Abstract
Background
Zoster vaccine is a single dose live, attenuated vaccine (ZVL) indicated for individuals ≥50 years-old for the prevention of herpes zoster (HZ). Safety data from clinical trials and post-licensure studies provided reassurance that ZVL is generally safe and well tolerated. The objective of this review was to provide worldwide post-marketing safety information following 10 years of use and >34 million doses distributed.
Methods
All post-marketing adverse experience (AE) reports received worldwide between 02-May-2006 and 01-May-2016 from healthcare professionals following vaccination with ZVL and submitted to the MSD AE global safety database, were analyzed.
Results
A total of 23,556 AE reports, 93% non-serious, were reported. Local injection site reactions (ISRs), with a median time-to-onset of 2 days, were the most frequently reported AEs followed by HZ. The majority of HZ reports were reported within 2 weeks of vaccination and considered, based on time-to-onset, pathogenesis of HZ, and data from clinical trials, to be caused by wild-type varicella-zoster virus (VZV). HZ confirmed by PCR analysis to be VZV Oka/Merck vaccine-strain was identified in an immunocompetent individual 8 months postvaccination and in 4 immunocompromised individuals. Disseminated HZ was reported very rarely (<1%) with 38% occurring in immunocompromised individuals. All reports of disseminated HZ confirmed by PCR as VZV Oka/Merck vaccine-strain were in individuals with immunosuppressive conditions and/or therapy at the time of vaccination.
Conclusions
The safety profile of ZVL, following 10 years of post-marketing use, was favorable and consistent with that observed in clinical trials and post-licensure studies.
Keywords: Herpes zoster, Zoster vaccine live, Shingles vaccine live, Safety, Post-marketing
1. Introduction
Herpes zoster (HZ) (shingles) is a painful rash caused by reactivation of varicella-zoster virus (VZV) that remains latent in the sensory nerve ganglia after primary VZV infection. VZV reactivation is observed when VZV immunity decreases, as occurs with aging or due to immunocompromising conditions or treatments. The risk of HZ increases with age, as does the risk of having postherpetic neuralgia (PHN), long-lasting pain due to HZ. Zoster vaccine (ZVL: ZOSTAVAX™; Merck & Co., Inc., Kenilworth, NJ, USA), is a single dose, lyophilized, live, attenuated VZV (Oka/Merck) vaccine indicated for individuals ≥50 years-old for the prevention of HZ and HZ-related PHN [1,2]. ZVL is currently licensed in >55 countries (refrigerated and/or frozen formulation) with >34 million doses distributed, worldwide. Two pivotal Phase III efficacy studies, Shingles Prevention Study (SPS) and ZOSTAVAX Efficacy and Safety Trial (ZEST), demonstrated an efficacy of ZVL against HZ of 51% in subjects ≥60 years-old and 69.8% in subjects 50–59 years-old [3,4]. Longer-term follow-up indicated that vaccine efficacy for the incidence of HZ declined over time and persisted through year 8 following vaccination [5,6]. Several post-marketing observational studies have confirmed the effectiveness of ZVL in routine use, with results consistent with clinical trial data [7–10].
The objective of this review is to summarize adverse experiences (AEs) reported worldwide to Merck, Sharp, & Dohme Corp. (MSD), following 10 years of post-marketing use. Safety data from previously published clinical trials and post-marketing observational studies are also discussed [3,4].
1.1. Overview of MSD clinical trial safety data
ZVL was well tolerated in over 58,000 vaccine recipients during the course of clinical development. The safety of ZVL was demonstrated in SPS and ZEST in adults ≥50 years-old who reported a diverse range of comorbid medical conditions, generally reflective of the adult population (Supplementary Annex-1) [3,4,11]. Supplementary Table 1 represents a summary of the safety results from the AE Monitoring substudy of SPS and ZEST. Within SPS and ZEST, the only statistically significant differences observed for systemic clinical AEs, were headache (in SPS and ZEST) and pain in the extremity (in ZEST).
In both studies, the overall incidence of vaccine-related injection-site reactions (ISRs) was significantly greater for subjects vaccinated with ZVL compared to placebo (Supplementary Table 2). Of note, most ISRs were reported as mild in intensity. Overall, AEs were reported at a higher rate among subjects 50–59 years-old than among those ≥60 years-old, mainly due to vaccine-related ISRs (pain, swelling, erythema, pruritus) [3,4,11].
Polymerase chain reaction (PCR) analysis was used to detect VZV and to differentiate VZV wild-type from Oka/Merck vaccine-strain. Across the ZVL clinical development program, all HZ/HZ-like rashes that were VZV positive by PCR analysis were wild-type VZV. VZV Oka/Merck vaccine-strain was not detected in any of the specimens. Of importance, during the 6 weeks following vaccination, subjects in the ZVL group (SPS and ZEST combined) had fewer confirmed cases of HZ overall compared to the placebo group (12 vs. 32), with a decline in the number of confirmed cases of HZ over the 6 week period (Fig. 1).
Fig. 1.
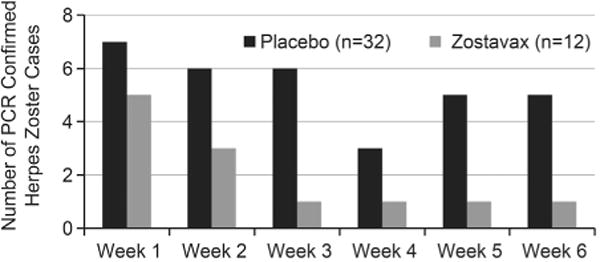
Confirmed herpes zoster cases from clinical trials in the first 6 weeks following vaccination (SPS and ZEST combined)a.
Varicella/varicella-like rashes reported across the clinical development program that were VZV positive by PCR analysis were associated with wild-type VZV, with the exception of lesion specimens collected from 2 subjects not enrolled in either SPS or ZEST with Oka/Merck vaccine-strain (Supplementary Annex-1).
In a post-marketing, placebo-controlled clinical study evaluating the general safety of ZVL in adults ≥60 years-old over 6 months, the safety profile of serious AEs (SAEs) during primary (Day 1–42) and secondary (Day 1–182) follow-up periods was similar in ZVL and placebo groups [12]. In other clinical trials, the safety profile of ZVL in distinct populations and across various formulations was generally similar to that seen in the AE Monitoring Substudy of the SPS [3,13–20].
