Abstract
The liver possesses an extraordinary ability to regenerate after injury. Hepatocyte-driven liver regeneration is the default pathway in response to mild-to-moderate acute liver damage. When replication of mature hepatocytes is blocked, facultative hepatic progenitor cells (HPCs), also referred to as oval cells (OCs) in rodents, are activated. HPC/OCs have the ability to proliferate clonogenically and differentiate into several lineages including hepatocytes and bile ductal epithelia. This is a conserved liver injury response that has been studied in many species ranging from mammals (rat, mouse, and human) to fish. In addition, improper HPC/OC activation is closely associated with fibrotic responses, characterized by myofibroblast activation and extracellular matrix production, in many chronic liver diseases. Matrix remodeling and metalloprotease activities play an important role in the regulation of HPC/OC proliferation and fibrosis progression. Thus, understanding molecular mechanisms underlying HPC/OC activation has therapeutic implications for rational design of anti-fibrotic therapies.
Keywords: Liver regeneration, Hepatic progenitor cells (HPCs), Oval cells (OCs), Liver injury, Hepatic fibrosis
1. Introduction
The liver is a vital organ within the body. A broad range of hepatic insults such as hepatitis virus, alcohol, fat accumulation, and drug toxicity can damage the liver to result in steatohepatitis, fibrosis, cirrhosis, and even development of hepatocellular carcinoma. Liver diseases have been a major health concern worldwide because of their high prevalence and poor long-term clinical outcome. This is particularly true in China, where approximately 300 million people suffer from liver diseases.1 Liver transplantation is the only curative option for end-stage liver disease, but sources of donors are limited. Thus, there is an urgent need to develop alternative therapeutic strategies. Hepatic stem/progenitor cells (HPCs), also known as oval cells (OCs) in rodents, are one potential suitable source for liver cell replacement. These cells can be activated through orchestrated signaling networks mediated by a plethora of growth factors, cytokines, and enzymes in response to severe liver injury and/or compromised hepatocyte function. Abnormal amplification of HPC/OCs is also associated with pathological scarring processes and may contribute to the development of liver cancer. Crosstalk among damaged epithelial components, pro-fibrotic mesenchymal elements, and pro-inflammatory immune cells, as well as specialized extracellular matrix (ECM) in the HPC niche, is of fundamental importance in sustaining HPC activation and liver fibrosis in disease conditions. This review covers experimental and clinical studies of HPC activation and associated liver fibrosis, with emphasis on mediators of ECM regulation in the HPC niche.
2. HPC/OC characteristics and origins
Liver stem/progenitor cells include unique populations that are able to differentiate into hepatic parenchymal cells, hepatocytes, and/or bile ductular epithelial cells. During development, hepatoblasts appear in the foregut endoderm, where they give rise to both hepatocytes and cholangiocytes.2 Similarly, adult livers contain so-called HPC/OCs, a heterogeneous population of transit-amplifying cells that expand during severe liver damage upon inhibition of hepatocyte proliferation.3 HPC activation in the form of ductular reactions has been observed in many pathophysiological processes of human liver diseases.4 In rodents, HPC/OCs represent small hepatobiliary reactive cells approximately 10 µm in diameter with a large nuclear-to-cytoplasm ratio and oval-shaped nucleus (hence their name).5 HPC/OCs represent dynamic cell populations that constantly change their phenotype depending on the injured cell type and consequent state of differentiation.6 Despite lacking specific markers for HPC/OCs, a broad panel of surface antigens and intracellular proteins has been reported in these cells and their progeny (see Table 1).7–24 Moreover, the bi-potential ability of HPC/OCs is evidenced by detection of markers for both bile ductular epithelial cells and hepatocytes in numerous experimental models and human studies.8,19 HPC/OCs are thought to differentiate into hepatocytes to restore lost liver mass and function in response to hepatocyte damage, such as that occurring with severe viral hepatitis or massive hepatocyte loss in human patients.7,25,26 In contrast, HPC/OCs mediate biliary regeneration in circumstances where bile ductular epithelium is the most damaged cell type, including diseases such as primary biliary cirrhosis or primary sclerosing cholangitis.27 Recent studies using genetic lineage-tracing techniques in transgenic mice have challenged the contribution of HPC/OCs to liver regeneration.28,29 Nevertheless, HPC/OC activation is often associated with myofibroblast cell activation and liver fibrosis during chronic liver disease.30–32 In human studies, HPC/OCs accumulate as injuries become more severe, indicating HPC proliferation increases with progressively worsening liver injury and fibrosis.32 Correlation between degree of HPC activation and the severity of liver disease has been shown in both acute and chronic human liver diseases.31
Table 1.
Summary of markers immune-reactive for HPC/OC.
