Abstract
Usnic acid is a prominent secondary lichen metabolite that has been used for various purposes worldwide. Crude extracts of usnic acid or pure usnic acid have been marketed in the United States as dietary supplements to aid in weight loss. The US Food and Drug Administration (FDA) received 21 reports of liver toxicity related to the ingestion of dietary supplements that contain usnic acid. This prompted the FDA to issue a warning about one such supplement, LipoKinetix, in 2001 (http://www.cfsan.fda.gov/~dms/ds-lipo.html). Subsequently, usnic acid and Usnea barbata lichen were nominated by the National Toxicology Program (NTP) for toxicity evaluations. At present, a toxicological evaluation of usnic acid is being conducted by the NTP. This review focuses on the recent findings of usnic acid-induced toxicities and their underlying mechanisms of action.
Keywords: Weight loss dietary supplement, usnic acid and Usnea barbata toxicity
INTRODUCTION
In the United States, dietary and herbal supplements are extensively used, and under the Dietary Supplement Health and Education Act (DSHEA) of 1994 they are not required to undergo rigorous safety evaluation prior to being marketed. An estimated 83 million Americans use alternative medical therapies, including herbal and dietary supplements. It is reported that one-quarter of adults use an herb to treat medical illness and that Americans spend $4–12 billion annually on herbal products (1, 2). Although herbs are believed to be safe because of the accumulated knowledge of their prior use in traditional medicine, toxic effects do occur, particularly when they are used in non-traditional ways or when they are used in novel combinations with other herbs (1, 2).
Usnic acid is an abundant constituent of several lichen species (3, 4). It has a long history of use in traditional medicine as an antimicrobial agent. However, during the past decade, usnic acid has been marketed as a dietary supplement for weight loss because of its ability to increase fat metabolism and to raise basal metabolic rate. This novel use and reported associated human toxicity has stimulated recent research into the mechanisms of action of usnic acid and the biology of the lichens that produce it.
CHEMISTRY OF USNIC ACID
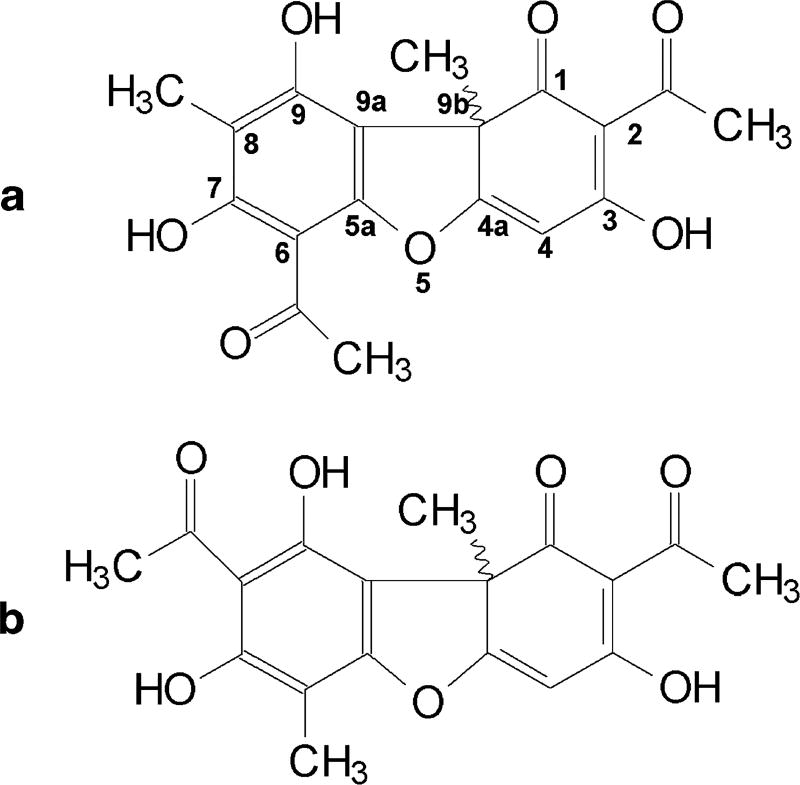
Usnic acid was first isolated as a prominent secondary lichen metabolite by the German scientist Knop in 1844 (5). When extracted from the lichen, it is yellow and crystalline in appearance. Usnic acid [full name, 2,6-diacetyl-7,9-dihydroxy-8, 9 b-dimethyl-dibenzofuran-1,3(2H,9bH)-dione] exists in two enantiomers; (+) D-usnic acid and (−) L-usnic acid, indicating an R or S projection of the angular-CH3 group at position 9b (Figure 1a). The enantiomers have been identified as showing different biological activities (6). In addition, two other natural isomers—(+) and (−) isousnic acids [2,8-diacetyl-7,9-dihydroxy-6,9b-dimethyldibenzofuran-1,3(2H,9bH)-dione]— are also found in lichens (Figure 1b).
Figure 1.
Structure of usnic (a) and isousnic (b) acids.
Usnic acid can be chemically synthesized from methylphloroacetophenone by oxidative coupling followed by hydrolysis in sulfuric acid (7). In 1969, Taguchi and coworkers (8) confirmed that methylphloroacetophenone, which is produced from acetyl CoA, was also an intermediate in the biosynthesis of usnic acid in lichens.
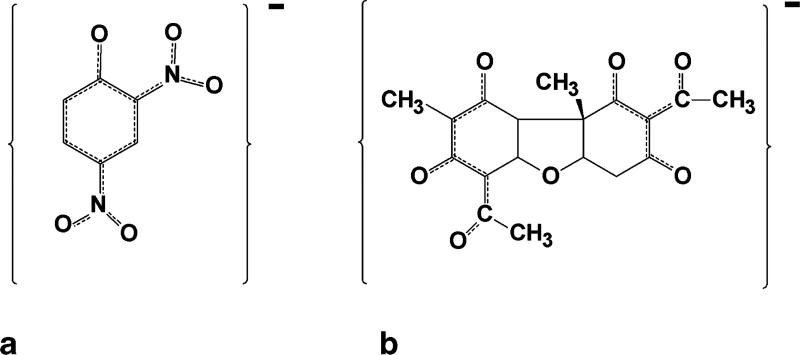
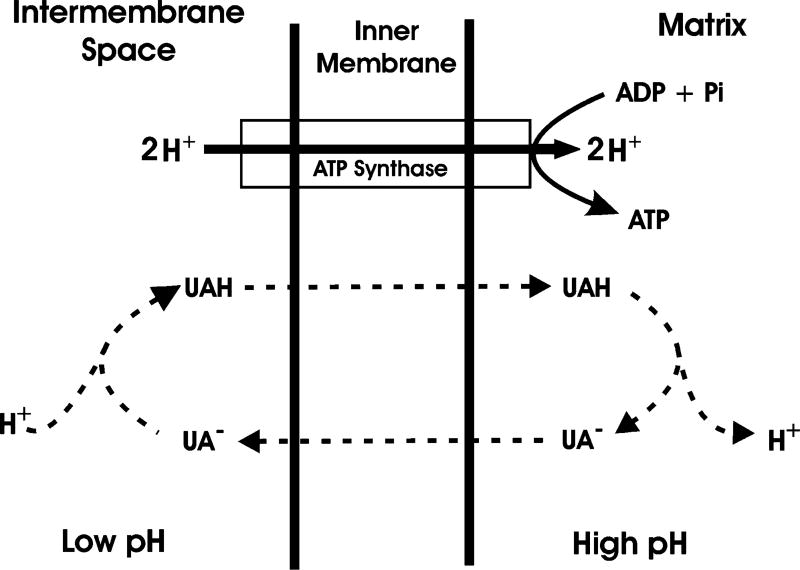
Of the three hydroxyl groups present in the usnic acid molecule, the enolic hydroxyl at the 3 position (Figure 1a) has the strongest acidic character (pKa 4.4) due to the inductive effect of the keto group, whereas the hydroxyl groups at positions 9 and 7 are less acidic with pKa values of 8.8 and 10.7, respectively (3). Usnic acid is highly lipophilic in both neutral and anionic forms because of its β-triketone groups that absorb the negative charge of the anion by resonance stabilization (9) (Figure 2). This lipophilicity of usnic acid and the usneate anion allows usnic acid to behave as a membrane uncoupler in a similar manner to 2,4-dinitrophenol (10, 11). According to Chemiosmotic theory, such molecules easily diffuse through biological membranes in their charged and neutral forms, which results in the breakdown or uncoupling of ion gradients (12). For example, usnic acid can pass through the inner-mitochondrial membranes by passive diffusion into the matrix where it is ionized, releasing a proton into the matrix. The resulting usneate anion can then diffuse back into the inter-membrane space, where it binds to a proton on the acidic side of the inner-membrane proton gradient to re-form usnic acid, which can then diffuse back into the matrix. The resulting cycle (Figure 3) causes proton leakage that eventually can dissipate the proton gradient across the inner-membrane, disrupting the tight coupling between electron transport and adenosine triphosphate (ATP) synthesis. This mitochondrial uncoupling xx activity of usnic acid has been demonstrated in vitro in several studies (13–16), and as discussed later, it is thought to play a major role in usnic acid hepatotoxicity. However, usnic acid also produces the same uncoupling actions on bacterial cell membranes; this forms the basis for its antimicrobial activity.
