Abstract
Purpose
The literature on hypoglossal nerve stimulation (HNS) for the treatment of moderate-to-severe obstructive sleep apnea (OSA) was reviewed from 2014, the time of FDA approval for the Inspire Systems device, to 2017 for themes that might be useful conceptually and practically in the consideration of this new non-anatomic surgical therapy.
Recent Findings
there are now further follow-up articles since the 12-month results for Apnea Reduction (STAR) trial of the Inspire device, and post-approval publications which report similar and/0r improved AHI outcomes. Other emerging themes include drug-induced sedation endoscopy (DISE) as a tool in assessment of eligibility and a more detailed understanding of mechanisms for an HNS effects.
Summary
The post-STAR literature provides guidelines for an integrated coordination of medicine and surgery to appropriately screen and manage patients.
Keywords: Hypoglossal nerve stimulation, Obstructive sleep apnea, Clinical management OSA, Neurotheraputics
Introduction
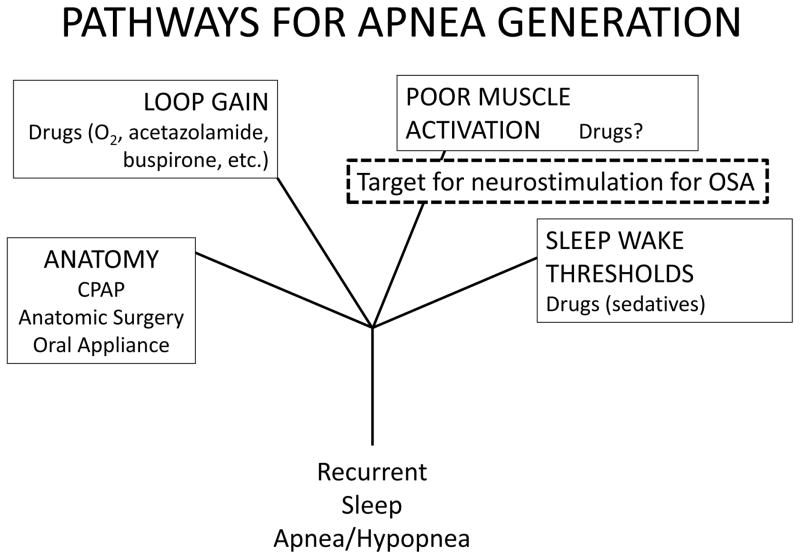
Sleep apnea syndrome is complex both biologically and physiologically in that no one pathway explains all of it manifestations. There is now four general proximate pathways and targets for therapy by which one might develop recurrent apneas and hypopneas, the signature features of sleep disordered breathing (Figure 1).
Figure 1.
There are four general pathways that contribute to the development of recurrent obstructive apneas during sleep 12; under the heading for each pathway there are listed current of potential (e.g. “drugs?”) treatments that might act in a management of the syndrome related to multiple obstructive apneas. These pathways are not mutually exclusive, and treatment of one may uncover the impact of others. For instance, CPAP therapy may not address a high loop gain or an intrinsic problem with arousal thresholds, and this can complicate treatment responses. HNS addresses the pathway with poor muscle activation, mitigating the fall in muscle activity that occurs at the onset of an apnea.
These pathways alone and in combination affect the appearance and number of events 1, 2.
Foundational therapies for moderate-to-severe obstructive sleep apnea (OSA) are continuous positive airway pressure (CPAP), oral airway appliances, upper airway anatomic surgery, and bariatric surgery 3, all which work primarily on the anatomic problems of a small and compliant upper airway. Of these, CPAP is considered a primary, first-line approach. In those who use this therapy there are dose-dependent improvement in symptoms of disturbed sleep and quality of life, and a lower blood pressure; mortality and stroke risk are reduced 4. However, a third of patients are unable to use or tolerate CPAP therapy 5. Fitted oral appliances worn at night keep the oro- and naso-pharynx open, and are viable, alternative approaches, also improving quality of life and blood pressure 6. Bariatric surgery and significant weight loss, when achieved by medical treatments, are efficacious in three-quarters of those who are morbidly obese, and produces its effect by increasing upper airway stability 7, 8.
Surgical approaches to the anatomy of the adult upper airway are described in a literature largely made of case series and, while effective in some, are not as predictably efficacious nor durable as one would like 9. Uvulopalatopharyngoplasty (UPPP), a common procedure for adult OSA, is safe and effective. For instance, a recent trial of UPPP plus tonsillectomy vs. watchful waiting at a single center showed that early surgery will reduce severe AHI by an average of 30–50%, yet the residual AHI may still be in the mild-to-moderate range (>5<20/hr) 10. There are across centers a number of ways a UPPP can be performed, determined in large part by a surgeon’s formative training and augmented by experience and progression of technique over time. Anatomic surgery has been refocused in the post-HNS era, and one result of HNS availability is an increasing recognition of the value of anatomic assessments in those who cannot or will not tolerate CPAP. This has led to procedures in those who may not meet the drug-induced sedation endoscopy (DISE) criteria for an implant because they exhibit a concentric collapse of the velopharynx during DISE. Procedures are being developed to address this anatomic trait 11. Additional discussion of DISE is presented later.
The approach of stimulation of the nerves and muscles as therapy for OSA dates to the 1980’s, and this history is available 12. This therapy directly addresses the inadequate muscle activation (Figure 1), and leaves anatomy, loop gain, and sleep arousal systems largely intact. Additionally, it works in both genders, in a broad age range, and in those with obesity and stable cardiovascular co-morbidities, all of which are the traditional demographic risk factors. The current approaches for this therapy comprises three devices. The Inspire Medical Systems (Maple Grove MN) distributes the only FDA-approved HNS device for OSA. It has a 3-electrode cuff placed on medial branch of CNXII. The Apnex Medical Inc. (St. Paul MN) device had a similar electrode design and placement and showed promise in Phase II trials 13 but failed FDA Phase III trials in terms of meeting efficacy standards; the company is no longer in existence. A third device (ImThera Medical, San Diego CA) is currently in a Phase III clinical trial; this device places a 6-electrode cuff on the trunk of CNXII 14, 15. All use an implantable, pacemaker-like pulse-generator in the upper chest with tunneled stimulation lead, implanted through a submental surgical approach. The Apnex device used a measure of impedance and the Inspire device uses a pressure sensor placed in the 4th or 5th intercostal space to detect intrathoracic pressure as a trigger for the stimulation period; the ImThera does not use a trigger and relies on its proprietary stimulation patterning 12.