| Marker name | Sources | Species | Author and references |
|---|---|---|---|
| Cytokeratin (K)7 | Adult biliary marker | Human | Xiao et al.7 |
| Cytokeratin (K)19 | Adult biliary marker | Human | Lee et al.8 |
| Cytokeratin (K)14 | Adult biliary marker | Human | Xiao et al.7 |
| Epithelial cell adhesion molecule (EPCAM) | Fetal hepatoblast and adult biliary marker | Mouse | Tanaka et al.9 |
| Human | Okabe et al.10 | ||
| Rat | Dan et al.11 | ||
| Schmelzer et al.12 | |||
| Yovchev et al.13,14 | |||
| Sry-like HMG box protein 9 (Sox9) | Fetal hepatoblast and adult biliary marker | Mouse | Furuyama et al.15 |
| Cytokeratin (K) 8 | Adult hepatocyte marker | Human | Xiao et al.7 |
| Cytokeratin (K) 18 | Adult hepatocyte marker | Human | Xiao et al.7 |
| c-Met | Adult hepatocyte marker | Human | Xiao et al.7 |
| α-fetoprotein protein | Fetal hepatoblast marker | Mouse | Nierhoff et al.16 |
| Rat | Kuhlmann et al.17 | ||
| Human | Rao et al.18 | ||
| albumin | Adult hepatocyte marker | Mouse | Fausto et al.19 |
| Rat | Kuijk et al.20 | ||
| Human | Xiao et al.7 | ||
| c-Kit | Fetal hepatoblast and adult hematopoietic marker | Mouse | Petersen et al.21 |
| Sca-1 | Fetal hepatoblast and adult hematopoietic marker | Mouse | Nierhoff et al.16 |
| Petersen et al.21 | |||
| Thy-1 (CD90) | Adult hematopoietic marker | Rat | Petersen et al.22 |
| Prominin/CD133 | Adult hematopoietic marker | Mouse | Suzuki et al.23 |
| C-X-C chemokine receptor type 4 (CXCR4) | Adult hematopoietic marker | Mouse | Cardinale et al.24 |
| Neural cell adhesion molecule (NCAM) | Adult neural cell marker | Human | Cardinale et al24 |
The origin of HPC/OCs is also a topic of contention with many different theories. Traditionally, they are considered to be small bile ductular epithelial cells located in the Canal of Hering, a transitional zone between the bile canaliculi and interlobular ductal systems in mammals.33 An extrahepatic origin of rat OCs, such as bone marrow, has also been suggested.34 In addition, it has been proposed that HPC/OCs may be derived from hepatic stellate cells (HSCs), as HSCs contain many of the same signaling pathways and express similar genes as undifferentiated cells in rats.35 Other recent studies have demonstrated that conversion from hepatocyte or bile ductular epithelial cells to HPCs is a reversible process after recovery from injury, suggesting HPC/OCs may originate from dedifferentiation of mature hepatocytes or bile ductular epithelial cells in response to liver damage.36 At least four distinct niches have been demonstrated: canal of Hering, intralobular bile ducts, peri-ductal cells, and peri-biliary hepatocytes.37 The liver is believed to regenerate through a multi-tiered, flexible system rather than a single HPC/OC location in response to severe damage. This multi-tiered, flexible system determines phenotypic expression, proliferative capability, and differentiation properties of HPC/OCs under specific pathologic or pathophysiologic circumstances. Taken together, ‘‘location is everything”, as Petersen and Shupe stated.38 This explains the diversity of HPC responses in different niches under distinct injury models.
3. Experimental models of HPC/OC activation
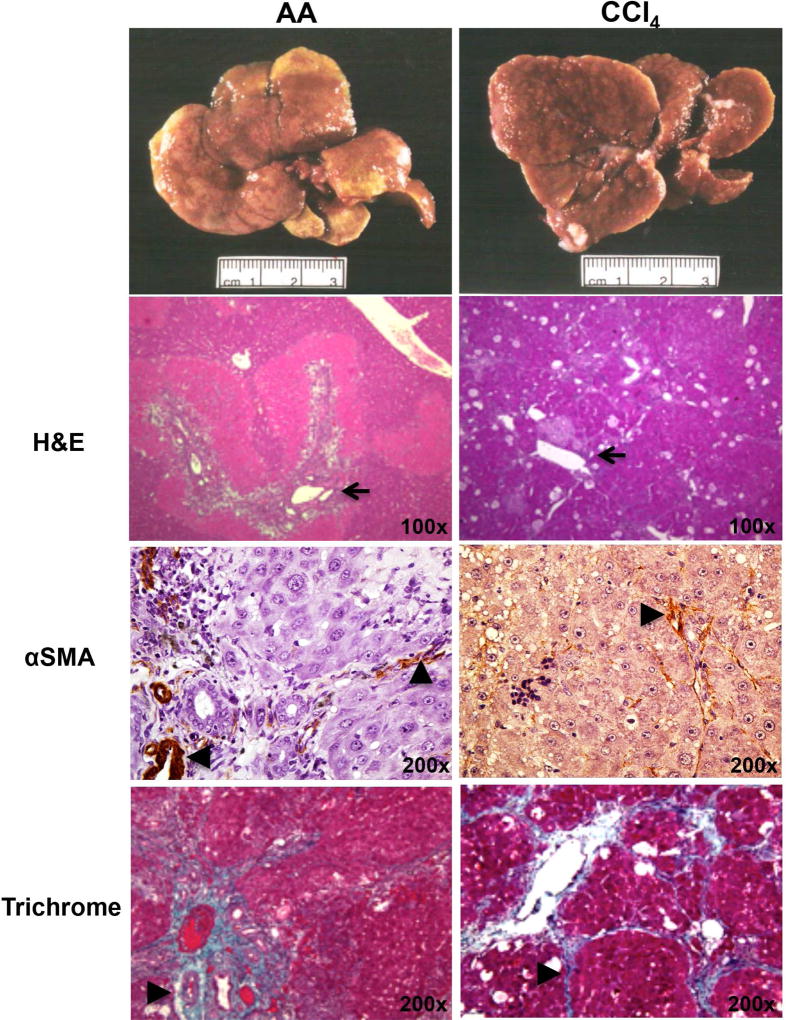
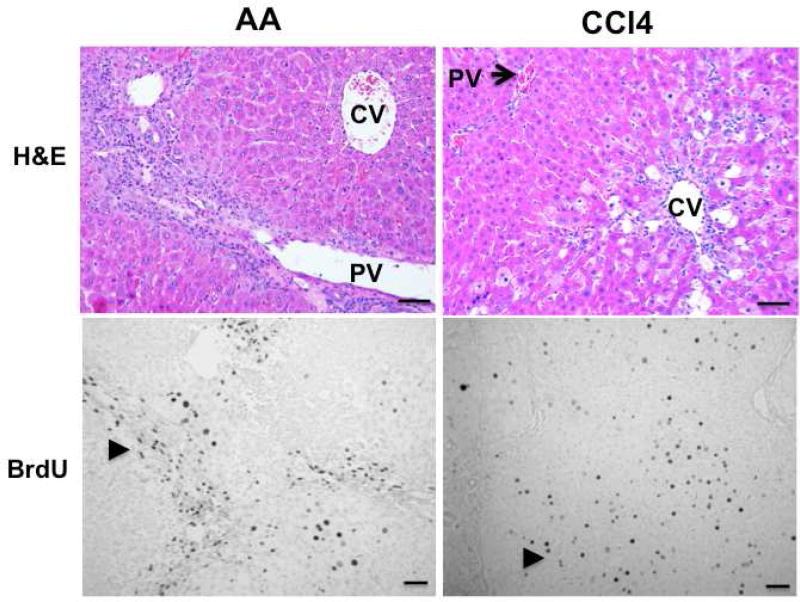
HPC/OCs can be activated in a number of animal models. In rats, surgical removal of two-thirds of the liver lobe by partial hepatectomy (PHx) has been combined with administration of hepatoxins such as 2-acetylaminofluorene (2-AAF) to trigger an HPC/OC response.39 In addition, bio-reactivation of chemicals can cause zonation-dependent regeneration. Allyl alcohol (AA) is metabolized to highly reactive aldehyde acrolein by alcohol dehydrogenase, which is principally localized in the periportal area.40 Cytochrome P450 enzymes can convert CCl4 into highly reactive metabolites that trigger lipid peroxidation, leading to hepatocyte death at centrilobular areas.41 As shown in Fig. 1, activation of α-smooth muscle actin (SMA)+ myofibroblast cells and collagen deposition are common features of both types of liver damage. However, AA induces extensive periportal proliferation, whereas CCl4 intoxication causes hepatocyte proliferation in central lobular parenchyma (Fig. 2). Combining 2-AAF implantation with either type of chronic liver injury triggers a robust HPC/OC response.42
Fig.1.
