Abstract
Malaria is caused by infection with Plasmodium parasites that have a complex life cycle. The parasite protein P47 is critical for disease transmission. P47 mediates mosquito immune evasion in both Plasmodium berghei (Pbs47) and Plasmodium falciparum (Pfs47), and has been shown to be important for optimal female gamete fertility in P. berghei. Pfs47 presents strong geographic structure in natural P. falciparum populations, consistent with natural selection of Pfs47 haplotypes by the mosquito immune system as the parasite adapted to new vector species worldwide. These key functions make Plasmodium P47 an attractive target to disrupt malaria transmission.
Keywords: malaria transmission, Plasmodium fertilization, Plasmodium falciparum, Plasmodium berghei, immune evasion, P47, Pfs47, mosquito immunity, complement-like system, female gamete
Introduction
Malaria is the most important human parasitic disease, with 212 million cases and 429,000 deaths in 2015 [1]. It is caused by Plasmodium parasites with a complex life cycle that alternates between a vertebrate host and a mosquito vector. Plasmodium falciparum and Plasmodium vivax are the most prevalent agents of human malaria and are transmitted by anopheline mosquitoes. While Plasmodium parasites have a rather restricted vertebrate host range, they have adapted to at least 70 different mosquito species [2], many of them evolutionarily distant from vectors in Africa, where human malarias originated [3,4].
Plasmodium undergoes obligatory sexual reproduction and multiple developmental stages in the mosquito [5–7]. Mosquitoes become infected when a female ingests a blood meal containing Plasmodium gametocytes (Fig 1). These develop into gametes in the mosquito midgut lumen and fuse to form a zygote, which subsequently matures into a motile ookinete and invades the mosquito midgut epithelium. If the ookinete succeeds in traversing the midgut epithelial cell, it transforms into an oocyst and replicates, generating thousands of sporozoites that are released into the mosquito hemolymph. Some sporozoites are able to invade the salivary gland and are transmitted to another person when the mosquito acquires a subsequent blood meal. To be transmitted, Plasmodium parasites must overcome many obstacles [8], such as physical barriers and antiplasmodial responses that target ookinetes [9] or oocysts [10,11]. In some incompatible parasite-vector combinations, the mosquito complement-like immune response eliminates most ookinetes [9,12,13].
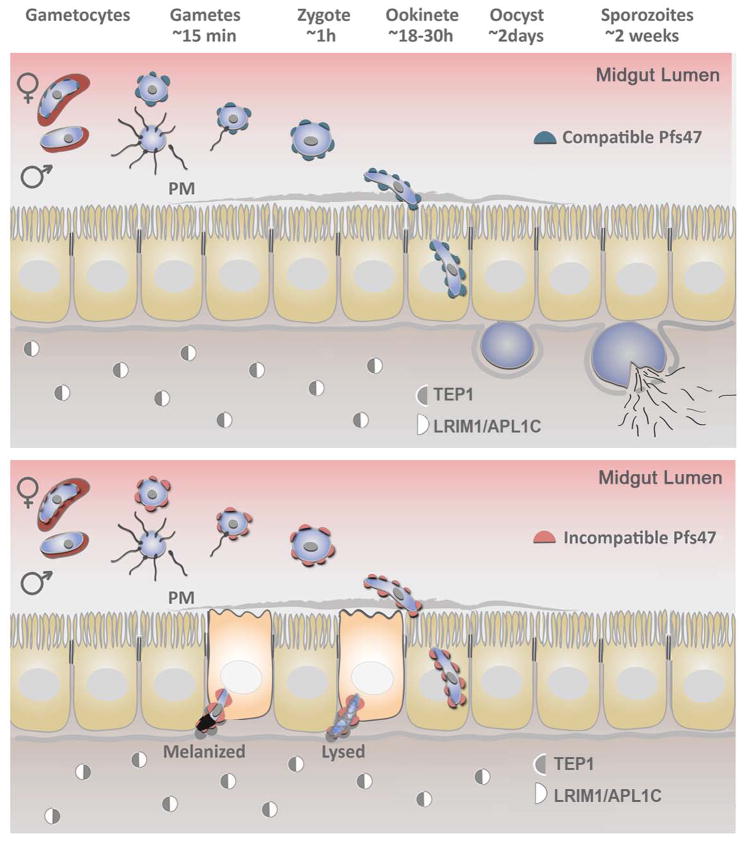
Figure 1. Role of Plasmodium P47 in parasite development in the mosquito.
