Abstract
Bacillus subtilis subsp. krictiensis ATCC55079 produces the cyclic lipopeptide antibiotics iturin A–F as well as several surfactins. Here, we analyzed and characterized the biosynthetic genes associated with iturin and surfactin production in this strain. We aligned the sequences of each iturin and surfactin synthetase ORF obtained from a genomic library screen and next generation sequencing. The resulting 37,249-bp and 37,645-bp sequences associated with iturin and surfactin production, respectively, contained several ORFs that are predicted to encode proteins involved in iturin and surfactin biosynthesis. These ORFs showed higher sequence homologies with the respective iturin and surfactin synthetase genes of B. methylotrophicus CAU B946 than with those of B. subtilis RB14 and B. subtilis ATCC6633. Moreover, comparative analysis of the secondary metabolites produced by the wild-type and surfactin-less mutant (with a spectinomycin resistance cassette inserted into the srfAB gene within the putative surfactin gene region) strains demonstrated that the mutant strain showed significantly higher antifungal activity against Fusarium oxysporum than the wild-type strain. In addition, the wild-type strain-specific surfactin high performance liquid chromatography (HPLC) peaks were not observed in the surfactin-less mutant strain. In contrast, the iturin A peak detected by HPLC and liquid chromatography-mass spectrometry (LC/MS) in the surfactin-less mutant strain was 30% greater than that in the wild-type strain. These results suggested that the gene cluster we identified is involved in surfactin biosynthesis, and the biosynthetic pathways for iturin and surfactin in Bacillus strains producing both iturin and surfactin may utilize a common pathway.
Introduction
The increasing prevalence of fungicide-resistant fungal strains and public concern over the harmful environmental effects of agrochemicals have prompt the concept of environmentally friendly biological control agents as alternatives to or complements of agrochemicals [1–3]. Various bacterial strains have been used as biological control agents to suppress plant diseases [4–7] and postharvest decay of fruits and vegetables [8, 9]. Bacillus species, which produce antibiotics that inhibit plant pathogens [6–8] and are environmentally safe, are among the most remarkable bacterial control agents [8]. In fact, Bacillus-based products constitute about half of the commercially available bacterial control agents [10].
Bacillus subtilis is a gram-positive bacterium that produces various nonribosomally synthesized cyclic lipopeptides. These compounds share a cyclic structure consisting of a β-amino or β-hydroxy fatty acid integrated into a peptide moiety [11]. The prominent differences among cyclic lipopeptides are the type and sequence of the amino acids in the peptide and the branching of the fatty acid chain. Cyclic lipopeptides are classified into three families, the iturin [10, 12], fengycin and plipastatin [13], and surfactin families [14]. Members of the iturin family, such as iturin, bacillomycin, and mycosubtilin, show potent antifungal activity and are heptapeptides linked to a β-amino fatty acid chain [15–17]. Members of the fengycin and plipastatin family are decapeptides with a β-hydroxy fatty acid, while members of the surfactin family are heptapeptides with a β-hydroxy fatty acid [6]. Several studies on iturin and surfactin [18], iturin and fengycin [6], and surfactin and fengycin [19] have shown that these lipopeptides have synergistic functions. Moreover, some Bacillus strains have been shown to simultaneously produce all three lipopeptide families [6, 19–22].
The biosynthesis genes that encode the proteins that produce various lipopeptides of the iturin family have been cloned and sequenced, including the mycosubtilin synthetase of B. subtilis ATCC6633 [15], the iturin A operon of B. subtilis RB14 [17], and the bacillomycin D operons of B. amyloliquefaciens FZB42 [21] and B. subtilis AU195 [16]. In addition, the gene clusters encoding proteins associated with iturin A and surfactin synthesis have been widely investigated [17, 23–26]. The iturin operon was reported to be more than 38 kb long and composed of four open reading frames, ituD, ituA, ituB, and ituC [17], while the surfactin gene cluster consisted of four open reading frames [26]. However, studies of the iturin and surfactin biosynthesis genes in Bacillus strains producing both iturin and surfactin are extremely limited, and their sequences differ significantly among strains [17, 25]. Therefore, further studies of iturin and surfactin biosynthesis, including the sequencing of complete iturin and surfactin biosynthesis genes are needed.
In a previous study, we reported that B. subtilis subsp. krictiensis ATCC55079 produces six kinds of iturins [27], has suppressive effects against various phytopathogenic fungi, and shows potential for use as a biological control agent [28, 29]. To compare the iturin and surfactin biosynthesis genes in this strain with the corresponding genes of other Bacillus strains, we analyzed the gene clusters associated with iturin and surfactin biosynthesis using a genomic library and next generation sequencing (NGS). To determine whether the identified genes are essential for iturin or surfactin biosynthesis, we generated a mutant strain with a spectinomycin resistant gene cassette inserted into the genes of the wild-type Bacillus strain by homologous recombination. Then, the secondary metabolites produced by the wild-type and srfAB mutant B. subtilis subsp. krictiensis ATCC55079 strains were analyzed by HPLC and LC-MS.
Materials and methods
Bacterial strains, plasmids, and culture conditions
Bacillus subtilis subsp. krictiensis ATCC55079, the iturin and surfactin-producing strain used in this study, was isolated from soil [27–29]. B. subtilis 168 [26] and Escherichia coli HB101 were obtained from the Korea Research Institute of Bioscience and Biotechnology (Daejeon, Korea). E. coli DH5α, plasmid pTZ18R (Amersham Pharmacia Biotech), and cosmid pLAFR3 were obtained from Seoul National University (Seoul, Korea) and were used for routine cloning and sequencing. Cosmid pLAFR3 and E. coli HB101 were utilized to construct a genomic library. The pBC KS(+) (Stratagene) vector and the mini-Tn10 delivery vector pIC333 were used for homologous recombination. All bacteria were stored at -70°C in 20% (vol/vol) glycerol. Fusarium oxysporum, Magnaporthe grisea, and Trichophyton mentagrophytes, which were used for the antifungal activity bioassays, were maintained at 25°C on potato dextrose agar and Sabouraud dextrose agar. The details for all the strains, cosmids, and plasmids used in this study are listed in Table 1.
Table 1. Strains and plasmids used in this study.
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Bacterial strains | ||
| Escherichia coli DH5α | F- Φ80dlacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17 (rk- mk+) supE44 relA1deoRΔ(lacZYA-argF) U169 | Promega, Madison, WI |
| Escherichia coli HB101 | F- hsdS20 (rB- mB-) recA13 ara-14 proA2 lacY1 galK2rpsL20 (Strr) xyl-5 mtl-1 supE44 thi-1 leuB6 | Promega, Madison, WI |
| Bacillus subtilis 168 | trpC2 | [26] |
| Bacillus subtilis subsp. krictiensis ATCC55079 | Wild-type | [28] |
| Fungi | ||
| Fusarium oxysporum | Wild-type | This study |
| Magnaporthe grisea | Wild-type | This study |
| Trichophyton mentagrophytes | Wild-type | This study |
| Plasmids | ||
| pTZ18R | Ampr plasmid carrying the T7 g10 promoter | [31] |
| pLAFR1 | pRK290 containing the cos site | [32] |
| pLAFR3 | pLAFR1 containing an HaeII fragment of pUC8 | [32] |
| pIC333 | Mini-Tn10 delivery vector | [33] |
| pJJ5, pJJ71, pJJ121, pJJ815 | Genomic library clones constructed using cosmid vector pLAFR3 | This study |
| pJJ121E2 | 16.4-kb EcoRI-digested pJJ121 fragment inserted into pTZ18R | This study |
| pJJ121E3 | 4.8-kb EcoRI-digested pJJ121 fragment inserted into pTZ18R | This study |
| pJJ815E4 | 3.7-kb EcoRI-digested pJJ815 fragment | This study |
| pJJ121E2-2 | 7.9-kb EcoRI and SalI-digested pJJ121E2 fragment inserted into pBC KS(+) | This study |
| pJJ121E2-1 | BamHI and XbaI-digested pIC333 fragment inserted into the ClaI site of pJJ121E2-2 | This study |
All B. subtilis strains and E. coli DH5α were incubated at 30°C overnight in Luria-Bertani (LB) broth without or with spectinomycin (100 μg/mL; Sigma). The medium used to culture the Bacillus cyclic lipopeptide-producing strains was a complex medium containing sucrose [30.0 g/L], soytone [10.0 g/L], yeast extract [5.0 g/L], K2HPO4 [0.5 g/L], MgSO4 [2.0 g/L], MnCl2 [4.0 mg/L], CaCl2 [5.0 mg/L], and FeSO4·7H2O [25.0 mg/L] in distilled water, and adjusted to pH 7.0. For transformation, Spizizen’s minimal medium [30] containing 50% glucose [10 ml/L], 2% casein hydrolysate [10.0 ml/L], 10% yeast extract [10.0 ml/L], 1 M MgCl2 [6.0 g/L], KH2PO4 [6.0 g/L], K2HPO4 [14.0 g/L], (NH4)SO4 [2.0 g/L], Na3 citrate·2H2O [1.0 g/L], and MgSO4 [0.2 g/L] was used.
