Abstract
Diagnosis of deep-seated bacterial infection is difficult, as neither standard anatomical imaging nor radiolabeled, autologous leukocytes distinguish sterile inflammation from infection. Two recent imaging efforts are receiving attention: 1) radioactive derivatives of sorbitol show good specificity with Gram-negative infections, and 2) success from combining anatomical and functional imaging for cancer diagnosis has rekindled interest in 99mTc-fluoroquinolone-based imaging. With the latter, computed tomography (CT) would be combined with single-photon-emission-computed tomography (SPECT) to detect a 99mTc-fluoroquinolone-bacterial interaction. The present minireview provides a framework for advancing with fluoroquinolone-based imaging by identifying gaps in our understanding of the process. One issue is the reliance of 99mTc labeling on reduction of sodium pertechnetate, which can lead to colloid formation and loss of specificity. Specificity problems may be reduced by altering quinolone structure (an example is switching from ciprofloxacin to sitafloxacin). Another issue is the uncharacterized nature of 99mTc-ciprofloxacin binding to, or sequestration in, bacteria -- specific interactions with DNA gyrase, an intracellular fluoroquinolone target, are unlikely. Replacing the C6-F of the fluoroquinolone with 18F provides an alternative to pertechnetate that may lead to imaging based on drug interactions with gyrase. Gyrase-based imaging requires knowledge of fluoroquinolone action, which we update. We conclude that quinolone-based probes show promise for diagnosis of bacterial infection, but improvements in specificity and sensitivity are needed. Those improvements require optimization of quinolone structure and reduction of pertechnetate, chemistry efforts that can be accelerated by refining microbiological assays.
Introduction
Diagnosis of deep-seated infection is problematic, in part because invasive methods, such as fine-needle aspiration, are not easily applied to critically ill patients. Furthermore, imaging technologies, such as X-ray, ultrasonography, computed tomography, and magnetic resonance imaging, are inherently non-specific and require morphological changes that may not be obvious at early stages of infection. Traditional detector- based leukocyte imaging fails to provide the fine- structure detail needed to accurately locate, and effectively treat, infection. To address these issues, attention has turned to a combination of computed tomography (CT) and leukocyte scintigraphy (radiolabeled leukocyte single-photon emission computed tomography [SPECT]) and to metabolic imaging by 18-fluorodeoxyglucose positron emission tomography (18FDG-PET). However, these leukocyte and metabolic imaging methods still fail to distinguish infection from sterile inflammation. Thus, a bacterium-specific test is needed.
Two recent efforts are encouraging. One, which continues the focus on metabolic substrates, has revealed that a derivative of sorbitol specifically enters Gram-negative bacteria. This work has entered initial phases of human testing.1 The second effort involves early work suggesting that 99mTc-labeled ciprofloxacin might serve as a functional probe for both Gram-negative and Gram-positive infections. Examples of poor specificity tempered early enthusiasm, but a novel approach has emerged from cancer diagnosis2 that could revitalize fluoroquinolone-based infection imaging. The strategy involves combining a functional assay, such as a radiolabeled, bacterium-specific test using SPECT, with an imaging method, such as CT, to accurately locate infection. Consequently, work on 99mTc-fluoroquinolone-based imaging is continuing.3 To help focus efforts at quinolone refinement, we review why 99mTc-ciprofloxacin has been considered a viable imaging agent, consider aspects of imaging that lack an adequate knowledge base, and suggest ways to move forward.
Ciprofloxacin is an early member of the fluoroquinolone-class of antimicrobials. The agent was introduced into clinical practice in the 1980s, at about the same time that imaging became popular for agents radiolabeled with a metastable isotope of technetium (99mTc). By the late 1990s, promising results were obtained from coupling 99mTc to antibiotics, antibodies, peptide analogues, and anticancer drugs. The widespread availability and broad-spectrum activity of ciprofloxacin led to this antibacterial being complexed to 99mTc and examined as a functional assay for infection. The use of ciprofloxacin was even commercialized with a diagnostic kit called Infecton®.
Among the potential applications was endocarditis caused by Staphylococcus aureus. It was hoped that information from echocardiograms could be augmented by ciprofloxacin-based imaging to assess infection in heart valves and pacemakers.4 Another potential application involved distinguishing sternal infection from inflammation, particularly following coronal bypass surgery.5 Clinical studies, when added to work with animal models of infection, were encouraging. However, conflicting results subsequently emerged.6 Since little effort had been made to understand the mechanism underlying bacterial imaging by ciprofloxacin, improving the system required trial-and-error studies using animal models for testing – rapid, reliable microbiological assays are not in place. Consequently, early excitement about imaging with 99mTc-ciprofloxacin dissipated. Nevertheless, quinolones remain a viable choice due to their broad spectrum activity – they must interact physically with many different bacterial pathogens.
Technetium-Ciprofloxacin-Based Imaging
Diagnostic procedures have typically involved intravenous injection of a single dose (370–740 MBq) of 99mTc-ciprofloxacin (~1 mg). At this dose, peak serum concentration is about 0.08 μg/mL, approximately 50-times below that achieved with therapeutic doses of ciprofloxacin. Thus, a single dose is unlikely to have therapeutic value. Since the peak concentration of 99mTc-ciprofloxacin is above the minimal inhibitory concentration (MIC) for ciprofloxacin with some bacteria, 99mTc-ciprofloxacin could, in principle, exert some selective pressure for emergence of resistance. Indeed, selective pressure can be exerted at concentrations well below MIC. However, emergence of fluoroquinolone resistance is largely an epidemiological problem that is rarely seen with individual patients (most resistance issues derive from bacterial strains that are resistant prior to infection, which is discussed below).
Images of 99mTc-ciprofloxacin are acquired using a dual-headed gamma camera with 1-min exposures at intervals of 5, 30, 60, and 360 min; in some cases, a 24-hr reading is included to reduce the relative signal from non-specific labeling. 99mTc-ciprofloxacin concentrates in kidneys and is excreted through the urinary system; it also concentrates in uninfected liver and spleen. Consequently, many tissues cannot be imaged. However, bones and soft tissue have background levels low enough to allow sites of infection to be distinguished, especially when labeling is measured at various times after administration of the imaging agent. Indeed, early reports of 99mTc-ciprofloxacin imaging were promising. For example, in a multinational study involving more than 800 patients, imaging with 99mTc-ciprofloxacin showed a sensitivity of 85% and specificity for infection of almost 82%.4 Examples of potential applications are listed in Table 1.
Table 1.