Plasmodium infection of the mosquito is initiated when the mosquito takes a blood meal containing gametocytes. These gametocytes mature into gametes in the gut lumen and, within minutes, fuse to form a zygote. P47 is expressed of the surface of female gametocytes and gametes, and in P. berghei it is required for optimal fertilization. The zygote develops into a motile ookinete that, one day after blood feeding, traverses mosquito midgut epithelial cells. P47 is also expressed on the surface of ookinetes, and in P. falciparum and P. berghei, it allows parasite evasion of the mosquito immune system. Vector-compatible Pfs47 haplotypes (dark blue) inhibit JNK signaling and the induction of two key enzymes, NADPH-oxidase 5 (NOX5) and heme-peroxidase 2 (HPX2), that potentiate epithelial nitration in the invaded midgut cell. Successful ookinetes reach the basal membrane (BM) and form an oocyst. About two weeks later, oocysts release thousands of sporozoites into the hemolymph. Some sporozoites invade the salivary gland and are injected into a new vertebrate host when the mosquito takes another blood meal. Parasite lines that carry a vector-incompatible Pfs47 haplotype (red) trigger the midgut protein nitration response leading to detection and binding of the thioester-containing protein TEP1 to the parasite surface. TEP1 forms a complex that eliminates the parasite through lysis or melanization. TEP1 is stabilized in the hemolymph by leucine-rich repeat proteins LRIM1 and APL1C. PM, Peritrophic matrix.
Ookinete midgut invasion activates a strong epithelial nitration response [14,15] that triggers local release of hemocyte-derived microvesicles [16] that, in turn, promote mosquito complement-like activation. The Anopheles gambiae thioester containing protein (TEP1), a homolog of complement factor C3 in vertebrates [17], is stabilized in the hemolymph by interacting with the leucine-rich repeat proteins LRIM1 and APL1C [18,19] (Fig. 1). When activated, TEP1 binds to the ookinete surface and triggers the formation of a complex that kills the parasites. Here we review the known biological functions of P47 critical for malaria transmission: Plasmodium fertilization and parasite evasion of the mosquito immune system. The importance of immune evasion for the adaptation of Plasmodium falciparum to different mosquito species during the globalization of malaria will be discussed.
P47 organization
P47 is one of 14 members of the six-cysteine (6-Cys) protein family [20]. The characteristic 6-Cys domain (also called s48/45 domain) was initially identified in Pfs230 [21]. Although the protein sequence homology is low (14–36% amino acid identity), this family is characterized by containing anywhere from 1 to 14 copies of the 6-Cys motif domain, and have clear orthologs in all Plasmodium species that have been analyzed. Members of the 6-Cys family are secreted, or membrane-anchored proteins, and are expressed at different stages of the parasite’s life cycle. Some of them are important for sporozoite liver invasion, while others are involved in fertilization [20] or mosquito immune evasion [22].
The P47 gene was initially identified in P. falciparum (Pfs47) based on sequence homology to other 6-Cys members, such as Pfs230 and Pfs48/45, two of the leading transmission-blocking vaccine targets [23–25]. The Pfs47 (PF3D7_1346800) gene is localized in chromosome 13, adjacent to Pfs48/45 (PF3D7_1346700). Both genes lack introns and have a similar domain organization, consisting of three 6-Cys domains, yet share low sequence homology (26% amino acid identity). Pfs48/45 is expressed on the surface of both male and female gametocytes and gametes, and is required for male fertility [26].
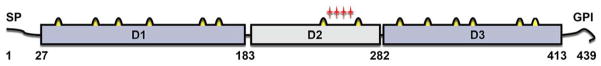
Pfs47 has a signal peptide and a putative GPI anchor sequence (Fig. 2), domains 1 and 3 are characteristic 6-Cys domains, while domain 2 is a degenerate s48/45 domain with only two cysteines (Fig 2) [27]. Although there are clear Pfs47 orthologs in other Plasmodium species, the sequence homology is low (Fig. S1). For example, P. falciparum and P. vivax have 42% amino acid identity. Analysis of P47 sequences from several rodent malaria parasites indicates that P47 is evolving under positive selection [28,29] with exceptionally high ratio of nonsynonymous to synonymous substitutions (dN/dS) in the second domain [28].
Figure 2.
Structural organization of Plasmodium falciparum Pfs47 protein (drawn to scale). Pfs47 has three domains (D1–D3). The D1 and D3 domains have 6 cysteines (in yellow), while the D2 domain only has 2 cysteines. Red stars denote the four amino acid differences between the West African P. falciparum line (GB4) that evades the immune system of A. gambiae L3–5 mosquitoes and the Brazilian strain (7G8) that is eliminated. SP, predicted signal peptide; GPI, glycosylphosphatidylinositol-anchoring signal; numbers indicate amino acid position.
Biological function of P47
Fertilization
P47 is localized on the surface of female gametocytes (Fig. 1) and gametes, as well as of zygotes and ookinetes [22,30,31]. It does not seem to be essential for fertilization in P. falciparum because gametocyte cultures in which the Pfs47 gene was disrupted (Pfs47-KO) efficiently infected A. stephensi Nijmegen mosquitoes [31]. Furthermore, three anti-Pfs47 monoclonal antibodies did not inhibit A. stephensi infection with wild-type P. falciparum parasites [31]. Later studies in P. berghei found that P47 was required for female fertility under in vitro culture conditions, and disruption of the gene also significantly impaired fertilization in vivo [28]. Although Pfs47 is not essential for fertilization when mosquitoes are fed large number of cultured P. falciparum gametocytes, it may play an important role in optimizing fertilization under in vivo conditions, as observed in the P. berghei system, in which much smaller numbers of gametocytes are ingested by female mosquitoes.