Comparison of AA- or CCl4- induced liver fibrosis in rats. AA (7.4 mg/kg) and CCl4 (300 mg/kg) were given to rats through IP injection for 90 days to induce chronic injury based on previous reports.40,41 H&E, αSMA and Trichrome staining were carried out. Damaged and fibrotic areas (indicated by arrows or arrowheads) are shown.
Fig. 2. AA or CCl4 administration causes periportal or central lobular proliferation, respectively.
AA (7.4 mg/kg) and CCl4 (300 mg/kg) were administered to rats through IP injection for 45 days. BrdU (100 mg/kg body weight) was injected into animals 2 hours before sacrifice. H&E staining was performed to monitor histological changes in damaged livers. Proliferating cells were labeled with monoclonal mouse BrdU antibody followed by 3, 3’-diaminobenzidine (DAB) detection. Arrowheads indicate proliferating cells and damaged areas. Scale bar: 100 µm. PV: portal vein; CV: central vein.
There is remarkable heterogeneity of HPC/OCs in rat and mouse models. For instance, 2-AAF is unable to trigger an HPC/OC response in mice.43 Instead, mouse HPC/OCs are activated using different dietary or toxin models, including the choline-deficient ethionine-supplemented diet, 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) diet, and phenobarbital/cocaine.44,45 The methionine-choline deficient (MCD) diet is also well known to induce fatty liver with HPC proliferation in mice.46 These toxins can interfere with cellular and molecular mechanisms of liver regeneration through membrane damage, inflammatory reactions, or even activation of non-parenchymal cells such as Kupffer cells.47 Recently, genetic murine models of HPC activation have been developed. For example, a system using β-napthoflavone for inducible deletion of Mdm2, an E3 ubiquitin-protein ligase that degrades TRP53 (p53), was successful in over 98% of hepatocytes using an AhCre system, and resulted in upregulation of p53, induction of p53-mediated hepatocyte death, and senescence of nearly all hepatocytes expressing p21.48 These transgenic mice will be very valuable tools to model HPC activation, hepatocyte injury, and senescence in rats and human patients.
With regard to laboratory use, zebrafish are unique specimens for the study of liver regeneration for many reasons. First, they possess extensive regenerative capabilities. Second, as a result of the high genetic conservation of vertebrates, liver structure and signaling pathways are relatively conserved between mammals and zebrafish.49 Third, each mating event results in hundreds of offspring that are easily genetically manipulated and transparent for observation of embryonic development. Finally, numerous methods for studying zebrafish liver regeneration exist, including 1/3 PHx, ethanol models, and ablation of hepatocytes in transgenic animals with nitroreductase, which converts metronidazole into a toxin that activates a fibrogenic response.49–51
4. HPC/OC niche
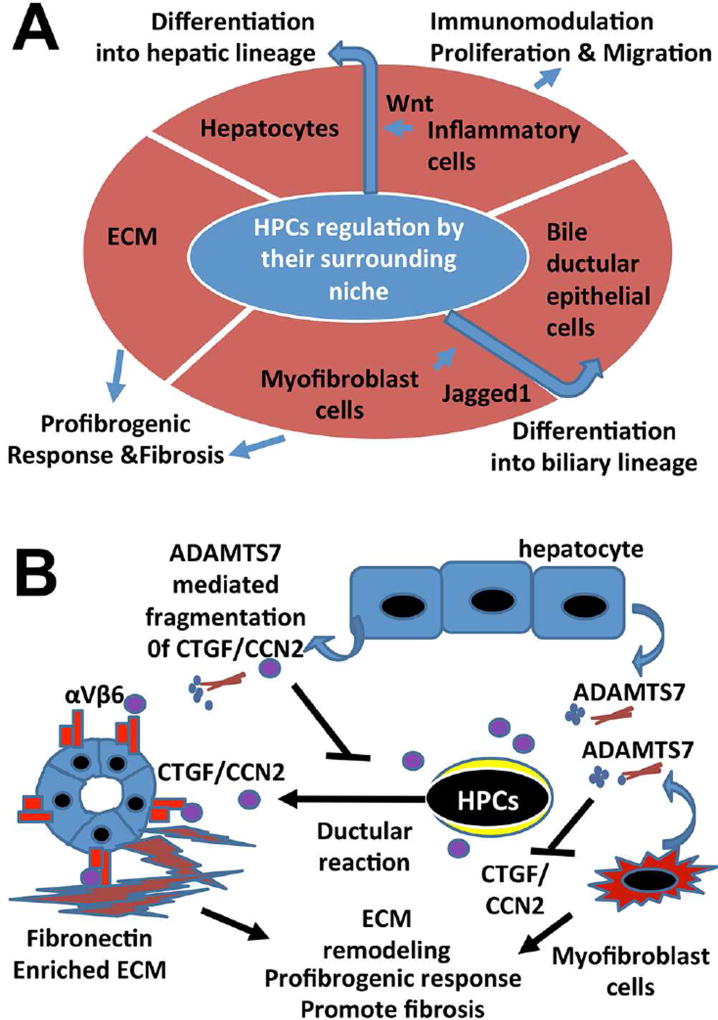
The HPC niche consists of multiple types of stromal cells and surrounding ECM that forms a special microenvironment controlling the characteristics of HPC/OCs and balance of HPC/OC activation, proliferation, and differentiation (Fig. 3A). The significance of the microenvironment in control of cell behavior and fate has been demonstrated in elegant liver transplantation studies. Nonfunctional cells collected from cirrhotic rats can recover full metabolic and proliferative function after engraftment into non-cirrhotic animals.52 Stromal cells, an important component of the HPC/OC compartment, include damaged epithelial cellular elements (bile ductular epithelial cells and hepatocytes), activated HSCs/myofibroblasts, and macrophages, as well as other inflammatory cells recruited after liver injury. Immune cells, such as macrophages and lymphocytes, have been shown to stimulate and initiate regenerative responses in experimental models.53 In response to debris from damaged hepatocytes, macrophages can promote HPC/OCs towards hepatocyte differentiation through Wnt signaling.54 This is in contrast to biliary injury in which HSCs are activated and promote HPC/OC differentiation into biliary duct epithelial cells through Jagged1/Notch pathways.54 In addition, activated HSCs/myofibroblasts form intimate contact with HPC/OCs and produce ECM to support HPC cell adhesion and migration.5 However, production of excessive matrix proteins, such as collagen, by activated HSCs/myofibroblasts causes disorganization of hepatic microarchitecture and interferes with blood/fluid exchange, ultimately contributing to the pathogenesis of liver fibrosis and cirrhosis.
Fig.3. HPC/OC niche and its damage responses.