Examples of clinical studies with 99mTc-labeled ciprofloxacin
| Infection type | No. Patients | Sensitivity (%) | Specificity (%) | Ref. |
|---|---|---|---|---|
| Foot infection/type 2 diabetes mellitus | 25 | 66 | 86 | 7 |
| Gastrointestinal/abdominal infections | 21 | 79 | 91 | 8 |
| Orthopedic infection | 22 | 85 | 92 | 9 |
| Osteoarticular infection | 27 | 100 | 38 | 6 |
| Osteoarticular infection | 94 | 98 | 100 | 10 |
| Osteomyelitis | 48 | 81 | 87 | 11 |
| Osteomyelitis | 45 | 97 | 80 | 5 |
| Postoperative spine infection | 22 | 78 | 69 | 12 |
| Prosthetic joint infections | 16 | 86 | 78 | 13 |
| Pulmonary tuberculosis | 21 | 80 | 90 | 14 |
| Septic arthritis | 25 | 94 | 47 | 15 |
| Spinal infection | 11 | 82 | 87 | 16 |
| Tuberculous osteomyelitis | 14 | 93 | 71 | 17 |
In principle, 99mTc-fluoroquinolone-based imaging should be superior to labeled autologous leukocytes for identifying sites of infection, because leucocyte-based imaging reflects only inflammation. Moreover, recovering leucocytes from patients for labeling and administration requires additional laboratory procedures, thereby making 99mTc-fluoroquinolone-based imaging easier to perform in a clinical setting.
Experimentally, comparisons have been performed between 99mTc-labeled leukocytes and labeled ciprofloxacin for a variety of infections of joints, abdomen, heart, skin, and soft tissues. In one study,18 the two imaging agents showed concordance for 39/57 cases, but for 18 they did not. In nine of the discordant cases, 99mTc-ciprofloxacin scored positive while leucocytes were negative; eight cases showed labeled ciprofloxacin to be correct at identifying infections not reported by leukocytes. These data supported the assertion that bacteria-specific labeling was superior. The other nine cases scored negative with 99mTc-ciprofloxacin but positive with leucocytes. Of these, four were true negative; the other five had been treated with antibiotics that may have cleared bacteria from the site but left inflammation. Such results stimulated interest in 99mTc-ciprofloxacin as a radiodiagnostic. However, not every study showed the antimicrobial probe to be superior to labeled leukocytes.6 Thus, the method is not robust enough for general clinical use.
Combining fluoroquinolone-based imaging with CT is likely to bring a new level of sensitivity to the detail afforded by CT. Indeed, CT alone has not performed as well as 99mTc-ciprofloxacin in studies with infected animals. For example, with diagnosis of mature, acute pancreas infection of swine, imaging by 99mTc-ciprofloxacin exhibited 94% sensitivity, 92% specificity, and 93% accuracy. For CT, the parameters were 13%, 100% and 50%.19 Thus, 99mTc-fluoroquinolone-based imaging, commonly performed by SPECT, could add value to anatomical imaging by CT.
Interaction of 99mTc-Ciprofloxacin with Bacterial Cells
The early attention to clinical applications was not paralleled by similar effort on microbiology. Thus, many basic outstanding questions remain. For example, can 99mTc-ciprofloxacin-based imaging demonstrate resolution of infection? In essence, can dead cells be distinguished from live ones, and if so, what is the basis for the distinction? In some reports, heat-killed bacteria (Escherichia coli and S. aureus) exhibited properties similar to those seen with live bacteria.20, 21 However, in other studies heat-killed S. aureus constituted a negative control for specific imaging of infection in animal models.22 Such contradictions must be resolved and methods for generating relevant “dead” bacterial populations established. For example, bacteria killed by antibacterial agents are likely to be more germane than bacteria killed by heat treatment.
Another issue is that 99mTc-ciprofloxacin behavior differs from that of its component parts when tests are performed with bacteria. For example, pure ciprofloxacin does not compete with 99mTc-ciprofloxacin for binding to S. aureus.21 Moreover, when two commonly studied efflux pumps are over-expressed in cultured S. aureus (NorA) or Pseudomonas aeruginosa (MexAB-OprM), no effect on accumulation of 99mTc-ciprofloxacin is observed.23 However, both pumps do reduce the intracellular concentration of free ciprofloxacin. 99mTc by itself is not taken up by the cells. Thus, studies of bacterial interactions with 99mTc-fluoroquinolones cannot be advanced by examining only the quinolone or the 99mTc component.
An approach for studying binding mechanism is to obtain mutants that fail to carry out binding. Such mutants should allow growth under conditions in which a 99mTc-fluoroquinolone blocks wild-type growth. To our knowledge, this avenue has not been explored.
A third issue concerns DNA gyrase, the intracellular target of ciprofloxacin. Part of the initial enthusiasm for 99mTc-ciprofloxacin as an imaging agent rested on the broad-spectrum nature of the antimicrobial, which was due to the widespread distribution of gyrase. It has become clear that gyrase is not the target of 99mTc-ciprofloxacin, as seen with a biochemical test in which the formation of complexes containing ciprofloxacin, gyrase, and plasmid DNA was examined. These ternary complexes (cleaved complexes) are the hallmark of quinolone action, as they rapidly block DNA replication and interfere with the movement of transcription complexes. When ciprofloxacin was complexed to rhenium, which is chemically similar to technetium, and then used to study formation of the drug-gyrase-DNA complexes,24 the Re-ciprofloxacin reagent was at least 100-fold less active than free ciprofloxacin (interaction with gyrase could be even lower than reported, because release of free ciprofloxacin from the Re-ciprofloxacin complex could have contributed to the apparent, low-level activity).
Finding little or no cleaved-complex-forming activity is consistent with how 99mTc and Re form complexes with ciprofloxacin – they interact with the C3-carboxyl and C4-keto substituents of fluoroquinolones (see Figure 1 for quinolone numbering and for location of radiolabel interaction with ciprofloxacin). The C3 and C4 structural moieties are central to the binding of quinolones to type II DNA topoisomerases25 --complexing of fluorquinolone with 99mTc or Re would exclude binding to gyrase-DNA complexes.
Figure 1.