Immune evasion
An A. gambiae strain (L3–5), genetically selected to be highly refractory to P. cynomolgi infection, also eliminated most P. falciparum strains from Asia or the Americas but, interestingly, some parasite strains from West Africa survived infection of the mosquito [32]. Later studies showed that the African strains that survive, evade the mosquito complement-like system [13]. A genetic cross between the Brazilian 7G8 P. falciparum strain that is eliminated and the African GB4 strains that survives in A. gambiae L3–5, was used to identify the gene that made the African parasites “invisible” to the mosquito immune system. A combination of genetic mapping, linkage group selection and functional genetics identified Pfs47 as a gene required for P. falciparum to evade immune detection [22]. There are only four amino acid differences between the Pfs47 proteins in the 7G8 and GB4 strains (Fig. 2), all present between the two cysteines in domain 2, that are key determinants of parasite survival in A. gambiae L3–5 [22,33]. In contrast, both parasite lines (GB4 and 7G8) readily infect the A. gambiae G3 strain, indicating that mosquito genetic factors also determine how effective different Pfs47 haplotypes are in promoting evasion of mosquito immunity [22]. It is clear, however, that Pfs47 greatly enhances P. falciparum parasite survival in both A. gambiae G3 and L3–5 strains, because the great majority of P. falciparum (NF54) parasites in which the Pfs47 gene has been disrupted are readily eliminated in both mosquito strains [22]. Pfs47 appears to prevent elimination of the parasite by disrupting c-Jun N-terminal kinase (JNK) signaling [34], a pathway that is essential to trigger epithelial nitration [34,35]. Lack of nitration precludes the release of hemocyte-derived microvesicles [16] and prevents local TEP1 activation and binding on the ookinete surface [22,34]. Interestingly, a Pfs47-KO line readily infects the A. stephensi (Nijmegen strain) mosquitoes [31] [22]. It is possible that because the A. stephensi Nijmegen strain was genetically selected for high infectivity with P. falciparum [36], this colony was fixed for some polymorphism that disrupts antiplasmodial immunity. Alternatively, some vector species may be naturally very permissive to many different P47 haplotypes.
Similar to Pfs47, recent studies show that Pbs47 is also required for P. berghei to evade the complement-like system in A. gambiae [37]. Interestingly, P. berghei is particularly susceptible to melanization by A. gambiae mosquitoes carrying the TEP1-R1 allele [9], but this is not the case for P. falciparum. For example, when the G3 (TEP1 S3/S3) and L3–5 (TEP1 R1/R1) mosquitoes were mixed in a hybrid colony and allowed to mate for many generations, there was a strong association between P. berghei elimination and the TEP R1 allele, with melanization frequencies of 98% in TEP1 R1/R1 homozygous, 73% in R1/S3 hybrids and 10% in S3/S3 females. In contrast, P. falciparum 7G8 parasites, that are very effectively melanized by the L3–5 strain, were not melanized at all by mosquitoes of this hybrid colony [13]; indicating that, besides TEP1 R1, there are other gene(s) in L3–5 mosquitoes that are required to trigger P. falciparum melanization. However, it is possible that TEP1 R1 could enhance lysis of P. falciparum 7G8 parasites. For example, a genetic cross between a A. gambiae M colony fixed for TEP1rB (Mali-NIH) and one for fixed for TEP1s (Yaoundé) showed a similar dominant effect of TEP1 rB on P. berghei melanization and lack of melanization of the ND37 clone obtained from P. falciparum NF54. The number of ND37 oocysts was significantly lower (a reduction of about 40% in the mean number of oocysts) in TEP1s/rB compared to TEP1 s/s females, suggesting that TEP1 rB, or some gene in close proximity, promotes P. falciparum lysis. The effect of TEP1 rB in P. falciparum is less dramatic than when the same mosquitoes are infected with P. berghei [38].
P47 population structure and selection by mosquito vectors
Population structure studies of Pfs47, based on a limited number of laboratory and field isolates, revealed a strong geographic structure [39], similar to what had previously been described for Pfs48/45 [40]. Genotyping of 35 P. falciparum oocysts from field-infected A. gambiae from Tanzania showed high inbreeding coefficients for Pfs47 and Pfs48/45 suggestive of assortative mating; while Pfs47 single nucleotide polymorphism (SNP) analysis revealed a modest, but significant, difference in the Pfs47 haplotypes present in field-infected A. gambiae vs. A. funestus mosquitoes. This suggests that Pfs47 is under natural selection by these two vectors [39]. Furthermore, Pfs47 is one of the P. falciparum genes with the highest SNP differentiation between Africa, Asia and Oceania (based on whole genome population genetic analysis of 227 isolates) [41]. P. vivax P47 (Pvs47) is also polymorphic [42], and is one of the genes with the highest population differentiation between continents (based on whole genome sequences of 195 isolates) [43]. Analysis of 516 Cambodian isolates detected 22 different Pfs47 protein haplotypes closely-related to all other Asian isolates [44], in agreement with clustering of certain haplotypes in different continents.