The Phase II study 16 and Phase III STAR trial 17 for the Inspire device identified the proximal medial branch (vs. trunk) of the hypoglossal nerve (CN XII) as the better placement site for tongue stabilization and/or protrusion. When this implantation was considered for severe OSA and in patients who were morbidly obese, there was a greater likelihood of failure 16. As a result, there is in a BMI upper limit (32 kg/m2, up to 35 kg/m2 in a black box warning) and AHI limit (65/hr) in the FDA labelling, as such candidates are less likely to respond. Also employed in the Phase II trial was drug induced sedation endoscopy or DISE. DISE is a procedure where the upper airway, especially the velo- and oro-pharynx, are examined while in an anesthetized state for the pattern of collapse 18. European centers already were familiar with this procedure as a pre-surgical planning tool. In the Phase II trial, HNS treatment was successful in eight of 10 patients with an anterior-posterior (AP) pattern of velopharyngeal collapse on DISE, while HNS was successful in less than half of those with concentric collapse 16. Complete concentric collapse at the level of the velopharynx during DISE is an exclusion criterion at the present time.
Beyond the 12-months of the STAR Trial
The Inspire-sponsored FDA Phase III Stimulation Therapy for Apnea Reduction (the STAR trial) has to date the most information on outcomes with this therapy: safety and efficacy 19–21. The STAR trial was a prospective cohort study which enrolled 126 CPAP-intolerant OSA patients, all of whom had activation of the therapy about 1 month after implantation. Important features are the exclusion criteria. In the STAR trial exclusion criteria included body mass index (BMI) > 32 kg/m2; AHI <20 or >50, or central and/or mixed apnea index present >20% within the AHI value; and a complete concentric collapse at the level of the velopharynx observed with DISE.
A BMI of <32 kg/m2 (FDA black box warning for those <35>32 kg/m2) and an upper limit of an AHI of 65/hr appear in the FDA labeling. Both are based on success criteria empirically described in the Phase II for the Phase III trials. They are empiric criteria; however, these traits reflect a lower critical closing pressure (Pcrit) and/or respiratory control gain that might otherwise affect AHI values (Figure 1). It should be noted that more than half of patients with BMI >32 kg/m2demonstrate AP collapse pattern on DISE 18, 22, 23, so that this criteria of a BMI is being reconsidered 24. Concerning age, while the FDA approval lists this therapy as for those >18 years, there is no upper age limit. The Inspire Phase II and Phase III studies in all the devices excluded those in which there was active cardiopulmonary disease and chronic cardiopulmonary, metabolic or renal disease of such severity where one might expect only a marginal benefit of treating the AHI and/or OSA symptoms. A retrospective analysis of STAR trial responders reported a trend that non-responders might be younger and less likely to have had prior upper airway surgery for OSA 20.
The STAR cohort were adults, aged ~55 years, with mean BMI of 28.4 kg/m2, and baseline AHI of 32/hr. About 80-% were male and 88% Caucasian. The primary endpoints were AHI and a 4% oxygen-desaturation index (ODI). The secondary, patient-based endpoints were sleepiness and sleep-related quality of life. At 12 months, 66% of participants were responders by AHI criteria with a median <10/hr, and 75% were responders by ODI criteria. No control group was included in the study; however, at 12 months, 46 patients who had responded well were randomly assigned to either continue therapy or to a one-week withdrawal. Withdrawal of therapy for a week resulted an increase in AHI and symptoms towards pre-treatment levels 21. For those who like the more formal approach of a randomized “sham” arm similar to drug-trials, in these device-oriented treatment studies, the design is either a delayed turning on of therapy or this approach of therapy withdrawal.
At 36-months, 98 of 126 patients completed follow up and agreed to a voluntary PSG 20. The 36-month PSG group did not differ in baseline characteristics to the original cohort; however, in aggregate this group included a smaller percentage of 12-month non-responders than the 12-month group. Mean AHI decreased 62%, from a baseline ~30/hr to ~11/hr. Seventy-four percent achieved response as defined by the AHI Sher criteria 9.
The STAR patient-oriented endpoints were the Epworth Sleepiness Scale (ESS) and Functional Outcomes of Sleep Questionnaire (FOSQ) scores. Descriptions of snoring as reported by bed partner were collected. At 36 months, 77% had an ESS <11 and 63% had a FOSQ score >17; both indicating improvement to a population “normal” range. Soft or no snoring, as reported by a bed partner, was 17% at baseline rose to 81% at 36 months.
At a 48-month interim visit which did not include a PSG, 91 of the 126 agreed to a visit designed to check symptoms, use, and amplitude levels 25. ESS and FOSQ were improved from baseline without change from the 12-, 24- or 36-month follow-up. Similarly, the effects on snoring were similar to prior data points after 12-months. Functional amplitudes were unchanged, as was the threshold for sensation. Throughout this time, HNS therapy compliance based on self-report was substantially higher (~80% nightly use) than pre-therapy CPAP (~20%). An indirect measure of energy used over this period was consistent with this estimate. Newer models will have use time provided in an adherence format, similar to that expected for CPAP.
Reported adverse events attributed to the device are minimal and not life threatening. Two devices were explanted at the patient’s request, one due to discomfort and the other due to septic arthritis; the device was not infected. Between 12 and 48 months two patients required procedure to address sensing lead displacement. The only complication specific to the device was an initial temporary tongue weakness reported in 17% of participants, most which resolved spontaneously.