Figure 2.
Structures of the monoanionic forms of 2,4-dinitrophenol (a) and usnic acid (b) showing the resonance stabilization of their negative charges by delocalization of π orbital electrons as shown by the dashed lines, as described by Mitchell (12).
Figure 3.
Mechanism of mitochondrial uncoupling as proposed by Mitchell (12); chemicals with membrane uncoupling activity, such as usnic acid, are lipophilic and can diffuse through biological membranes in both their ionized and unionized forms; they can therefore transport protons across the inner mitochondrial membrane by diffusion, resulting in a reduced proton gradient to drive ATP synthesis.
BOTANY OF USNEA LICHENS
Lichens are symbiotic organisms of fungi and algae that comprise about 17,000 species, which synthesize numerous metabolites (4, 16). Lichens and their metabolites exert a wide variety of biological functions and have been used in perfumery, cosmetics, ecological applications, and pharmaceuticals. The significance of lichens and their metabolites was summarized in a review article by Huneck (17). It is estimated that lichens cover approximately 8% of the earth surface. Usnic acid has been identified in many lichen genera including species of Alectoria, Cladonia, Evemia, Lecanora, Parmelia, Ramalina, and Usnea. Traditionally, Usnea species such as the pendulous “beard” lichens, U. barbata, U. florida and U. longissima have been used as a source of usnic acid in herbal medicine and their usnic acid content ranges from 1–3% of dry weight (4, 18). Usnea sold in the United States (Figure 4) is often sold as U. barbata but can consist of several Usnea species, and if collected from American forests is unlikely to contain U. barbata, since this species is not native to North America (19, 20). U. filipendula, U. longissima, and U. scabrata are American Usnea species that are morphologically similar to Eurasian U. barbata and all contain usnic acid (20). Usnic acid is synthesized within the mycobiont (fungal part) of the lichen and is then deposited onto the outer surface of the photobiont (6).
Figure 4.
Typical Usnea lichen samples obtained commercially in the United States.
HISTORY OF USE OF USNEA LICHENS AND USNIC ACID
The first recorded use of the Usnea species in Traditional Chinese Medicine (TCM) dates to 101 B.C., when it was used as an antimicrobial agent under the Chinese name of Song Lo. Song Lo tea or decoction for internal and external use has also been recorded for detoxification of the liver, treatment of malaria, wounds, snake bite, cough, and so on. The common TCM dosages are 6–9 g of dried lichen, which corresponds with approximately 60–120 mg usnic acid per day (21). However, despite its long history, Song Lo is classified as a rarely used herb in TCM (only 500 are commonly used among over 6,000 herbs recorded in TCM).
Usnea species have been used as antimicrobial agents in many countries and were being developed as a modern pharmaceutical just prior to the advent of the penicillin antibiotics (4). The crude extracts of usnic acid rich lichens (e.g., Usnea species) have been used throughout the world to treat various ailments, such as pulmonary tuberculosis, pain, fever, wounds, athlete’s foot, and other dermal lesions. They have also been used as expectorants, in antibiotic salves, deodorants, and herbal tinctures (3, 4, 22, 23).
Thus, Usnea species, or purified usnic acid, are currently constituents in a variety of products worldwide. For example, lichen extract and purified usnic acid have been widely used in perfumery, cosmetics, sunscreens, and personal hygiene products such as toothpaste, mouthwash, shampoo, and deodorant. In Germany, pure usnic acid has been formulated and used in cosmetics and pharmaceuticals under the trade names of “Omnigran a, Granobil, and Usnagren A” (24). In Finland, “ramalina thrausta” was used internally to treat sore throat and toothache and externally to treat wounds and athlete’s foot (3, 23). In Italy, usnic acid has been used in vaginal creams, foot creams, powders, and shampoo (25). In Argentina, “Barba del la Piedra” (Usnea densirostra) has been sold to treat many ailments (26). In these preparations, usnic acid is employed either as the active principle or has functioned as a preservative. In the United States, Usnea can be obtained in bulk powder or as dried lichen from several herbal supply companies. It is widely available in dietary supplement stores either alone or in combination with other herbs such as Echinacea as tinctures in alcoholic or alcohol-free preparations.
CLINICAL PHARMACOLOGY
Antimicrobial Activity
During the 1980s, interest in usnic acid as an antimicrobial was renewed because of increasing experience of multidrug resistance caused by overuse of synthetic antibiotics (4). It has been shown that both the optical enantiomers of usnic acid are active against Gram-positive bacteria and mycobacteria (3), and several research studies and clinical trials have confirmed the antibacterial properties of usnic acid. For example, in preliminary clinical trials, a mouthwash containing 1% (+)-usnic acid was administered to volunteers. The samples of oral bacterial flora were examined at regular intervals. It was reported that the growth of Streptococcus mutans involved in the etiology of dental caries, was selectively suppressed (27). Using standardized assays, the in vitro susceptibility of pathogenic Gram positive and anaerobic bacteria toward usnic acid has been confirmed (3). Usnic acid has been shown to suppress the growth of Gram-positive organisms that are mainly responsible for body odor. Ethoxydiglycol extracts of lichens containing 10% usnic acid on a wet weight basis have been demonstrated to have preservative potential in moisturizing cream (3). Usnic acid was found to be effective against Mycobacteriurn aureurn (28). In in vitro assays, usnic acid and its salt inhibited the growth of Mycobacteriurn tuberculosis at relatively low concentrations (29). Partially purified usnic acid from Song Lo has also been therapeutically tested in China for tuberculosis and chronic bronchitis (21). For tuberculosis, patients received usnic acid tablets 90 mg/day (or 1.5 mg/kg/day). Thirty patients were treated for an average of 71 days. Side effects reported included stomachache and elevated liver enzymes. Some required suspension of usnic acid treatment for a week after taking the usnic acid tablets for about 3 months. For bronchitis, 91 patients were treated by one of the four preparations (2 × 100 ml decoction of 30 g Song Lo/day, crude crystalline material of Song Lo, sodium usneate, and unspecified partially purified Song Lo extract, at equalized dosages of 30 mg usnic acid equivalent three times per day). Ten days was considered a treatment period. Side effects of dry mouth, dizziness, and nausea were reported (21). Other recent studies have shown that usnic acid is active against methicillin-resistant Staphylococcus aureus (30, 31), and its potential use in the sterilization of surgical implants is being investigated (32).
Antimycotic Activity
During a short-term treatment with usnic acid salt, 65 patients with Tineapedis exhibited a significant improvement in their clinical conditions (4).
Antiprotozoal Activity
Usnic acid (−) exhibited a significant inhibitory effect against the pathogenic protozoan Trichornonas vaginalis at comparatively lower concentrations than metronidazole (33). The compound also showed leishmanicidal properties both in vitro and in vivo studies; intralesional administration produced a reduction in lesion weight and parasite body burden (34).