(A) The HPC/OC niche consists of a specialized pro-fibrogenic extracellular matrix, epithelial elements (hepatocytes, cholangiocytes and their intermediate state), inflammatory cells, and myofibroblasts. Based on the type of injury, HPC/OCs can differentiate into either hepatocytes or bile ductular epithelial cells. Phagocytosis of hepatocyte debris by liver macrophages results in expression of Wnt3a. Wnt3a in close proximity to HPC/OCs activates Wnt pathways, leading to transcriptional activation of Numb (a Notch pathway inhibitor) and progenitor differentiation into hepatocytes.54 In biliary injury, HPC/OC activation in the form of a ductular reaction is often associated with myofibroblast activation and extensive collagen deposition, leading to liver fibrosis. Myofibroblasts express Jagged1 that activates Notch receptors in HPC/OCs leading to differentiation towards a biliary lineage.54 (B) CTGF/CCN2 acts in an autocrine manner to promote HPC/OC activation, ductular reaction and biliary fibrosis by binding to the integrin αvβ6 receptor on HPC/OCs and cholangiocytes in DDC-induced murine liver injury.70 ADAMTS7 protease can be secreted from hepatocytes and myofibroblasts in the HPC/OC niche to regulate HPC/OC activation and biliary fibrosis by CTGF/CCN2 fragmentation.72
5. Signaling pathways involved in HPC activation
Many factors and signals produced by HPC/OCs and stromal cells regulate HPC proliferation, migration, and differentiation. These signaling pathways are outlined in the following sections.
5.1. Pathways for HPC/OC proliferation
Hepatocyte growth factor (HGF)/c-Met signaling is a critical regulator of liver regeneration, as it activates a variety of other signaling pathways involved in proliferation, motility, migration, and invasion.55 Deletion of c-Met receptors in murine studies illustrates their importance in liver regeneration. In response to DDC-induced liver injury, c-Met–deficient mice exhibit a reduced HPC/OC response, impaired HPC/OC migration, and decreased hepatocytic differentiation in vivo.56 Other important regulators are in the Hippo pathway, which controls the activity of the transcriptional co-activator Yes-associated protein (YAP). Phosphorylation of YAP by kinases in the Hippo signaling pathway can cause cytoplasmic retention of YAP, thus impeding its ability to access target gene promoters. YAP transcriptionally regulates connective tissue growth factor (CTGF, also termed CCN2) during cell adhesion and has emerging roles in controlling HSC activation.57 Ectopic activation of YAP in differentiated hepatocytes can result in their dedifferentiation, which drives liver overgrowth and HPC/OC appearance.58,59 Knocking out key components in the Hippo pathway, such as WW45, restrains HPC/OC proliferation, liver size, and the development of liver cancer.60
5.2. Pathways for HPC/OC and macrophage interaction
Tumor necrosis-like factor weak inducer of apoptosis (TWEAK), a member of the pro-inflammatory tumor necrosis factor family, is a regulator of HPC and macrophage interactions. It is strongly induced in monocytes, T lymphocytes and macrophages, and promotes HPC proliferation through its specific receptor, fibroblast growth factor-inducible 14 (Fn14).61 This signal is powerful enough that injection of TWEAK can result in HPC/OC proliferation in unharmed mouse liver. In addition, chemokine stromal cell-derived factor 1 (SDF-1) has been shown to be upregulated in a wide range of human chronic liver diseases.62,63 It binds to its receptor, CXCR4, and plays a variety of roles including recruitment of bone marrow stromal cells (BMSCs) to damaged liver. CXCR4 is expressed by many different cell types, including BMSCs and inflammatory cells. SDF-1 is produced by HPC/OCs and attracts CXCR4+ inflammatory cells that also express TWEAK.63 Finally, activation of the Wnt/beta-catenin signaling pathway plays a significant role in HPC/OC expansion during liver diseases, including hepatitis C and cirrhosis.54 In hepatocytic diseases of humans and rodents, numerous macrophages infiltrate the periportal areas as part of the HPC/OC niche. As shown in Fig 3A, phagocytosis of hepatocyte debris by hepatic macrophages results in expression of Wnt3a. Wnt3a in close proximity to HPCs activates Wnt pathways leading to transcriptional activation of Numb (a Notch pathway inhibitor) and differentiation of progenitors into hepatocytes.54
5.3. Pathways for HPC/OC and myofibroblast interaction
Fibroblast growth factor 7 (FGF7) can regulate HPC/OC and myofibroblast interactions. The signal originating from activated HSCs is received by FGFR2-IIIb receptors located on hepatocytes.64 Fgf7-deficient mice displayed significantly reduced OC proliferation and higher mortality when placed on a DDC diet or subjected to bile duct ligation.65 Forced expression of FGF7 in knockout mice was able to reverse damage by decreasing levels of hepatocyte injury and cholestasis markers weeks after liver injury, showing a potential role for FGF7 in liver treatment.