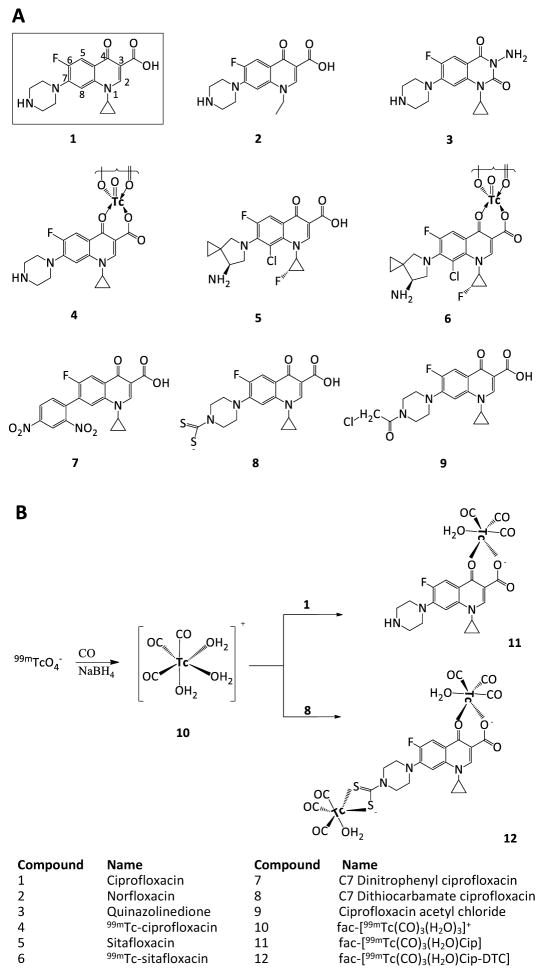
Aspects of fluoroquinolone structure. Panel A. Structures of quinolone-class molecules. Quinolones have a characteristic dual-ring structure (numbering is as shown for ciprofloxacin). Ciprofloxacin differs from norfloxacin by replacement of the N1-ethyl with an N1-cyclopropyl group. The quinazolinedione shown is identical to ciprofloxacin except at the 2 and 3 positions. The absence of the C3 carboxyl prevents the dione from interacting strongly with amino acids in helix-4 of GyrA and ParC, which in turn makes diones insensitive to resistance substitutions in helix-4. 99mTc is shown interacting at the C3 carboxylate and C4 carbonyl substitutents of ciprofloxacin. Panel B. Labeling of ciprofloxacin (1) and dithiocarbamate ciprofloxacin (8) with fac-[99mTc(OH2)3(CO)3]+ (10). A carbonyl water complex of technetium (compound 10) is formed by reacting 99mTcO4 with carbon monoxide and sodium borohydride. Compound 10 then complexes with either ciprofloxacin (1) or compound 8, in both cases at the fluoroquinolone C3 and C4 positions.
Infection-imaging studies should also argue against gyrase being the target of 99mTc-ciprofloxacin, but insufficient data have been collected to make the case. For example, in an early study, imaging was unaffected by bacterial resistance to ciprofloxacin.26 Resistance commonly maps to genes encoding gyrase, and thus the experiment would have argued against gyrase being a target. However, increased efflux, which is not related to gyrase, can also cause resistance; the study failed to establish the source of resistance and therefore failed to address the target issue. A similar problem arose in another study in which clinical isolates of S. aureus were used to generate a foot infection in rats.27 When 99mTc-ciprofloxacin was injected into the rats, radioactive regions in infected feet were detected only when the infecting bacteria were susceptible to ciprofloxacin. As in the experiment cited above, the molecular basis for resistance was not examined. Nevertheless, the rat study does argue for specificity, since an effect of bacterial resistance implicates infection rather than inflammation or increased blood flow as the source of the signal.
We conclude that the mechanism by which 99mTc-ciprofloxacin interacts with bacteria is a mystery. Our current speculation is that the 99mTc moiety of the imaging agent allows bacterial cells to sequester the agent through a mechanism that is distinct from that used for the pure drug. In vitro tests are now needed to show how drug structure, bacterial species, and physiological status of the bacteria influence sequestration of 99mTc-labeled fluoroquinolones. Such information is expected to be important for designing an effective quinolone-based imaging agent.
Preparation and Chemical Properties of 99mTc-Labeled Quinolones
Technetium, along with manganese and rhenium, is positioned in group VIIB of the periodic table with the transition metals. As a member of group VIIB, the electronic configuration of technetium is 1s22s22p63s23p63d104s24p64d65s1. It readily loses the seven electrons of the 4d and 5s orbitals to attain a +7 oxidation state; the neutral state is as pertechnetate (TcO4−). Technetium accepts electrons in these orbitals in reactions with reducing agents, gaining oxidation states from +1 to +6. In these states, technetium forms complexes with a variety of ligands.
More than 50 isotopes of Tc have been reported, with half-lives ranging from a few seconds to millions of years (98Tc has a half life of 4.2 million years). Of these isotopes,99mTc is the most important for medical imaging.28 99mTc forms when natural molybdenum (98Mo, ~24% purity) is irradiated with slow neutrons to produce inexpensive, but low specific-activity 99Mo. 99Mo forms 99mTc (6-hr half-life; +7 oxidation state), which emits a 140-keV γ-ray that is readily detected by SPECT, an integrated form of radioactive tracer detection.
A principal factor in the formation and activity of 99mTc-labeled fluoroquinolones is the 99mTc oxidation state -- it needs to be lowered to react with ligands and to create a stable radiodiagnostic. To achieve that, 99mTcO4−1 is treated with a reducing agent when incubated with ligand (fluoroquinolone).
Obtaining stable complexes requires reducing the oxidation state of 99mTc by different amounts for different ligands. For example, 99mTc is reduced to its +1 oxidation state when formed as technetium sestamibi; it is +3 when the ligands are dimercaptosuccinic acid, 2,6-dimethylphenyl-carbamoylmethyl iminodiacetic acid analogues, furifosmin or teboroxime, and the oxidation state for stable complexes is +4 when the ligand is diethylenetriaminepentaacetic acid or methylene diphosphonate. It is +5 with citrate, mercapto-acetylglycylglycylglycine, gluconate, or tetrofosmin.29–33 Stable complexes with ciprofloxacin and other fluoroquinolones form when 99mTc is reduced to the +5 oxidation state and a d2 electronic configuration.