Antiviral Activity
In a cancer chemoprevention assay, (+)-usnic acid isolated from Usnea longissima was found to be significantly effective against tumor-promoter–induced Epstein-Barr virus with an ED5O of 1.0 µg/mL (35). (+)-Usnic acid also inhibited the cytopathologic effects of herpes simplex type I and polio type 1 viruses in the infected kidney cells of the African green monkey (3). In a clinical trial, the effect of an intravaginal formulation containing usnic acid and zinc sulfate as an adjuvant therapy to radio surgical treatment was evaluated in 100 women with genital infections of human papilloma virus. The treatment significantly improved the time of re-epithelization one month after the radio surgery (36).
Antiproliferative Activity
(−)-Usnic acid caused moderate inhibition in the murine P388 leukemia assay and also exhibited cytotoxic activity against cultured Ll210 cells; it was inferred that p-tri-ketone moiety was essential for the optimum activity (37). On the other hand, (+)-usnic acid (50 µg/mL) reduced the cell counts of leukemic (K − 562) and endometrial carcinoma cell culture (HEC-50) (10, 38).
Anti-Inflammatory Activity
In an acute rat paw edema and a chronic rat cotton pellet assay at 100 mg/kg oral dose level, the anti-inflammatory action of (+)-usnic acid was comparable with ibuprofen at the same dose level (39).
Analgesic and Antipyretic Activity
In two mice studies, the analgesic and antipyretic effects of usnic acid were evaluated (22). At 100 mg/kg oral dose level, usnic acid exhibited a significant analgesic effect as indicated in acetic acid-induced writhing and tail pressure tests. At oral dose levels up to 300 mg/kg, usnic acid also expressed significant antipyretic activity determined through lipopolysaccharide-induced hyperthermia.
METABOLISM OF USNIC ACID
The data related to in vivo usnic acid regarding the absorption, distribution, metabolism, and excretion (ADME) are limited.
A protein binding of usnic acid in rabbit plasma and bovine serum albumin revealed that usnic acid was extensively bound to protein with approximately 99.2% of bound form (40). A tissue disposition study in rats following 25 mg/kg (i.p.) administration showed that usnic acid distributed into various tissues and usnic acid levels were high in lung, liver, followed by blood with mean tissue/plasma ratios of 1.777, 1.503, and 1.192, respectively (40).
A pharmacokinetic study of usnic acid was conducted in rabbits following intravenous or oral administration of 5 and 20 mg/kg (29, 41). Mean terminal half-life of usnic acid level in plasma was 10.7 ± 4.6 hours with intravenous administration of 5 mg/kg. A longer half-life of 18.9 ± 2.9 hours was observed following oral administration. The oral maximum plasma concentration was 32.5 ± 6.8 µg/ml and was achieved in 12.2 ± 3.8 hours.
A recent study determined the in vitro metabolism characteristics of usnic acid in humans using human plasma, hepatocytes, and liver subcellular fractions (42). To identify metabolites, usnic acid was incubated in human liver S9 fractions and samples were applied to LC/MS (liquid chromatograph/mass spectrometry) ion chromatograms. Various metabolites, including three oxidized metabolites and two glucuronide conjugates, were characterized. For example, hydroxylations of two methyl ketones were observed (M2 and M3, MH+16 = 359 Da). These two major oxidized metabolites were regio-isomeric hydroxyketones and were not differentiated by LC/MS. The third identified oxidized metabolite is M1, the monohydroxylated metabolite (M1). Two isomeric glucuronide metabolites (MH+176, M4 and M5) of parent usnic acid were identified in the study. In this in vitro study, the authors also reported that the half-life of usnic acid in human liver microsomes was 19.3 min with an intrinsic clearance of 45.24 ml/min/kg. This predicted a human hepatic clearance of 13.86 ml/min/kg.
Phase I metabolizing enzymes (cytochrome P450, CYP isoforms) involved in metabolism of usnic acid were investigated using human liver microsomes pre-incubated with usnic acid and several CYP inhibitors. The inhibitors, used to determine the CYP isoforms responsible for catalyzing the turnover of usnic acid, included furafylline (CYP1A2), thioTEPA (CYP2B6), quercetin (CYP2C8), sulfaphenazole (CYP2C9), (s)-(+)—3-benzylnirvanol (CYP2C19), quinidine (CYP2D6), and ketoconazole (CYP3A4/5). Among the inhibitors investigated, only furafylline, the inhibitor of CYP1A2, showed the effect on the turnover rate of usnic acid by increasing its half-life to ten times. This suggested that the oxidative metabolism of usnic acid was mainly mediated by CYP1A2. Usnic acid was a weak inhibitor of CYP2D6, a potent inhibitor of CYP2C19 and CYP2C9, a less potent inhibition of CYP2C8, and CYP2C18 occurred because the enzyme induction/inhibition study demonstrated that the IC50 (the concentration causing 50% inhibition of enzyme activity) was 9.06 nM, 94.03 nM, 1.9 µM, and 6.3 µM for CYPs 2C19, 2C9, 2C8, and 2C18, respectively (42).
Using 12 recombinant human UDP-glucuronosyltransferase isoforms, the authors assessed the roles of each in the formation of usnic acid glucuronide conjugates and found that the usnic acid glucuronide conjugation was primary mediated by isoforms, UGT1A1, and UGT1A3, with minor contribution of UGT1A8 (42).
IN VITRO TOXICITY DATA
Several in vitro toxicological studies of usnic acid have been conducted. Pramyothin et al. (15) studied hepatotoxic effects of usnic acid in isolated rat hepatocytes. Treatment with 100 or 1000 µM of usnic acid in rat primary hepatocytes for 1 hour induced the release of hepatic transaminases (AST and ALT), decreased the content of reduced glutathione, and caused loss of cell membrane integrity. The study also compared usnic acid toxic effects with a well-known hepatotoxin, carbon tetrachloride (CCl4). Treatment with usnic acid and CCl4 exhibited similar cellular responses, suggesting that usnic acid may have the same hepatotoxic mechanisms as presented by CCl4(15). Another toxicological study was performed in mouse primary hepatocytes (14). Treatment with 5 µM usnic acid for 16 hours in mouse primary hepatocytes resulted in 98% cell death, which appeared to be associated with cell necrosis rather than apoptosis. The study also demonstrated that usnic acid treatment was associated with inhibition and uncoupling of the electron transport chain in mitochondria. Usnic acid treatment triggered oxidative stress by increasing free radical generation, and the oxidative stress appears to be critical in usnic acid-induced hepatotoxicity (14).
In addition to hepatocytes, usnic acid has been reported to induce toxicity in other normal and malignant cell types (43, 44). For example, the nontransformed human keratinocyte cell line, HaCaT, was used for testing the sensitivity to lichen metabolites, including usnic acid (43). The study demonstrated that usnic acid exerted antiproliferative action and inhibited cell growth with an IC50 value of 2.1 µM. Inhibition of cell growth was due to a cytostatic effect and not a cytotoxic effect, as LDH (lactate dehydrogenase) release only marginally increased after treatment with 2 µM usnic acid. Another in vitro study investigated toxicity of usnic acid using human lymphocytes and two cell lines, V79 (Chinese hamster lung fibroblast) and A549 (human lung carcinoma epithelial) (44). The results revealed that both enantiomers of usnic acid had significant cytotoxic effects to the cultured human lymphocytes and cell lines. A low dose of (+)-usnic acid (~1.8 µM) induced cytotoxicity in A549 cells, while the same dose of usnic acid did not show toxicity in V79 cells, indicating that cancerous cells (A549) were more sensitive to usnic acid than normal cells (V79). Moreover, the study also demonstrated that usnic acid-induced apoptosis in human lymphocytes in a dose-dependent manner.