TGF-β signaling stimulates several processes including cell differentiation, ECM synthesis, cell growth, cell death, and apoptosis. In liver regeneration, TGF-β induces expression of ECM proteins, such as CTGF, in most types of fibrosis.66 CTGF belongs to the Cyr61/CTGF/Nov (CCN) protein family and can potentiate the fibrogenic activity of TGF-β through direct interaction.67,68 We have found that CTGF is highly expressed in murine and rat HPC/OCs.69,70 It binds to integrins on the surface of many cell types and is able to promote cell attachment and migration on provisional matrix.71 Deletion of this molecule affects HPC/OC activation and biliary fibrosis during DD- induced liver injury in mice.70 In addition, we have identified a disintegrin and metalloproteinase with thrombospondin type I repeat 7 (ADAMTS7) as a novel CTGF protease produced by myofibroblasts and hepatocytes in the HPC/OC niche.72 Knocking out ADAMTS7 is associated with abnormal an HPC/OC response and biliary fibrosis in the DDC mouse model. As shown in Fig. 3B, this represents a new mechanism for regulation of CTGF activity during HPC/OC activation, bile ductular reaction, and biliary fibrosis. Given that CTGF is over expressed in many types of fibrotic disorders,67 there is a clear clinical relevance of ADAMTS7 activity for targeting overexpressed CTGF in fibrotic disorders. Hedgehog (Hh) pathway activation leads to an increase in HPC/OCs, repair-related inflammation, vascular remodeling, and liver regeneration, while also contributing to fibrosis and carcinogenesis.73 Deletion of an obligate intermediate of the Hh pathway, Smoothened (SMO), in cells expressing the myofibroblast-associated gene αSMA can prevent induction of Hh-related transcription factors and inhibit expression of myofibroblast genes.74 As SMO deletion prevents aggregation of myofibroblasts and liver epithelial progenitors, it is a demonstrated regulator of both fibrotic and regenerative responses.
In biliary diseases, the Notch pathway is implicated in differentiation of HPC/OCs towards biliary epithelial cells through interaction with myofibroblasts.54 As shown in Fig 3A, myofibroblasts express Jagged1, which coordinates with Notch on the surface of HPC/OCs to activate Notch signaling, resulting in a biliary phenotype in response to biliary liver damage typically observed in primary biliary cirrhosis and primary sclerosing cholangitis.54,75 Disruption of Notch signaling in male rats with a γ-secretase inhibitor after 2-AAF treatment followed by 70% PHx demonstrated that the Notch pathway is essential for producing properly functioning hepatocytes.76 Deletion of the RBP-J effector of Notch signaling abolishes this YAP-mediated trans-differentiation between hepatocytes and HPC/OCs.59 These phenomena demonstrate both the considerable degree of plasticity of multiple liver cell types and ambiguous function of HPC/OCs in liver regeneration.
6. Conclusion
HPC/OCs, a heterogeneous set of proliferating epithelial cells, exhibit various intermediate states and are activated when hepatocyte function is compromised during severe liver damage. The origin and contribution of HPC/OCs to damaged liver is controversial and may vary based on extent, type, and location of hepatic injury. Nevertheless, HPC activation is closely associated with fibrotic responses in many experimental animal models and chronic liver diseases. New technologies, including genetic tools such as lineage tracing and knockout mouse models, as well as the development of various approaches to isolate cells of interest, have been developed to characterize molecular targets for HPC/OC activation. Understanding mechanisms underlying HPC/OC activation and liver fibrosis is critical for in vivo and in vitro manipulation of HPC/OC expansion and differentiation in large numbers. These studies also provide a fundamental basis for the development of therapeutic strategies against related chronic liver diseases.
Acknowledgments
We would like to thank Ms. Alicia Brown for immunohistochemical analysis in Figs. 1 and 2. We thank Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author’s contributions
AB and LP designed the concept of the review, prepared table, and wrote the review. JZ, QC, SW and BEP generated figures and provided critical comments on the paper.
Conflict of interest
The authors declare that they have no conflict of interest.