Genomic DNA library construction
Genomic DNA was isolated from Bacillus subtilis subsp. krictiensis ATCC55079 according to a previously described method [30]. The genomic library was constructed using the cosmid vector pLAFR3 and Gigapack III XL packaging extract (Stratagene). Genomic DNA fragments greater than 20 kb in length, which were obtained by partial digestion with Sau3AI, were ligated to pLAFR3 that was digested with BamHI and dephosphorylated. The ligation mixture was packaged with Gigapack III XL packaging extract and transfected into E. coli HB101 cells according to the manufacturer’s protocol.
Cloning putative surfactin biosynthesis genes from the genomic library
To obtain the surfactin biosynthesis genes from the wild-type B. subtilis genomic library, two PCR primers were designed using the sequence of the surfactin biosynthesis genes of B. subtilis 168, which was used for the Bacillus genome project and contains a surfactin synthetase gene. The primers were synthesized and purified by Bioneer and the PCR was performed on an i-Cycler (Bio-Rad). To clone the putative surfactin biosynthesis genes from the genomic DNA of B. subtilis 168 and B. subtilis subsp. krictiensis, we amplified the genes by PCR using primers B9 (5′- GCAAAATTTCCGGACAGCGGGATAT-3ʹ) and B10 (5′-TCGATCCGGCCGATGTATTCGAT-3ʹ). Approximately 100 ng of genomic DNA was added to a 50 μL reaction mixture containing 10 mM Tris-HCl (pH 9.0), 40 mM KCl, 1.5 mM MgCl2, 10pmol each of primer, 250 μM dNTPs, and 1 unit of Taq polymerase (AccuPower PCR PreMix, Bioneer). The reactions were performed with the following cycling conditions: an initial denaturation step for 5 min at 95°C, 30 cycles of denaturation for 1 min at 95°C, annealing for 1 min at 42°C, and extension for 2 min at 72°C, with a final extension for 5 min at 72°C. Then, an aliquot (20 μL) of the amplification products was separated on a 1% agarose gel (SeaKem LE agarose, Lonza) in 1× TAE buffer (40 mM Tris-acetate, 1 mM EDTA, pH 8.0). A DNA fragment of approximately 1.8 kb, which is the same size as the products obtained from B. subtilis 168, was detected in the wild-type B. subtilis subsp. krictiensis strain. The sequence of this product was determined and compared to that of other cyclic lipopeptide synthetase genes. Then, this 1.8-kb gene product was used as a probe in a colony hybridization experiment to select clones containing the putative surfactin biosynthesis genes from a genomic library of the wild-type B. subtilis strain. For the hybridization experiment, the 1.8-kb PCR amplification product from B. subtilis subsp. krictiensis was purified using the QIAquick gel extraction kit (Qiagen) and then labeled with [α-32P]-dCTP using the Prime-a-gene labeling system (Promega). Colony hybridization and Southern hybridization with the labeled probe were performed as described previously [34].
Sequencing of the cosmid clone containing the putative surfactin biosynthesis genes
Positive cosmid clones, pJJ815 and pJJ121, were selected by colony hybridization and Southern hybridization. Restriction enzyme maps of the cosmid clones were constructed by digestion with EcoRI and SmaI, and then EcoRI digested fragments of the cosmid clones were subcloned into the high-copy vector pTZ18R. The sequences of both strands of the vector constructs were confirmed by sequencing on an ABI 3730XL capillary DNA sequencer (Solgent), and the nucleotide sequencing results were analyzed by using NCBI BLAST and CLUSTALW.
Disruption of the region containing putative surfactin biosynthesis genes by homologous recombination
A mutant in which the putative surfactin biosynthesis gene was disrupted (a surfactin-less mutant) was constructed by double crossover homologous recombination. To generate this mutant, a 7.9-kb EcoRI and SalI-digested pJJ121E2 fragment containing the putative surfactin biosynthesis genes was cloned into the pBC KS(+) vector to construct pJJ121E2-2. Then, a BamHI and XbaI fragment containing a spectinomycin resistance gene cassette was excised from pIC333 and Cla I sites were introduced by PCR. Then, the BamHI-XbaI fragment containing the ClaI sites was ligated into the ClaI site of the putative surfactin biosynthesis gene in pJJ121E2-2, to create pJJ121E2-1, which contains a surfactin biosynthesis gene disrupted by a spectinomycin resistance gene cassette. The pJJ121E2-1 plasmid was transformed into wild-type B. subtilis subsp. krictiensis grown in Spizizen’s minimal medium as follows. Wild-type B. subtilis subsp. krictiensis was inoculated into 2 mL of Spizizen’s medium and cultivated at 30°C with shaking at 200 rpm for 16 h. An aliquot of this culture was inoculated into fresh Spizizen’s medium and grown at 30°C with shaking at 200 rpm for 16 h until the cultures reached an absorbance at 580 nm (A580) of 1.0. Then, the wild-type strain was transformed with pJJ121E2-1 plasmid, which contains a mini-Tn10 transposon, to replace the internal 7.9-kb region of the srfAB fragment in the genome with the fragment in pJJ121E2 containing a spectinomycin gene cassette via homologous recombination by selecting spectinomycin-resistant transformants. For the transformation, 1 μg of pJJ121E2-1 was added to 0.5 mL of culture. After incubation at 30°C with shaking at 200 rpm for 60 min, the mixture was spread on an LB agar plate containing 100 μg/mL spectinomycin and incubated at 30°C for 24 h. Transformants that grew on LB agar containing 100 μg/mL spectinomycin were selected and subjected to Southern blot analysis to verify integration of the vector in the chromosome. Genomic DNAs from several transformants were digested with ClaI, separated on a 0.7% agarose gel, and blotted to a nylon membrane (Amersham Pharmacia Biotech). Southern hybridization was performed with a spectinomycin-resistance gene probe labeled with DIG-11-dUTP [34] for 16 h at 65°C. DNA labeling and detection were performed using a DIG DNA labeling and detection kit (Roche, Germany) according to the manufacturer’s instructions.
Comparison of the antifungal activities of the wild-type and surfactin-less mutant B. subtilis subsp. krictiensis strains
The antifungal activities in the culture broth from the wild-type and surfactin-less mutant strains were determined by the agar diffusion method [35]. Mycelial or spore suspensions of test fungi were mixed with a soft agar overlay of 0.8% potato dextrose or Sabouraud dextrose agar and added to potato dextrose agar and Sabouraud dextrose agar plates, respectively. After solidification of the agar overlay, the plates were used in the bioassay. Sterile, stainless steel cylinders (8 mm outer diameter × 10 mm long, Fisher) were placed on the surface of the agar plates, and test samples of the culture broth from the wild-type and mutant strains were loaded into the sterile cylinders, and the plates were incubated at 25°C for 2 days. Then, the diameter of the inhibitory zone on the plates was measured and recorded in millimeters. In addition, commercially available iturin A and surfactin were used on plates containing the test fungi as positive controls.