MECHANISM OF ACTION OF USNIC ACID
Several studies using well-established methods have evaluated the effects of usnic acid on mitochondrial function in vitro. As early as 1950, Johnson and colleagues (45) studied the effect of usnic acid using rat kidney and liver homogenates. They reported that usnic acid, at the level of 1 µM, stimulated oxygen consumption in the presence of several substrates. At higher usnic acid concentrations (8–30 µM), phosphate uptake was reduced without a corresponding fall in oxygen consumption. This suggested uncoupling of oxidative phosphorylation. Pramyothin and coworkers (15) utilized primary rat hepatocytes to evaluate usnic acid hepatotoxic effect and demonstrated that usnic acid-induced loss of cell membrane integrity accompanied with elevated ac- tivities of AST and ALT. In the same study, stimulated respiration was also assessed in isolated rat liver mitochondria using various substrates such as glutamate plus malate or succinate. The results showed that usnic acid stimulated oxygen consumption by inducing maximal stimulation of respiration (in the absence of ADP, state-4) (3~7 fold dependent on substrates), suggesting an uncoupling of oxidative phosphorylation, which is in agreement with the observation by Johnson et al. (45).
Uncoupling of oxidative phosphorylation can also be assessed by a decrease in ADP/O ratios; the ratio of phosphate consumed (phosphorylation of ADP to ATP) to oxygen consumed. In a study using mouse liver mitochondria, it was reported that usnic acid at a concentration as low as 0.75 µM inhibited the ADP/O ratio by 50%, causing maximal stimulation of 100% of state-4 respiration and 300% induction of Mg2+-ATPase activity. The uncoupling effect was dose-dependent, and a higher concentration of usnic acid exhibited a complete uncoupling of oxidative phosphorylation (13).
Han et al. (14) demonstrated in mitochondria isolated from rat liver a similar uncoupling effect of usnic acid in the presence of bovine serum albumin (BSA) in buffer. However, usnic acid acted as both an uncoupler and an inhibitor of mitochondria in the absence of BSA in buffer in primary mouse hepatocytes and rat liver mitochondria. The inhibition of usnic acid on mitochondria further caused oxidative stress with an increase in hydrogen peroxide generation. These data suggest that adverse hepatic effects of usnic acid on mitochondrial function may not be limited to uncoupling of oxidative phosphorylation.
The ability of usnic acid to interfere with transmembrane ion gradients and mitochondrial function may also contribute to its reported antimicrobial properties (30). Usnic acid concentrations of 0.1 mg/ml (approximately 300 µM) stimulated oxygen consumption by the mitochondria-containing fungus Sac- charomyces cerevisiae, while concentrations above 100 mM inhibited oxygen consumption (10). This is strikingly similar to the bi-phasic concentration-response to usnic acid observed in mammalian tissue homogenates and mitochondria.
GENOTOXICITY EVALUATIONS OF USNIC ACID
It is known that some natural plants contain many compounds that are genotoxic (46). Using the Ames Salmonella/microsome assay, Shibamoto et al. (16) tested the mutagenicity of usnic acid along with two other lichen constituents; physodic acid (5′ carboxy-3,4′-dihydroxy-5-methylcaproyl-6′-pentyl-6-carboxy-diphenyl ether 2′,6- lactone) and physodalic acid (3′-acetoxyl-5′-carboxy-3,4′-dihydroxy-2-formyl-5,6′-dimethl-3 methylacetoxy-6-carboxy-diphenyl ether 2′,6-lactone) in two Salmonella typhimurium strains (TA98 and TA100) with or without S9 addition. Physodalic acid exhibited a clear dose-related mutagenicity in TA100, the tester strain; the addition of the S9 mix increased mutagenicity by four fold at the high dose (400 per plate). In contrast, usnic and physodalic acids showed no mutagenicity in tested strains, including TA98 and TA100 without or with S9 addition at a highest dose of 200 µg per plate for both chemicals. Koparal and coworkers (44) evaluated (+)-usnic acid and (−)-usnic acid genotoxicity in human lymphocytes from two healthy male donors in vitro using the cytokinesis-blocked micronucleus (CBMN) assay. The results obtained from their study suggest that even though the number of micronuclei was higher in both usnic acid enantiomers treated human lymphocytes in comparison with those in the control, the induction was not significant statistically. The authors concluded that both (+) - and (−)-usnic acid were non-genotoxic, as shown by the absence of micronucleus induction in human lymphocytes. Oral administration of a single dose of either 100 or 200 mg/kg usnic acid caused a slight increase in micronucleated erythrocytes in the mice 24 and 48 hours after treatment, which returned to control levels by 72 hours (47). The increasing of micronucleated erythrocytes occurred in the mice administrated with (+)-usnic acid without affecting DNA synthesis suggests an effect of usnic acid on spindle apparatus.
IN VIVO TOXICITY DATA
The in vivo toxicities of usnic acid have been reported in both animals and plants, even though data are sparse. In several experimental animal or wild animal species, such as guinea pigs, mice, rats, domestic sheep, cow elks, and mosquitoes, either general toxicity or organ-specific toxicity, or both, have been reported. It was reported in 1950 (48) that in female guinea pigs with tuberculosis, subcutaneous injection of usnic acid (20 mg per animal for 6 days, followed by 10 mg per animal for 24 days) caused a slight weight loss in the first week and a significant inhibition of weight gain during the next 3 weeks. Even after the discontinuation of usnic acid, weight gain was still considerably inhibited for at least 2 weeks. This is the first report showing that usnic acid could cause weight loss with the possibility of general toxicity, although this possibility was largely ignored in the ensuing decades. Of note, no apparent organ-specific toxicities in the liver, spleen, or lung were observed in this report (48). In healthy male Swiss mice, treatment of usnic acid intraperitoneally for 15 mg/kg for 15 days caused no apparent general toxicity, as evidenced by the negative observations in clinical signs or changes of body weight (49, 50). However, strong hepatotoxicity, including elevated serum transaminase activity and extensive liver necrosis, were observed. No toxicity in other organs such as the kidney and spleen were detected in the study. The similar pattern of toxicity was also revealed in the tumor-bearing mice (49, 50). In male Wistar albino rats, usnic acid (i.p., 50 or 200 mg/kg for 5 days) induced remarkable swelling of the liver mitochondria and endoplasmic reticulum assessed by electron microscopy, although no changes in serum transaminase activity were observed, indicating that mild hepatotoxicity occurred (15). With same strain of rat, another group reported that usnic acid at dosage of 500 and 1000 mg/kg by oral administration did not produce toxic effects at 24 h, but higher dosages of 2000 mg/kg showed some toxicity (51). Unfortunately, there was no further information regarding usnic acid-induced toxicity described in the report (51). Feeding domestic sheep with usnic acid of 323–776 mg/kg for a maximum of 9 days induced several clinical signs such as lethargy and anorexia, or even death, with the estimated median toxic dose between 485 and 647 mg/kg (52). Other toxicity indices, such as serum lactate dehydrogenase, aspartate aminotransferase and creatine kinase, were also increased. A complete postmortem examination revealed that pathological changes occurred exclusively in the skeletal muscle (52). This is in sharp contrast with mice, rats, and humans, in which the liver is considered to be the most vulnerable organ with usnic acid insults. Usnic acid is also the assumed toxicant and was associated with some 400–500 cow elk deaths occurred in Wyoming in 2004 (52, 53). Necropsy revealed extensive muscle damage such as muscle pallor and streaking, particularly in the semitendinosus, semimebranous, and pelvic limbs. Histological examination showed necrosis, rupture, inflammation, and degeneration of myofiber. However, a causative relationship between muscle damage and usnic acid exposure is yet to be established. Usnic acid also serves as a strong toxicant toward certain insects such as mosquitoes. It has been reported that usnic acid (5 and 10 ppm) killed all the larvae of certain species of mosquitoes on their 3rd–4th stages, suggesting that it might be developed as a novel natural insecticide (54). In addition to the toxicity toward animals, usnic acid displays phytotoxicity as well. It exerts the toxic effects on the growth of onion and lettuce, possibly by the inhibition of plant p-hydroxyphenylpyruvate dioxygenase, indicating the potential usage as an herbicide (6).