References
- 1.Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of china. Hepatology. 2014;60:2099–2108. doi: 10.1002/hep.27406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shin D, Monga SP. Cellular and molecular basis of liver development. Compr Physiol. 2013;3:799–815. doi: 10.1002/cphy.c120022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shin S, Kaestner KH. The origin, biology, and therapeutic potential of facultative adult hepatic progenitor cells. Curr Top Dev Biol. 2014;107:269–292. doi: 10.1016/B978-0-12-416022-4.00010-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gouw AS, Clouston AD, Theise ND. Ductular reactions in human liver: diversity at the interface. Hepatology. 2011;54:1853–1863. doi: 10.1002/hep.24613. [DOI] [PubMed] [Google Scholar]
- 5.Farber E. Similarities in the sequence of early histological changes induced in the liver of the rat by ethionine, 2-acetylamino-fluorene, and 3'-methyl-4-dimethylaminoazobenzene. Cancer Res. 1956;16:142–148. [PubMed] [Google Scholar]
- 6.Boulter L, Lu WY, Forbes SJ. Differentiation of progenitors in the liver: a matter of local choice. J Clin Invest. 2013;123:1867–1873. doi: 10.1172/JCI66026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao JC, Ruck P, Adam A, Wang T-X, Kaiserling E. Small epithelial cells in human liver cirrhosis exhibit features of hepatic stem-like cells: immunohistochemical, electron microscopic and immunoelectron microscopic findings. Histopathology. 2003;42:141–149. doi: 10.1046/j.1365-2559.2003.01544.x. [DOI] [PubMed] [Google Scholar]
- 8.Lee JS, Heo J, Libbrecht L, et al. A novel prognostic subtype of human hepatocellular carcinoma derived from hepatic progenitor cells. Nat Med. 2006;12:410–416. doi: 10.1038/nm1377. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka M, Okabe M, Suzuki K, et al. Mouse hepatoblasts at distinct developmental stages are characterized by expression of EpCAM and DLK1: drastic change of EpCAM expression during liver development. Mech Dev. 2009;126:665–676. doi: 10.1016/j.mod.2009.06.939. [DOI] [PubMed] [Google Scholar]
- 10.Okabe M, Tsukahara Y, Tanaka M, et al. Potential hepatic stem cells reside in EpCAM+ cells of normal and injured mouse liver. Development. 2009;136:1951–1960. doi: 10.1242/dev.031369. [DOI] [PubMed] [Google Scholar]
- 11.Dan YY, Riehle KJ, Lazaro C, et al. Isolation of multipotent progenitor cells from human fetal liver capable of differentiating into liver and mesenchymal lineages. Proc Natl Acad Sci U S A. 2006;103:9912–9917. doi: 10.1073/pnas.0603824103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmelzer E, Zhang L, Bruce A, et al. Human hepatic stem cells from fetal and postnatal donors. J Exp Med. 2007;204:1973–1987. doi: 10.1084/jem.20061603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yovchev MI, Grozdanov PN, Joseph B, Gupta S, Dabeva MD. Novel hepatic progenitor cell surface markers in the adult rat liver. Hepatology. 2007;45:139–149. doi: 10.1002/hep.21448. [DOI] [PubMed] [Google Scholar]
- 14.Yovchev MI, Grozdanov PN, Zhou H, Racherla H, Guha C, Dabeva MD. Identification of adult hepatic progenitor cells capable of repopulating injured rat liver. Hepatology. 2008;47:636–647. doi: 10.1002/hep.22047. [DOI] [PubMed] [Google Scholar]
- 15.Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43:34–41. doi: 10.1038/ng.722. [DOI] [PubMed] [Google Scholar]
- 16.Nierhoff D, Ogawa A, Oertel M, Chen YQ, Shafritz DA. Purification and characterization of mouse fetal liver epithelial cells with high in vivo repopulation capacity. Hepatology. 2005;42:130–139. doi: 10.1002/hep.20735. [DOI] [PubMed] [Google Scholar]
- 17.Kuhlmann WD, Peschke P. Hepatic progenitor cells, stem cells, and AFP expression in models of liver injury. Int J Exp Pathol. 2006;87:343–359. doi: 10.1111/j.1365-2613.2006.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao MS, Khan AA, Parveen N, Habeeb MA, Habibullah CM, Pande G. Characterization of hepatic progenitors from human fetal liver during second trimester. World J Gastroenterol. 2008;14:5730–5737. doi: 10.3748/wjg.14.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fausto N. Liver regeneration and repair: hepatocytes, progenitor cells, and stem cells. Hepatology. 2004;39:1477–1487. doi: 10.1002/hep.20214. [DOI] [PubMed] [Google Scholar]
- 20.Kuijk EW, Rasmussen S, Blokzijl F, et al. Generation and characterization of rat liver stem cell lines and their engraftment in a rat model of liver failure. Sci Rep. 2016;6:22154. doi: 10.1038/srep22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petersen BE, Grossbard B, Hatch H, Pi L, Deng J, Scott EW. Mouse A6-positive hepatic oval cells also express several hematopoietic stem cell markers. Hepatology. 2003;37:632–640. doi: 10.1053/jhep.2003.50104. [DOI] [PubMed] [Google Scholar]
- 22.Petersen BE, Goff JP, Greenberger JS, Michalopoulos GK. Hepatic oval cells express the hematopoietic stem cell marker Thy-1 in the rat. Hepatology. 1998;27:433–445. doi: 10.1002/hep.510270218. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki A, Sekiya S, Onishi M, et al. Flow cytometric isolation and clonal identification of self-renewing bipotent hepatic progenitor cells in adult mouse liver. Hepatology. 2008;48:1964–1978. doi: 10.1002/hep.22558. [DOI] [PubMed] [Google Scholar]
- 24.Cardinale V, Wang Y, Carpino G, et al. Multipotent stem/progenitor cells in human biliary tree give rise to hepatocytes, cholangiocytes, and pancreatic islets. Hepatology. 2011;54:2159–2172. doi: 10.1002/hep.24590. [DOI] [PubMed] [Google Scholar]
- 25.Santoni-Rugiu E, Jelnes P, Thorgeirsson SS, Bisgaard HC. Progenitor cells in liver regeneration: molecular responses controlling their activation and expansion. APMIS. 2005;113:876–902. doi: 10.1111/j.1600-0463.2005.apm_386.x. [DOI] [PubMed] [Google Scholar]