Consistent with these previous reports, analysis of 364 Pfs47 sequences from P. falciparum isolates collected around the world identified 47 DNA haplotypes that exhibit a high dN/dS, suggestive of natural selection [45]. The 42 Pfs47 protein sequence haplotypes identified, share 97.7–99.8% amino acid identity, with Domain 2 being the most polymorphic region of the protein. Phylogenetic analysis showed that the haplotypes cluster into two main clades. The largest clade includes 32 haplotypes that are more frequent in Africa, one exclusive to Papua New Guinea and three that are the only ones detected in the Americas, consistent with the African origin of P. falciparum [46]. The smaller clade, includes six haplotypes that are frequent in Asia but were not detected in the Americas. In summary, both Pfs47 and Pvs47 haplotypes present a marked geographical population structure at a continental level not observed in most other genes, suggesting that this population structure is the product of a natural selection process that favors certain P47 haplotypes in a given continent [45].
Immune evasion and globalization of P. falciparum malaria
Direct comparison of the compatibility between three major malaria vectors from Africa (A. gambiae), Southeast Asia (A. dirus) and the Americas (A. albimanus), with P. falciparum isolates collected from these continents, supports the hypothesis that Pfs47 has been important for the adaptation of P. falciparum to evolutionarily distant Anopheline vectors. Anopheline mosquitoes had higher compatibility (i.e. higher infection intensity and prevalence) when infected with P. falciparum from the same geographic region, suggesting that P. falciparum underwent natural selection while adapting to different vectors [45]. The mosquito immune system was shown to be a major determinant of parasite-vector compatibility, because disruption of the mosquito complement system greatly enhanced infection in combinations with low compatibility. Furthermore, genetic replacement of the Pfs47 haplotype in an African P. falciparum line with Pfs47 haplotypes from other geographic regions was sufficient to change the compatibility with these anopheline vectors by allowing the parasite to evade the mosquito complement-like system [45]. Taken together, these studies indicate that the mosquito immune system has been an important barrier for adaptation of P. falciparum to distant anopheline species through selection of Pfs47, which may have influenced the parasite’s population structure and the epidemiology of malaria. Based on these findings, the “lock-and-key theory” of P. falciparum globalization was proposed, in which Pfs47 is the “key” that interacts with a mosquito receptor (“lock”), disrupting the antiplasmodial response [45]. The receptors are predicted to be different in evolutionary distant vectors and to select parasites that carry a compatible Pfs47 haplotype (the correct key), by allowing them to evade immune detection.
This model has important implications for the spread of the kelch propeller domain protein (K13) mutations that mediate delayed parasite clearance in response to artemisinin, because the K13 and Pfs47 genes are likely to be genetically linked, due to their close proximity (151 kb) [47]. P. falciparum lines with these K13 mutations are associated with the two most frequent Pfs47 haplotypes in Asia [44]. African mosquito vectors can be infected with these lines [44,45], but the level of infection in A. gambiae is significantly lower than in A. dirus (Asian) mosquitoes [45]. This would suggest that the K13 mutations could readily spread from Asia to Africa if the parasite is under drug pressure, which was the case for the spread of chloroquine resistance from Asia to Africa [48]. These Asian lines have very low compatibility with A. albimanus (New World vector), suggesting that the K13 mutations are less likely to spread from Asia to the New World [45].
Infections of A. gambiae (Ngousso and L3–5 strains) with three different African P. falciparum isolates, showed that most parasites from two of the isolates survived in both mosquito strains. However, most parasites from the third isolate (NF165) were eliminated by the mosquito complement-like system in both the Ngousso and L3–5 strains [49]. This was unexpected, because the predicted protein sequence of Pfs47 Domain 2 from NF165 is identical to that of Pfs47 from GB4, a strain that evades mosquito immunity. Two potential explanations for this discrepancy could be that NF165 may have other amino acid differences outside Domain 2 (only 59% of the Pfs47 gene was sequenced) that may be major determinants of compatibility in some parasite strains, or that, besides Pfs47, other genes may be also be required for effective immune evasion. Although the great majority of Pfs47-KO parasites are eliminated by the mosquito complement-like system in both G3 and L3–5 mosquitoes, the fact that some Pfs47-KO parasites can infect A. gambiae mosquitoes indicates that Pfs47 is not absolutely essential for parasite survival [22, 45].
Although it is apparent that the immune system of some anophelines selects certain Pfs47 haplotypes, there is no evidence of fertilization incompatibility between P. falciparum strains from different continents, as genetic crosses readily produce hybrid progeny [50,51]. The human immune system is another possible selective force on Pfs47, as gametocytes not ingested by mosquitoes can elicit immunity [52,53], and antibodies following immunization with recombinant P. vivax Pvs47 have transmission-blocking activity [54]. This has led us to re-evaluate Pfs47 as a potential transmission-blocking vaccine target.