1.2. Overview of safety from post-marketing observational studies
The real-world safety of ZVL was evaluated in approximately 29,000 vaccinated individuals ≥60 years-old in a post-licensure cohort study [21]. No safety concerns were identified in this study of ZVL in routine conditions of use. The safety of ZVL in routine conditions of use was also examined by the United States (U.S.) Centers for Disease Control and Prevention (CDC) in a large post-licensure observational study conducted in 8 managed care organizations participating in the Vaccine Safety Datalink network [22,23].
Both post-licensure observational safety studies supported the findings from the pre-licensure clinical trials and provided reassurance that ZVL is generally safe and well tolerated in routine conditions of use (Supplementary Annex-2).
2. Methods
2.1. Description of MSD post-marketing safety database
The MSD AE Reporting and Review System (MARRS), a passive, spontaneous, and voluntary database for ZVL, contains reports of all post-marketing AEs submitted by healthcare providers (HCPs); reports from the literature (Supplementary Annex-3) and non-interventional studies are also included.
A report may contain ≥1 AE and includes all AEs reported by that individual. An SAE, defined using regulatory and not medical criteria, is an AE that results in death, is life threatening, requires inpatient hospitalization or prolongation of existing hospitalization, results in persistent or significant disability or incapacity, is a congenital anomaly/birth defect, or is a medically important event or reaction [24–26].
Reports of AEs temporally related to ZVL administration are added to the MARRS without regard to the likelihood of a causal relationship, coded in the terminology used by the reporter, and mapped to terms in a standard Medical Dictionary for Regulatory Activities (MedDRA). Demographic, medical/clinical, and laboratory information provided by the reporter is variable, inconsistent, and often incomplete resulting in variability for several of the parameters discussed in this report. Physician assessed causality is frequently not reported. The clinical and laboratory information reported and entered into the MARRS is generally not further confirmed.
All post-marketing reports in the MARRS database temporally associated with ZVL from market introduction (02-May-2006) through 01-May-2016 have been included in the analysis. The AEs discussed are MedDRA preferred terms that have been mapped from the reporter term (Supplementary Annex-3) and include the most common as well as AEs likely of interest to HCPs. Percents were calculated using the number of reports received for a specific AE over the total number of reports received.
Time-to-onset was calculated from the date of vaccination (day zero) to first occurrence of the AE in post-marketing reports containing full date information; outcome represents the outcome of the AE at the time of the report.
Reports with an AE of rash, to include HZ, HZ-like, varicella, and varicella-like rash, were evaluated between days 0–42 (6 weeks) postvaccination. AE reports of HZ with time-to-onset >6 weeks postvaccination were considered reports of vaccine failure. Results of PCR analysis and/or VZV strain identification performed by independent and academic laboratories are reported [27,28].
The National Childhood Vaccine Injury Act of 1986 requires HCPs within the U.S. to report certain AEs following the administration of vaccines to the Vaccine Adverse Event Reporting System (VAERS), which is co-managed by the CDC and Food and Drug Administration [29]. All reports received are submitted to VAERS.
3. Results
3.1. Overview of post-marketing adverse experience reports
Following 10 years of post-marketing use, a total of 23,556 reports, containing a total of 45,898 AEs, were reported (Table 1). The majority of the reports were non-serious (93%), reported in females (70%), and originated from the U.S. (83%). Within the EU, 86% of the reports originated from the United Kingdom.
Table 1.
Demographic profile of adverse experience reports received worldwide following 10 years of post-marketing use of ZVL.
| Characteristic | n/N (%) |
|---|---|
| Report seriousness | N = 23,556 |
| Non-serious | 22,009/23,556 (93) |
| Serious | 1547/23,556 (7) |
| Region (country of incidence) | N = 23,556 |
| United States | 19,581/23,556 (83) |
| Canada | 1870/23,556 (8) |
| European Union | 1155/23,556 (5) |
| Rest of World | 950/23,556 (4) |
| Age (reports with age reported) | N = 13,259 |
| <18 yearsa | 559/13,259 (4) |
| 18–49 yearsb | 549/13,259 (4) |
| 50–59 years | 1726/13,259 (13) |
| ≥60 years | 10,425/13,259 (79) |
| Median age, years (range) | 65 (<1a –112) |
| Gender (reports with gender reported) | N = 16,135 |
| Male | 4915/16,135 (30) |
| Female | 11,220/16,135 (70) |
Reports of suspected secondary transmission of varicella or medication error/off label use for administration outside the indicated age.
Reports of medication error/off label use for administration outside the indicated age.
Among the 13,259 post-marketing reports with age reported, 79% were from individuals ≥60 years-old and 13% from individuals 50–59 years-old. The remaining 8% (97% non-serious) were individuals <50 years-old with AEs of medication error predominating.
Among the 45,898 AEs reported, 4607 (8.5%) were SAEs (Table 2). The AEs of ISR, HZ and rash were reported most frequently; while ophthalmic HZ (HZO) and AEs suggestive of disseminated HZ, were reported very rarely.
Table 2.
Commonly reported clinical adverse experiences and adverse experiences of ophthalmic and disseminated herpes zoster reported postvaccination with ZVL following 10 years of post-marketing use.