Neuromuscular Insights from HNS therapy
In sleep, patients may exhibit collapse at level of the velum, oropharynx, tongue base, and epiglottis, alone or in combination; if one site was targeted for anatomic surgery, post-procedure another site may appear to contribute to the production of obstructive events during sleep 26–28.
HNS initially was projected to have efficacy in patients only with obstruction at the tongue base, and in retrospect it would have been astounding if HNS had no effect on oropharyngeal patency. The pattern of obstruction at the level of the velum during DISE became a critical feature, and to date cannot be predicted on the basis of other tests 23. There is one consensus statement on DISE procedures 18 and experience is increasing. DISE is subject to the vagaries of anesthetic level, but at any given level there can be generally good interrater reliability in deciding whether or not there is a predominant AP or concentric collapse 29. Higher BMI and higher AHI have been identified as parameters associated with a non-AP of the velum on DISE, but the correlation is modest at best 30. The factors that produce this observed effect deserve attention.
Awake endoscopy or with DISE, HNS will open the retropalatal as well as oropharyngeal level of the upper airway 31. In a subgroup studied at or near the midpoint of the STAR trial, the results were divided between “responders” and “non-responders” at 12-months. Both groups opened the oro-pharynx to the same degree but those who subsequently had a good AHI response at 12-months to HNS therapy had a larger increase in retropalatal area compared to non-responders. Extending these observations was a study revealing increased opening at the retropalatal level and an obvious activation of the geniohyoid muscle showed a better reduction in AHI. The authors believed that a bilateral protrusion of the tongue base was responsible for opening at the level of the soft palate 32. Both groups suggested that the endoscopic effect could be by palatoglossal coupling, due to a linkage of the muscles within the soft palate to those of the tongue body.
Others have published imaging studies to identify the effect of HNS on structures outside the airway. One case series compared the actions of HNS to pressure forcing. HNS moved the hyoid arch forward along with the tongue associated with velo- and oro-pharyngeal opening 33. Opening the airway with positive pressure does not produce a change in hyoid position, indicating that it is not just airway size that changes the position of the hyoid arch. These studies speculated that there was an indirect, mechanical coupling to the velo-pharyngeal airway wall. This is consistent with the anatomic displacements reported for an oral appliance 34 and for maxillomandibular advancement 35, as both result in retropalatal and retroglossal airway volume increases, and while not measured, inspection of the images suggests an anterior positioning of the hyoid arch and tongue base.
One post-approval study has confirmed an improvement in sleep architecture during the diagnostic polysomnography. In 26 patient with reductions from 34/hr to 9/hr at 2 months, there appeared a reduction of N1-sleepand arousals, and an increase in REM sleep, to levels above age-matched norms, indicating that HNS when successful can improve sleep stage expression shortly after it is turned on 36.
The VOTE Classification
The VOTE classification is a method for characterizing DISE findings that focuses on 3-dimensional features specific to the velopharynx (V), oropharynx (O), Tongue (T), and epiglottis (E), sites identified as relevant to sleep disordered breathing 37, 38. There was an existing paradigm- nose, oropharynx, hypopharynx, and larynx (NOHL) reporting, but in comparison it appears that the VOTE classification is more “comprehensive” especially for functional changes at the level of the epiglottis and pharynx. In this report the VOTE classification correlated with OSA severity and identified a number of affected sites; this study anesthetic level was described with bispectral assessments (BIS) 39. In the use of VOTE in a DISE setting, the interobserver agreement between an experienced observer and a learner does have a learning curve, but the problem area is more often at the tongue base, rather than the velopharynx or the epiglottis 40. The DISE can be used for planning other treatments that might be offered to CPAP-intolerant patients 41. The VOTE system then provides a standardized qualitative framework to describe DISE results, in a setting without proprioceptive feedback; in contrast the Müller maneuver is focused on the oropharynx 42.
In regard to which anesthesia is optimal to identify reproducible and actionable results, one group reported that propofol by continuous infusion, at a level of “medium” sedation led to the perceived better decisions regarding surgical treatment 43. Others, however, have compared propofol and, after recovery, with midazolam, finding that using a continuous perfusion, there is a good agreement; in this report, outpatient physical exam did not correlate with drug-induced sleep findings 44. However, others believe that more work is needed to standardize the anesthesia side of DISE 45.
Studies examining the influence of head rotation, on “severity” and patterning of anterior-posterior (AP) and concentric collapse (CC), suggested that at the level of the velum there is a qualitatively less “severe” AP collapse with head rotation 46.
DISE with CPAP could be useful in understanding why the patient failed CPAP treatment, for instance because of a floppy epiglottis. This approach could identify a pattern or place of airway collapse that may require varying pressures different from the one the patient is using, as well as anatomical factors that may be corrected to help with compliance 47,
However, one should stay tuned to this line of work. While the field may go beyond NOHL and VOTE to more quantitative methods for describing results, the DISE will be justified in the planning for other surgical procedures, such as mandibular-maxillary advancement or robotic approaches to the base of the tongue 48, or intervention with oral appliances49, especially when there is CPAP failure 50.
Clinical Management in the Post-Approval Era
Four larger post-STAR 12-month series have been published, two based on a single center and two from a European post-approval registry at 6-month and 12-month follow-up post-implant. These centers used the FDA DISE and AHI criteria for inclusion, but were less stringent in regard to BMI criteria. A summary of these studies is shown in Table 1, beginning with the 36-month outcomes of the STAR trial.
Table 1.
STAR follow-up and Post-Approval Studies
| Study | Cohort | # | Overall Response | Summary of Outcomes |
|---|---|---|---|---|
| Woodson et al, 2016 | STAR 36-months | 98/126 | 74% | AHI from 30.4/h to 11.4/h, ESS 77%<11 |
| Kent et al, 2016 | UPMC | 20 | 95% (AHI < 15) | AHI from 33/h to 5/h, ESS from 10 to 6, 7 hrs/night use |
| Heiser et al, 2016 | Munich | 31 | 97% (50% reduce) | AHI from 33/h to 7/h, ESS from 13 to 6, 6.6 hrs/night use |
| Heiser et al, 2017* | German Multi-center | 60 | 70% (AHI < 15) | AHI from 28.6/h to 8.3/h, Lowest desaturation from 77% to 90%, 6.2 hrs/night use |
This is the 6-month timepoint and there is a 12-month follow-up study (Steffen et al 2017) with similar outcomes.