Comparative analysis of the secondary metabolites produced by the wild-type and surfactin-less mutant B. subtilis strains
To assess iturin and surfactin production, the Bacillus subtilis strains were grown in the complex medium described above at 30°C for 3 days. The cells were removed by centrifugation at 8,000 × g for 10 min, and the supernatants were adjusted to pH 3 and incubated overnight at 4°C to precipitate the lipopeptides. The precipitates were centrifuged, dissolved in 1M Tris-HCl buffer (pH 7.4), and extracted three times with butanol. The butanol layers were evaporated in vacuo, dissolved in methanol, and then filtered through a 0.45-μm filter. The secondary metabolites obtained from the culture broth of the wild-type B. subtilis subsp. krictiensis and surfactin-less mutant strains were analyzed by high performance liquid chromatography (HPLC; Agilent 1100) with a C18 column (YMC-pack Pro, 4.6 × 250 mm, 5 μm; YMC). The peaks at 210 nm were detected with a UV detector. The column was eluted with a gradient of CH3CN (A)/0.05% trifluoroacetic acid in water (B) at a flow rate of 1 mL/min as follows: 20–60% A/80-40% B (v/v) for 50 min, 60–80% A/40-20% B (v/v) for 5 min, 80–100% A/20-0% B (v/v) for 30 min, 100% A/0% B (v/v) for 3 min, and 20% A/80% B (v/v) for 2 min. Authentic iturin and surfactin (Sigma) were used as references.
To determine the molecular weights of the iturin and surfactin peaks from wild-type B. subtilis subsp. krictiensis that were detected by HPLC, butanol extract-evaporated culture broth was analyzed by using a Nanospace SI-2 HPLC (Shiseido, Tokyo, Japan) and an LCQ Deca XP ion trap mass spectrometer (Thermo Finnigan, San Jose, CA) equipped with an electrospray ionization interface at the Korea Basic Science Institute (Seoul). The column used was a Phenomenex C18 column (1.0 × 150 mm, 5 μm; Phenomenex, U. S. A.), which was eluted with CH3CN containing 0.1% formic acid (A) and water containing 0.1% formic acid (B) at a flow rate of 50 μL/min as follows: 35% A/65% B (v/v) for 5 min, 35–100% A /65-0% B (v/v) for 75 min, 100% A /0% B (v/v) for 5 min, 100–35% A /0-65% B (v/v) for 5 min, and 35% A/65% B (v/v) for 10 min. Mass spectra were obtained in positive ion mode with m/z values ranging from 50 to 2,000.
Next-generation sequencing of iturin biosynthesis genes
Whole genome sequence of wild-type B. subtilis subsp. krictiensis was obtained to identify the iturin biosynthesis genes by sequencing on an Illumina Hiseq 2500 sequencer (Teragen Etex Bio Institute, Korea), and the nucleotide sequencing results were analyzed with A5 software (ver. 2015522).
Nucleotide sequence accession numbers
The nucleotide sequences of the iturin and surfactin biosynthesis genes from B. subtilis subsp. krictiensis have been deposited in GenBank (accession numbers KU170613 and KC454625, respectively).
Results
Cloning and organization of cosmid clones containing putative surfactin biosynthesis genes from a genomic library of wild-type B. subtilis
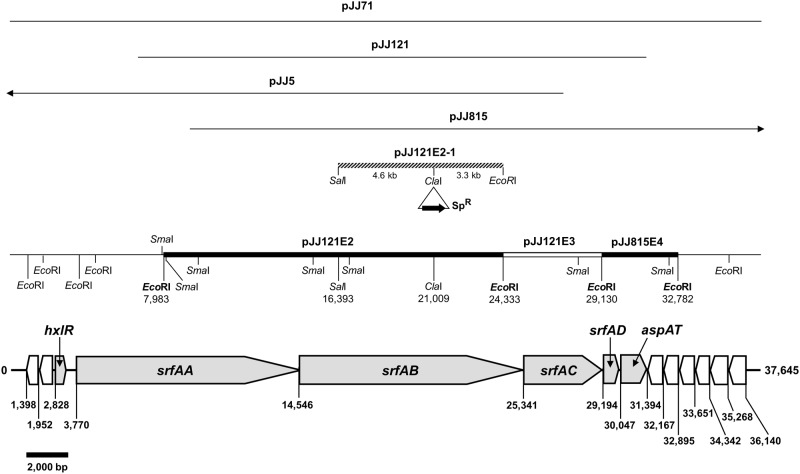
To clone the biosynthesis genes responsible for surfactin production, a screening was performed of a genomic library from wild-type B. subtilis subsp. krictiensis ATCC55079. The structures, molecular weights, and lengths of the biosynthetic genes of iturin and surfactin are very similar. In addition, some Bacillus strains produce both iturin and surfactin simultaneously [17, 36]. Based on these observations, surfactin and iturin biosynthesis are thought to share a common pathway up to the established steps, after which, these two cyclic lipopeptides are synthesized via separate pathways. Therefore, we attempted to clone the surfactin biosynthesis genes of B. subtilis subsp. krictiensis using the sequences of the surfactin biosynthesis genes from B. subtilis 168 [26], which was used in the Bacillus genome sequencing project. The1.8-kb products amplified from wild-type B. subtilis subsp. krictiensis and B. subtilis 168 genomic DNA showed 66–99% homology to the sequences of several cyclic lipopeptide synthetase genes, such as surfactin synthetase, peptide synthetase, and lichenysin synthetase. Several positive cosmid clones with 30-kb–40-kb inserts were identified via colony hybridization with a radiolabeled 1.8-kb PCR product as a probe, and four clones (pJJ5, pJJ71, pJJ121, and pJJ815) were finally selected and used to construct a restriction enzyme map of the cosmid clones (Fig 1).
Fig 1. Organization of the ORFs and restriction map of the putative surfactin biosynthesis genes cloned from Bacillus subtilis subsp. krictiensis ATCC55079.
The surfactin genes are designated as srfAA, srfAB, srfAC, and srfAD. The spectinomycin resistance gene from the mini-Tn10 in pIC333 is designated as SpR.
