular type, which has been previously reported as the predominant one. Finally, we did not find any alterations in pigmentation or cellular components of the ocular epithelium or posterior segment that has been previously reported [3]. Essentially, the oncogenic mutation in this model appears to be capable of driving almost all melanocytes to hyperproliferation and subsequent neoplasia, with more neoplasia in some sites than others depending on whether additional mutational events are required. Why we are seeing a shift in phenotype between the animal facilities is unclear and further investigation is undoubtedly warranted. Although these mice were maintained on a C57BL/6J background, we cannot account for genetic drift of the C57BL6/J colonies between the different institutes, which could influence tumour growth [4]. It is tempting to speculate that environmental factors such as differences in the housing conditions of the mice between the two facilities (detailed in Supplementary Data Table 1) may be playing a role, as these factors have been shown to affect tumour growth [4]; indeed the presence of Harderian gland hyperplasia and adenoma formation has been observed in aged mice from other lines in our Facility. Similarly the microbiota of the mice, which would undoubtedly differ between the two facilities, affects many aspects of physiology, most importantly tumor-immune cell interactions, and thus can play a major role in regulating the initiation, progression and dissemination of cancer [5].
Supplementary Material
Source of Funding
This study was supported by funding from Cancer Research UK to DJA (C20510/A1301), ERC CombatCancer to DJA (319661) and Wellcome Trust to DJA (077012/Z/05/Z).
Footnotes
Females were predominantly used in this study as litters from this mouse line were typically small and mice carrying the Hgf allele came through at sub-Mendelian ratios, so to avoid having singly housed males (in the case of only one male from a litter carrying the Hgf allele) we mostly kept females for tumour watch studies, as they could be group housed regardless of their age.
Statement of Conflict of Interests
All authors declare they have no conflict of interests.
References
- 1.Tormo D, Ferrer A, Gaffal E, Wenzel J, Basner-Tschakarjan E, Steitz J, Heukamp LC, Gütgemann I, Buettner R, Malumbres M, Barbacid M, et al. Rapid growth of invasive metastatic melanoma in carcinogen-treated hepatocyte growth factor/scatter factor-transgenic mice carrying an oncogenic CDK4 mutation. Am J Pathol. 2006;169(2):665–72. doi: 10.2353/ajpath.2006.060017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Landsberg J, Gaffal E, Cron M, Kohlmeyer J, Renn M, Tüting T. Autochthonous primary and metastatic melanomas in Hgf-Cdk4 R24C mice evade T-cell-mediated immune surveillance. Pigment Cell Melanoma Res. 2010;23(5):649–60. doi: 10.1111/j.1755-148X.2010.00744.x. [DOI] [PubMed] [Google Scholar]
- 3.Kilian MM, Herwig MC, Holz FG, Tüting T, Loeffler KU. Overexpression of hepatocyte growth factor and an oncogenic CDK4 variant in mice alters corneal stroma morphology but does not lead to spontaneous ocular melanoma. Melanoma Res. 2016;26(1):89–91. doi: 10.1097/CMR.0000000000000206. [DOI] [PubMed] [Google Scholar]
- 4.Gopinath C. Spontaneous tumour rates: their use to support rodent bioassays. Toxicol Pathol. 1994;22(2):160–4. doi: 10.1177/019262339402200209. [DOI] [PubMed] [Google Scholar]
- 5.Roy S, Trinchieri G. Microbiota: a key orchestrator of cancer therapy. Nat Rev Cancer. 2017;17(5):271–285. doi: 10.1038/nrc.2017.13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