Conclusions
Plasmodium P47 is critical for successful malaria transmission. In P. berghei, P47 is required for optimal fertilization, but in P. falciparum this requirement is not well established. In both P. falciparum and P. berghei, P47 is also important for mosquito immune evasion. P47 has one of the strongest signatures of natural selection and population structure in the P. falciparum and P. vivax genomes. The immune system of mosquitoes from different continents appears to be one of the forces that have selected Pfs47 haplotypes as malaria became global. It is unclear whether all anopheline species exert selection on the parasite. The nature of the mosquito Pfs47 receptor, and the mechanism by which it disrupts JNK signaling also remain to be determined. The importance of P47 for malaria transmission warrants further studies to assess its feasibility as a target to block malaria transmission.
Supplementary Material
Highlights.
The Plasmodium P47 protein is critical for successful malaria transmission.
In P. berghei, P47 is required for optimal fertilization.
P47 is also important for mosquito immune evasion in P. falciparum and P. berghei.
P47 has a strong geographic structure in P. falciparum and P. vivax populations.
The mosquito immune system selects Pfs47 haplotypes in different continents.
Acknowledgments
The authors acknowledge the critical comments of the reviewers that helped to improve the manuscript and the editorial assistance by Adeline Williams.
Funding
This work was supported by the Intramural Research Program of the Division of Intramural Research Z01AI000947, National Institute of Allergy and Infectious Diseases (NIAID), NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.WHO. World Malaria Report 2016. Geneva: World Health Organization; 2016. [Google Scholar]
- 2.Sinka ME, Bangs MJ, Manguin S, Rubio-Palis Y, Chareonviriyaphap T, Coetzee M, Mbogo CM, Hemingway J, Patil AP, Temperley WH, et al. A global map of dominant malaria vectors. Parasit Vectors. 2012;5:69. doi: 10.1186/1756-3305-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Molina-Cruz A, Barillas-Mury C. The remarkable journey of adaptation of the Plasmodium falciparum malaria parasite to New World anopheline mosquitoes. Memórias do Instituto Oswaldo Cruz. 2014;109:662–667. doi: 10.1590/0074-0276130553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molina-Cruz A, Zilversmit MM, Neafsey DE, Hartl DL, Barillas-Mury C. Mosquito Vectors and the Globalization of Plasmodium falciparum Malaria. Annu Rev Genet. 2016;50:447–465. doi: 10.1146/annurev-genet-120215-035211. [DOI] [PubMed] [Google Scholar]
- 5.Vlachou D, Schlegelmilch T, Runn E, Mendes A, Kafatos FC. The developmental migration of Plasmodium in mosquitoes. Current Opinion in Genetics & Development. 2006;16:384–391. doi: 10.1016/j.gde.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 6.Sinden RE. The cell biology of malaria infection of mosquito: advances and opportunities. Cell Microbiol. 2015;17:451–466. doi: 10.1111/cmi.12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bennink S, Kiesow MJ, Pradel G. The development of malaria parasites in the mosquito midgut. Cell Microbiol. 2016;18:905–918. doi: 10.1111/cmi.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith RC, Vega-Rodriguez J, Jacobs-Lorena M. The Plasmodium bottleneck: malaria parasite losses in the mosquito vector. Mem Inst Oswaldo Cruz. 2014;109:644–661. doi: 10.1590/0074-0276130597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blandin S, Shiao SH, Moita LF, Janse CJ, Waters AP, Kafatos FC, Levashina EA. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae. Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- 10.Gupta L, Molina-Cruz A, Kumar S, Rodrigues J, Dixit R, Zamora RE, Barillas-Mury C. The STAT pathway mediates late-phase immunity against Plasmodium in the mosquito Anopheles gambiae. Cell Host Microbe. 2009;5:498–507. doi: 10.1016/j.chom.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith RC, Barillas-Mury C, Jacobs-Lorena M. Hemocyte differentiation mediates the mosquito late-phase immune response against Plasmodium in Anopheles gambiae. Proc Natl Acad Sci U S A. 2015;112:E3412–3420. doi: 10.1073/pnas.1420078112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaramillo-Gutierrez G, Rodrigues J, Ndikuyeze G, Povelones M, Molina-Cruz A, Barillas-Mury C. Mosquito immune responses and compatibility between Plasmodium parasites and anopheline mosquitoes. BMC Microbiol. 2009;9:154. doi: 10.1186/1471-2180-9-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molina-Cruz A, Dejong RJ, Ortega C, Haile A, Abban E, Rodrigues J, Jaramillo-Gutierrez G, Barillas-Mury C. Some strains of Plasmodium falciparum, a human malaria parasite, evade the complement-like system of Anopheles gambiae mosquitoes. Proc Natl Acad Sci U S A. 2012 doi: 10.1073/pnas.1121183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar S, Gupta L, Han YS, Barillas-Mury C. Inducible peroxidases mediate nitration of anopheles midgut cells undergoing apoptosis in response to Plasmodium invasion. J Biol Chem. 2004;279:53475–53482. doi: 10.1074/jbc.M409905200. [DOI] [PubMed] [Google Scholar]