| Adverse experiences | Total AEs N = 45,898 n(%) | Serious AEs N = 4607 n(%) |
|---|---|---|
| Injection/vaccination site reactionsa | 9396 (20.5) | 192 (4.2) |
| Herpes zoster | 3943 (8.6) | 373 (8.1) |
| Rashb | 1922 (4.2) | 163 (3.5) |
| Erythema (not at the injection site) | 628 (1.4) | 48 (1.0) |
| Pain | 614 (1.3) | 85 (1.8) |
| Pruritus | 536 (1.2) | 40 (0.9) |
| Headache | 471 (1.0) | 64 (1.4) |
| Pyrexia | 454 (0.99) | 69 (1.4) |
| Pain in extremity | 439 (0.96) | 59 (0.97) |
| Peripheral swelling | 308 (0.67) | 24 (0.52) |
| Blister | 291 (0.63) | 39 (0.85) |
| Paraesthesia | 238 (0.52) | 43 (0.93) |
| Malaise | 229 (0.50) | 38 (0.82) |
| Nausea | 222 (0.48) | 51 (1.1) |
| Cellulitis | 218 (0.47) | 33 (0.72) |
| Varicella | 217 (0.47) | 28 (0.61) |
| Arthalgia | 215 (0.47) | 44 (0.96) |
| Urticaria | 213 (0.46) | 23 (0.50) |
| Ophthalmic HZ | 143 (0.31) | 32 (0.69) |
| HZ disseminated/HZ cutaneous disseminated | 15 (<0.1) | 10 (0.22) |
| Necrotizing retinitis/herpes zoster necrotizing retinitis | 4 (<0.1) | 4 (<0.1) |
| Acute disseminated encephalomyelitis (ADEM) | 3 (<0.1) | 3 (<0.1) |
Injection Site: abscess, atrophy, bruising, cellulitis, cyst, dermatitis, discharge, discolouration, dryness, eczema, erosion, erythema, exfoliation, haematoma, hypersensitivity, hypoaesthesia, induration, inflammation, irritation, macule, mass, necrosis, nodule, oedema, pain, pallor, papule, paraesthesia, pruritus, reaction, streaking, swelling, ulcer, urticaria, warmth. Vaccination site: abscess, bruising, cellulitis, discolouration, discomfort, erythema, haematoma, hypersensitivity, induration, inflammation, irritation, nodule, oedema, papule, paraesthesia, pruritus, rash, reaction, swelling, urticaria, warmth.
Rash terms: rash, vesicular, pruritic, papular, pustular, erythematous, generalized, macular, maculo-papular, morbilliform, and papular.
3.2. Overview of post-marketing reports positive by PCR for VZV Oka/Merck vaccine strain
VZV Oka/Merck vaccine-strain was identified by PCR assay in 14 specimens with the AEs of: ISR (n = 5), varicella (n = 3), HZ (n = 4), and disseminated HZ (n = 2) (Table 3). Thirteen specimens (93%) were from skin lesions. Immunosuppressive conditions and/or therapy at the time of vaccination was reported in 5 (38%) reports with one fatal outcome [30].
Table 3.
Reports of VZV Oka/Merck vaccine-strain identified through PCR analysis.
| Age/gender | VZV AE | Potentially IS therapy | Significant medical history | Source of specimen | Time-to-onset from vaccination to AE (days) |
|---|---|---|---|---|---|
| 61/F | Injection-site reaction | NR | None | Skin lesion | 2 |
| 64/M | Injection-site rash | NR | None | Skin lesion | 2 |
| 59/F | Injection-site reaction | NR | None | Skin lesion | 2 |
| 60/F | Injection-site reaction | NR | None | Skin lesion | 2 |
| 61/M | Varicella | NR | No history of varicella and no recent exposure to varicella; ~50 varicella lesions reported pv | Skin lesion | 17 |
| 54/M | Varicella | Prednisone | Myelofibrosis | Skin lesion | NR |
| 40/F | Varicella | NR | No history of varicella; Off label use for age | Skin lesion | 18 |
| 68/F | HZ | NR | None [31] | Skin lesion | ~240 |
| 53/M | HZ | NR | Recurrent episodes of HZ following initial pv episode; Diagnosed with grade 3 follicular lymphoma 13 months pv | Skin lesion | 137 |
| 73/F | HZ | Prednisone | Rheumatoid arthritis | Skin lesion | 25 |
| 66/F | Injection-site erythema, HZa | NR | None | Skin lesion | 6 |
| 80/F | HZ, Necrotizing retinopathys | Methotrexate | Rheumatoid arthritis; HZ reported on day 14 pv and antivirals not prescribed; Necrotizing retinopathy reported 2 months pv | Vitreous fluid | 14 |
| 64/M | DHZ | Methotrexate, Prednisone | Sjogren’s syndrome (see Table 4 for details) | Skin lesion | 56 |
| 79/M | DHZ, Pneumonitis | Cyclophosphamide FludarabineRituximab (5 months prior) | Chronic lymphocytic leukemia [30] | Skin lesion (VZV + BAL specimen but strain ID was not done) | ~37 |
Abbreviations: VZV = varicella-zoster virus; AE = adverse experience; PCR = polymerase chain reaction; M = male; F = female; IS = immunosuppressive; NR = not reported; HZ = herpes zoster; DHZ = disseminated HZ; BAL = bronchoaveolar lavage; ID = identification; pv = postvaccination.
This report of HZ at the injection site was interpreted and counted as ISR and not HZ.
3.3. Summary of post-marketing reports of common and selected adverse experiences
1. Injection-site reactions
There were 4355 post-marketing reports (9396 AEs; 192 SAEs) of ISR (Supplementary Annex-3): erythema (27.1%), swelling (15.4%), pain (12.6%), warmth (9.4%), and pruritus (8.3%) reported most frequently. Median time-to-onset of the 60.5% (2639/4355) of reports with time-to-onset reported was 2 days (range 1– 1051 days). Of the 74% (3213/4355) of reports with outcome reported, 61% recovered.
2. HZ and HZ-like rash
There were 3810 post-marketing reports (3943 AEs; 373 SAEs) with an AE of HZ. Time-to-onset was reported in 45% (1717/3810) of the reports. Of these, 53% (907/1717) reported HZ within 2 weeks and 65% (1121/1717) within 6 weeks postvaccination. A steady decline in the number of reports of HZ was observed between Week 1 and Week 6 postvaccination (Fig. 2). Of the 58% (2191/3810) with outcome reported, 64% recovered. Two reports listed HZ as a cause of death (without confirming the presence of VZV); only 1 provided clinical information (Supplementary Table-3).
Fig. 2.
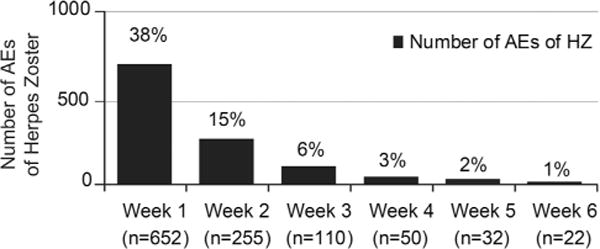
Post-marketing adverse experiences of herpes zoster reported weeks 1 through 6 postvaccination with ZVL (N = 1717).