Sequencing of cosmid clones containing putative surfactin biosynthesis genes
To determine the sequence of the putative surfactin biosynthesis genes of B. subtilis subsp. krictiensis, the selected cosmid clones were subcloned into a pTZ18R vector, and the sequence of the subclones was determined. The sequence of the putative surfactin biosynthesis genes from B. subtilis subsp. krictiensis was 37,645 bp in length and contained 14 open reading frames (ORFs; Fig 1). Six of the ORFs are in the same orientation, whereas the others are in the opposite orientation. Two ORFs, hxlB and hxlA, located upstream of the putative surfactin region have homology to the B. subtilis 168 genes hxlB (72%) and hxlA (79%) [26], and the B. methylotrophicus CAU B946 (formerly B. amyloliquefaciens subsp. plantarum CAU B946) genes hxlB (99%) and hxlA (99%) [25, 37], respectively. These genes are thought to encode the sugar phosphate isomerase (hxlB) involved in capsule formation and a sugar phosphate synthase (hxlA), which are key enzymes in the ribulose monophosphate pathway, in which compounds containing carbon-carbon bonds are synthesized from single carbon units [38]. The third ORF, designated hxlR, is separated from hxlA by 217 bp and has significant homology to the hxlAB genes in the surfactin synthetase operon of B. subtilis 168 (79%), an HTH-type transcriptional activator, hxlRI, of B. methylotrophicus CAU B946 (99%), and hxlR of B. subtilis TU-B-10 (79%) [39]. The fourth ORF, located 589 bp downstream of hxlR, is 10,755 bp and is designated srfAA. SrfAA showed 74% and 99% homology to the surfactin synthetase genes (srfAA) of B. subtilis 168 [26] and B. methylotrophicus CAU B946 [25, 37], respectively. The next gene, designated srfAB, which was 10,760 bp in size, showed 74% and 99% homology to the surfactin synthetases (srfAB) of B. subtilis 168 and B. methylotrophicus CAU B946, respectively. SrfAC, which is located 35 bp downstream of srfAB, showed 87% and 99% homology to the surfactin synthetases (srfAC) of B. subtilis 168 and B. methylotrophicus CAU B946. The seventh ORF, designated srfAD, exhibited 75% and 99% homology to the surfactin synthetase thioesterases (srfAD) of B. subtilis 168 and B. methylotrophicus CAU B946, respectively. The next gene, aspAT, shared more than 98% homology with the aspartate aminotransferases aspB3 [24] and aspAT of B. subtilis and B. methylotrophicus CAU B946, respectively. The sfp gene, located in 37 bp downstream of aspAT, encodes a 4′-phosphopantetheinyl transferase and showed 72% and 73% homology to the sfp of B. subtilis TU-B-10 and B. subtilis 168, respectively, and 99% homology to B. methylotrophicus CAU B946. The next gene, yczE, which is predicted to encode an inner membrane protein regulating antibiotic production, showed 74% homology to yczE of B. subtilis 168 and >98% homology to yczE of both B. subtilis [24] and B. methylotrophicus CAU B946. The next three genes, yckI, yckJ, and yckK, which encode amino acid ABC transporter proteins, showed 81–87% homology to the tcy genes of B. subtilis 168 and 99–100% homology to the yck genes of B. methylotrophicus CAU B946. The final ORF exhibited 75% homology to bsdA and yclA of B. subtilis 168 and B. subtilis TU-B-10 [39], respectively, and 99% homology to yclA of B. methylotrophicus CAU B946. Interestingly, the sequences of srfAA, srfAB, srfAC, and srfAD in wild-type B. subtilis subsp. krictiensis showed relatively low homology (74–87%) to the surfactin synthetase operon of B. subtilis 168 and very high homology (99%) to the surfactin synthetase operon of B. methylotrophicus CAU B946. Although the surfactin biosynthesis genes cloned from wild-type B. subtilis subsp. krictiensis are highly homologous to the surfactin synthetase in B. methylotrophicus CAU B946 [25, 37], B. subtilis subsp. krictiensis has already been shown to produce various iturin compounds by NMR and MS analyses and amino acid composition determination [27, 28] as well as several surfactins. Moreover, the in vitro antifungal activities of iturin and surfactin against F. oxysporum clearly differed (Fig 2A). Based on the differences in antifungal activity and the high levels of sequence homology between wild-type B. subtilis subsp. krictiensis and B. methylotrophicus CAU B946, we decided to further investigate the putative surfactin biosynthesis genes from the wild-type B. subtilis subsp. krictiensis strain to determine whether these genes were directly involved in surfactin biosynthesis.
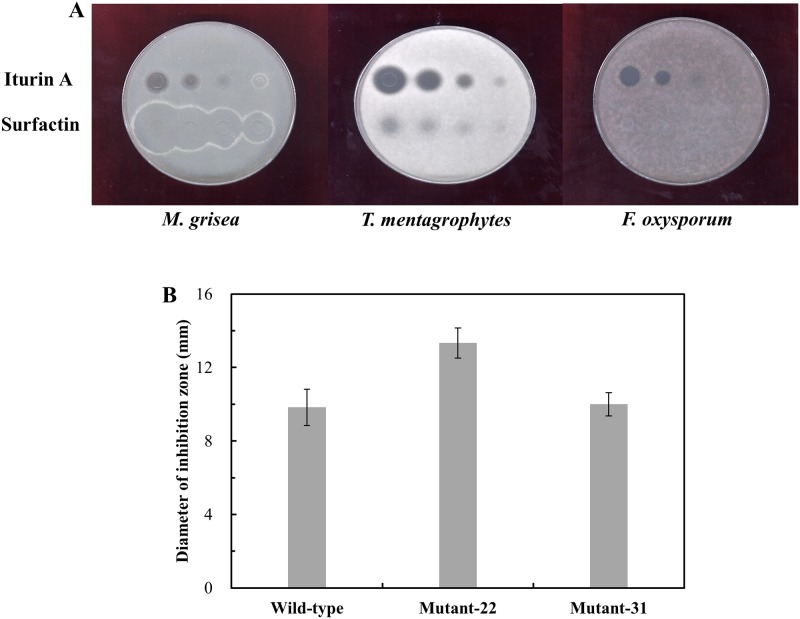
Fig 2. Comparison of the antifungal activities of the wild-type and surfactin-less mutants B. subtilis subsp. krictiensis ATCC55079 strains against F. oxysporum.
(A) Comparison of the antifungal activities of authentic iturin (top) and surfactin (bottom) against various fungi. Two-fold serially dilutions of iturin and surfactin were loaded into PDA plates containing M. grisea (concentration range, 1.56–12.5 μg/ml; left), F. oxysporum (6.25–50 μg/mL; right), and a Sabouraud plate containing T. mentagrophytes (3.12–25 μg/mL; center). (B) Comparison of the antifungal activities of the wild-type, mutant-22, and mutant-31strains. Antifungal activities against F. oxysporum were examined using culture broth (250 μL) from each strain. Data are expressed as means ± SD for separate experiments performed in sextuplicate.
Construction of a surfactin-less B. subtilis subsp. krictiensis mutant
To disrupt the srfAB gene in B. subtilis subsp. krictiensis, the pJJ121E2-1 plasmid containing the spectinomycin resistance gene was constructed by homologous recombination. The pJJ121E2-1 plasmid was transformed into wild-type B. subtilis subsp. krictiensis, and several transformants that grew on LB agar plates containing spectinomycin (which should be srfAB disruption mutants) were selected. The antifungal activities of these transformants against F. oxysporum were examined. Two colonies were finally selected, which were designated mutant-22 and -31, and were used for further studies. The mutant-22 and -31 showed inhibition zones of average 13.3 ± 0.8 mm and 10.0 ± 0.6 mm against F. oxysporum, respectively, whereas the wild-type strain showed zones of average 9.8 ± 0.9 mm. Interestingly, mutant-22 showed significantly higher antifungal activities against F. oxysporum than the wild-type B. subtilis subsp. krictiensis strain (Fig 2B), whereas mutant-31 showed antifungal activity similar to that of the wild-type strain. The antifungal activities of commercially available iturin A and surfactin against F. oxysporum were examined as positive controls. As shown in Fig 2A, authentic iturin A showed potent, dose-dependent, inhibitory activity against F. oxysporum in the range of 6.25 to 50 μg/mL, whereas surfactin exhibited no inhibitory activity. This result suggested that the antifungal activity of wild-type B. subtilis subsp. krictiensis was due to iturin, whereas the increase in the antifungal activity of mutant-22 was caused by disruption of the surfactin biosynthesis genes.
To confirm the double-crossover in the chromosome of the mutant-22 and -31 strains, genomic DNAs were probed with a spectinomycin resistance gene fragment in a Southern hybridization. For the mutant-22 strain, a DNA band was detected, migrating at the same size as the spectinomycin resistance gene (1.5 kb), whereas no band was detected for wild-type B. subtilis subsp. krictiensis DNA (S1 Fig), which confirmed that the spectinomycin resistant gene was inserted into the chromosomal DNA of the mutant-22 strain. However, for mutant-31, the DNA band detected with spectinomycin resistance gene probe showed a different migration rate, suggesting that the spectinomycin gene was incorrectly inserted into the chromosomal DNA of this strain.