- 15.Oliveira GdA, Lieberman J, Barillas-Mury C. Epithelial Nitration by a Peroxidase/NOX5 System Mediates Mosquito Antiplasmodial Immunity. Science. 2012;335:856–859. doi: 10.1126/science.1209678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castillo JC, Ferreira ABB, Trisnadi N, Barillas-Mury C. Activation of mosquito complement antiplasmodial response requires cellular immunity. Science Immunology. 2017:2. doi: 10.1126/sciimmunol.aal1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baxter RH, Chang CI, Chelliah Y, Blandin S, Levashina EA, Deisenhofer J. Structural basis for conserved complement factor-like function in the antimalarial protein TEP1. Proc Natl Acad Sci U S A. 2007;104:11615–11620. doi: 10.1073/pnas.0704967104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fraiture M, Baxter RH, Steinert S, Chelliah Y, Frolet C, Quispe-Tintaya W, Hoffmann JA, Blandin SA, Levashina EA. Two mosquito LRR proteins function as complement control factors in the TEP1-mediated killing of Plasmodium. Cell Host Microbe. 2009;5:273–284. doi: 10.1016/j.chom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 19.Povelones M, Waterhouse RM, Kafatos FC, Christophides GK. Leucine-rich repeat protein complex activates mosquito complement in defense against Plasmodium parasites. Science. 2009;324:258–261. doi: 10.1126/science.1171400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arredondo SA, Kappe SH. The s48/45 six-cysteine proteins: mediators of interaction throughout the Plasmodium life cycle. Int J Parasitol. 2016 doi: 10.1016/j.ijpara.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williamson KC, Criscio MD, Kaslow DC. Cloning and expression of the gene for Plasmodium falciparum transmission-blocking target antigen, Pfs230. Mol Biochem Parasitol. 1993;58:355–358. doi: 10.1016/0166-6851(93)90058-6. [DOI] [PubMed] [Google Scholar]
- 22••.Molina-Cruz A, Garver LS, Alabaster A, Bangiolo L, Haile A, Winikor J, Ortega C, van Schaijk BCL, Sauerwein RW, Taylor-Salmon E, et al. The Human Malaria Parasite Pfs47 Gene Mediates Evasion of the Mosquito Immune System. Science. 2013;340:984–987. doi: 10.1126/science.1235264. This study identified the first P. falciparum gene that actively modulates the immune response of the host and makes the parasite “invisible” to the mosquito immune system. Pfs47 inhibits the nitration response in parasite-invaded midgut cells, preventing effective activation of the mosquito complement system. This study revealed that Pfs47 is a strong parasite survival factor that greatly enhances human malaria transmission. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sauerwein RW, Bousema T. Transmission blocking malaria vaccines: Assays and candidates in clinical development. Vaccine. 2015;33:7476–7482. doi: 10.1016/j.vaccine.2015.08.073. [DOI] [PubMed] [Google Scholar]
- 24.Nikolaeva D, Draper SJ, Biswas S. Toward the development of effective transmission-blocking vaccines for malaria. Expert Rev Vaccines. 2015;14:653–680. doi: 10.1586/14760584.2015.993383. [DOI] [PubMed] [Google Scholar]
- 25.Templeton TJ, Kaslow DC. Identification of additional members define a Plasmodium falciparum gene superfamily which includes Pfs48/45 and Pfs230. Mol Biochem Parasitol. 1999;101:223–227. doi: 10.1016/s0166-6851(99)00066-3. [DOI] [PubMed] [Google Scholar]
- 26.van Dijk MR, Janse CJ, Thompson J, Waters AP, Braks JA, Dodemont HJ, Stunnenberg HG, van Gemert GJ, Sauerwein RW, Eling W. A central role for P48/45 in malaria parasite male gamete fertility. Cell. 2001;104:153–164. doi: 10.1016/s0092-8674(01)00199-4. [DOI] [PubMed] [Google Scholar]
- 27.Gerloff DL, Creasey A, Maslau S, Carter R. Structural models for the protein family characterized by gamete surface protein Pfs230 of Plasmodium falciparum. Proc Natl Acad Sci U S A. 2005;102:13598–13603. doi: 10.1073/pnas.0502378102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28•.van Dijk MR, van Schaijk BC, Khan SM, van Dooren MW, Ramesar J, Kaczanowski S, van Gemert GJ, Kroeze H, Stunnenberg HG, Eling WM, et al. Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog. 2010;6:e1000853. doi: 10.1371/journal.ppat.1000853. This study found that P230 and P47 play a critical role during fertilization in Plasmodium berghei. Mutant P. berghei parasites that lack P230 or P47 have reduced fertily. Mating experiments showed that P230 is a male fertility factor, while P47 is requried for female fertility. Low levels of fertilization still ocurr despite a major decrease in zygote formation in the mutant parasites, indicating that the gametes can use an alternative fertilization strategy that is independent of these genes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramiro RS, Khan SM, Franke-Fayard B, Janse CJ, Obbard DJ, Reece SE. Hybridization and pre-zygotic reproductive barriers in Plasmodium. Proc Biol Sci. 2015;282:20143027. doi: 10.1098/rspb.2014.