3. Varicella and Varicella-like rash
There were 221 post-marketing reports (221 AEs; 29 SAEs) with an AE of varicella. Median time-to-onset of the 47% (103/221) of reports with time-to-onset reported was 11 days (range: 1–691 days) postvaccination. Results of a PCR analysis were reported in 6/221 reports, of which 5 were from lesion specimens and 1 from cerebrospinal fluid. PCR analysis of the 5 lesions was reported as Oka/Merck vaccine-strain (n = 3), wild-type VZV (n = 1), and inadequate specimen (n = 1) (Table 3). The specimen from CSF was reported as wild-type VZV. Of the 63% (139/221) of reports with patient outcome reported, 66.2% recovered with no reports of fatal outcome.
4. Rash (non-HZ and non-varicella)
There were 1922 post-marketing reports (2238 AEs, 185 SAEs) containing one or more rash terms. Median time-to-onset of the 57% (1091/1922) of reports with time-to-onset reported was 4 days (range: 1–3728 days) postvaccination. Outcome was reported in 67% (1296/1922) of the reports with 66% recovered.
5. Hypersensitivity/anaphylaxis
There were 178 post-marketing reports (190 AEs; 80 SAEs) with AEs of hypersensitivity or anaphylaxis (Supplementary Annex-3). Median time-to-onset of the 62% (110/178) of reports with time-to-onset reported was 3 days postvaccination (range: 1–366 days). Among the 110 reports, time-to-onset was reported as ≤24 h post-vaccination in 37.2%, >24 to ≤48 h in 20% and >48 to 72 h in 25%. Outcome was reported in 57% (102/178) of the reports with 70% (71/102) recovered and 2 reports of death (Supplementary Table-3).
6. Disseminated HZ
There were 18 post-marketing reports (19 AEs; 14 SAEs) with AEs of or suggestive of disseminated HZ (Supplementary Annex-3) with visceral involvement reported in 4/18 reports (Table 4).
Table 4.
Summary of adverse experience reports of disseminated herpes zoster reported postvaccination with and without visceral involvement.
| Age (years)/gender | Disseminated and other reported AEs | Time-to-onset pv (days) for disseminated AEs | Medically significant case information | IS concomitant therapies | PCR analysis for VZV | AE recovery status at time of report |
|---|---|---|---|---|---|---|
| Disseminated HZ with visceral involvement (n = 4) | ||||||
| 66/F | HZ meningoencephalitis, Uveitis, Vaccination failure, Drug interaction | ~330 | Literature report of patient with h/o progressive relapsing multiple sclerosis; ~11 months pv HZ meningoencephalitis was diagnosed; PCR analysis of spinal fluid detected VZV but strain identification was not performed [31,32] | High-dose IV methylprednisolone Fingolimod | VZV detected in spinal fluid (strain identification not done) | Recovered |
| 77/F | Varicella zoster pneumonia, Myasthenia gravis, Anaphylactic reaction, Transient ischaemic attack, Endotracheal intubation, Gastrointestinal tube insertion | NR | H/o type 2 diabetes mellitus, hypertension, and breast cancer (remission); Day 3 pv admitted to the intensive care unit and intubated for respiratory distress thought to be secondary to an allergic reaction to ZVL; Day 58 pv discharged from rehabilitation unit with diagnoses of anaphylaxis, varicella pneumonia, hypoxia, and ischemic brain injury; ~84 days pv follow-up for ptosis of the eye, weakness, low voice, and esophageal dysphagia and gastroesophageal reflux resulted in diagnosis of myasthenia gravis; no report of bronchoscopy or specimens for viral culture or PCR analysis | NR | Not recovered | |
| 79/M | HZ disseminated*, VZV infection*, Pneumonitis**, HZ meningoencephalitis, Acute hepatic failure, Respiratory failure, Multi-organ failure, Medication error | *~37 **~42 |
H/o chronic lymphocytic lymphoma; therapy with cyclophosphamide, fludarabine and rituximab received ~ 5 months pre-vaccination; ~2 weeks pv patient felt unwell and received antibiotics for flu like symptoms, and lethargy; ~37 days pv he developed a pustular rash and was hospitalized and treated with IV acyclovir for VZV infections; ~42 days pv patient was intubated with respiratory failure and soon after developed fulminant liver failure which progressed to multi-organ failure [30] | VZV Oka-vaccine- strain from skin lesion; VZV from plasma and BAL without report of strain identification | Died ~ 62 days pv from multi-organ failure | |
| 80/F | HZ necrotizing retinopathy, Photopsia, Rash vesicular, Vitreous floater | ~60 | H/o anti-glutamic acid decarboxylase antibody positive, osteoporosis and rheumatoid arthritis and diabetes mellitus; ~2 weeks pv HZ of the torso; ~2 months pv flashes and floaters in her left eye; vitrectomy confirmed acute retinal necrosis; PCR analysis of vitreous fluid confirmed Oka vaccine-strain VZV and oral valacyclovir was begun | Methotrexate | VZV Oka vaccine- strain from vitreous fluid of left eye | Recovered with sequelae |
| Disseminated HZ without visceral involvement (n = 14) | ||||||
| 56/M | HZ disseminated, Injection site pain | ~35 | H/o renal transplant; | Mycophenolate mofetil Prednisone | NR | NR |
| 60/M | HZ disseminated, Disease complication, Drug prescribing error, Medication error | NR | H/o cancer (unspecified) and immunosuppressed | NR | Died | |
| 61/M | HZ disseminated | 1 | Day of vaccination, patient experienced itching, and varicellalike lesions over body; valacyclovir prescribed | NR | Recovered | |
| 64/M | HZ disseminated, Headache, Rash, Rash pruritic | 8 | H/o tubular adenoma; IV acyclovir for 3 days | NR | Recovered | |