Comparative analysis of the secondary metabolites in the wild-type and mutant-22 strains by HPLC and LC/MS
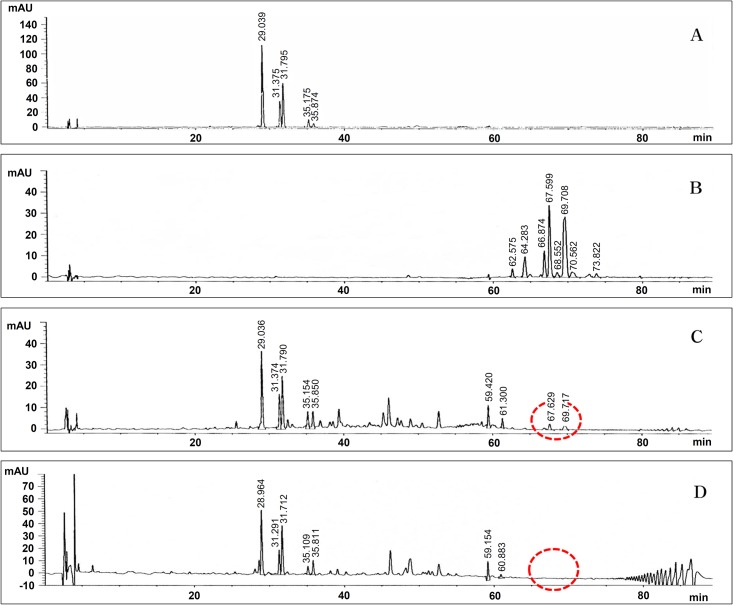
To examine the differences in the cyclic lipopeptides produced by the wild-type and mutant-22 B. subtilis subsp. krictiensis strains, the secondary metabolites extracted from the culture broth of these two strains were analyzed by HPLC. Six iturin compounds peaks were detected in the wild-type strain, and the patterns of these peaks were very similar to those of commercially available authentic iturin A (Fig 3A and 3C) and the same as those of iturin A–F (molecular weights of 1042, 1056, 1056, 1070, 1070, and 1084, respectively), which were previously isolated and identified by various instrumental analyses in our laboratory [27]. Several iturin peaks with the same retention times as the wild-type strain were also observed in mutant-22. However, the small amounts of two surfactin peaks with retention times of 67 and 69 min detected in the wild-type strain were not detected in the mutant-22 strain (Fig 3D), and iturin production by this strain was slightly higher than that of the wild-type strain when the same amount of butanol extract was used in the HPLC analysis (Fig 3D).
Fig 3. Qualitative HPLC analyses of the iturin and surfactin compounds produced by the wild-type and mutant-22 B. subtilis subsp. krictiensis strains.
A: Authentic iturin A (500 μg/mL), B: Authentic surfactin (500 μg/mL), C: Wild-type B. subtilis subsp. krictiensis ATCC55079, D: Surfactin-less mutant-22 strain.
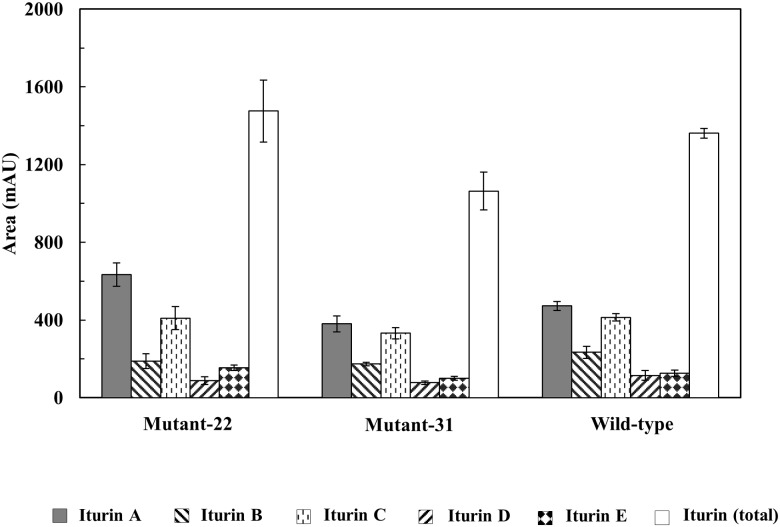
To analyze the amount of iturin production by the mutant-22 strain, in which a spectinomycin resistance gene was inserted into srfAB, the HPLC peak areas of iturin A to E in the wild-type and mutants strains were compared. Iturin F peak areas producing very small amounts in these strains were excluded in the comparative analysis. Interestingly, iturin A production by the mutant-22 strain was markedly higher (by 30 percent), whereas the total iturin production by the mutant-22 strain was just slightly higher than that of the wild-type strain (Fig 4).
Fig 4. Quantitative analyses of iturin A–E produced by wild-type B. subtilis subsp. krictiensis and the surfactin-less mutants-22 and -31 strains.
Data are expressed as means ± SD for separate experiments in quadruplicate.
In addition, iturin E production was also 20 percent higher, while the iturin B, C, and D production by the mutant strain was lower than that of the wild-type strain. Among these iturins, the iturin A, D, and E show strong antifungal activities [40, 41], whereas iturin B and C have no antifungal activity [41]. This suggests that the increased antifungal activity against F. oxysporum of mutant-22 (Fig 2B) was due to the increase in iturin A production, considering the small amount of iturin E produced.
To further investigate the iturin and surfactin peaks detected in the wild-type B. subtilis subsp. krictiensis strain, the molecular weights of these peaks were determined by LC-MS. The mass spectra of the six iturin peaks (iturin A–F) detected by HPLC showed quasi-molecular ion peaks [M+H]+ at m/z 1,043.5, 1,057.5, 1,057.5, 1,071.5, 1,071.5, and 1,085.5 (Table 2; see S2–S8 Figs), corresponding to molecular weights of 1,042, 1,056, 1,056, 1,070, 1,070, and 1,084, respectively, which was 14 mass units higher than the values of iturin A. The molecular weights of these peaks corresponded to the previously reported molecular masses of iturin A–F [27, 28]. In addition, the mass spectra of various surfactin peaks showed quasi-molecular ion peaks [M+H]+ at m/z 1,008.4, 1,022.5, 1,022.5, and 1,036.5), corresponding to molecular weights of 1,007, 1,021, 1,021, and 1,035, respectively (Table 2; see S9–S13 Figs). These results suggested that the identified gene clusters in the genome of B. subtilis subsp. krictiensis were involved in surfactin biosynthesis, even though the mutant-22 strain showed higher antifungal activity against F. oxysporum than the wild-type strain.