Among the early reducing agents tested were ascorbic acid and ferrous iron. Although they reduced 99mTcO4−1, reduction tended to be incomplete.34 Sodium borohydride (NaBH4) and sodium dithionite (Na2S2O4) were effective in alkaline medium (pH >8) at high concentration, but the yield of the 99mTc-labeled radiopharmaceutical was low.35 Eventually formamidine sulphonic acid (FSA) and stannous salts, such as stannous tartrate and stannous chloride, emerged as preferred reductants among the many examined for safety, water solubility, restricted presence of free pertechnetate, and suppression of 99mTc-colloid formation (colloids are thought to reduce specificity).20, 26, 35
Preparation of 99mTc-labeled quinolone, using FSA or stannous tartrate as the reducing agent, requires a heating step, which makes preparation more complex than when SnCl2.2H2O is used.36, 37 This disadvantage of FSA and stannous tartrate led to SnCl2.2H2O being chosen as the reductant in the cold kits sold for preparation of 99mTc-ciprofloxacin.20
Although complexes of 99mTc-ciprofloxacin have been used for imaging since 1993,38 the structure of the radiopharmaceutical remains poorly defined.39 For most 99mTc-labeled compounds used clinically,28, 34 including the fluoroquinolones, the +5 oxidation state can be stabilized by forming a technetium(V)-oxo core (TcO+3 (13), TcO2+ (14)) or by forming a nitride core TcN+2 (15) (Figure 2) through a tetradentate ligand or through a pair of bidentate ligands.40, 41 X-ray crystallography and spectroscopic analysis of other metal-quinolone structures, such as Cu(II)-ciprofloxacin42 and iron(II)-norfloxacin,43, indicate that the quinolone is bidentately bonded to 99mTc through the ring carbonyl oxygen and one of the carboxylic oxygens (Figure 3).28, 29 Structures bearing an oxo or a nitride core both have square planar pyramid geometry, with the C4-oxygen of two ciprofloxacin molecules located at the geometrical apex; an oxygen or nitrogen of the oxo or nitrido cores, respectively, locates at the pyramid of the Tc core (Figure 2).29, 44 Since almost all fluoroquinolones have the same C3 and C4 moieties, the general labeling principles are likely to be common for the class.
Figure 2.
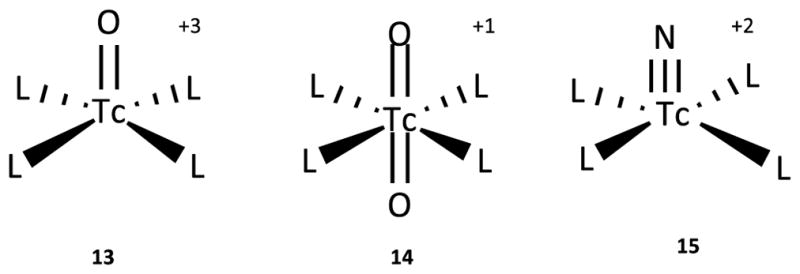
Structure of technetium cores. Oxo cores (13 & 14) and a nitride core (15) are shown complexed with 99mTc (V). “L” represents ligands that coordinate with the Tc cores to form the apex of the structure; the oxygen or nitrogen of the core form the pyramid base. In the case of ciprofloxacin labeling, the four ligand positions are occupied by two bidentate ciprofloxacin molecules, as shown in Figure 3.
Fig. 3.
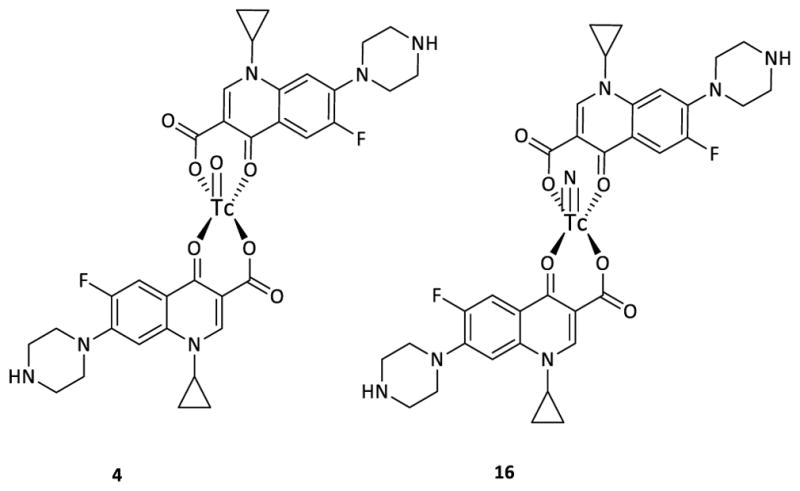
Bidentate interaction of fluoroquinolone with Tc. Structures of 99mTc-ciprofloxacin are shown using TcO+3 (4) and TcN+2 (16) cores that complex with ciprofloxacin through a two-bidentate coordination. Both structures have a square planar pyramid geometry, with the C4-oxygen of ciprofloxacin at the geometrical apex and an oxygen or nitrogen at the pyramid base of the Tc core.
Typically, the radiosynthesis of 99mTc-ciprofloxacin is performed by the addition of 99mTcO4−1 saline solution (~ 5 mCi in 2 mL) to a vial containing reducing agent (SnCl2.2H2O; ~ 50 μg) and ciprofloxacin (2 mg) at acidic pH and room temperature. With gentle shaking, the radiosynthesis reaction is complete in 5–10 min.20 For complete reduction and maximum radiochemical yield, the reducing agent is present in large excess, with the ratio of SnCl2.2H2O to99mTcO4−1 as high as 108 to 109.34
When FSA is used to label ciprofloxacin with 99mTc, a >98% yield of 99mTc-ciprofloxacin is achieved; infection-localization specificity is 70% with abdominal infection and 90% with skeletal infection.26 These values compare favorably with the use of stannous tartrate (80% specificity with deep-seated infection4) and stannous chloride (70% with arthroplasty infection45). Thus, several reducing agents have emerged as suitable for generating 99mTc-fluoroquinolone-based imaging agents.
For nuclear diagnostics, simple, rapid preparation of imaging agents is highly desirable. To overcome the need for additional purification following reduction by stannous salts, compound 10 (also called Tc-tricarbonyl core) has been introduced (see Figure 1B). Compound 10 is synthesized by direct reduction of 99mTcO4−1 using an aqueous solution of sodium borohydride in the presence of carbon monoxide. The complexing of compound 10 with ciprofloxacin, which occurs with 99mTc in the low +1 oxidation state and in a low-spin d6 electronic configuration, has the advantage of straightforward synthesis. Moreover, the three water ligands are readily substituted by a variety of biomolecules, the Tc-tricarbonyl core is largely inert, and colloid formation is suppressed.46 Thus, the Tc-tricarbonyl core constitutes a potential platform for developing stable and specific 99mTc(CO)3-labeled probes.