| 64/M | HZ disseminated, Medication error, Pneumonia (community acquired) | 56 | H/o Sjogren’s syndrome, cryoglobinemia, sicca syndrome, and leukocytoclastic vasculitis; wrist lesions on Day 56 pv followed by erythematous papules, vesicles, and vesiculopustular in different stages of evolution noted on head, trunk, buttocks and extremities on Day 64; T max 98° with no report of pain; oral anti-viral therapy followed by IV on Day 68 | Prednisone Methrotrexate | VZV Oka vaccine-strain | Recovered |
| 74/M | HZ disseminated, Ophthalmic HZ, Brain edema, Nasal edema, Nervous system disorder | 51 | Day 7 pv patient experienced swelling of sinuses; Day 9 pv eye swelling and the diagnosis of HZ; Day 51 pv hospitalized for central nervous system disorder with poor balance/memory and difficulty walking-no image studies or laboratory tests reported | NR | Recovered | |
| NR/M | HZ disseminated | ~365 | ~1 year pv experienced dissemination of the herpes virus. Limited information reported | NR | Not recovered | |
| NR/NR | HZ disseminated | NR | Limited information reported | NR | NR | |
| NR/M | HZ disseminated, Secondary transmission | NR | Report of secondary transmission; report is for husband (recipient of bone marrow transplant) of vaccinee; husband hospitalized with disseminated herpes zoster | NR | Recovered | |
| 79/F | HZ cutaneous disseminated, Post herpetic neuralgia | 5 | H/o chronic obstructive pulmonary disease and ischaemic heart disease; HZ, dermatomes T 7 and 8 | NR | Recovered | |
| 78/F | HZ cutaneous disseminated, Post-herpetic neuralgia (PHN), Rash (vesicular, erythematous), Infection via vaccinee | NR | Report of secondary transmission; report is for wife of vaccinee who developed HZ at C2-4 and PHN; no AEs reported in the vaccinee | NR | Not recovered | |
| 80/F | HZ cutaneous disseminated, Rash vesicular | 9 | Day 9 pv HZ at dermatomes T 4 and T8 | Budesomide formoterol | NR | Recovered |
| 83/F | HZ disseminated, Overdose | ~60 | H/o chronic obstructive disease, type 2 diabetes mellitus, hypercholesterolemia, osteoarthritis, gout, and hypertension; ~2 months pv treated with valacyclovir for a blistering weeping rash over entire body; ~5 months pv evaluated for blistering rash on palms of hand and edema of the right leg; physical examination revealed “various lesions” on upper and lower extremities and disseminated varicella zoster was diagnosed | Inadequate specimen (not confirmed) | NR | |
| 85/F | HZ disseminated | ~1460 | ~4 years pv diagnosed with disseminated HZ and hospitalized for 10 days | NR | Recovered | |
Abbreviations: VZV = varicella-zoster virus; AE = adverse experience; pv = postvaccination; IS = immunosuppressive; PCR = polymerase chain reaction; M = male; F = female; HZ = herpes zoster; NR = not reported; BAL = broncho-alveolar lavage; ~approximately.
Among the 4 reports with visceral involvement, 3 reported a history of immunosuppressive conditions and/or concomitant use of immunosuppressant therapies. VZV Oka/Merck vaccine-strain, confirmed by PCR analysis, was reported in 2 reports of which, one was a report of fatal outcome [30] (Supplementary Table-3).
Among the 14 reports without visceral involvement, 4 reported a history of immunosuppressive conditions and/or concomitant use of immunosuppressive therapies. Among the 4 reports, a lesion specimen from 1 report was confirmed by PCR as VZV Oka/Merck vaccine-strain. The clinical description, however, suggest varicella and not disseminated HZ, as the cause of the rash (Table 4).
7. Ophthalmic HZ (HZO)
There were 141 post-marketing reports (143 AEs, 32 SAEs) of HZO with a median age of 71 years (range: 42–90 years) reported among the 74% (105/141) of reports with age reported. Median time-to-onset of the 40% (57/141) of reports with time-to-onset reported was 56 days (range: 1–4596 days) postvaccination. Analysis by PCR was reported in 3/141 reports with wild-type strain confirmed in all. Among the 55% (78/141) of reports with outcome, 63% recovered.
At the time of vaccination, a prior history of at least 1 episode of HZO was reported in 7.1% (10/141) of the reports with half of the reports sourced from a single literature article [33] that provided limited information (Supplementary Table-4).
8. Central nervous system experiences
There were 498 post-marketing reports (509 AEs; 100 SAEs) involving CNS experiences (Supplementary Annex-3). The majority of the AEs were headache (93.0%; 471/509) followed by encephalitis (2.7%; 14/509) (Supplementary Annex-5a), and ataxia (1.8%; 9/509); 3 were AEs of acute disseminated encephalomyelitis (ADEM) (Supplementary Annex-5b). Among the 68% (340/498) of reports with time-to-onset reported, the median time-to-onset of CNS events was 2 days postvaccination (range: 1–2002 days). VZV PCR analysis of cerebrospinal fluid from the 4/14 reports of encephalitis were: wild-type (n = 2), negative (n = 1) and inadequate specimen (n = 1). Reports of ADEM did not report results of PCR or image studies. Outcome was reported in 74% (371/498) of the reports with 61% (227/371) recovered and 2 reports of death. (Supplementary Annex-3).
9. HZ Oticus (Ramsay Hunt syndrome)
There were 14 post-marketing reports (14 AEs; 4 SAEs) with an AE of HZ oticus with only 14% (2/14) reporting an AE of VIIth nerve palsy. Median time-to-onset among the 50% (7/14) of reports with time-to-onset reported was 18 day (range: 2–221 days). No reports included results of PCR. Among the 68% (9/14) of reports with outcome reported, 44% (4/9) were recovered at the time of the report. There were no reports of fatal outcome.
10. Fatal outcome reports
Fatal outcome, temporally but not causally, associated with vaccination with ZVL, was reported in 0.3% (74/23,556) of the post-marketing reports. Among the 74% (55/74) of reports with age reported, the majority (55%; 30/55) were in individuals ≥75 years-old.