Table 2. Cyclic lipopeptide products of the wild-type B. subtilis subsp. krictiensis ATCC55079 strain as detected by LC-MSa.
| Product and observed mass peaks (m/z) | Retention time (min) | Assignment | |
|---|---|---|---|
| Iturin | 1043.5, 1041.5 | 22.61 | C14- Iturin A [M + H]+, [M - H]- |
| 1057.5, 1055.4 | 24.14 | C15- Iturin B [M + H]+, [M - H]- | |
| 1057.5, 1055.5 | 26.15 | C15- Iturin C [M + H]+, [M - H]- | |
| 1071.5, 1069.5 | 26.44 | C16- Iturin D [M + H]+, [M - H]- | |
| 1071.5, 1069.5 | 27.20 | C16- Iturin E [M + H]+, [M - H]- | |
| 1085.5, 1083.5 | 28.07 | C17- Iturin F [M + H]+, [M - H]- | |
| Surfactin | 1008.5, 1006.6 | 66.05 | C13- surfactin [M + H]+, [M - H]- |
| 1022.5, 1020.7 | 70.88 | C14- surfactin [M + H]+, [M - H]- | |
| 1022.5, 1020.7 | 71.93 | C14- surfactin [M + H]+, [M - H]- | |
| 1036.5, 1034.5 | 75.23 | C15- surfactin [M + H]+, [M - H]- | |
Sequencing and organization of iturin biosynthesis genes from wild-type B. subtilis
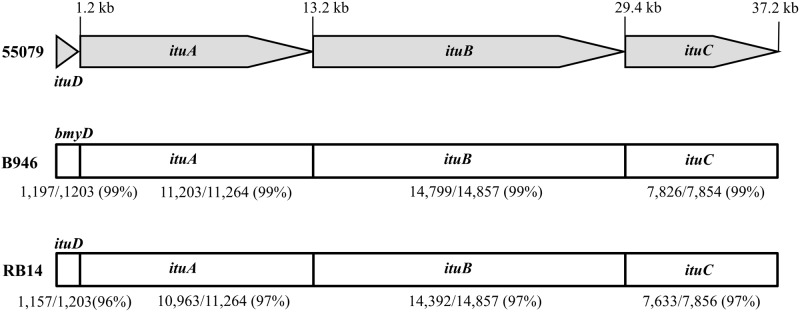
We sequenced the whole genome of B. subtilis subsp. krictiensis to identify the iturin biosynthesis genes by next generation sequencing. The sequence of the iturin biosynthesis genes in B. subtilis subsp. krictiensis was 37,249 bp in length and contained four iturin biosynthesis genes (Fig 5). ItuD, which is 1,203 bp in length, and showed 96% and 99% homology to the malonyl-CoA transacylase gene of B. subtilis RB14, which contains a complete 38-kb iturin A operon [17], and B. methylotrophicus CAU B946 [25, 37], respectively. The next gene, designated ituA, was 11,951 bp in length and exhibited 97% and 99% homology to the iturin synthetase A of B. subtilis RB14 and B. methylotrophicus CAU B946, respectively. The next gene, designated ituC, which is located in 89 bp downstream of ituB and is 7,853 bp in length, showed 97% and 99% homology to iturin synthetase C genes of B. subtilis RB14 and B. methylotrophicus CAU B946 (Fig 5), respectively. The sequences of ituD, ituA, ituB, and ituC in B. subtilis subsp. Krictiensis showed relatively high homologies (97–99%) to the iturin synthetase operons of B. subtilis RB14 and B. methylotrophicus CAU B946. Although the iturin biosynthesis genes from B. subtilis subsp. krictiensis are more highly homologous to the iturin synthetase in B. methylotrophicus CAU B946 than to those of B. subtilis RB14, the slight differences in the sequences of the ituA and ituB genes, which were 11,952 and 14,858 bp in length, respectively, may reflect a species-specific difference between the two strains.
Fig 5. Sequence homologies of the iturin synthetase genes from B. subtilis subsp. krictiensis ATCC55079 with other iturin synthetase genes.
The organization and positions of the homologous gene clusters in B. subtilis RB14 and B. methylotrophicus CAU B946 were drawn by referring to references 17 and 25.
Discussion
B. subtilis subsp. krictiensis ATCC55079 produces the potent antifungal cyclic lipopeptides iturin A–F [27, 28]. Recently, we showed, through HPLC and LC/MS analyses, that this strain also produces a small amount of surfactin. Here, we described the identification of the surfactin and iturin biosynthesis genes in this strain, which are located in an approximately 37-kb region. The identified surfactin genes included four ORFs that we designated srfAA, srfAB, srfAC, and srfAD. Each ORF showed relatively high homology (74–99%) to the surfactin synthetase genes of B. subtilis 168 [26] and B. methylotrophicus CAU B946, which was recently in a genome announcement [25, 37]. To obtain evidence that these putative genes are involved in surfactin biosynthesis, we disrupted srfAB in B. subtilis subsp. krictiensis ATCC55079 using a mini-Tn10 transposon-bearing plasmid. The resulting mutant strain (mutant-22) did not produce surfactin, but produced significantly more iturin A than the wild-type strain (Fig 2B). Based on the loss of surfactin production observed in the mutant-22 strain (Fig 3D), we concluded that the putative surfactin biosynthesis genes identified in this study are indispensable for the production of several surfactins.
SrfAA, srfAB, and srfAC encode the condensation and adenylation domains of the large peptide synthetase responsible for the biosynthesis of the peptide chain, as has been observed for other cyclic lipopeptide antibiotics [25, 26], and srfAD encodes the thioesterase domain. In addition, other ORFs, including the aspartate aminotransferase gene (aspAT), 4′-phosphopantetheine transferase gene (sfp), and yczE, which encodes the inner membrane protein that regulates antibiotic production, are also located adjacent to srfAD. These observations are consistent with a previous report that the 4′-phosphopantetheine transferase (sfp) gene, which is defective in the surfactin-producing B. subtilis 168 strain, is essential for the production of iturin A and surfactin in B. subtilis RB14 [42, 43] and fengycin in B. amyloliquefaciens FZB42 [44]. Moreover, they are also in agreement with previous results showing that sfp and yczE encode essential factors for the production of bacillomycin D, a member of the iturin family, in B. amyloliquefaciens FZB42 [21, 44]. These results suggest that the production of non-ribosomal cyclic lipopeptide antibiotics in Bacillus might be controlled by a common regulatory system [43]. However, the modules in the conserved domains of these surfactin biosynthesis genes were more similar to those in the surfactin operons of B. methylotrophicus CAU B946 [25, 37] than those of B. subtilis 168 [26]. In contrast, the iturin biosynthesis genes of B. subtilis subsp. krictiensis are also closely related, but not identical, to those of B. methylotrophicus CAU B946 and B. subtilis RB14, which contains a complete 38-kb iturin A operon. Based on these results, the different homologies among the iturin and surfactin biosynthesis genes in B. subtilis subsp. krictiensis, B. subtilis RB14, B. subtilis 168, and B. methylotrophicus CAU B946 might be due to differences in species specificity.
Because the surfactin-less mutant-22 strain, which has a disrupted surfactin biosynthesis gene, exhibited a 30% increase in iturin A production compare to that in the wild-type strain, iturin biosynthesis in B. subtilis subsp. krictiensis might also occur through an alternative pathway other than the one involving genes encoded by the iturin A and surfactin operons previously described [17, 26].
Although we do not have any experimental data on the regulatory mechanism underlying the increased iturin production in the surfactin-less mutant strain, we propose two possible explanations. First, the enhanced iturin production in the mutant strain might be due to increased iturin biosynthesis using the substrate remaining in medium after blocking surfactin biosynthesis. Second, positive or negative regulators of cyclic lipopeptides, such as the ComP/ComA two-component system, DegU, Sfp or YczE, PerR, and Rap proteins and Phr peptides, might be involved in a mechanism that increases iturin production in the surfactin-less mutant strain. ComP/ComA, Sfp, PerR, and Phr are positive regulators of srfA transcription [11, 45–48], and overexpression of the comA and sigA genes was shown to improve iturin production [49]. In addition, DegU and YczE positively regulate the synthesis of bacillomycin D in Bacillus amyloliquefaciens strain [50]. Thus, changes in the expression levels of regulators of the iturin operon may enhance iturin production in the surfactin-less mutant. However, at present, identification of the regulators responsible for the increased iturin production in the surfactin-less mutant strain and the detailed mechanism remain to be investigated.