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khan SM, Franke-Fayard B, Mair GR, Lasonder E, Janse CJ, Mann M, Waters AP. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell. 2005;121:675–687. doi: 10.1016/j.cell.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 31.van Schaijk BC, van Dijk MR, van de Vegte-Bolmer M, van Gemert GJ, van Dooren MW, Eksi S, Roeffen WF, Janse CJ, Waters AP, Sauerwein RW. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol Biochem Parasitol. 2006;149:216–222. doi: 10.1016/j.molbiopara.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 32.Collins FH, Sakai RK, Vernick KD, Paskewitz S, Seeley DC, Miller LH, Collins WE, Campbell CC, Gwadz RW. Genetic selection of a Plasmodium-refractory strain of the malaria vector Anopheles gambiae. Science. 1986;234:607–610. doi: 10.1126/science.3532325. [DOI] [PubMed] [Google Scholar]
- 33.Canepa GE, Molina-Cruz A, Barillas-Mury C. Molecular Analysis of Pfs47-Mediated Plasmodium Evasion of Mosquito Immunity. PLoS One. 2016;11:e0168279. doi: 10.1371/journal.pone.0168279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34•.Ramphul UN, Garver LS, Molina-Cruz A, Canepa GE, Barillas-Mury C. Plasmodium falciparum evades mosquito immunity by disrupting JNK-mediated apoptosis of invaded midgut cells. Proceedings of the National Academy of Sciences. 2015;112:1273–1280. doi: 10.1073/pnas.1423586112. This study showed that Pfs47 alters the cell death pathway of invaded midgut cells by disrupting JNK signaling, preventing activation of caspases and epithelial nitration responses in parasite-invaded midgut cells. The presence of Pfs47 results in broad and profound changes in gene expression in response to infection that are already evident 14 hours before ookinetes invade the mosquito midgut. The inability to activate JNK signaling precludes effective activation of the mosquito complement system and greatly enhances parasite survival. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garver LS, de Almeida Oliveira G, Barillas-Mury C. The JNK pathway is a key mediator of Anopheles gambiae antiplasmodial immunity. PLoS Pathog. 2013;9:e1003622. doi: 10.1371/journal.ppat.1003622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Feldmann AM, Ponnudurai T. Selection of Anopheles stephensi for refractoriness and susceptibility to Plasmodium falciparum. Med Vet Entomol. 1989;3:41–52. doi: 10.1111/j.1365-2915.1989.tb00473.x. [DOI] [PubMed] [Google Scholar]
- 37••.Ukegbu CV, Giorgalli M, Yassine H, Ramirez JL, Taxiarchi C, Barillas–Mury C, Christophides GK, Vlachou D. Plasmodium berghei P47 is essential for ookinete protection from the Anopheles gambiae complement-like response. Scientific Reports. 2017;7:6026. doi: 10.1038/s41598-017-05917-6. This study shows that in P. berghei, P47 is expressed in female gametocytes, gametes and ookinetes, and has two different functions. In gametocytes, P47 is required for optimal fertilization and ookinete formation in the midgut lumen. P47 is also essential to protect ookinetes from the mosquito complement-like response, as they come in contact with mosquito hemolymph. Coinfection experiments showed that P47 from wild-type parasites does not confer immune protection to coinvading ookinetes lacking P47, and that lack of P47 in the surface of ookinetes does not affect co-invading ookinetes expressing P47. The protection of the ookinete by P47 is not due to a sytemic mechanism but a mechanism that acts locally on the infected midgut cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White BJ, Lawniczak MKN, Cheng C, Coulibaly MB, Wilson MD, Sagnon NF, Costantini C, Simard F, Christophides GK, Besansky NJ. Adaptive divergence between incipient species of Anopheles gambiae increases resistance to Plasmodium. Proceedings of the National Academy of Sciences. 2011;108:244–249. doi: 10.1073/pnas.1013648108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anthony TG, Polley SD, Vogler AP, Conway DJ. Evidence of non-neutral polymorphism in Plasmodium falciparum gamete surface protein genes Pfs47 and Pfs48/45. Mol Biochem Parasitol. 2007;156:117–123. doi: 10.1016/j.molbiopara.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 40.Conway DJ, Machado RL, Singh B, Dessert P, Mikes ZS, Povoa MM, Oduola AM, Roper C. Extreme geographical fixation of variation in the Plasmodium falciparum gamete surface protein gene Pfs48/45 compared with microsatellite loci. Mol Biochem Parasitol. 2001;115:145–156. doi: 10.1016/s0166-6851(01)00278-x. [DOI] [PubMed] [Google Scholar]