CASE REPORTS OF HEPATOTOXICITY IN HUMANS
The idea of utilizing chemicals with mitochondrial uncoupling activity for weight loss originated in the early 1930s after it was noticed that munition workers exposed to 2,4-dinitrophenol lost weight (55). Subsequently, 2,4-dinitrophenol was formulated into an anti-obesity drug that was prescribed by some physicians or directly marketed to the public with some claims of efficacy. However, many serious side effects were also recorded, including liver, heart, and muscle toxicity and cataract formation so that in 1938, the FDA finally declared 2,4-dinitrophenol too toxic to be used under any circumstances (55). Following this, reports of 2,4-dinitrophenol misuse became less frequent. Interest in uncoupling chemicals has resurfaced primarily in the body-building community with the advent of the Internet and the passage of DSHEA resulting in the clandestine trade of 2,4-dinitrophenol (56, 57) and the open marketing of usnic acid and other natural products in dietary supplements formulated for weight loss (21). Such formulations generally contain relatively high usnic acid concentrations, either alone or in combination with other ingredients, and their use has been reported to be associated with hepatotoxicity.
In 2000, Favreau et al. (58) reported that seven previously healthy patients developed acute hepatitis after ingesting LipoKinetix (Syntrax, Cape Girardeau, MO) and recovered spontaneously after discontinuing its use. Subsequently, two more cases of acute hepatitis were reported after taking LipoKinetix with one resulting in a liver transplant (59). LipoKinetix was a multi-ingredient product; one capsule contained 25 mg of norephedrin hydrochloride, 100 mg of usnic acid, 100 µg of 3,5-diiodothyronine, 3 mg of yohimbine hydrochloride, and 100 mg of caffeine. It was sold as a dietary supplement to promote weight loss. The manufacturer claimed that LipoKinetix “affects oxidative phosphorylation in such a way that an incredible amount of fatty acids are burned,” therefore promoting weight loss. The recommended dosage of LipoKinetix was 1–2 capsules 3 times per day, which is 3–6 times higher than usnic acid dosages used in TCM. Production and sale of LipoKinetix was terminated in 2001, but Syntrax continued to produce a product with similar ingredients but without usnic acid, which was called AdipoKinetix.
UCP-1 (BDC Nutrition, Richmond, KY) was marketed as a weight-loss product containing 150 mg of usnic acid, 525 mg of L-carnitine, and 1050 mg of calcium pyruvate per capsule. The recommended dosage ofUCP-1 was 3 capsules 3 times per day. Sanchez et al. (60) reported the development of severe liver failure in two patients who were taking the recommend dosage of UCP-1. One resulted in a liver transplant. Druazo et al. (61) also reported one case of a healthy woman who, after taking pure usnic acid (Industrial Strength AAA Services, Frazer Park, CA) for weight loss, presented with liver failure requiring a transplant. The recommended dosage of pure usnic acid from this manufacturer was 500 mg per day.
To date, the FDA has received at least 21 adverse event reports, including 1 death attributed to the weight loss dietary supplement containing usnic acid (LipoKinetix andUCP-1) or pure usnic acid. Twelve cases associated with hepatotoxicity appeared in the literature (60–62), and are summarized in Table 1. These include 8 females and 4 males. The median age of the patients was 31 years. Two patients required liver transplantation and the others ultimately recovered. While the total number of people who have experimented with weight loss supplements containing usnic acid is unknown, the manufacturer of LipoKinetix has claimed to have sold over 30,000 bottles of the supplement (63).
Table 1.
A summary of the cases associated with hepatotoxicity.
| Case 1 | Case 2 | Case 3 | Case 4 | |
| Age | 38 | 38 | 28 | 32 |
| Sex | Female | Male | Female | Female |
| Ethnicity | Asian | Asian | Caucasian | unknown |
| Agent | UCP-1 | UCP-1 | Pure usnic acid | LipoKinetix |
| Dose of usnic acid (mg/day) | 1350 | 1350 | 500 | 300–600 |
| Duration of use | 3 months | 3 months | 2 weeks | 8 weeks |
| Jaundice | Yes | No | Yes | |
| Abdominal discomfort | Yes | No | Yes | |
| Total bilirubin (mg/dL) | 23 | 0.6 | 28 | 35 |
| AST level (U/L) | 1536 | 451 | 1016 | 1110 |
| ALT level (U/L) | 1636 | 1462 | 449 | 1500 |
| Pathology | Shrunken liver parenchymal necrosis | Severe acute panacinar hepatitis, | Shrunken liver hepatic necrosis | Centrilobular necrosis, apoptotic cells |
| Outcome | Liver transplantation | Recovered | Liver transplantation | Liver transplantation |
| Reference | (60) | (60) | (61) | (59) |
| Case 5 | Case 6 | Case 7 | Case 8 | |
| Age | 32 | 20 | 32 | 31 |
| Sex | Female | Female | Female | Male |
| Ethnicity | unknown | Asian | Asian | Asian |
| Agent | LipoKinetix | LipoKinetix | LipoKinetix | LipoKinetix |
| Dose of usnicacid (mg/day) | 300–600 | 300–600 | 300–600 | 300–600 |
| Duration of use | 8 weeks | 14 days | 10 days | 30 days |
| Jaundice | Yes | Yes | Yes | |
| Abdominal discomfort | Yes | Yes | Yes | |
| Total bilirubin (mg/dL) | 20 | 14.6 | 11.5 | 4.6 |
| AST level (U/L) | 1600 | 2978 | 9360 | 1396 |
| ALT level (U/L) | 1420 | 2871 | 7040 | 438 |
| Pathology | Centrilobular necrosis, apoptotic cells | NA | NA | NA |
| Outcome | Recovered | Recovered | Recovered | Recovered |
| Reference | (59) | (58) | (58) | (58) |
| Case 9 | Case 10 | Case 11 | Case 12 | |
| Age | 28 | 23 | 26 | 31 |
| Sex | Female | Female | Male | Male |
| Ethnicity | Asian | Asian | Caucasian | Caucasian |
| Agent | LipoKinetix | LipoKinetix | LipoKinetix | LipoKinetix |
| Dose of usnic acid (mg/day) | 300–600 | 300–600 | 300–600 | 300–600 |
| Duration of use | 30 days | 21 days | 84 days | 63 days |
| Jaundice | No | No | Yes | Yes |
| Abdominal discomfort | Yes | Yes | Yes | Yes |
| Total bilirubin (mg/dL) | 3.9 | 2.2 | 6.1 | 2.4 |
| AST level (U/L) | 2068 | 3826 | 7550 | 564 |
| ALT level (U/L) | 2087 | 3826 | 14 | ALT level (U/L) |
| Pathology | NA | NA | NA | NA |
| Outcome | Recovered | Recovered | Recovered | Recovered |
| Reference | (58) | (58) | (58) | (58) |
FDA REGULATORY PERSPECTIVE
Based on the adverse events described above and first reported by Medwatch in November 2001, the Center for Food Safety and Applied Nutrition (CFSAN), Food and Drug Administration (FDA) issued a warning letter (64, 64), on November 19, 2001, titled “FDA Warns Consumers Not to Use the Dietary Supplement LipoKinetix” since it had been implicated in a number of serious liver injuries. After receiving additional reports of persons who developed liver injury or liver failure while using LipoKinetix, the FDA subsequently issued a strong recommendation to the manufacturer, Syntrax Innovation Inc., to withdraw the product from the market (Letter to Distributor on Hazardous Dietary Supplement LipoKinetix) (65).
In order to further understand the risk to human health of usnic acid and Usnea preparations, the Division of Dietary Supplement Programs/CFSAN/FDA nominated usnic acid to the NTP for the evaluation of short-term and long-term toxicity, in January of 2005 (21). Secondary goals of the nomination were to examine dermal toxicity of usnic acid and evaluation of usnic acid and Usnea lichens for their genotoxic, developmental, and reproductive toxicity. Studies were initiated in 2006 and are being conducted in our laboratories at the National Center for Toxicological Research (NCTR). They currently involve acute and subchronic rodent toxicity testing of both usnic acid and Usnea lichen preparations and associated in vitro and mechanistic studies. Fourteen-day acute toxicity studies have shown that usnic acid is hepatotoxic to both rats and mice exposed via feed to concentrations of 100 or 200 mg/kg/day, but that lower dosages are well tolerated (Table 2). Usnea lichen preparations containing equivalent concentrations of usnic acid produced greater toxicity than pure usnic acid (66).