When three potential imaging probes, compounds 4, 7 and 8 were compared for imaging a murine infection arising from injection of S. aureus into thigh muscle, the target to non-target ratio was 4.3, 1.8, and 4.5, respectively, for the three probes (infected and uninfected thigh muscles were compared).47 As a result of such work, labeling with 99mTc-tricarbonyl core is beginning to be employed for studies of other fluoroquinolones.22
Fluoroquinolone structure appears to affect the target to non-target ratio, as ciprofloxacin is less active than some other quinolone derivatives.3 Although head-to-head comparisons have not been reported, compound 6 (99mTc-labeled sitafloxacin) may be better at imaging S. aureus.22, 48 Independent confirmation of sitafloxacin work would encourage comparisons with the thousands of other quinolones that are available for optimizing the quinolone moiety of the imaging agent (note that the agents need not be good therapeutics to be good diagnostics).
Labeling Fluoroquinolones with Gallium
Recently positron emission tomography (PET) imaging was advanced by the introduction of PET radionuclide generators. In these systems, a long-lived parent radionuclide decays to a short-lived radionuclide suitable for PET. For example, the generator system for gallium-68 (68Ga, half-life = 67.7 min) results from the decay of long-lived germanium-68 (68Ge, half-life = 270.9 days). The 68Ge/68Ga generator is suitable in remote locations, similar to the 99Mo/99mTc generator used for production of 99mTc.49–51 An advantage of the 68Ge/68Ga generator system is the large number of reports describing on 68Ga-labeled molecules.52–56 In addition, PET offers greater sensitivity, spatial and temporal resolution, and more promising quantification capabilities than SPECT.57
The good sensitivity of PET imaging and the availability of in-house generation of 68Ga encouraged application to ciprofloxacin. In an initial report,58 bifunctional chelating agents that bind Ga were attached to the C7 end of ciprofloxacin using a propylamine linker. In this study, two derivatives of ciprofloxacin were generated, one with [2-(p-isothiocyanato)-1,4,7,10-tetraazacyclo-dodecane-1,4,7,10-tetraacetic acid] (p-SCN-Bz-DOTA), shown as compound 17 in Fig. 4, and the other with [2-(p-isothiocyanato)-1,4,7-triazacyclononane-1,4,7-triacetic acid] (p-SCN-Bz-NOTA), shown as compound 18 in Fig. 4.
Figure 4.
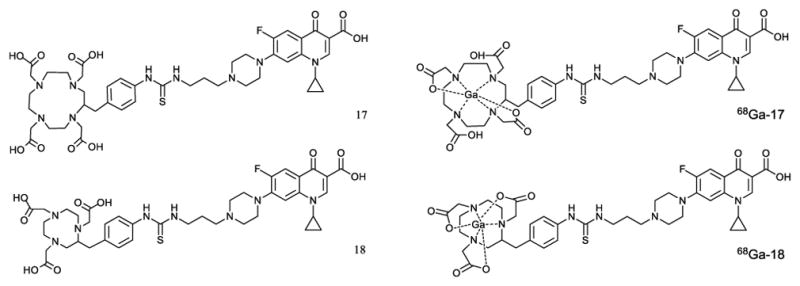
CP-PA-SCN-Bz-DOTA (17) and CP-PA-SCN-Bz-NOTA (18) ciprofloxacin derivatives for labeling with 68Ga (β+-emitter PET radionuclide). The structure of both compound 17 and 18 are shown as 68Ga-17 and 68Ga-18 in which compound 17 and 18 make 6 coordinate covalent bonds with 68Ga in its +3 oxidation state.
Binding to cultured S. aureus was 0.9–1.0% and 1.6–2.3%, respectively, for 68Ga-17 and 68Ga-18. These numbers were 3–7 times higher than with bacteria rendered non-viable by an unspecied process. As with 99mTc-ciprofloxacin work, ciprofloxacin failed to compete with the labeled compound for binding. In an animal infection experiment, S. aureus was injected into thigh muscle, and as a control turpentine was injected to create inflammation. For both probes the infection to control ratio was 2–3. This small difference in signal between infection site and an inflamed site suggest that considerable refinement is needed, much as discussed above for 99mTc-labeled quinolones.
Labeling Fluoroquinolones with 18Fluorine
Labeling at the C6-F position of ciprofloxacin with 18F provides a different approach for infection diagnosis by eliminating the pertechnetate and chelating moieties from consideration, since the 18F labeled probe is chemically identical to ciprofloxacin. Side-by-side testing of 18F-ciprofloxacin and 99mTc-ciprofloxacin or 68Ga-ciprofloxacin can now be performed to define the contribution of the 99mTc and 68Ga labeling moieties to interactions with bacterial cells.
Since 18F-ciprofloxacin preferentially localizes in infected tissue (up to 5-fold47, 59), fluoroquinolone alone can be an imaging agent. However, the large, uncharacterized binding of 18F-ciprofloxacin to bacteria (E. coli) masks specific binding to gyrase.59 Thus, even with bonafide ciprofloxacin, the nature of the interaction with bacterial cells is undefined.
We note that 18F-ciprofloxacin was initially disfavored for routine clinical use because production of 18F required a cyclotron and therefore distribution to remote areas was logistically difficult. Moreover, the labeling procedure, a nucleophilic 18F/19F exchange reaction, is lengthy (40 min), requires high temperature (175 °C), and involves complex handling.60 Recently, however, an automated method has been reported.60 If the 18F-labeling technology becomes widely used, which may involve solving the proximity problem between cyclcotron and PET facility, it may be important to revisit the potential of direct binding to gyrase. Toward that end, in the next section we provide a brief update on fluoroquinolone interactions with the target topoisomerases.
Fluoroquinolone Action
As pointed out above, the hallmark of quinolone action is the formation of ternary, cleaved complexes containing quinolone, DNA, and gyrase or topoisomerase IV. Formation of cleaved complexes, which is reversible,61 constitutes the mechanistic basis for MIC, a measure of growth inhibition. X-ray crystallography of cleaved complexes shows gyrase and topoisomerase IV as heterotetramers in which one fluoroquinolone molecule binds to each GyrA/GyrB dimer (ParC/ParE for topoisomerase IV).62, 63 These structures show binding of the drug at a pair of sites in which the drug intercalates into DNA; the C7 ring system extends into GyrB (ParE), and the C3–C4 keto/carboxyl interacts with helix-4 of GyrA (ParC) through a water-magnesium bridge.25 This important drug-target interaction at GyrA would be blocked by complexing of fluoroquinolone to 99mTc.