- 26.Falkowski O, An HJ, Ianus IA, et al. Regeneration of hepatocyte 'buds' in cirrhosis from intrabiliary stem cells. J Hepatol. 2003;39:357–364. doi: 10.1016/s0168-8278(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigo-Torres D, Affò S, Coll M, et al. The biliary epithelium gives rise to liver progenitor cells. Hepatology. 2014;60:1367–1377. doi: 10.1002/hep.27078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schaub JR, Malato Y, Gormond C, Willenbring H. Evidence against a stem cell origin of new hepatocytes in a common mouse model of chronic liver injury. Cell Rep. 2014;8:933–939. doi: 10.1016/j.celrep.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tanimizu N, Nishikawa Y, Ichinohe N, Akiyama H, Mitaka T. Sry HMG box protein 9-positive (Sox9+) epithelial cell adhesion molecule-negative (EpCAM−) biphenotypic cells derived from hepatocytes are involved in mouse liver regeneration. J Biol Chem. 2014;289:7589–7598. doi: 10.1074/jbc.M113.517243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Williams MJ, Clouston AD, Forbes SJ. Links between hepatic fibrosis, ductular reaction, and progenitor cell expansion. Gastroenterology. 2014;146:349–356. doi: 10.1053/j.gastro.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 31.Lowes KN, Brennan BA, Yeoh GC, Olynyk JK. Oval cell numbers in human chronic liver diseases are directly related to disease severity. Am J Pathol. 1999;154:537–541. doi: 10.1016/S0002-9440(10)65299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roskams T, De Vos R, Van Eyken P, Myazaki H, Van Damme B, Desmet V. Hepatic OV-6 expression in human liver disease and rat experiments: evidence for hepatic progenitor cells in man. J Hepatol. 1998;29:455–463. doi: 10.1016/s0168-8278(98)80065-2. [DOI] [PubMed] [Google Scholar]
- 33.Roskams TA, Theise ND, Balabaud C, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. doi: 10.1002/hep.20130. [DOI] [PubMed] [Google Scholar]
- 34.Petersen BE, Bowen WC, Patrene KD, et al. Bone marrow as a potential source of hepatic oval cells. Science. 1999;284:1168–1170. doi: 10.1126/science.284.5417.1168. [DOI] [PubMed] [Google Scholar]
- 35.Kordes C, Sawitza I, Götze S, Herebian D, Häussinger D. Hepatic stellate cells contribute to progenitor cells and liver regeneration. J Clin Invest. 2014;124:5503–5515. doi: 10.1172/JCI74119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tarlow BD, Pelz C, Naugler WE, et al. Bipotential adult liver progenitors are derived from chronically injured mature hepatocytes. Cell Stem Cell. 2014;15:605–618. doi: 10.1016/j.stem.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuwahara R, Kofman AV, Landis CS, Swenson ES, Barendswaard E, Theise ND. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology. 2008;47:1994–2002. doi: 10.1002/hep.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petersen B, Shupe T. Location is everything: the liver stem cell niche. Hepatology. 2008;47:1810–1812. doi: 10.1002/hep.22333. [DOI] [PubMed] [Google Scholar]
- 39.Dabeva MD, Alpini G, Hurston E, Shafritz DA. Models for hepatic progenitor cell activation. Proc Soc Exp Biol Med. 1993;204:242–252. doi: 10.3181/00379727-204-43660. [DOI] [PubMed] [Google Scholar]
- 40.Lee JH, Ilic Z, Sell S. Cell kinetics of repair after allyl alcohol-induced liver necrosis in mice. Int J Exp Pathol. 1996;77:63–72. doi: 10.1046/j.1365-2613.1996.00964.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang PY, Kaneko T, Tsukada H, Nakano M, Nakajima T, Sato A. Time courses of hepatic injuries induced by chloroform and by carbon tetrachloride: comparison of biochemical and histopathological changes. Arch Toxicol. 1997;71:638–645. doi: 10.1007/s002040050438. [DOI] [PubMed] [Google Scholar]
- 42.Petersen BE, Zajac VF, Michalopoulos GK. Hepatic oval cell activation in response to injury following chemically induced periportal or pericentral damage in rats. Hepatology. 1998;27:1030–1038. doi: 10.1002/hep.510270419. [DOI] [PubMed] [Google Scholar]
- 43.Jelnes P, Santoni-Rugiu E, Rasmussen M, et al. Remarkable heterogeneity displayed by oval cells in rat and mouse models of stem cell-mediated liver regeneration. Hepatology. 2007;45:1462–1470. doi: 10.1002/hep.21569. [DOI] [PubMed] [Google Scholar]
- 44.Akhurst B, Croager EJ, Farley-Roche CA, et al. A modified choline-deficient, ethionine-supplemented diet protocol effectively induces oval cells in mouse liver. Hepatology. 2001;34:519–522. doi: 10.1053/jhep.2001.26751. [DOI] [PubMed] [Google Scholar]
- 45.Fickert P, Stöger U, Fuchsbichler A, et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Machado MV, Michelotti GA, Xie G, et al. Mouse models of diet-induced nonalcoholic steatohepatitis reproduce the heterogeneity of the human disease. PLoS One. 2015;10:e0127991. doi: 10.1371/journal.pone.0127991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rosenberg D, Ilic Z, Yin L, Sell S. Proliferation of hepatic lineage cells of normal C57BL and interleukin-6 knockout mice after cocaine-induced periportal injury. Hepatology. 2000;31:948–955. doi: 10.1053/he.2000.5410. [DOI] [PubMed] [Google Scholar]
- 48.Lu WY, Bird TG, Boulter L, et al. Hepatic progenitor cells of biliary origin with liver repopulation capacity. Nat Cell Biol. 2015;17:971–983. doi: 10.1038/ncb3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chu J, Sadler KC. A new school in liver development: lessons from zebrafish. Hepatology. 2009;50:1656–1663. doi: 10.1002/hep.23157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goessling W, North TE, Lord AM, et al. APC mutant zebrafish uncover a changing temporal requirement for wnt signaling in liver development. Dev Biol. 2008;320:161–174. doi: 10.1016/j.ydbio.2008.05.526. [DOI] [PubMed] [Google Scholar]