None report results inferior to the STAR 12-month data. One from a US center 51, in 20 patients meeting the FDA criteria, mean AHI was reduced, with 95% achieving an AHI <15/h. Another was a single European tertiary referral center which reported results from 31 consecutive patients and outcomes at 2, 3, 6, and 12 months after HNS surgery 52, 53. The mean pre-implantation AHI of ~33/h could be reduced and was associated with parallel improvements in ODI, ESS and FOSQ. Patients maintained adherence to therapy use after 12 months; most reported improvement in sleep and daytime symptoms. The HNS usage time was ~43 +/− 12 hr/wk at 6-months. The median AHI was reduced at 6 months to approximately 8/hr, and no patient required surgical revision of the implanted system 54. A follow-up results at 12 months was similar 53.
Case reports of commercial implants start to emphasize the utility of this device in unique patients. One involved a case of a patient with persistent symptoms and findings of OSA, including an AHI >30/hr, despite a history of multiple multilevel procedures, including an uvulopalatopharyngoplasty (UPPP) with revision, a genioglossus advancement, and a maxillomandibular advancement; he responded to HNS with an AHI of <10/hr and sleepiness relief 55. Another case report found a successful co-use of HNS with an implantable defibrillator; however, if this is to be considered, there needs to be agreement from each device manufacturer that this approach will work 56. A third showed that the technology could be employed after radiotherapy for head and neck cancer distorted the anatomy 57. The technology was useful in the management of OSA in a patient with Down’s syndrome, where CPAP therapy adherence is very, very low 58. Finally there is a report of normalization of AHI which illustrated that HNS could be used in Stanford model of progression in those who did not respond to Phase I and II procedures used in this center 59.
It now appears that the upper limit of BMI has a more limited role in predicting outcomes. A favorable AP collapse on DISE can still present in those with BMI values >35 kg/m2. BMI has some correlation to higher critical closing pressure 60, but centers are now more focused on the results of DISE to rule out a complete concentric collapse. For implant success related to postoperative tongue motions, nerve integrity monitoring is used to predict correct cuff placement in one of the groups with the higher success rates 24. However, anecdotally in the STAR follow-up patients who gain weight, experience snoring and/or unrefreshing sleep instead of trying a change in the stimulator settings patients tend to choose weight loss and weight management.
Management Experiences
Post-FDA approval, new centers offering HNS have emerged. Success is based on leadership and cooperation among surgeons and sleep medicine specialists. For this therapy in the post-approval era, the process steps in considering a patient to the follow-up of the 10% of those who eventually qualify are becoming clearer (Table 2).
Table 2.
Arc of Therapy with HNS
| Steps in the Process | Issue | Discussion Points | Outcome |
|---|---|---|---|
| Evaluation of the patient for eligibility | Determination of “cannot or will not use CPAP” | Is this a true failure? Are there other options that are acceptable and have not been tried? | Either reestablish care management or refer to revaluation |
| PSG for eligibility | AHI in all positions and sleep states, and proportion of central/mixed to obstructive events | AHI <20/h or >65/h is the issue and whether there is a central (loop gain component). Is there an opportunity to manage medically? | Here it is eligible or ineligible because of the metrics of the PSG. |
| Surgical evaluation | Outpatient evaluation and discussion about the procedure, and decision about whether the patient generally has rational/realistic view of surgical management. | If HNS is not a therapy, is there an interest in going forward with anatomic surgery? Discussion of DISE and its rationale and cost benefit besides that of the office examination? | Decision for DISE and the results. |
| Implant | Assessment of co- morbidity and feasibility as an outpatient or inpatient procedure. | The “phenotype” of movement with placement of the HNS electrode is a crucial skill. | Surgical success and recovery |
| 1-month assessment | Assuming surgical recovery, determination of the stimulation of first sensation, functional amplitude, and upper limit of tolerance | Creating confidence that the stimulation is comfortable and that only at the extreme there is discomfort. | Setting the limits for patient exploring a range of amplitudes for the next month. |
| 2-month in-center titration PSG | Do the settings work in all positions and in NREM and REM sleep? | This PSG study may or may not provide the final word, as results have to be correlated with symptoms (snoring levels and restorative function of sleep) | What settings are best going forward? What range of amplitude is to be provided to the patient? What are the expectations? |
| Follow-up management | Have settings for the first sensation, functional amplitude, and upper a limit of amplitude changing with healing? Sleepiness (ESS) and quality of life (FOSQ) results. Any issues with implant. | Is this working? What issues are present with the device setting?… use? …result? As well as healing and pain with the incisions and implant. | Decisions on changing the amplitudes and on whether there is a need for further evaluation, either “guess and check” or a follow-up study (portable study or in- lab titration). |
| Follow-up 3- and 6- and then every 12-months | Check settings and adjust according to symptoms of snoring? Check weight and health maintenance. | Any concerns and problems. | If problems, try to address as outpatient before PSG reiteration? |
In some places this may be the same person; however, in many academic centers the interactions have historically been limited to referral and not co-decision making in a broader context. Surgeons benefit from hands-on training in simulator placement on cadavers, and physicians will need to review the purpose and manner of setting up pacing parameters of amplitude and coordination with breathing efforts. Besides physicians, the daytime health care associates should be able to handle inquiries and care issues once implanted. Referral populations are important to cultivate to assure a rapid accrual to develop expertise. In those being referred for HNS less than half will proceed through the inclusion and exclusion criteria. In the sleep diagnostic center, a sleep technologist will need to be trained in titration during a PSG. Hospital administrators and practice managers will need instruction on billing, reimbursement, and the ancillary care needs across patients, like a programming tablet for follow-up and polysomnography titrations. A large time gap between implants may result in require re-training and re-adjustments in the program if these key personnel are not engaged. Finally, financial considerations for the patient include the costs of assessment and DISE, and if a PSG has not been done in several years, a repeat all night sleep study to determine AHI in regard to NREM and REM sleep, proportion of central or mixed events (ideally <25%), and positional effects. Prior authorization, procurement logistics, DISE and operating room readiness, and scheduling are issues that are not encountered in the course of management with CPAP or oral appliance.