Table 2.
Acute toxicity of usnic acid or Usnea lichen in Fischer 344 rats and B6C3F1 mice.
| Rdt - Usnic dcid | Rat - Usnea Lichen | Mouse - Usnic Acid | Mouse - Usnea Lichen | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|||||||||||||
| Target Dose as mg/kg usnic acid in feed |
Dead/Moribund | Liver pdthology | Dead/Moribund | Liver pathology | Dead/Moribund | Liver pathology | Dead/Moribund | Liver pathology | ||||||||
|
|
|
|
|
|
|
|
|
|||||||||
| M | F | M | F | M | F | M | F | M | F | M | F | M | F | M | F | |
| 200 | 100% | 100% | 100% | 80% | — | — | — | — | 40% | 20% | 80% | 60% | 100% | 100% | — | — |
| 100 | 20% | 60% | 100% | 100% | 100% | 100% | 100% | 100% | 0% | 0% | 80% | 0% | 60% | 100% | 100% | 40% |
| 30 | 0% | 0% | 20% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 40% | 0% |
| 5 | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
| Control (0) | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% | 0% |
The animals were exposed to either pure (+)-usnic acid or equivalent concentrations of usnic acid (predominantly the (+) enantiomer) in Usnea lichen (U.scabrata and U. cavernosa) mixed in feed for 14 days. Liver lesions consisted of cell swelling or contraction, cytoplasmic vacuolization, clearing or clumping of organelles, increased cytoplasmic eosinophilia and are associated with factors leading to irreversible cellular degeneration and necrosis. Data taken from Ali et al. (66) and unpublished work.
In addition, screening and testing of usnic acid for its potential mutagenicity in bacteria has been conducted and completed by NTP. Three strains with different genetic backgrounds, including one E. coli strain and two Salmonella typhimurium strains (TA98 and TA100), were used for the study. No significant mutagenicity was observed in the tested strains (67).
Mechanistic studies conducted at NCTR have demonstrated that in mice exposed to usnic acid for 14 days (feed study, Table 2), genes coding for proteins involved in mitochondrial electron transport, fatty acid metabolism, and apoptosis are up-regulated, suggesting that the adaptive response to usnic acid hepatotoxicity requires increased β-oxidation of fat and induction of mitochondrial respiratory complexes I–IV to increase the proton flux to compensate for usnic acid’s uncoupling activity (68). Studies have also demonstrated that usnic acid was strongly toxic to primary cultured rat hepatocytes, with an IC50 of 10 µM after a 24-h exposure. Cell lethality was associated with the mitochondrial permeability transition (MPT) and mitochondrial depolarization. Similar effects were also observed with HepG2 human hepatoma cells (69). Our studies have confirmed the mitochondrial uncoupling effects of usnic acid using rat hepatocytes, in which usnic acid inhibited the activity of the respiratory complex V with an IC50 of 46 µM. Usnic acid displayed no effects on the activity of the respiratory complexes I or IV, but slightly inhibited the activity of the respiratory complexes II+III at 150 µM. We additionally found that usnic acid caused an abrupt depletion of cellular ATP content in rat hepatocytes. Within 1 min, ATP was depleted by 50%. Because ATP depletion was the earliest detectable toxicity endpoint, we speculate that the uncoupling and inhibition of oxidative phosphorylation and subsequent ATP depletion was the triggering event leading to hepatocyte cell death following usnic acid exposure in vitro (69).
Acknowledgments
This article is not an official guidance or policy statement of US Food and Drug Administration (FDA). No official support or endorsement by the US FDA is intended or should be inferred. This research was supported in part by Interagency Agreement (IAG 224-07-007) between the National Center for Toxicological Research/US FDA and the National Institute for Environmental Health Sciences/National Toxicology Program.
References
- 1.Willett KL, Roth RA, Walker L. Workshop overview: Hepatotoxicity assessment for botanical dietary supplements. Toxicol Sci. 2004;79:4–9. doi: 10.1093/toxsci/kfh075. [DOI] [PubMed] [Google Scholar]
- 2.Bent S, Ko R. Commonly used herbal medicines in the United States: a review. Am J Med. 2004;116:478–485. doi: 10.1016/j.amjmed.2003.10.036. [DOI] [PubMed] [Google Scholar]
- 3.Ingolfsdottir K. Usnic acid. Phytochemistry. 2002;61:729–736. doi: 10.1016/s0031-9422(02)00383-7. [DOI] [PubMed] [Google Scholar]
- 4.Cocchietto M, Skert N, Nimis PL, Sava G. A review on usnic acid, an interesting natural compound. Naturwissenschaften. 2002;89:137–146. doi: 10.1007/s00114-002-0305-3. [DOI] [PubMed] [Google Scholar]
- 5.Knop W. Untersuchung uber die Flechten. Justus Lieb Ann Chem. 1844;49:103–124. [Google Scholar]
- 6.Romagni JG, Meazza G, Nanayakkara NP, Dayan FE. The phytotoxic lichen metabolite, usnic acid, is a potent inhibitor of plant p-hydroxyphenylpyruvate dioxygenase. FEBS Lett. 2000;480:301–305. doi: 10.1016/s0014-5793(00)01907-4. [DOI] [PubMed] [Google Scholar]
- 7.Barton DHR, Deflorin AM, Edwards OE. The synthesis of usnic acid. J Chem Soc. 1956;1956:530–534. [Google Scholar]
- 8.Taguchi H, Sankawa U, Shibata S. Biosynthesis of natural products. VI. Biosynthesis of usnic acid in lichens. A general scheme of biosynthesis of usnic acid. Chem Pharm Bull (Tokyo) 1969;17:2054–2060. doi: 10.1248/cpb.17.2054. [DOI] [PubMed] [Google Scholar]
- 9.Sharma RK, Jannke PJ. Acidity of usnic acid. Indian Journal of Chemistry. 1966;4:16–18. [Google Scholar]
- 10.Cardarelli M, Serino G, Campanella L, Ercole P, De Cicco NF, Alesiani O, Rossiello F. Antimitotic effects of usnic acid on different biological systems. Cell Mol Life Sci. 1997;53:667–672. doi: 10.1007/s000180050086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Backor M, Gaburjakova J, Hudak J, Zeigler W. The biological role of secondary metabolites from lichens. 1. The influence of usnic acid on bimolecular lipid membranes. Pysiologia Plantarum. 1997;29:67–71. [Google Scholar]
- 12.Mitchell P. Vectorial chemistry and the molecular mechanics of chemiosmotic coupling: power transmission by proticity. Biochem Soc Trans. 1976;4:399–430. doi: 10.1042/bst0040399. [DOI] [PubMed] [Google Scholar]
- 13.Abo-Khatwa AN, al Robai AA, al Jawhari DA. Lichen acids as uncouplers of oxidative phosphorylation of mouse-liver mitochondria. Nat Toxins. 1996;4:96–102. doi: 10.1002/19960402nt7. [DOI] [PubMed] [Google Scholar]