In the review of reports, cause of death in 39.2% (29/74) of reports was either not reported or lacked sufficient information for adequate assessment. For the majority of the remaining 60.8% (45/74) of reports, cause of death was assessed to have been related to a preexisting or concurrent condition (20/45) or a cardiac disorder (11/45) (Supplementary Figure-1). Time-to-onset from vaccination to the fatal event for those with a cardiac disorder ranged from 1 day-9 months postvaccination. The remaining causes of death, infection (5/45), CNS event (4/45) and other causes (5/45), were each <1% and included individuals with immunosuppressive conditions and/or concomitant use of immunosuppressive therapies. Disseminated HZ, caused by Oka/Merck vaccine-strain, was among the causes of death in an immunosuppressed individual (Supplementary Table-3) [30].
4. Discussion/conclusion
Considering that the MSD safety database includes all post-marketing AE reports submitted by HCPs worldwide, including reports from health authorities, the literature and non-interventional studies, this is the first comprehensive review of the safety profile for ZVL following 10 years of marketed use and >34 million doses distributed worldwide.
The strength of post-marketing surveillance is that it provides information on real-world use to include populations excluded or not studied during clinical trials as well as the reporting of less common and/or rare AEs not observed during clinical trials [34]. These strengths are balanced by the limitations of post-marketing surveillance as a passive, spontaneous, voluntary, and incomplete reporting system from a population of unknown size; thereby prohibiting the calculation of AE incidence rates. The medical information, diagnosis and laboratory data in post-marketing reports is provided by the reporter, generally without confirmation. Additionally, causality cannot usually be established when the only evidence is a temporal association [35]. However, despite these limitations, this review confirmed that the overall safety profile of ZVL following post-marketing use is consistent with safety findings reported in pivotal clinical trials with the vast majority (93%) of the AE reported as non-serious and ISRs being the most common AE reported [3,4,11,36].
HZ accounted for 8.6% of the overall AEs and 8.1% of the SAEs from post-marketing (Table 2). A similar pattern of HZ occurrence postvaccination was observed in clinical trials and post-marketing, with the majority of reports occurring within 14 days postvaccination (Figs. 1 and 2). While PCR analysis results were rarely reported in post-marketing reports, HZ cases occurring in clinical trials, including within the first 6 weeks, were all confirmed as wild-type VZV. Given this, along with the mechanism of action and pathogenesis of HZ, it is reasonable to expect wild-type VZV as the most likely cause of HZ reported within 6 weeks of vaccination in reports received through post-marketing. Importantly, to date and in immunocompetent individuals, HZ caused by Oka/Merck vaccine-strain has not been detected within 6 weeks postvaccination. Following vaccination and latency, VZV Oka/Merck vaccine-strain rarely reactivates and causes HZ [31,37]. In the case report by Tseng, HZ caused by VZV Oka/Merck vaccine-strain, occured in an immunocompetent individual 8 months postvaccination with ZVL [31]. Per the authors, Oka/Merck vaccine-strain superinfection in the face of prior wild-type immunity was considered but the possibility that the case represented a rare VZV-naive 68-year-old person, with ZVL serving as her primary VZV infection, could not be ruled out. The reports of varicella caused by VZV Oka/Merck vaccine-strain were anticipated. Within the clinical development program 2 reports of VZV Oka/Merck vaccine-strain associated with varicella-like rashes have been reported [1,2].
Based on the efficacy of ZVL against HZ observed in clinical trials, vaccine failure, as assessed in the remaining 35% (596/1717) of reports with an AE of HZ reported >6 weeks following vaccination, is not unexpected.
ZVL contains gelatin and neomycin, which mechanistically is the most likely source for allergic reactions [38]. Hypersensitivity reactions and anaphylaxis have been reported rarely in clinical trials and accounted for 0.41% of the AEs in post-marketing. In clinical trials, symptoms were reported within minutes after vaccination and within a median time-to-onset of 3 days in post-marketing. The median time-to-onset in post-marketing surveillance is consistent with the CDC-sponsored post-marketing observational study where the risk of allergic reaction was increased in 1–7 days post-vaccination [23].
The potential risk of disseminated disease contraindicates ZVL [39], as with many live virus vaccines, in immunosuppressed or immunodeficient individuals including those on immunosuppressive therapy [1,2,40]. Many professional organizations and government immunization advisory committees have modified these recommendations when considering the benefits of preventing HZ and the risk of vaccine-induced AEs [40–46]. Over the 10 years of post-marketing use, disseminated HZ with fatal outcome has been reported in an immunocompromised individual [30]. However, the variable quality of spontaneous post-marketing reports, to include incomplete information, has hindered the analysis of several reports. None-the-less, 2 of the 18 reports of disseminated HZ were reports of secondary transmission of disseminated HZ and mechanistically implausible, and 6 were reports from individuals contraindicated to receive ZVL (Table 4). Oka/Merck vaccine-strain was detected from specimens obtained from 3 of 6 patients reported to receive ZVL despite contraindications, leaving no doubt as to causality and supporting the contraindication for vaccination in these patients.
In conclusion, the results of this review find that the safety profile of ZVL, following 10 years of post-marketing use and >34 million doses distributed, remains favorable and consistent with that observed in clinical trials and post-licensure studies [3,4,11,21,23]. AEs caused by Oka/Merck vaccine-strain have been reported rarely and primarily in individuals contraindicated to receive ZVL. Analysis by PCR with VZV-strain identification is recommended if unexpected AEs are observed following vaccination with ZVL.
Supplementary Material
Acknowledgments
A special thank you to: Jon Stek, MS (Merck & Co., Inc., Kenilworth, NJ, USA) and Karyn Davis, BA (Merck& Co., Inc., Kenilworth, NJ, USA) for their guidance and editorial support; Ann Marko, BSN (Merck & Co., Inc., Kenilworth, NJ, USA) for her collaboration through the VZV Identification Program; and Jason Chen, MD (Columbia University College of Physicians and Surgeons) and Sharon Steinberg (Columbia University College of Physicians and Surgeons) for their expertise in performing the PCR analysis and VZV strain-identification for several of the specimens.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.vaccine.2017.11.013.
Footnotes
Sponsor’s role
This research was funded by Merck & Co., Inc., Kenilworth, NJ, USA (sponsor). In conjunction with the external investigators, this research was designed, executed, and analyzed by the sponsor. Although the sponsor formally reviewed a penultimate draft of this manuscript, the opinions expressed are those of the authorship and may not necessarily reflect those of the sponsor. All co-authors approved the final version of the manuscript.