In the STAR trial the follow-up included multiple post-operative evaluations and retitrations during a PSG and in frequent office visits 17. As clinical experience has developed over time, there is still a need in the assessment phase for a detailed all-night study with PSG to capture all elements of respiratory disturbance expression (sleep stage, arousals, hypoxemia, ECG morphology, etc.) and ancillary even rare issues that are illuminated, such as PLMs, spike-and-wave discharges, non-atonic REM, delayed sleep phase, etc. There is probably a need a PSG sometime after implant (1–2 months) to examine the effect of stimulation on event type in all positions and in all stages of sleep, as well as to show improvements in oxygen saturation and heart rate by sleep state. However, management at follow-up can be managed by home sleep testing, portable studies of cardiopulmonary function over time without sleep, as shown in post-approval studies 19, 53.
The major costs associated with HNS are the cost of the device and the cost of the procedure. In one report, the estimated lifetime incremental cost effectiveness ratio (ICER) of $39,471 per quality-adjusted life year (QALY) for patients meeting the STAR inclusion criteria 61. This cost is less than the currently accepted cost-effectiveness threshold in the United States of $40–50K/QALY, but more than CPAP, which has an ICER of $15,915/QALY. For a patient perspective there may occur out-of-pocket costs for deductibles and co-pays for assessments prior to implantation. For instance, the DISE procedure may or may not result in the identification of an ideal candidate; however, the cost is still there in those are then considered for other therapy. Follow-up visits after the stimulator implant is coded not only for the visit but for programming, and adds cost, especially when deductibles are high. The center needs to plan for the inevitable discussions about all costs (assessment, implant, and follow-up) with this therapy.
Summary and Conclusion
While there was the Apnex technology and there is an on-going ImThera Phase III trial, currently it is the Inspire Medical Systems HNS device which is currently FDA-approved as neurostimulation therapy for OSA. In selected patients the device appears safe and durable with reasonable effectiveness compared to the absence of effective therapy before the implant. For HNS to most predictably reduce AHI in moderate-to-severe OSA patients, and to produce symptom relief, there is a set of inclusion and exclusion criteria, and many patients will not be candidates. For those with complete concentric collapse, procedures like lateral pharyngeal wall stabilization 11, 62 can improve AHI and/or change the closure to a favorable anterior-posterior collapse pattern 63. Post-approval studies show that several centers can achieve good results 64, indicating that the management approach can be transportable across centers with expertise 53, 54.
CPAP, the current first-line approach for OSA management, is non-invasive and can be effective; motivational sessions, equipment checks, and coaching are crucial in its adoption. Within the limitations of the cost per quality-adjusted life year (QALY) value (for estimates of cost-effectiveness), CPAP is more effective than no treatment and seems to become a cost-effective strategy after 2 years of use 65, 66. Likewise oral appliances are safe, and effective but no cost-effectiveness estimates are available beyond that of the device itself 66. The most expensive surgical option is mandibular-maxillary advancement but this like tracheostomy is a down-the-line therapy; while it is considered a cure, there is no rigorous objective assessments of cost-effectiveness 67. What HNS has done is to create another option in OSA management for the CPAP intolerant patient, identify individual factors in determining surgical success, focus definitions of upper airway function, and reenergize surgical interest in OSA. Limitations to address include the need for pre-implant assessments like DISE and MRI compatibility.
The post-STAR literature provides evidence for success with the commercial application of HNS for the treatment of CPAP-intolerant patients. There is now a literature from groups who report good results within a clinical management program especially when there is a coordination of medicine and surgery expertise to appropriately screen and manage patients.
Footnotes
Compliance with Ethics Guidelines
Conflict of Interest
Madeleine M. Strohl, Motoo Yamauchi, and Zhe Peng declare no conflicts of interest.
Kingman P. Strohl reports a grant from Inspire Medical Systems for STAR trial site PI and post-approval study PI.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
*Important reference
**Very important reference
- 1.Eckert DJ, White DP, Jordan AS, Malhotra A, Wellman A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. American journal of respiratory and critical care medicine. 2013;188:996–1004. doi: 10.1164/rccm.201303-0448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owens RL, Edwards BA, Eckert DJ, et al. An Integrative Model of Physiological Traits Can be Used to Predict Obstructive Sleep Apnea and Response to Non Positive Airway Pressure Therapy. Sleep. 2015;38:961–70. doi: 10.5665/sleep.4750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–47. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mannarino MR, Di Filippo F, Pirro M. Obstructive sleep apnea syndrome. European journal of internal medicine. 2012;23:586–93. doi: 10.1016/j.ejim.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep medicine reviews. 2011;15:343–56. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sutherland K, Vanderveken OM, Tsuda H, et al. Oral appliance treatment for obstructive sleep apnea: an update. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2014;10:215–27. doi: 10.5664/jcsm.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ashrafian H, Toma T, Rowland SP, et al. Bariatric Surgery or Non-Surgical Weight Loss for Obstructive Sleep Apnoea? A Systematic Review and Comparison of Meta-analyses. Obesity surgery. 2015;25:1239–50. doi: 10.1007/s11695-014-1533-2. [DOI] [PubMed] [Google Scholar]