- 14.Han D, Matsumaru K, Rettori D, Kaplowitz N. Usnic acid-induced necrosis of cultured mouse hepatocytes: inhibition of mitochondrial function and oxidative stress. Biochem Pharmacol. 2004;67:439–451. doi: 10.1016/j.bcp.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 15.Pramyothin P, Janthasoot W, Pongnimitprasert N, Phrukudom S, Ruangrungsi N. Hepatotoxic effect of (+)usnic acid from Usnea siamensis Wainio in rats, isolated rat hepatocytes and isolated rat liver mitochondria. J Ethnopharmacol. 2004;90:381–387. doi: 10.1016/j.jep.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 16.Shibamoto T, Wei CI. Mutagenicity of lichen constituents. Environ Mutagen. 1984;6:757–762. doi: 10.1002/em.2860060512. [DOI] [PubMed] [Google Scholar]
- 17.Huneck S. The significance of lichens and their metabolites. Naturwissenschaften. 1999;86:559–570. doi: 10.1007/s001140050676. [DOI] [PubMed] [Google Scholar]
- 18.Cansaran D, Kahya D, Yurdakulola E, Atakol O. Identification and quantitation of usnic acid from the lichen Usnea species of Anatolia and antimicrobial activity. Z Naturforsch [C] 2006;61:773–776. doi: 10.1515/znc-2006-11-1202. [DOI] [PubMed] [Google Scholar]
- 19.Esslinger TL. A cumulative checklist for the lichen-forming, lichenicolous and allied fungi of the continental United States and Canada. Fargo, North Dakota: North Dakota State University; 2007. [Google Scholar]
- 20.Brodo IM, Sharnoff SD, Sharnoff S. Lichens of North America. New Haven, CT: Yale University Press; 2001. [Google Scholar]
- 21.Frankos VH. NTP nomination for usnic acid and Usnea barbata. 2004 Available at: http://ntp-server.niehs.nih.gov/
- 22.Okuyama E, Umeyama K, Yamazaki M, Kinoshita Y, Yamamoto Y. Usnic acid and diffractaic acid as analgesic and antipyretic components of Usnea diffracta. Planta Med. 1995;61:113–115. doi: 10.1055/s-2006-958027. [DOI] [PubMed] [Google Scholar]
- 23.Vartia KO. The Lichens. New York: Academic Press; 1973. Antibiotics in lichens; pp. 547–561. [Google Scholar]
- 24.Sweetman SC. Martindale: The complete drug reference. London: Pharmaceutical Press: 2004. [Google Scholar]
- 25.Rafanelli S, Bacchilega R, Stanganelli I, Rafanelli A. Contact dermatitis from usnic acid in vaginal ovules. Contact Dermatitis. 1995;33:271–272. doi: 10.1111/j.1600-0536.1995.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 26.Correche ER, Carrasco M, Escudero ME, Velazquez L, de Guzman AMS, Giannini F, Enriz RD, Jauregui EA, Cenal JP, Giordano OS. Study of the cytotoxic and antimicrobial activities of usnic acid and derivatives. Fitoterapia. 1998;69:493–501. [Google Scholar]
- 27.Ghione M, Parrello D, Grasso L. Usnic acid revisited, its activity on oral flora. Chemioterapia. 1988;7:302–305. [PubMed] [Google Scholar]
- 28.Ingolfsdottir K, Chung GA, Skulason VG, Gissurarson SR, Vilhelmsdottir M. Antimycobacterial activity of lichenmetabolites in vitro. Eur J Pharm Sci. 1998;6:141–144. doi: 10.1016/s0928-0987(97)00078-x. [DOI] [PubMed] [Google Scholar]
- 29.Krishna DR, Venkataramana D. Pharmacokinetics of D(+)-usnic acid in rabbits after intravenous and oral administration. Drug Metab Dispos. 1992;20:909–911. [PubMed] [Google Scholar]
- 30.Lauterwein M, Oethinger M, Belsner K, Peters T, Marre R. In vitro activities of the lichen secondary metabolites vulpinic acid, (+)-usnic acid, and (−)-usnic acid against aerobic and anaerobic microorganisms. Antimicrob Agents Chemother. 1995;39:2541–2543. doi: 10.1128/aac.39.11.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elo H, Matikainen J, Pelttari E. Potent activity of the lichen antibiotic (+)-usnic acid against clinical isolates of vancomycin-resistant enterococci and methicillin-resistant Staphylococcus aureus. Naturwissenschaften. 2007;94:465–468. doi: 10.1007/s00114-006-0208-9. [DOI] [PubMed] [Google Scholar]
- 32.Francolini I, Norris P, Piozzi A, Donelli G, Stoodley P. Usnic acid, a natural antimicrobial agent able to inhibit bacterial biofilm formation on polymer surfaces. Antimicrob Agents Chemother. 2004;48:4360–4365. doi: 10.1128/AAC.48.11.4360-4365.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu J, Zhang M, Ding D, Tan T, Yan B. Effect of Cladonia alpestris on Trichomonas vaginalis in vitro. Chinese J Parasitic Dis. 1995;13:126–129. [PubMed] [Google Scholar]
- 34.Fournet A, Ferreira ME, Rojas dA, Torres dO, Inchausti A, Yaluff G, Quilhot W, Fernandez E, Hidalgo ME. Activity of compounds isolated from Chilean lichens against experimental cutaneous leishmaniasis. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol. 1997;116:51–54. doi: 10.1016/s0742-8413(96)00127-2. [DOI] [PubMed] [Google Scholar]
- 35.Yamamoto Y, Miura Y, Kinoshita Y, Higuchi M, Yamada Y, Murakami A, Ohigashi H, Koshimizu K. Screening of tissue cultures and thalli of lichens and some of their active constituents for inhibition of tumor promoter-induced Epstein-Barr virus activation. Chem Pharm Bull (Tokyo) 1995;43:1388–1390. doi: 10.1248/cpb.43.1388. [DOI] [PubMed] [Google Scholar]
- 36.Scirpa P, Scambia G, Masciullo V, Battaglia F, Foti E, Lopez R, Villa P, Malecore M, Mancuso S. A zinc sulfate and usnic acid preparation used as post-surgical adjuvant therapy in genital lesions by Human Papillomavirus. Minerva Ginecol. 1999;51:255–260. [PubMed] [Google Scholar]
- 37.Takai M, Uehara Y, Beisler JA. Usnic acid derivatives as potential antineoplastic agents. J Med Chem. 1979;22:1380–1384. doi: 10.1021/jm00197a019. [DOI] [PubMed] [Google Scholar]
- 38.Kristmundsdottir T, Aradottir HA, Ingolfsdottir K, Ogmundsdottir HM. Solubilization of the lichen metabolite (+)-usnic acid for testing in tissue culture. J Pharm Pharmacol. 2002;54:1447–1452. doi: 10.1211/002235702225. [DOI] [PubMed] [Google Scholar]
- 39.Vijayakumar CS, Viswanathan S, Reddy MK, Parvathavarthini S, Kundu AB, Sukumar E. Anti-inflammatory activity of (+)-usnic acid. Fitoterapia. 2000;71:564–566. doi: 10.1016/s0367-326x(00)00209-4. [DOI] [PubMed] [Google Scholar]
- 40.Krishna DR, Ramana DV, Mamidi NV. In vitro protein binding and tissue distribution of D(+) usnic acid. Drug Metabol Drug Interact. 1995;12:53–63. doi: 10.1515/dmdi.1995.12.1.53. [DOI] [PubMed] [Google Scholar]
- 41.Venkataramana D, Krishna DR. Pharmacokinetics of d(+)-usnic acid in rabbits after intravenous administration. Eur J Drug Metab Pharmacokinet. 1993;18:161–163. doi: 10.1007/BF03188791. [DOI] [PubMed] [Google Scholar]
- 42.Foti RS, Dickmann LJ, Davis JA, Greene RJ, Hill JJ, Howard ML, Pearson JT, Rock DA, Tay JC, Wahlstrom JL, et al. Metabolism and related human risk factors for hepatic damage by usnic acid containing nutritional supplements. Xenobiotica. 2008;38:264–280. doi: 10.1080/00498250701802514. [DOI] [PubMed] [Google Scholar]