- 41.Manske M, Miotto O, Campino S, Auburn S, Almagro-Garcia J, Maslen G, O’Brien J, Djimde A, Doumbo O, Zongo I, et al. Analysis of Plasmodium falciparum diversity in natural infections by deep sequencing. Nature. 2012;487:375–379. doi: 10.1038/nature11174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woo MK, Kim KA, Kim J, Oh JS, Han ET, An SS, Lim CS. Sequence polymorphisms in Pvs48/45 and Pvs47 gametocyte and gamete surface proteins in Plasmodium vivax isolated in Korea. Mem Inst Oswaldo Cruz. 2013:108. doi: 10.1590/S0074-02762013000300015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hupalo DN, Luo Z, Melnikov A, Sutton PL, Rogov P, Escalante A, Vallejo AF, Herrera S, Arevalo-Herrera M, Fan Q, et al. Population genomics studies identify signatures of global dispersal and drug resistance in Plasmodium vivax. Nat Genet. 2016;48:953–958. doi: 10.1038/ng.3588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.St Laurent B, Miller B, Burton TA, Amaratunga C, Men S, Sovannaroth S, Fay MP, Miotto O, Gwadz RW, Anderson JM, et al. Artemisinin-resistant Plasmodium falciparum clinical isolates can infect diverse mosquito vectors of Southeast Asia and Africa. Nat Commun. 2015;6:8614. doi: 10.1038/ncomms9614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45••.Molina-Cruz A, Canepa GE, Kamath N, Pavlovic NV, Mu J, Ramphul UN, Ramirez JL, Barillas-Mury C. Plasmodium evasion of mosquito immunity and global malaria transmission: The lock-and-key theory. Proc Natl Acad Sci U S A. 2015;112:15178–15183. doi: 10.1073/pnas.1520426112. This study found that P. falciparum isolates from Africa, Asia or the Americas have low compatibility to malaria vectors from a different continent, and that parasite elimination in incompatible combinations is mediated by the mosquito immune system. Global analysis of Pfs47 from natural populations identified 42 different haplotypes that exhibit strong geographic population structure and much lower haplotype diversity outside Africa. Replacement of the Pfs47 haplotypes in a P. falciparum isolate was sufficient to make it compatible to a different mosquito species. A new working model of how mosquito immunity shapes natural populations of P. falciparum, “the lock and key theory”, was proposed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu W, Li Y, Learn GH, Rudicell RS, Robertson JD, Keele BF, Ndjango JB, Sanz CM, Morgan DB, Locatelli S, et al. Origin of the human malaria parasite Plasmodium falciparum in gorillas. Nature. 2010;467:420–425. doi: 10.1038/nature09442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ariey F, Witkowski B, Amaratunga C, Beghain J, Langlois AC, Khim N, Kim S, Duru V, Bouchier C, Ma L, et al. A molecular marker of artemisinin-resistant Plasmodium falciparum malaria. Nature. 2014;505:50–55. doi: 10.1038/nature12876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wootton JC, Feng X, Ferdig MT, Cooper RA, Mu J, Baruch DI, Magill AJ, Su XZ. Genetic diversity and chloroquine selective sweeps in Plasmodium falciparum. Nature. 2002;418:320–323. doi: 10.1038/nature00813. [DOI] [PubMed] [Google Scholar]
- 49.Eldering M, Morlais I, van Gemert GJ, van de Vegte-Bolmer M, Graumans W, Siebelink-Stoter R, Vos M, Abate L, Roeffen W, Bousema T, et al. Variation in susceptibility of African Plasmodium falciparum malaria parasites to TEP1 mediated killing in Anopheles gambiae mosquitoes. Sci Rep. 2016;6:20440. doi: 10.1038/srep20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Su X, Hayton K, Wellems TE. Genetic linkage and association analyses for trait mapping in Plasmodium falciparum. Nat Rev Genet. 2007;8:497–506. doi: 10.1038/nrg2126. [DOI] [PubMed] [Google Scholar]
- 51.Hayton K, Gaur D, Liu A, Takahashi J, Henschen B, Singh S, Lambert L, Furuya T, Bouttenot R, Doll M, et al. Erythrocyte binding protein PfRH5 polymorphisms determine species-specific pathways of Plasmodium falciparum invasion. Cell Host Microbe. 2008;4:40–51. doi: 10.1016/j.chom.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Skinner J, Huang C-Y, Waisberg M, Felgner PL, Doumbo OK, Ongoiba A, Kayentao K, Traore B, Crompton PD, Williamson KC. Plasmodium falciparum Gametocyte-Specific Antibody Profiling Reveals Boosting through Natural Infection and Identifies Potential Markers of Gametocyte Exposure. Infection and Immunity. 2015;83:4229–4236. doi: 10.1128/IAI.00644-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Paul NH, Vengesai A, Mduluza T, Chipeta J, Midzi N, Bansal GP, Kumar N. Prevalence of Plasmodium falciparum transmission reducing immunity among primary school children in a malaria moderate transmission region in Zimbabwe. Acta Trop. 2016;163:103–108. doi: 10.1016/j.actatropica.2016.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tachibana M, Suwanabun N, Kaneko O, Iriko H, Otsuki H, Sattabongkot J, Kaneko A, Herrera S, Torii M, Tsuboi T. Plasmodium vivax gametocyte proteins, Pvs48/45 and Pvs47, induce transmission-reducing antibodies by DNA immunization. Vaccine. 2015;33:1901–1908. doi: 10.1016/j.vaccine.2015.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.