- 8.Sarkhosh K, Switzer NJ, El-Hadi M, Birch DW, Shi X, Karmali S. The impact of bariatric surgery on obstructive sleep apnea: a systematic review. Obesity surgery. 2013;23:414–23. doi: 10.1007/s11695-012-0862-2. [DOI] [PubMed] [Google Scholar]
- 9.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–77. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 10.Sommer UJ, Heiser C, Gahleitner C, et al. Tonsillectomy with Uvulopalatopharyngoplasty in Obstructive Sleep Apnea. Deutsches Arzteblatt international. 2016;113:1–8. doi: 10.3238/arztebl.2016.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elbassiouny AM. Soft palatal webbing flap palatopharyngoplasty for both soft palatal and oropharyngeal lateral wall collapse in the treatment of snoring and obstructive sleep apnea: a new innovative technique without tonsillectomy. Sleep & breathing = Schlaf & Atmung. 2015;19:481–7. doi: 10.1007/s11325-014-1067-9. [DOI] [PubMed] [Google Scholar]
- 12*.Strohl KP, Baskin J, Lance C, et al. Origins of and implementation concepts for upper airway stimulation therapy for obstructive sleep apnea. Respiratory investigation. 2016;54:241–9. doi: 10.1016/j.resinv.2016.01.006. This review provides details on the history of stimulation and on the differences between devices assessed for commercial use. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kezirian EJ, Goding GS, Jr, Malhotra A, et al. Hypoglossal nerve stimulation improves obstructive sleep apnea: 12-month outcomes. Journal of sleep research. 2014;23:77–83. doi: 10.1111/jsr.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zaidi FN, Meadows P, Jacobowitz O, Davidson TM. Tongue anatomy and physiology, the scientific basis for a novel targeted neurostimulation system designed for the treatment of obstructive sleep apnea. Neuromodulation: journal of the International Neuromodulation Society. 2013;16:376–86. doi: 10.1111/j.1525-1403.2012.00514.x. discussion 86. [DOI] [PubMed] [Google Scholar]
- 15.Friedman M, Jacobowitz O, Hwang MS, et al. Targeted hypoglossal nerve stimulation for the treatment of obstructive sleep apnea: Six-month results. The Laryngoscope. 2016;126:2618–23. doi: 10.1002/lary.25909. [DOI] [PubMed] [Google Scholar]
- 16.Van de Heyning PH, Badr MS, Baskin JZ, et al. Implanted upper airway stimulation device for obstructive sleep apnea. The Laryngoscope. 2012;122:1626–33. doi: 10.1002/lary.23301. [DOI] [PubMed] [Google Scholar]
- 17.Strollo PJ, Jr, Soose RJ, Maurer JT, et al. Upper-airway stimulation for obstructive sleep apnea. The New England journal of medicine. 2014;370:139–49. doi: 10.1056/NEJMoa1308659. [DOI] [PubMed] [Google Scholar]
- 18.De Vito A, Carrasco Llatas M, Vanni A, et al. European position paper on drug-induced sedation endoscopy (DISE) Sleep & breathing = Schlaf & Atmung. 2014 doi: 10.1007/s11325-014-0989-6. [DOI] [PubMed] [Google Scholar]
- 19.Soose RJ, Woodson BT, Gillespie MB, et al. Upper Airway Stimulation for Obstructive Sleep Apnea: Self-Reported Outcomes at 24 Months. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2016;12:43–8. doi: 10.5664/jcsm.5390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20**.Woodson BT, Soose RJ, Gillespie MB, et al. Three-Year Outcomes of Cranial Nerve Stimulation for Obstructive Sleep Apnea: The STAR Trial. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2016;154:181–8. doi: 10.1177/0194599815616618. This is the most recent follow-up from the STAR trial. It includes information on the variability over time in the effectiveness of the device, as well as safety issues. [DOI] [PubMed] [Google Scholar]
- 21.Woodson BT, Gillespie MB, Soose RJ, et al. Randomized Controlled Withdrawal Study of Upper Airway Stimulation on OSA: Short- and Long-term Effect. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2014;151:880–7. doi: 10.1177/0194599814544445. [DOI] [PubMed] [Google Scholar]
- 22.Aktas O, Erdur O, Cirik AA, Kayhan FT. The role of drug-induced sleep endoscopy in surgical planning for obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2014 doi: 10.1007/s00405-014-3162-8. [DOI] [PubMed] [Google Scholar]
- 23.Vroegop AV, Vanderveken OM, Boudewyns AN, et al. Drug-induced sleep endoscopy in sleep-disordered breathing: Report on 1,249 cases. The Laryngoscope. 2014;124:797–802. doi: 10.1002/lary.24479. [DOI] [PubMed] [Google Scholar]
- 24.Heiser C, Hofauer B. Predictive Success Factors in Selective Upper Airway Stimulation. ORL; journal for oto-rhino-laryngology and its related specialties. 2017;79:121–8. doi: 10.1159/000455728. [DOI] [PubMed] [Google Scholar]
- 25.Gillespie MB, Soose RJ, Woodson BT, et al. Upper Airway Stimulation for Obstructive Sleep Apnea: Patient-Reported Outcomes after 48 Months of Follow-up. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2017 doi: 10.1177/0194599817691491. 194599817691491. [DOI] [PubMed] [Google Scholar]
- 26.Hudgel DW. Variable site of airway narrowing among obstructive sleep apnea patients. Journal of applied physiology (Bethesda, Md: 1985) 1986;61:1403–9. doi: 10.1152/jappl.1986.61.4.1403. [DOI] [PubMed] [Google Scholar]