This is the first report of increased antifungal activity in a surfactin-less mutant, which contains a disrupted surfactin biosynthesis gene, which is involved in iturin and surfactin production. However, the ORFs responsible for surfactin biosynthesis in B. subtilis subsp. krictiensis showed low level homology to the surfactin operon of B. subtilis 168 [26] and high level homology to the surfactin synthetase of B. methylotrophicus CAU B946 [25, 37]. In addition, B. subtilis subsp. krictiensis exhibited high level homologies to the iturin operons of B. subtilis RB14 and B. methylotrophicus CAU B946. We confirmed that the production of these two cyclic lipopeptide antibiotics (iturin and surfactin) may utilize a common pathway up to the previously established steps, which could provide an alternative approach for cloning genes for the production of nonribosomal cyclic lipopeptide antibiotics. Any interaction between the iturin and surfactin biosynthesis genes in Bacillus strains producing both iturin and surfactin in the production of cyclic lipopeptides needs to be investigated in future studies.
Supporting information
Lanes: 1, genomic DNA from wild-type B. subtilis subsp. krictiensis digested with ClaI; 2, 3, 21, 22, 23, and 31, genomic DNAs from various transformants digested with ClaI; pJJ121E2-1, the spectinomycin resistance gene from the mini-Tn10 of pIC333 digested with XbaI and BamHI.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Acknowledgments
We thank the Korea Basic Science Institute (Seoul) for performing the LC/MS analyses. The Institute did not provide any funding.
Data Availability
The nucleotide sequences of the iturin and surfactin biosynthesis genes from B. subtilis subsp. krictiensis have been deposited in GenBank (accession numbers KU170613 and KC454625, respectively).
Funding Statement
This work was supported by a grant from the Bioindustry Technology Development Program funded by the Ministry of Agriculture, Food, and Rural Affairs (Grant No. 111048-03) to SUK. The funder had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. Green Biotech Co. provided support in the form of salaries for author MSC, but did not have any additional role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific role of this author is articulated in the ‘author contributions’ section.
References
- 1.Fernández-Ortuño D, Pérez-Garcia A, López-Ruiz F, Romero D, De Vicente A, Torés JA, et al. Occurrence and distribution of resistance to QoI fungicides in populations of Podosphaera fusca in south central Spain. Eur J Plant Pathol. 2006; 115: 215–222. [Google Scholar]
- 2.McGrath MT. Fungicide resistance in cucurbit powdery mildew: Experience and challenges. Plant Dis. 2001; 85: 236–245. [DOI] [PubMed] [Google Scholar]
- 3.Kiss L. A review of fungal antagonists of powdery mildews and their potential as biological agents. Pest Manage Sci. 2003; 59: 475–483. [DOI] [PubMed] [Google Scholar]
- 4.De Boer M, Bom P, Kindt F, Keurentjes JJB, Van Der Sluis I, Van Loon LC, et al. Control of Fusarium wilt of radish by combining Pseudomonas putida strains that have different disease-suppressive mechanisms. Phytopathology 2003; 93: 626–632. doi: 10.1094/PHYTO.2003.93.5.626 [DOI] [PubMed] [Google Scholar]
- 5.Cazorla FM, Duckett SB, Bergström ET, Noreen S, Odijk R, Lugtenberg BJJ, et al. Biocontrol of avocado dematophora root rot by antagonistic Pseudomonas fluorescens PCL1606 correlates with the production of 2-hexyl 5-propyl resorcinol. Mol Plant Microbe Interact. 2006; 19: 418–428. doi: 10.1094/MPMI-19-0418 [DOI] [PubMed] [Google Scholar]
- 6.Romero D, De Vicente A, Rakotoaly RH, Dufour SE, Veening JW, Arrebola E, et al. The iturin and fengycin families of lipopeptides are key factors in antagonism of Bacillus subtilis toward Podosphaera fusca. Mol Plant Microbe Interact. 2007; 20: 430–440. doi: 10.1094/MPMI-20-4-0430 [DOI] [PubMed] [Google Scholar]
- 7.Leclère V, Bécher M, Adam A, Guez JS, Wathelet B, Ongena M, et al. Mycosubtilin overproduction by Bacillus subtilis BBG100 enhances the organism’s antagonistic and biocontrol activities. Appl Environ Microbiol. 2005; 71: 4577–4584. doi: 10.1128/AEM.71.8.4577-4584.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arrebola E, Jacobs R, Korsten L. Iturin A is the principal inhibitor in the biocontrol activity of Bacillus amyloliquefaciens PPCB004 against postharvest fungal pathogens. J Appl Microbiol. 2010; 108: 386–395. doi: 10.1111/j.1365-2672.2009.04438.x [DOI] [PubMed] [Google Scholar]
- 9.Sharma RR, Singh D, Singh R. Biological control of postharvest diseases of fruits and vegetables by microbial antagonist: A review. Biol Control. 2009; 50: 205–221. [Google Scholar]
- 10.Ongena M, Jacques P. Bacillus lipopeptides: versatile weapons for plant disease biocontrol. Trends Microbiol. 2007; 16: 115–125. [DOI] [PubMed] [Google Scholar]
- 11.Roongsawang N, Washio K, Morikawa M. Diversity of nonribosomal peptide synthetases involved in the biosynthesis of lipopeptide biosurfactants. Int J Mol Sci. 2011; 12: 141–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peypoux F, Guinand M, Michel G, Delcambe L, Das BC, Lederer E. Structure of iturin A, a peptidolipid antibiotic from Bacillus subtilis. Biochemistry 1978; 17: 3992–3996. [DOI] [PubMed] [Google Scholar]
- 13.Umezawa H, Aoyagi T, Nishikiori T, Okuyama A, Yamagishi Y, Hamada M, et al. Plipastatins: new inhibitors of phospholipase A2, produced by Bacillus cereus BMG202-fF67. I. Taxonomy, production, isolation and preliminary characterization. J Antibiot. 1986; 39: 737–744. [DOI] [PubMed] [Google Scholar]
- 14.Peypoux F, Bonmatin JM, Wallach J. Recent trends in the biochemistry of surfactin. Appl Microbiol Biotechnol. 1999; 51: 553–563. [DOI] [PubMed] [Google Scholar]
- 15.Duitman E H, Hamoen L. W., Rembold M, Venema G, Seitz H, Saenger W, et al. The mycosubtilin synthetase of Bacillus subtilis ATCC6633: A multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. Proc Natl Acad Sci. 1999; 96: 13294–13299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moyne AL, Cleveland TE, Tuzun S. Molecular characterization and analysis of the operon encoding the antifungal lipopeptide bacillomycin D. FEMS Microbiol Lett. 2004; 234: 43–49. doi: 10.1016/j.femsle.2004.03.011 [DOI] [PubMed] [Google Scholar]
- 17.Tsuge K, Akiyama T, Shoda M. Cloning, sequencing, and characterization of the iturin A operon. J Bacteriol. 2001; 183: 6265–6273. doi: 10.1128/JB.183.21.6265-6273.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magnet-Dana R, Thimon L, Peypoux F, Ptak M. Surfactin/iturin A interactions may explain the synergistic effect of surfactin on the biological properties of iturin A. Biochimie. 1992; 74: 1047–1051. [DOI] [PubMed] [Google Scholar]
- 19.Ongena M, Jourdan E, Adam A, Paquot M, Brans A, Joris B, et al. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ Microbiol. 2007; 9: 1084–1090. doi: 10.1111/j.1462-2920.2006.01202.x [DOI] [PubMed] [Google Scholar]
- 20.Roongsawang N, Thaniyavarn J. Isolation and characterization of a halotolerant Bacillus subtilis BBK-1 which produces three kinds of lipopeptides: bacillomycin L, plipastatin, and surfactin. Extremophiles 2002; 6: 499–506. doi: 10.1007/s00792-002-0287-2 [DOI] [PubMed] [Google Scholar]
- 21.Koumoutsi A, Chen XH, Henne A, Liesesang H, Hitzeroth G, Franke P, et al. Structural and functional characterization of gene clusters directing nonribosomal synthesis of bioactive cyclic lipopeptides in Bacillus amyloliquefaciens strain FZB42. J Bacteriol. 2004; 186: 1084–1096. doi: 10.1128/JB.186.4.1084-1096.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel H, Tscheka C, Edwards K, Karlsson G, Heerklotz H. All-or-none membrane permeabilization by fengycin-type lipopeptides from Bacillus subtilis QST713. Biochem Biophys Acta 2011;1808: 2000–2008. doi: 10.1016/j.bbamem.2011.04.008 [DOI] [PubMed] [Google Scholar]
- 23.Huang CC, Ano T, Shoda M. Nucleotide sequence and characteristics of the gene, lpa-14, responsible for biosynthesis of the lipopeptide antibiotics iturin A and surfactin from Bacillus subtilis RB14. J Ferment Bioeng. 1993; 76: 445–450. [Google Scholar]
- 24.Yao S, Gao X, Fuchsbauer N, Hillen W, Vater J, Wang J. Cloning, sequencing, and characterization of the genetic region relevant to biosynthesis of the lipopeptides iturin A and surfactin in Bacillus subtilis. Curr Microbiol. 2003; 47: 272–277. [DOI] [PubMed] [Google Scholar]
- 25.Blom J, Rueckert C, Niu B, Wang Q, Boriss R. The complete genome of Bacillus amyloliquefaciens subsp. plantarum CAU B946 contains a gene cluster for nonribosomal synthesis of iturin A. J Bacteriol. 2012; 194: 1845–1846. doi: 10.1128/JB.06762-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kunst F, Ogasawara N, Moszer I, Albertini AM, Alloni G, Azevedo V, et al. The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature 1997; 390: 249–256. doi: 10.1038/36786 [DOI] [PubMed] [Google Scholar]
- 27.Kim SK, Lee NK, Jeong TS, Kim YK, Choi JJ, Bok SH. Structure determination of antifungal KRF-001 produced by Bacillus subtilis subsp. krictiensis. Kor J Appl Microbiol Biotechnol. 1991; 19: 598–603. [Google Scholar]
- 28.Bok SH, Kim SU, Son KH, Kim SK, Kim YK, Lee HW, et al. Culture of Bacillus subtilis. US patent 5,155,041. 1992.