Quinolone resistance arises from failure of the compounds to avidly form cleaved complexes. One mechanism is restriction of intracellular drug concentration (mutations that reduce uptake or increase efflux). Another is blockage of the drug-target interaction, largely by amino acid substitutions in GyrA and ParC that interfere with formation of the water-Mg2+ bridge between quinolone and enzyme.25
The importance of the drug-enzyme bridge is emphasized by the behavior of fluoroquinolone-like compounds called quinazolinediones (for structure see Figure 1A, compound 3). These agents lack the C3-carboxyl, cannot form the water-Mg2+ bridge with amino acids in helix-4 of GyrA and ParC, and require higher concentrations to form cleaved complexes.64 Nevertheless, diones bypass the effects of existing resistance substitutions in GyrA and would therefore be unaffected by widespread fluoroquinolone resistance. Thus, they might be useful substitutes for ciprofloxacin as an 18F-based imaging agent. We note that gyrase-based resistance is unlikely to affect imaging by 99mTc-labeled compounds, because the compounds do not target gyrase, as pointed out above. Thus, diones would not confer an advantage for 99mTc-based diagnosis. Moreover, diones lack the C3 carboxyl group used to form 99mTc complexes.
Recent drug-enzyme cross-linking experiments identified a second binding interaction between fluoroquinolones and gyrase.61 The key observation is that a C7 acetyl chloride derivative of ciprofloxacin (compound 9) forms cross-links with Cys residues at two distant locations, one in GyrB, as expected from X-ray crystallography, and one in GyrA near the site where the water-Mg bridge forms between quinolone and enzyme. Cross-linking at two distant sites by the same species of quinolone is most easily explained by two drug-gyrase binding modes. In support of this idea, we recently found that an aryl group appended to the C7 piperazinyl ring of ciprofloxacin, which according to X-ray structures should extend into GyrB, suppresses the protective effect of GyrA resistance substitutions.65 The most active example found to date is a C7 dinitrophenyl derivative of ciprofloxacin (compound 7), a compound that selectively enriches cells carrying an uncommon gyrA resistance mutation. To our knowledge, C7-aryl derivatives of ciprofloxacin have not been examined as potential imaging agents.
Rapid lethal action by quinolones differs mechanistically from blocking growth, although both derive from formation of cleaved complexes. While lethal action is important therapeutically, it is irrelevant for imaging and is not discussed.
Moving Forward
As an imaging agent, 99mTc-ciprofloxacin suffers from low specificity; consequently, its use has been controversial.66–68 Variations in 99mTc-ciprofloxacin preparation, including colloid content and subjective evaluation of images, also contribute to uncertainty. Nevertheless, additional studies are encouraged by promising early clinical work with 99mTc-ciprofloxacin, apparent improvement arising from the substitution of sitafloxacin for ciprofloxacin, and the potential to improve resolution by combining CT with SPECT. These “proof-of-principle” studies now need to shift to careful comparative work with 99mTc-labeled fluoroquinolones to 1) determine how the agents interact with bacterial cells, 2) learn whether that mechanism differs among pathogen species, and 3) identify structural changes in the probes that increase the contribution of specific interactions. The same considerations apply to 68Ga-labeled probes.
Efforts are being made to develop inorganic, organic, and organometallic derivatives of 99mTc-ciprofloxacin,69 and many of the derivatives have imaging potential in animal models. They may also exhibit antibacterial activity, but that is not a positive feature, as it can contribute to the emergence of fluoroquinolone resistance. Indeed, structural modifications that improve binding to bacteria but eliminate potential therapeutic activity are preferred.
Refinement of 18F-labeled fluoroquinolones into gyrase-targeting agents remains a possibility, since these probes are structurally identical to the unlabeled antibacterial. Such work requires reducing non-gyrase binding, which is currently uncharacterized.
Concluding Remarks
99mTc offers many advantages as a radiodiagnostic, among which are ready availability, straightforward methods for preparation, and relative low cost. Moreover, the need for detecting deep-seated infection will continue to increase as antibiotic resistance limits the ability to diagnose by empirical trial-and-error treatment. Thus, a demand exists, especially in countries where expensive instrumentation is not readily available.
Considerable clinical data is now available for 99mTc-ciprofloxacin that can serve as a comparator for new derivatives. We also have methods for complexing fluoroquinolones to 99mTc, but those methods need chemistry refinement to avoid colloid formation and loss of specificity. Current tests for success (human and animal trials) are too cumbersome and expensive for rapid refinement of preparation methods and structural refinement. Intermediate assays, such as microbiological tests, are hampered by inadequate knowledge of binding mechanism. That lack of understanding also precludes the development of rapid biochemical assays. On the other hand, many options exist for optimizing the quinolone component of the complex: structure can affect many properties of quinolones, and thousands of these compounds have been synthesized. Thus, opportunities exist for refining the fluoroquinolones into useful imaging agents.
Acknowledgments
We thank the following for critical comments and assistance with the manuscript: Marila Gennaro, Robert Kerns, Gan Luan, Arkady Mustaev, Rashid Rasheed, Nusrat Jabeen Raza, and Bo Shopsin. The work was supported by grants from the National Institutes of Health (R01 AI073491) and from the Higher Education Commission, Islamabad (5612/Punjab/NRPU/R&D/HEC/2016).