Author contributions
English D. Willis, Meredith Woodward, Elizabeth Brown, Zoran Popmihajlov and Patricia Saddier: study concept/design, data analysis/interpretation, and manuscript preparation.
Paula W. Annunziato, Neal A. Halsey, Anne A. Gershon: data analysis/interpretation and manuscript preparation.
Potential conflicts of interest
EW, MW, EB, ZP, PS, PA are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and may own stock or stock options in the company. NH reports having served on a safety monitoring committee for one of the post-licensure safety studies included in this manuscript and currently serving on a post-licensure safety monitoring committee for HPV vaccine produced by Merck. AG reports research grants from NIH R01 and from DSMB for GSK Subunit zoster vaccine outside the submitted work.
References
- 1.U.S. Food and Drug Administration. Approved Products: Zostavax. 2016 http://wwwfdagov/downloads/biologicsbloodvaccines/vaccines/approvedproducts/ucm132831pdf. [Accessed 23 March 2017]
- 2.European Medicines Agency. Summary of Product Characterstics: Zostavax. 2016 < http://wwwemaeuropaeu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000674/WC500053462pdf>. [Accessed 23 March 2017]
- 3.Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–84. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 4.Schmader KE, Levin MJ, Gnann Jr JW, et al. Efficacy, safety, and tolerability of herpes zoster vaccine in persons aged 50–59 years. Clin Infect Dis. 2012;54:922–8. doi: 10.1093/cid/cir970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmader KE, Oxman MN, Levin MJ, et al. Persistence of the efficacy of zoster vaccine in the shingles prevention study and the short-term persistence substudy. Clin Infect Dis. 2012;55:1320–8. doi: 10.1093/cid/cis638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison VA, Johnson GR, Schmader KE, et al. Long-term persistence of zoster vaccine efficacy. Clin Infect Dis. 2015;60:900–9. doi: 10.1093/cid/ciu918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tseng HF, Smith N, Harpaz R, Bialek SR, Sy LS, Jacobsen SJ. Herpes zoster vaccine in older adults and the risk of subsequent herpes zoster disease. JAMA. 2011;305:160–6. doi: 10.1001/jama.2010.1983. [DOI] [PubMed] [Google Scholar]
- 8.Langan SM, Smeeth L, Margolis DJ, Thomas SL. Herpes zoster vaccine effectiveness against incident herpes zoster and post-herpetic neuralgia in an older US population: a cohort study. PLoS Med. 2013;10:e1001420. doi: 10.1371/journal.pmed.1001420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marin M, Yawn BP, Hales CM, et al. Herpes zoster vaccine effectiveness and manifestations of herpes zoster and associated pain by vaccination status. Hum Vaccin Immunother. 2015;11:1157–64. doi: 10.1080/21645515.2015.1016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tseng HF, Luo Y, Shi J, et al. Effectiveness of herpes zoster vaccine in patients 60 years and older with end-stage renal disease. Clin Infect Dis. 2016;62:462–7. doi: 10.1093/cid/civ930. [DOI] [PubMed] [Google Scholar]
- 11.Simberkoff MS, Arbeit RD, Johnson GR, et al. Safety of herpes zoster vaccine in the shingles prevention study: a randomized trial. Ann Int Med. 2010;152:545–54. doi: 10.7326/0003-4819-152-9-201005040-00004. [DOI] [PubMed] [Google Scholar]
- 12.Murray AV, Reisinger KS, Kerzner B, et al. Safety and tolerability of zoster vaccine in adults >/=60 years old. Hum Vaccin. 2011;7:1130–6. doi: 10.4161/hv.7.11.17982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell AF, Parrino J, Fisher Jr CL, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects on chronic/maintenance corticosteroids. Vaccine. 2015;33:3129–34. doi: 10.1016/j.vaccine.2015.04.090. [DOI] [PubMed] [Google Scholar]
- 14.Mills R, Tyring SK, Levin MJ, et al. Safety, tolerability, and immunogenicity of zoster vaccine in subjects with a history of herpes zoster. Vaccine. 2010;28:4204–9. doi: 10.1016/j.vaccine.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 15.Macaladad N, Marcano T, Guzman M, et al. Safety and immunogenicity of a zoster vaccine in varicella-zoster virus seronegative and low-seropositive healthy adults. Vaccine. 2007;25:2139–44. doi: 10.1016/j.vaccine.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Benson CHL, Andersen J, Jiang J, Bozzolo D, Annunziato P, Read S, et al. Zostavax is generally safe and immunogenic in HIV+ adults virologically suppressed on ART: results of a phase 2, randomized, double-blind, placebo-controlled trial CROI. 2012 Abstract #96. [Google Scholar]
- 17.Kerzner B, Murray AV, Cheng E, et al. Safety and immunogenicity profile of the concomitant administration of ZOSTAVAX and inactivated influenza vaccine in adults aged 50 and older. J Am Geriatr Soc. 2007;55:1499–507. doi: 10.1111/j.1532-5415.2007.01397.x. [DOI] [PubMed] [Google Scholar]
- 18.MacIntyre CR, Egerton T, McCaughey M, et al. Concomitant administration of zoster and pneumococcal vaccines in adults >/=60 years old. Hum Vaccin. 2010;6:894–902. doi: 10.4161/hv.6.11.12852. [DOI] [PubMed] [Google Scholar]
- 19.Gilderman LI, Lawless JF, Nolen TM, et al. A double-blind, randomized, controlled, multicenter safety and immunogenicity study of a refrigerator-stable formulation of Zostavax. Clin Vaccine Immunol. 2008;15:314–9. doi: 10.1128/CVI.00310-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyring SK, Diaz-Mitoma F, Padget LG, et al. Safety and tolerability of a high-potency zoster vaccine in adults >/= 50 or years of age. Vaccine. 2007;25:1877–83. doi: 10.1016/j.vaccine.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 21.Baxter R, Tran TN, Hansen J, et al. Safety of Zostavax–a cohort study in a managed care organization. Vaccine. 2012;30:6636–41. doi: 10.1016/j.vaccine.2012.08.070. [DOI] [PubMed] [Google Scholar]