- 27.Hudgel DW, Harasick T, Katz RL, Witt WJ, Abelson TI. Uvulopalatopharyngoplasty in obstructive apnea. Value of preoperative localization of site of upper airway narrowing during sleep. The American review of respiratory disease. 1991;143:942–6. doi: 10.1164/ajrccm/143.5_Pt_1.942. [DOI] [PubMed] [Google Scholar]
- 28.Hudgel DW, Hendricks C. Palate and hypopharynx--sites of inspiratory narrowing of the upper airway during sleep. The American review of respiratory disease. 1988;138:1542–7. doi: 10.1164/ajrccm/138.6.1542. [DOI] [PubMed] [Google Scholar]
- 29.Kezirian EJ, White DP, Malhotra A, Ma W, McCulloch CE, Goldberg AN. Interrater reliability of drug-induced sleep endoscopy. Archives of otolaryngology--head & neck surgery. 2010;136:393–7. doi: 10.1001/archoto.2010.26. [DOI] [PubMed] [Google Scholar]
- 30.Steffen A, Frenzel H, Wollenberg B, Konig IR. Patient selection for upper airway stimulation: is concentric collapse in sleep endoscopy predictable? Sleep & breathing = Schlaf & Atmung. 2015;19:1373–6. doi: 10.1007/s11325-015-1277-9. [DOI] [PubMed] [Google Scholar]
- 31.Safiruddin F, Vanderveken OM, de Vries N, et al. Effect of upper-airway stimulation for obstructive sleep apnoea on airway dimensions. The European respiratory journal. 2014 doi: 10.1183/09031936.00059414. [DOI] [PubMed] [Google Scholar]
- 32.Heiser C, Edenharter G, Bas M, Wirth M, Hofauer B. Palatoglossus coupling in selective upper airway stimulation. The Laryngoscope. 2017 doi: 10.1002/lary.26487. [DOI] [PubMed] [Google Scholar]
- 33.ElShebiny T, Venkat D, Strohl K, Hans MG, Alonso A, Palomo JM. Hyoid Arch Displacement with Hypoglossal Nerve Stimulation. American journal of respiratory and critical care medicine. 2017 doi: 10.1164/rccm.201612-2521LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ryan CF, Love LL, Peat D, Fleetham JA, Lowe AA. Mandibular advancement oral appliance therapy for obstructive sleep apnoea: effect on awake calibre of the velopharynx. Thorax. 1999;54:972–7. doi: 10.1136/thx.54.11.972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaghi S, Holty JE, Certal V, et al. Maxillomandibular Advancement for Treatment of Obstructive Sleep Apnea: A Meta-analysis. JAMA otolaryngology-- head & neck surgery. 2016;142:58–66. doi: 10.1001/jamaoto.2015.2678. [DOI] [PubMed] [Google Scholar]
- 36.Hofauer B, Philip P, Wirth M, Knopf A, Heiser C. Effects of upper-airway stimulation on sleep architecture in patients with obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2017 doi: 10.1007/s11325-017-1519-0. [DOI] [PubMed] [Google Scholar]
- 37.Kezirian EJ, Hohenhorst W, de Vries N. Drug-induced sleep endoscopy: the VOTE classification. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2011;268:1233–6. doi: 10.1007/s00405-011-1633-8. [DOI] [PubMed] [Google Scholar]
- 38.Hudgel DW, Harasick T. Fluctuation in timing of upper airway and chest wall inspiratory muscle activity in obstructive sleep apnea. Journal of applied physiology (Bethesda, Md: 1985) 1990;69:443–50. doi: 10.1152/jappl.1990.69.2.443. [DOI] [PubMed] [Google Scholar]
- 39*.da Cunha Viana A, Jr, Mendes DL, de Andrade Lemes LN, Thuler LC, Neves DD, de Araujo-Melo MH. Drug-induced sleep endoscopy in the obstructive sleep apnea: comparison between NOHL and VOTE classifications. Eur Arch Otorhinolaryngol. 2017;274:627–35. doi: 10.1007/s00405-016-4081-7. This is a recent article that describes fairly well the rationale for the use of DISE in the evaluation of patients not only for HNS but for other therapy. [DOI] [PubMed] [Google Scholar]
- 40.Carrasco-Llatas M, Zerpa-Zerpa V, Dalmau-Galofre J. Reliability of drug-induced sedation endoscopy: interobserver agreement. Sleep & breathing = Schlaf & Atmung. 2017;21:173–9. doi: 10.1007/s11325-016-1426-9. [DOI] [PubMed] [Google Scholar]
- 41.Charakorn N, Kezirian EJ. Drug-Induced Sleep Endoscopy. Otolaryngologic clinics of North America. 2016;49:1359–72. doi: 10.1016/j.otc.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 42.Yegin Y, Celik M, Kaya KH, Koc AK, Kayhan FT. Comparison of drug-induced sleep endoscopy and Muller’s maneuver in diagnosing obstructive sleep apnea using a VOTE classification system. Brazilian journal of otorhinolaryngology. 2016 doi: 10.1016/j.bjorl.2016.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heiser C, Fthenakis P, Hapfelmeier A, et al. Drug-induced sleep endoscopy with target-controlled infusion using propofol and monitored depth of sedation to determine treatment strategies in obstructive sleep apnea. Sleep & breathing = Schlaf & Atmung. 2017 doi: 10.1007/s11325-017-1491-8. [DOI] [PubMed] [Google Scholar]
- 44.Carrasco Llatas M, Agostini Porras G, Cuesta Gonzalez MT, et al. Drug-induced sleep endoscopy: a two drug comparison and simultaneous polysomnography. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2014;271:181–7. doi: 10.1007/s00405-013-2548-3. [DOI] [PubMed] [Google Scholar]
- 45.Capasso R, Rosa T, Tsou DY, et al. Variable Findings for Drug-Induced Sleep Endoscopy in Obstructive Sleep Apnea with Propofol versus Dexmedetomidine. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2016;154:765–70. doi: 10.1177/0194599815625972. [DOI] [PubMed] [Google Scholar]
- 46.Safiruddin F, Koutsourelakis I, de Vries N. Upper airway collapse during drug induced sleep endoscopy: head rotation in supine position compared with lateral head and trunk position. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery. 2015;272:485–8. doi: 10.1007/s00405-014-3215-z. [DOI] [PubMed] [Google Scholar]