References
- 1.Yao S, Xing H, Zhu W, Wu Z, Zhang Y, Ma Y, Liu Y, Huo L, Zhu Z, Li Z, Li F. Nucl Med Biol. 2016;43:206–214. doi: 10.1016/j.nucmedbio.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 2.Seo Y, Aparici C, Hasegawa B. Semin Nucl Med. 2008;38:177–198. doi: 10.1053/j.semnuclmed.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auletta S, Galli F, Lauri C, Martinelli D, Santino I, Signore A. Clin Transl Imaging. 2016;4:229–252. doi: 10.1007/s40336-016-0185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britton K, Wareham D, Das S, Solanki K, Amaral H, Bhatnagar A, Katamihardja A, Malamitsi J, Moustafa H, Soroa V, Sundram F, Padhy A. J Clin Pathol. 2002;55:817–823. doi: 10.1136/jcp.55.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Malamitsi J, Giamarellou H, Kanellakopoulou K, Dounis E, Grecka V, Christakopoulos J, Koratzanis G, Antoniadou A, Panoutsopoulos G, Batsakis C, Proukakis C. Clin Microbiol Infect. 2003;9:101–109. doi: 10.1046/j.1469-0691.2003.00506.x. [DOI] [PubMed] [Google Scholar]
- 6.Sarda L, Cremieux A, Lebellec Y, Meulemans A, Lebtahi R, Hayem G, Genin R, Delahaye N, Huten D, LeGuludec D. J Nucl Med. 2003;44:920–926. [PubMed] [Google Scholar]
- 7.Dutta P, Bhansali A, Mittal B, Singh B, Masoodi SR. Foot Ankle Int. 2006;27:716–722. doi: 10.1177/107110070602700911. [DOI] [PubMed] [Google Scholar]
- 8.Artiko V, Davidovic B, Nikolic N, Petrovic M, Vlajkovic M, Pesko P, Knezevic S, Dukic V, Stefanovic B, Tulic C, Popovic N, Milosavljevic T, Obradovic V. Hepatogastroenterology. 2005;52:491–495. [PubMed] [Google Scholar]
- 9.Yapar Z, Kibar M, Yapar A, Togrul E, Kayaselcuk U, Sarpel Y. Eur J Nucl Med. 2001;28:822–830. doi: 10.1007/s002590100555. [DOI] [PubMed] [Google Scholar]
- 10.Mora-Rios F, Isunza-Ramirez A, Lopez-Marmolejo A, Palma-Rosillo R, Guizar-Cuevas S, Mora-Magana I, VV-P Acta Ortop Mex. 2010;24:248–251. [PubMed] [Google Scholar]
- 11.Sundram F, Wong W, Ang E, Goh A, Ng D, Yu S. Ann Acad Med Singapore. 2000;29:699–703. [PubMed] [Google Scholar]
- 12.Gemmel F, DeWinter F, VanLaere K, Vogelaers D, Uyttendaele D, Dierckx R. Nucl Med Commun. 2004;25:277–283. doi: 10.1097/00006231-200403000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Larikka M, Ahonen A, Niemela O, Puronto O, Junila J, Hamalainen M, Britton K, Syrjala H. Nucl Med Commun. 2002;23:167–170. doi: 10.1097/00006231-200202000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Lee M, Yoon M, Hwang K, Choe W. Nucl Med Mol Imaging. 2010;44:116–122. doi: 10.1007/s13139-010-0021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pucar D, Jankovic Z, Dugonjic S, Popovic Z. Vojnosanit Pregl. 2009;66:395–398. doi: 10.2298/vsp0905395p. [DOI] [PubMed] [Google Scholar]
- 16.Falagas M, Valotassiou V, Papadouli D, Papadopoulos A, Malamitsi J. Clin Orthop Relat Res. 2006;444:34–37. doi: 10.1097/01.blo.0000201173.02883.6e. [DOI] [PubMed] [Google Scholar]
- 17.Sharma R, Tewari K, Bhatnagar A, Mondal A, Mishra A, Singh A, Chopra M, Rawat H, Kashyap R, Tripathi RP. Clin Nucl Med. 2007;32:367–370. doi: 10.1097/01.rlu.0000259322.31974.e8. [DOI] [PubMed] [Google Scholar]
- 18.Vinjamuri S, Solanki K, Bomanji J, Siraj Q, Britton K, Hall A, O’Shaughnessy E, Das S. Lancet. 1996;347:233–235. doi: 10.1016/s0140-6736(96)90407-9. [DOI] [PubMed] [Google Scholar]
- 19.Wang J, Sun G, Zhang J, Shao C, Zuo C, Hao J, Zheng J, Feng X. World J Gastroenterol. 2013;19:4897–4906. doi: 10.3748/wjg.v19.i30.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander K, Drost WT, Mattoon JS, Kowalski JJ, Funk JA, Crabtree AC. Can J Vet Res. 2005;69:272–277. [PMC free article] [PubMed] [Google Scholar]
- 21.Siaens R, Rennen H, Boerman O, Dierckx R, Slegers G. J Nucl Med. 2004;45:2088–2094. [PubMed] [Google Scholar]
- 22.Shah S, Khan A, Khan M. J Radioanal Nucl Chem. 2011;288:131–136. [Google Scholar]
- 23.Sierra J, Rodriguez-Puig D, Soriano A, Mensa J, Piera C, Vila J. Antimicrob Agents Chemother. 2008;52:2691–2692. doi: 10.1128/AAC.00217-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lecina J, Cortes P, Llagostera M, Piera C, Suades J. Bioorg Med Chem Lett. 2014;22:3262–3269. doi: 10.1016/j.bmc.2014.04.058. [DOI] [PubMed] [Google Scholar]
- 25.Aldred K, Kerns R, Osheroff N. Biochemistry. 2014;53:1565–1574. doi: 10.1021/bi5000564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Britton KE, Vinjamuri S, Hall AV, Solanki K, Siraj QH, Bomanji J, Das S. Eur J Nucl Med. 1997;24:553–556. doi: 10.1007/BF01267688. [DOI] [PubMed] [Google Scholar]
- 27.Doroudi A, Erfani M, Rahmatian M, Saadati S, Ahmadi F, Kiasat A, Khodayar M, Meghdadi H, Rezaee S. IOSR J Pharmacy. 2014;4:10–15. [Google Scholar]
- 28.Alberto R, Abram U. In: Handbook of Nuclear Chemistry. Vértes A, Nagy S, Klencsár Z, Lovas RG, Rösch F, editors. Springer; US, Boston, MA: 2011. pp. 2073–2120. [DOI] [Google Scholar]
- 29.Saha GB. Fundamentals of Nuclear Pharmacy. 6. Springer-Verlag; New York, London: 2010. [Google Scholar]
- 30.Kelly JD, Forster AM, Higley B. J Nucl Med. 1993;34:222. [PubMed] [Google Scholar]
- 31.Qadir MA, Wattoo FH, Yaseen M, Atta S, Wattoo MHS, Ahmad SA, Gulzar A. Alex J Med. 2015;51:47–52. [Google Scholar]
- 32.Naqvi SAR, Ishfaq MM, Khan ZA, Nagra SA, Bukhari IH, Hussain AI, Mahmood N, Shahzad SA, Haque A, Bokhari TH. Turk J Chem. 2012;36:267–277. [Google Scholar]
- 33.Nayak DK, Baishya R, Halder KK, Sen T, Sarkar BR, Ganguly S, Das MK, Debnath MC. Metallomics. 2012;4:1197–1208. doi: 10.1039/c2mt20132a. [DOI] [PubMed] [Google Scholar]