- 51.Huang M, Chang A, Choi M, Zhou D, Anania FA, Shin CH. Antagonistic interaction between Wnt and Notch activity modulates the regenerative capacity of a zebrafish fibrotic liver model. Hepatology. 2014;60:1753–1766. doi: 10.1002/hep.27285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu L, Yannam GR, Nishikawa T, et al. The microenvironment in hepatocyte regeneration and function in rats with advanced cirrhosis. Hepatology. 2012;55:1529–1539. doi: 10.1002/hep.24815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viebahn CS, Benseler V, Holz LE, et al. Invading macrophages play a major role in the liver progenitor cell response to chronic liver injury. J Hepatol. 2010;53:500–507. doi: 10.1016/j.jhep.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 54.Boulter L, Govaere O, Bird TG, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Organ SL, Tsao MS. An overview of the c-MET signaling pathway. Ther Adv Med Oncol. 2011;3:S7–S19. doi: 10.1177/1758834011422556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishikawa T, Factor VM, Marquardt JU, et al. Hepatocyte growth factor/c-met signaling is required for stem-cell-mediated liver regeneration in mice. Hepatology. 2012;55:1215–1226. doi: 10.1002/hep.24796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhao B, Ye X, Yu J, et al. TEAD mediates YAP-dependent gene induction and growth control. Genes Dev. 2008;22:1962–1971. doi: 10.1101/gad.1664408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mannaerts I, Leite SB, Verhulst S, et al. The Hippo pathway effector YAP controls mouse hepatic stellate cell activation. J Hepatol. 2015;63:679–688. doi: 10.1016/j.jhep.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 59.Yimlamai D, Christodoulou C, Galli GG, et al. Hippo pathway activity influences liver cell fate. Cell. 2014;157:1324–1338. doi: 10.1016/j.cell.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee KP, Lee JH, Kim TS, et al. The Hippo-Salvador pathway restrains hepatic oval cell proliferation, liver size, and liver tumorigenesis. Proc Natl Acad Sci U S A. 2010;107:8248–8253. doi: 10.1073/pnas.0912203107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tirnitz-Parker JE, Viebahn CS, Jakubowski A, et al. Tumor necrosis factor-like weak inducer of apoptosis is a mitogen for liver progenitor cells. Hepatology. 2010;52:291–302. doi: 10.1002/hep.23663. [DOI] [PubMed] [Google Scholar]
- 62.Hatch HM, Zheng D, Jorgensen ML, Petersen BE. SDF-1alpha/CXCR4: a mechanism for hepatic oval cell activation and bone marrow stem cell recruitment to the injured liver of rats. Cloning Stem Cells. 2002;4:339–351. doi: 10.1089/153623002321025014. [DOI] [PubMed] [Google Scholar]
- 63.Hao NB, Li CZ, Lü MH, et al. SDF-1/CXCR4 axis promotes MSCs to repair liver injury partially through trans-differentiation and fusion with hepatocytes. Stem Cells Int. 2015;2015:960387. doi: 10.1155/2015/960387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Steiling H, Mühlbauer M, Bataille F, Schölmerich J, Werner S, Hellerbrand C. Activated hepatic stellate cells express keratinocyte growth factor in chronic liver disease. Am J Pathol. 2004;165:1233–1241. doi: 10.1016/S0002-9440(10)63383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Takase HM, Itoh T, Ino S, et al. FGF7 is a functional niche signal required for stimulation of adult liver progenitor cells that support liver regeneration. Genes Dev. 2013;27:169–181. doi: 10.1101/gad.204776.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ihn H. Pathogenesis of fibrosis: role of TGF-beta and CTGF. Curr Opin Rheumatol. 2002;14:681–685. doi: 10.1097/00002281-200211000-00009. [DOI] [PubMed] [Google Scholar]
- 67.Brigstock DR. Connective tissue growth factor (CCN2, CTGF) and organ fibrosis: lessons from transgenic animals. J Cell Commun Signal. 2009;4:1–4. doi: 10.1007/s12079-009-0071-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abreu JG, Ketpura NI, Reversade B, De Robertis EM. Connective-tissue growth factor (CTGF) modulates cell signalling by BMP and TGF-beta. Nat Cell Biol. 2002;4:599–604. doi: 10.1038/ncb826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pi L, Oh SH, Shupe T, Petersen BE. Role of connective tissue growth factor in oval cell response during liver regeneration after 2-AAF/PHx in rats. Gastroenterology. 2005;128:2077–2088. doi: 10.1053/j.gastro.2005.03.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pi L, Robinson PM, Jorgensen M, et al. Connective tissue growth factor and integrin αvβ6: a new pair of regulators critical for ductular reaction and biliary fibrosis in mice. Hepatology. 2015;61:678–691. doi: 10.1002/hep.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pi L, Ding X, Jorgensen M, et al. Connective tissue growth factor with a novel fibronectin binding site promotes cell adhesion and migration during rat oval cell activation. Hepatology. 2008;47:996–1004. doi: 10.1002/hep.22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pi L, Jorgensen M, Oh SH, et al. A disintegrin and metalloprotease with thrombospondin type I motif 7: a new protease for connective tissue growth factor in hepatic progenitor/oval cell niche. Am J Pathol. 2015;185:1552–1563. doi: 10.1016/j.ajpath.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Choi SS, Omenetti A, Syn WK, Diehl AM. The role of hedgehog signaling in fibrogenic liver repair. Int J Biochem Cell Biol. 2010;43:238–244. doi: 10.1016/j.biocel.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Michelotti GA, Xie G, Swiderska M, et al. Smoothened is a master regulator of adult liver repair. J Clin Invest. 2013;123:2380–2394. doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Geisler F, Strazzabosco M. Emerging roles of Notch signaling in liver disease. Hepatology. 2015;61:382–392. doi: 10.1002/hep.27268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Darwiche H, Oh SH, Steiger-Luther NC, et al. Inhibition of Notch signaling affects hepatic oval cell response in rat model of 2AAF-PH. Hepat Med. 2011;3:89–98. doi: 10.2147/HMER.S12368. [DOI] [PMC free article] [PubMed] [Google Scholar]