- 47.Torre C, Liu SY, Kushida CA, Nekhendzy V, Huon LK, Capasso R. Impact of continuous positive airway pressure in patients with obstructive sleep apnea during drug-induced sleep endoscopy. Clinical otolaryngology: official journal of ENT-UK; official journal of Netherlands Society for Oto-Rhino-Laryngology & Cervico-Facial Surgery. 2017 doi: 10.1111/coa.12851. [DOI] [PubMed] [Google Scholar]
- 48.Meraj TS, Muenz DG, Glazer TA, Harvey RS, Spector ME, Hoff PT. Does drug-induced sleep endoscopy predict surgical success in transoral robotic multilevel surgery in obstructive sleep apnea? The Laryngoscope. 2017;127:971–6. doi: 10.1002/lary.26255. [DOI] [PubMed] [Google Scholar]
- 49.Liu SY, Huon LK, Iwasaki T, et al. Efficacy of Maxillomandibular Advancement Examined with Drug-Induced Sleep Endoscopy and Computational Fluid Dynamics Airflow Modeling. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2016;154:189–95. doi: 10.1177/0194599815611603. [DOI] [PubMed] [Google Scholar]
- 50.Kent DT, Rogers R, Soose RJ. Drug-Induced Sedation Endoscopy in the Evaluation of OSA Patients with Incomplete Oral Appliance Therapy Response. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2015;153:302–7. doi: 10.1177/0194599815586978. [DOI] [PubMed] [Google Scholar]
- 51.Kent DT, Lee JJ, Strollo PJ, Jr, Soose RJ. Upper Airway Stimulation for OSA: Early Adherence and Outcome Results of One Center. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2016;155:188–93. doi: 10.1177/0194599816636619. [DOI] [PubMed] [Google Scholar]
- 52.Heiser C, Knopf A, Bas M, Gahleitner C, Hofauer B. Selective upper airway stimulation for obstructive sleep apnea: a single center clinical experience. Eur Arch Otorhinolaryngol. 2017;274:1727–34. doi: 10.1007/s00405-016-4297-6. [DOI] [PubMed] [Google Scholar]
- 53.Steffen A, Sommer JU, Hofauer B, Maurer JT, Hasselbacher K, Heiser C. Outcome after one year of upper airway stimulation for obstructive sleep apnea in a multicenter German post-market study. The Laryngoscope. 2017 doi: 10.1002/lary.26688. [DOI] [PubMed] [Google Scholar]
- 54.Heiser C, Maurer JT, Hofauer B, Sommer JU, Seitz A, Steffen A. Outcomes of Upper Airway Stimulation for Obstructive Sleep Apnea in a Multicenter German Postmarket Study. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2017;156:378–84. doi: 10.1177/0194599816683378. [DOI] [PubMed] [Google Scholar]
- 55.Strohl M, Strohl K, Palomo JM, Ponsky D. Hypoglossal nerve stimulation rescue surgery after multiple multilevel procedures for obstructive sleep apnea. American journal of otolaryngology. 2016;37:51–3. doi: 10.1016/j.amjoto.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 56.Ong AA, O’Brien TX, Nguyen SA, Gillespie MB. Implantation of a defibrillator in a patient with an upper airway stimulation device. The Laryngoscope. 2016;126:E86–9. doi: 10.1002/lary.25683. [DOI] [PubMed] [Google Scholar]
- 57.Zheng Z, Hu S, Chernobilsky B. Hypoglossal Nerve Upper Airway Stimulator Implantation after Radiotherapy for Head and Neck Malignancy. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2017 doi: 10.1177/0194599817703928. 194599817703928. [DOI] [PubMed] [Google Scholar]
- 58.Diercks GR, Keamy D, Kinane TB, et al. Hypoglossal Nerve Stimulator Implantation in an Adolescent With Down Syndrome and Sleep Apnea. Pediatrics. 2016:137. doi: 10.1542/peds.2015-3663. [DOI] [PubMed] [Google Scholar]
- 59.Liu SY, Riley RW. Continuing the Original Stanford Sleep Surgery Protocol From Upper Airway Reconstruction to Upper Airway Stimulation: Our First Successful Case. Journal of oral and maxillofacial surgery: official journal of the American Association of Oral and Maxillofacial Surgeons. 2017 doi: 10.1016/j.joms.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 60.Strohl KP, Butler JP, Malhotra A. Mechanical properties of the upper airway. Comprehensive Physiology. 2012;2:1853–72. doi: 10.1002/cphy.c110053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pietzsch JB, Liu S, Garner AM, Kezirian EJ, Strollo PJ. Long-Term Cost-Effectiveness of Upper Airway Stimulation for the Treatment of Obstructive Sleep Apnea: A Model-Based Projection Based on the STAR Trial. Sleep. 2015;38:735–44. doi: 10.5665/sleep.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Korhan I, Gode S, Midilli R, Basoglu OK. The influence of the lateral pharyngeal wall anatomy on snoring and sleep apnoea. JPMA The Journal of the Pakistan Medical Association. 2015;65:125–30. [PubMed] [Google Scholar]
- 63.Dedhia RC, Strollo PJ, Soose RJ. Upper Airway Stimulation for Obstructive Sleep Apnea: Past, Present, and Future. Sleep. 2015;38:899–906. doi: 10.5665/sleep.4736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Heiser C, Thaler E, Boon M, Soose RJ, Woodson BT. Updates of operative techniques for upper airway stimulation. The Laryngoscope. 2016;126(Suppl 7):S12–6. doi: 10.1002/lary.26158. [DOI] [PubMed] [Google Scholar]
- 65.Guest JF, Helter MT, Morga A, Stradling JR. Cost-effectiveness of using continuous positive airway pressure in the treatment of severe obstructive sleep apnoea/hypopnoea syndrome in the UK. Thorax. 2008;63:860–5. doi: 10.1136/thx.2007.086454. [DOI] [PubMed] [Google Scholar]
- 66.McDaid C, Griffin S, Weatherly H, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: a systematic review and economic analysis. Health technology assessment (Winchester, England) 2009;13:iii–iv. xi–xiv, 1–119, 43–274. doi: 10.3310/hta13040. [DOI] [PubMed] [Google Scholar]
- 67.Camacho M, Teixeira J, Abdullatif J, et al. Maxillomandibular advancement and tracheostomy for morbidly obese obstructive sleep apnea: a systematic review and meta-analysis. Otolaryngology--head and neck surgery: official journal of American Academy of Otolaryngology-Head and Neck Surgery. 2015;152:619–30. doi: 10.1177/0194599814568284. [DOI] [PubMed] [Google Scholar]