- 22.Chen RT, Glasser JW, Rhodes PH, et al. The Vaccine Safety Datalink Team Vaccine Safety Datalink project: a new tool for improving vaccine safety monitoring in the United States. Pediatrics. 1997;99:765–73. doi: 10.1542/peds.99.6.765. [DOI] [PubMed] [Google Scholar]
- 23.Tseng HF, Liu A, Sy L, et al. Safety of zoster vaccine in adults from a large managed-care cohort: a Vaccine Safety Datalink study. J Int Med. 2012;271:510–20. doi: 10.1111/j.1365-2796.2011.02474.x. [DOI] [PubMed] [Google Scholar]
- 24.International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. E2D post-approval safety data management: definitions and standards for expedited reporting. 2016 < http://wwwichorg/products/guidelines/efficacy/efficacy-single/article/post-approval-safety-data-management-definitions-and-standards-for-expedited-reportinghtml>. [Accessed 2 November 2016]
- 25.U.S. Food and Drug Administration. The FDA safety information and adverse event reporting program. 2016 < http://wwwfdagov/Safety/MedWatch/HowToReport/ucm053087htm>. [Accessed 2 November 2016]
- 26.U.S. Food and Drug Administration. Draft guidance for industry: postmarketing safety reporting for human drug and biological products including vaccines. 2016 < http://wwwfdagov/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/Vaccines/ucm074850.htm>. [Accessed 2 November 2016]
- 27.LaRussa P, Lungu O, Hardy I, Gershon A, Steinberg SP, Silverstein S. Restriction fragment length polymorphism of polymerase chain reaction products from vaccine and wild-type varicella-zoster virus isolates. J Virol. 1992;66:1016–20. doi: 10.1128/jvi.66.2.1016-1020.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gershon ATM, Sewar J. In: Live attenuated varicella vaccine Vaccines. 6th. Plotkin S, Orenstein W, Offit P, editors. W.B. Saunders; Philadelphia: 2013. [Google Scholar]
- 29.Vaccine Adverse Event Reporting System. 2017 < https://vaershhsgov/index>. [Accessed 10 March 2017]
- 30.Costa E, Buxton J, Brown J, Templeton KE, Breuer J, Johannessen I. Fatal disseminated varicella zoster infection following zoster vaccination in an immunocompromised patient. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2015-212688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tseng HF, Schmid DS, Harpaz R, et al. Herpes zoster caused by vaccine-strain varicella zoster virus in an immunocompetent recipient of zoster vaccine. Clin Infect Dis. 2014;58:1125–8. doi: 10.1093/cid/ciu058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Issa NP, Hentati A. VZV encephalitis that developed in an immunized patient during fingolimod therapy. Neurology. 2015;84:99–100. doi: 10.1212/WNL.0000000000001109. [DOI] [PubMed] [Google Scholar]
- 33.Taylor E. Exacerbation of herpes zoster ophthalmicus (HZO) following zoster vaccine, live, attenuated Oka/Merck: a case series. Pharmacoepidemiol Drug Saf. 2013:10–01. 441. [Google Scholar]
- 34.Kennedy DGS, Lillie R. Spontaneous reporting in the United States. In: Strom BL, editor. Pharmacoepidemiology. 3rd. Chicester, United Kingdom: Wiley; 2000. pp. 151–74. [Google Scholar]
- 35.Halsey NEK, Dekker C, Klein N, Baxter R, LaRussa P, Marchant C, et al. Algorithm to assess causality after individual adverse events following immunizations. Vaccine. 2012;30:5791–8. doi: 10.1016/j.vaccine.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 36.Morrison VA, Oxman MN, Levin MJ, et al. Safety of zoster vaccine in elderly adults following documented herpes zoster. J Infect Dis. 2013;208:559–63. doi: 10.1093/infdis/jit182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galea SA, Sweet A, Beninger P, et al. The safety profile of varicella vaccine: a 10-year review. J Infect Dis. 2008;197(Suppl 2):S165–9. doi: 10.1086/522125. [DOI] [PubMed] [Google Scholar]
- 38.Stratton K, Ford A, Rusch E, Clayton EW, editors. Institute of Medicine of the National Academies. Adverse effects of vaccines: evidence and causality. National Academies Press; Washington, DC: 2012. [PubMed] [Google Scholar]
- 39.Stratman E. Visceral zoster as the presenting feature of disseminated herpes zoster. J Am Acad Dermatol. 2002;46:771–4. doi: 10.1067/mjd.2002.119091. [DOI] [PubMed] [Google Scholar]
- 40.Rubin LG, Levin MJ, Ljungman P, et al. 2013 IDSA clinical practice guideline for vaccination of the immunocompromised host. Clin Infect Dis. 2014;58:e44–e100. doi: 10.1093/cid/cit684. [DOI] [PubMed] [Google Scholar]
- 41.van Assen S, Agmon-Levin N, Elkayam O, et al. EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2011;70:414–22. doi: 10.1136/ard.2010.137216. [DOI] [PubMed] [Google Scholar]
- 42.Singh JA, Saag KG, Bridges SL, Jr, et al. 2015 American college of rheumatology guideline for the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2016;68:1–25. doi: 10.1002/acr.22783. [DOI] [PubMed] [Google Scholar]
- 43.National Comprehensive Cancer Network, Inc. NCCN Guidelines for supportive care: survivorship. 2013 < https://wwwnccnorg/professionals/physician_gls/f_guidelinesasp#supportive>. [Accessed 17 October 2016]
- 44.Government of Canada Advisory Committee Statement/National Advisory Committee on Immunization. Update on the use of herpes zoster vaccine. 2014 < http://publicationsgcca/site/eng/9698776/publicationhtml>. [Accessed: 15 December 2015]
- 45.Public Health England. Shingles (herpes zoster) Green Book; 2015. Chapter 28a. < https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/448815/2904130_Green_Book_Chapter_28a_v1_0_0W_July2015.PDF> [Accessed: 28 July 2015] [Google Scholar]
- 46.Australian Government Department of Health. Zoster (herpes zoster) 10th. Australian Immunisation Handbook; 2015. Part 4.24< http://www.immunise.health.gov.au/internet/immunise/publishing.nsf/Content/Handbook10-homeĥandbookpart4ĥandbook-4-24#4–1> [Accessed: 1 October 2015] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