- 43.Kumar KC, Muller K. Lichen metabolites. 2. Antiproliferative and cytotoxic activity of gyrophoric, usnic, and diffractaic acid on human keratinocyte growth. J Nat Prod. 1999;62:821–823. doi: 10.1021/np980378z. [DOI] [PubMed] [Google Scholar]
- 44.Koparal AT, Tuylu BA, Turk H. In vitro cytotoxic activities of (+)-usnic acid and (−)-usnic acid on V79, A549, and human lymphocyte cells and their non-genotoxicity on human lymphocytes. Nat Prod Res. 2006;20:1300–1307. doi: 10.1080/14786410601101910. [DOI] [PubMed] [Google Scholar]
- 45.Johnson RB, Feldott G. The mode of action of the antibiotic, usnic acid. Arch Biochem. 1950;28:317–323. [PubMed] [Google Scholar]
- 46.Brown JP. A review of the genetic effects of naturally occurring flavonoids, anthraquinones and related compounds. Mutat Res. 1980;75:243–277. doi: 10.1016/0165-1110(80)90029-9. [DOI] [PubMed] [Google Scholar]
- 47.al Bekairi AM, Qureshi S, Chaudhry MA, Krishna DR, Shah AH. Mitodepressive, clastogenic and biochemical effects of (+)-usnic acid in mice. J Ethnopharmacol. 1991;33:217–220. doi: 10.1016/0378-8741(91)90079-s. [DOI] [PubMed] [Google Scholar]
- 48.Marshak A, Kuschner M. The action of streptomycin and usnic acid on the development of tuberculosis in guinea pigs. Public Health Rep. 1950;65:131–144. [PMC free article] [PubMed] [Google Scholar]
- 49.Ribeiro-Costa RM, Alves AJ, Santos NP, Nascimento SC, Goncalves EC, Silva NH, Honda NK, Santos-Magalhaes NS. In vitro and in vivo properties of usnic acid encapsulated into PLGA-microspheres. J Microencapsul. 2004;21:371–384. doi: 10.1080/02652040410001673919. [DOI] [PubMed] [Google Scholar]
- 50.da Silva Santos NP, Nascimento SC, Wanderley MS, Pontes-Filho NT, da Silva JF, de Castro CM, Pereira EC, da Silva NH, Honda NK, Santos-Magalhaes NS. Nanoencapsulation of usnic acid: An attempt to improve antitumour activity and reduce hepatotoxicity. Eur J Pharm Biopharm. 2006;64:154–160. doi: 10.1016/j.ejpb.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 51.Odabasoglu F, Cakir A, Suleyman H, Aslan A, Bayir Y, Halici M, Kazaz C. Gastro-protective and antioxidant effects of usnic acid on indomethacin-induced gastric ulcer in rats. J Ethnopharmacol. 2006;103:59–65. doi: 10.1016/j.jep.2005.06.043. [DOI] [PubMed] [Google Scholar]
- 52.Dailey RN, Montgomery DL, Ingram JT, Siemion R, Vasquez M, Raisbeck MF. Toxicity of the lichen secondary metabolite (+)-usnic acid in domestic sheep. Vet Pathol. 2008;45:19–25. doi: 10.1354/vp.45-1-19. [DOI] [PubMed] [Google Scholar]
- 53.Cook WE, Raisbeck MF, Cornish TE, Williams ES, Brown B, Hiatt G, Kreeger TJ. Paresis and death in elk (Cervus elaphus) due to lichen intoxication in Wyoming. J Wildl Dis. 2007;43:498–503. doi: 10.7589/0090-3558-43.3.498. [DOI] [PubMed] [Google Scholar]
- 54.Cetin H, Tufan-Cetin O, Turk AO, Tay T, Candan M, Yanikoglu A, Sumbul H. Insecticidal activity of major lichen compounds, (−)- and (+)-usnic acid, against the larvae of house mosquito, Culex pipiens L. Parasitol Res. 2008;102:1277–1279. doi: 10.1007/s00436-008-0905-8. [DOI] [PubMed] [Google Scholar]
- 55.Colman E. Dinitrophenol and obesity: an early twentieth-century regulatory dilemma. Regul Toxicol Pharmacol. 2007;48:115–117. doi: 10.1016/j.yrtph.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 56.Miranda EJ, McIntyre IM, Parker DR, Gary RD, Logan BK. Two deaths attributed to the use of 2,4-dinitrophenol. J Anal Toxicol. 2006;30:219–222. doi: 10.1093/jat/30.3.219. [DOI] [PubMed] [Google Scholar]
- 57.Hsiao AL, Santucci KA, Seo-Mayer P, Mariappan MR, Hodsdon ME, Banasiak KJ, Baum CR. Pediatric fatality following ingestion of dinitrophenol: postmortem identification of a “dietary supplement”. Clin Toxicol. 2005;43:281–285. doi: 10.1081/clt-58946. [DOI] [PubMed] [Google Scholar]
- 58.Favreau JT, Ryu ML, Braunstein G, Orshansky G, Park SS, Coody GL, Love LA, Fong TL. Severe hepatotoxicity associated with the dietary supplement LipoKinetix. Ann Intern Med. 2002;136:590–595. doi: 10.7326/0003-4819-136-8-200204160-00008. [DOI] [PubMed] [Google Scholar]
- 59.Neff GW, Reddy KR, Durazo FA, Meyer D, Marrero R, Kaplowitz N. Severe hepatotoxicity associated with the use of weight loss diet supplements containing ma huang or usnic acid. J Hepatol. 2004;41:1062–1064. doi: 10.1016/j.jhep.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 60.Sanchez W, Maple JT, Burgart LJ, Kamath PS. Severe hepatotoxicity associated with use of a dietary supplement containing usnic acid. Mayo Clin Proc. 2006;81:541–544. doi: 10.4065/81.4.541. [DOI] [PubMed] [Google Scholar]
- 61.Durazo FA, Lassman C, Han SH, Saab S, Lee NP, Kawano M, Saggi B, Gordon S, Farmer DG, Yersiz H, et al. Fulminant liver failure due to usnic acid for weight loss. Am J Gastroenterol. 2004;99:950–952. doi: 10.1111/j.1572-0241.2004.04165.x. [DOI] [PubMed] [Google Scholar]
- 62.Estes JD, Stolpman D, Olyaei A, Corless CL, Ham JM, Schwartz JM, Orloff SL. High prevalence of potentially hepatotoxic herbal supplement use in patients with fulminant hepatic failure. Arch Surg. 2003;138:852–858. doi: 10.1001/archsurg.138.8.852. [DOI] [PubMed] [Google Scholar]
- 63.Anon FDA. Warns Against Dietary Supplement. 2001 Available at http://www.obgyn.net/newsrx/general_health-Nutriceuticals-20011217-11.asp.
- 64.CFSAN. FDA warns consumers not to use the dietary supplement LipoKenetix. 2001 Available at: http://www.cfsan.fda.gov/~dms/ds-lipo.html.
- 65.CFSAN. Letter to distributor of hazardous dietary supplement, LipoKinetix. 2001 Available at: http://www.cfsan.fda.gov/~dms/ds-ltr26.html.
- 66.Ali AA, Lewis SM, Mukherjee M, Olson G, Allaben WT, Leakey JE. Acute hepatotoxicity of usnic acid in Fischer 344 rats and B6C3F1 mice. Toxicol Sci. 2008;102(suppl 1):146. (Abstract) [Google Scholar]
- 67.NTP. Genotoxicity test of usnic acid in Salmonella. 2006 Available at: http://ntp-apps.niehs.nih.gov/ntp_tox/index.cfm?fuseaction=salmonella.overallresults&cas_no=125-46-2&endpointlist=SA.
- 68.Joseph A, Lee T, Moland CL, Branham WS, Fuscoe JC, Leakey JEA, Allaben WT, Lewis SM, Ali AA, Desai VG. Effect of usnic acid on mitochondrial functions as measured by mitochondria-specific oligonucleotide microarray in liver of B6C3F1 mice. Mitochondrion. 2008 doi: 10.1016/j.mito.2009.02.002. (In Press) [DOI] [PubMed] [Google Scholar]
- 69.Guo L, Shi Q, Blann E, Leakey J, Svancara DM, Natoli EJ, Jr, Nadanaciva S, Billis P, Banker MJ, Dykens JA, Will Y. Mitochondrial toxicity of usnic acid: Mechanistic activity contributes to its idiosyncratic hepatotoxicity. 2008 (submitted) [Google Scholar]