- 34.Kowalsky RJ. Technetium Radiopharmaceutical Chemistry. The University of New Mexico Health Sciences Center College of Pharmacy; Albuquerque, New Mexico: 2006. [Google Scholar]
- 35.Geskovski N, Kuzmanovska S, Simonoska Crcarevska M, Calis S, Dimchevska S, Petrusevska M, Zdravkovski P, Goracinova K. J Labelled Comp Radiopharm. 2013;56:689–695. doi: 10.1002/jlcr.3097. [DOI] [PubMed] [Google Scholar]
- 36.Solanki KK. Imaging of Infections. 5425935. US patent. 1995
- 37.Siaens RH, Rennen HJ, Boerman OC, Dierckx R, Slegers G. J Nucl Med. 2004;45:2088–2094. [PubMed] [Google Scholar]
- 38.Solanki K, Bomanji J, Siraj Q, Small M, Britton K. J Nucl Med. 1993;34:119. [Google Scholar]
- 39.Malamitsi J, Giamarellou H, Kanellakopoulou K, Dounis E, Grecka V, Christakopoulos J, Koratzanis G, Antoniadou A, Panoutsopoulos G, Batsakis C, Proukakis C. Clin Microbiol Infect. 2003;9:101–109. doi: 10.1046/j.1469-0691.2003.00506.x. [DOI] [PubMed] [Google Scholar]
- 40.Drevenšek P, Zupančič T, Pihlar B, Jerala R, Kolitsch U, Plaper A, Turel I. J Inorg Biochem. 2005;99:432–442. doi: 10.1016/j.jinorgbio.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 41.Jones AG, Davison A. J Nucl Med. 1982;23:1041. [PubMed] [Google Scholar]
- 42.Wu G, Wang G, Fu X, Zhu L. Molecules. 2003;8:287–296. [Google Scholar]
- 43.Uivarosi V. Molecules. 2013;18:11153–11197. doi: 10.3390/molecules180911153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anacona JR, Toledo C. Transit Metal Chem. 2001;26:228–231. [Google Scholar]
- 45.Fuster D, Soriano A, Garcia S, Piera C, Suades J, Rodrıguez D, Martinez J, Mensa J, Campos F, Pons F. Nucl Med Commun. 2011;32:44–51. doi: 10.1097/MNM.0b013e328340e6fb. [DOI] [PubMed] [Google Scholar]
- 46.Lipowska M, He H, Xu X, Taylor A, Marzilli P, Marzilli L. Inorg Chem. 2010;49:3141–3151. doi: 10.1021/ic9017568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang J, Zhang S, Guo H, Wang X. Bioorg Med Chem Lett. 2010;20:3781–3784. doi: 10.1016/j.bmcl.2010.04.057. [DOI] [PubMed] [Google Scholar]
- 48.Shah S, Khan A, Khan M. J Radioanal Nucl Chem. 2011;287:827–832. [Google Scholar]
- 49.Morgat C, Hindie E, Mishra AK, Allard M, Fernandez P. Cancer biotherapy & radiopharmaceuticals. 2013;28:85–97. doi: 10.1089/cbr.2012.1244. [DOI] [PubMed] [Google Scholar]
- 50.Fani M, André JP, Maecke HR. Contrast Media Mol Imaging. 2008;3:53–63. doi: 10.1002/cmmi.232. [DOI] [PubMed] [Google Scholar]
- 51.Mirzadeh S, Lambrecht RM. J Radioanal Nucl Chem. 1996;202:7–102. [Google Scholar]
- 52.Autio A, Jalkanen S, Roivainen A. EJNMMI Research. 2013;3:1–1. doi: 10.1186/2191-219X-3-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Poeppel TD, Binse I, Petersenn S, Lahner H, Schott M, Antoch G, Brandau W, Bockisch A, Boy C. Recent results in cancer research. Fortschritte der Krebsforschung Progres dans les recherches sur le cancer. 2013;194:353–371. doi: 10.1007/978-3-642-27994-2_18. [DOI] [PubMed] [Google Scholar]
- 54.Sollini M, Erba PA, Fraternali A, Casali M, Di Paolo ML, Froio A, Frasoldati A, Versari A. Scientific World J. 2014;2014:194123. doi: 10.1155/2014/194123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang J, Li D, Lang L, Zhu Z, Wang L, Wu P, Niu G, Li F, Chen X. J nucl med. 2016;57:9–14. doi: 10.2967/jnumed.115.165316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liolios C, Schäfer M, Haberkorn U, Eder M, Kopka K. Bioconjugate Chem. 2016;27:737–751. doi: 10.1021/acs.bioconjchem.5b00687. [DOI] [PubMed] [Google Scholar]
- 57.James ML, Gambhir SS. Physiological reviews. 2012;92:897–965. doi: 10.1152/physrev.00049.2010. [DOI] [PubMed] [Google Scholar]
- 58.Satpati D, Arjun C, Krishnamohan R, Samuel G, Banerjee S. Chem Biol Drug Des. 2016;87:680–686. doi: 10.1111/cbdd.12701. [DOI] [PubMed] [Google Scholar]
- 59.Langer O, Brunner M, Zeitlinger M, Ziegler S, Müller U, Dobrozemsky G, Lackner E, Joukhadar C, Mitterhauser M, Wadsak W, Minar E, Dudczak R, Kletter K, Müller M. Eur J Nucl Med Mol Imaging. 2005;32:143–150. doi: 10.1007/s00259-004-1646-2. [DOI] [PubMed] [Google Scholar]
- 60.Stanek J, Mairinger S, Wanek T, Kuntner C, Müller M, Langer O. Appl Radiat Isot. 2015;99:133–137. doi: 10.1016/j.apradiso.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 61.Mustaev A, Malik M, Zhao X, Kurepina N, Luan G, Oppegard L, Hiasa H, Marks K, Kerns R, Berger J, Drlica K. J Biol Chem. 2014;289:12300–12312. doi: 10.1074/jbc.M113.529164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Laponogov I, Pan X, Veselkov D, McAuley K, Fisher L, Sanderson M. PLoS One. 2010;5:e11338. doi: 10.1371/journal.pone.0011338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Blower T, Williamson B, Kerns R, Berger J. Proc Natl Acad Sci USA. 2016;113:1706–1713. doi: 10.1073/pnas.1525047113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Drlica K, Mustaev A, Towle T, Luan G, Kerns R, Berger J. ACS Chem Biol. 2014;9:2895–2904. doi: 10.1021/cb500629k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Malik M, Mustaev A, Schwanz H, Luan G, Shah N, Oppegard L, deSouza E, Hiasa H, Zhao X, Kerns R, Drlica K. Nucleic Acids Res. 2016;44:3304–3316. doi: 10.1093/nar/gkw161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Welling M, Ferro-Flores G, Pirmettis I, Brouwer C. Anti-Infective Agents in Med Chem. 2009;8:272–287. [Google Scholar]
- 67.Benitez A, Roca M, Martin-Comin J. Q J Nucl Med Mol Imaging. 2006;50:147–152. [PubMed] [Google Scholar]
- 68.Palestro C. J Nuclear Med. 2003;44:927–929. [PubMed] [Google Scholar]
- 69.Mirshojaei S. J Radioanal Nucl Chem. 2015;304:975–988. [Google Scholar]