- 29.Kim SU, Lee JW, Lee SH, Bok SH. Identification of bacteria having antifungal activity isolated from soils and its biological activity. Kor J Appl Microbiol Biotechnol. 1991; 19: 337–342. [Google Scholar]
- 30.Cutting SM, Vander Horn PB. Genetic analysis In: Harwood CR, Cutting SM, editors. Molecular Biological Methods for Bacillus. John Wiley & Sons; 1990. pp. 65–548 [Google Scholar]
- 31.Mead DA, Szczsna-Skorupa E, Kemper B. Single-stranded DNA blue T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986; 1: 67–74. [DOI] [PubMed] [Google Scholar]
- 32.Staskawicz B, Dahlbeck D, Keen N, Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987; 169: 5789–5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinmetz M, Richter R. Easy cloning of mini-Tn10 insertions from the Bacillus subtilis chromosome. J Bacteriol. 1994; 176: 1761–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual, 2nd ed Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1989. pp. 9.31–9.57. [Google Scholar]
- 35.Finn RK. Theory of agar diffusion methods for bioassay. Anal Chem. 1959; 31: 975–977. [Google Scholar]
- 36.Grover M, Nain L, Singh SB, Saxena AK. Molecular and biochemical approaches for characterization of antifungal trait of a potent biocontrol agent Bacillus subtilis RP24. Curr Microbiol. 2010; 60: 99–106. doi: 10.1007/s00284-009-9508-6 [DOI] [PubMed] [Google Scholar]
- 37.Dunlap CA, Kim SJ, Kwon SW, Rooney AP. Phylogenomic analysis shows that Bacillus amyloliquifaciens subsp. plantarum is a later heterotypic synonym of Bacillus methylotrophicus. IntJ Syst Evol Microbiol. 2015; 65: 2104–2109. [DOI] [PubMed] [Google Scholar]
- 38.Yasueda H, Kawahara Y, Sugimoto S. Bacillus subtilis yckG and yckF encode two key enzymes of the ribulose monophosphate pathway used by methylotrophs, and yckH is required for their expression. J Bacteriol. 1999; 181: 7154–7160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Earl AM, Eppinger M, Fricke Wf, Rosovitz MJ, Rasko DA, Daugherty S, et al. Whole-genome sequence of Bacillus subtilis and close relatives. J Bacteriol. 2012; 194: 2378–2379. doi: 10.1128/JB.05675-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Besson F, Michel G. Isolation and characterization of new iturins: iturin D and iturin E. J Antibiot. 1987; 40: 437–442. [DOI] [PubMed] [Google Scholar]
- 41.Besson F, Peypoux F, Michel G, Delcambe L. Identification of antibiotics of iturin group in various strains of Bacillus subtilis. J Antibiot. 1978; 31: 284–288. [DOI] [PubMed] [Google Scholar]
- 42.Tsuge K, Inoue S, Ano T, Itaya M, Shoda M. Horizontal transfer of iturin A operon, itu, to Bacillus subtilis 168 and conversion into an iturin A producer. Antimicrob Agents Chemother. 2005; 49: 4641–4648. doi: 10.1128/AAC.49.11.4641-4648.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huang CC, Ano T, Shoda M. Nucleotide sequence and characteristics of the gene, lpa-14, for biosynthesis of the lipopeptide antibiotics iturin A and surfactin from Bacillus subtilis RB14. J Ferm Bioeng. 1993; 76: 445–450. [Google Scholar]
- 44.Chen XH, Koumoutsi A, Scholz R, Eisenreich A, Schneider K, Heinemeyer I, et al. Comparative analysis of the complete genome sequence of the plant growth-promoting bacterium Bacillus amyloliquefaciens FZB42. Nature Biotechnol. 2007; 25: 1007–1014. [DOI] [PubMed] [Google Scholar]
- 45.Wang X, Luo C, Liu Y, Nie Y, Liu Y, Zhang R, Chen Z. Three non-aspartate amino acid mutations in the ComA response regulator receiver motif severely decrease surfactin production, competence development, and spore formation in Bacillus subtilis. J Microbiol Biotechnol. 2010; 20: 301–310. [PubMed] [Google Scholar]
- 46.Quadri LE, Weinreb PH, Lei M, Nakano MM, Zuber P, Walsh CT. Characterization of Sfp, a Bacillus subtilis phosphopantetheinyl transferase for peptidyl carrier protein domains in peptide synthetases. Biochemistry 1998; 37: 1585–1595. doi: 10.1021/bi9719861 [DOI] [PubMed] [Google Scholar]
- 47.Hayashi K, Ohsawa T, Kobayashi K, Ogasawara N, Ogura M. The H2O2 stress-responsive regulator PerR positively regulates srfA expression in Bacillus subtilis. J Bacteriol. 2005; 187: 6659–6667. doi: 10.1128/JB.187.19.6659-6667.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yang Y, Wu HJ, Lin L, Zhu QQ, Borriss R, Gao XW. A plasmid-born Rap-Phr system regulates surfactin production, sporulation and genetic competence in the heterologous host, Bacillus subtilis OKB105. Appl Microbiol Biotechnol. 2015; 99: 7241–7252. doi: 10.1007/s00253-015-6604-3 [DOI] [PubMed] [Google Scholar]
- 49.Zhang Z, Ding ZT, Zhong J, Zhou JY, Shu D, Luo D, Yang J, Tan H. Improvement of iturin A production in Bacillus subtilis ZK0 by overexpression of the comA and sigA genes. Lett Appl Microbiol. 2017; 64: 452–458. doi: 10.1111/lam.12739 [DOI] [PubMed] [Google Scholar]
- 50.Koumoutsi A, Chen XH, Vater J, Borriss R. DegU and YczE positively regulate the synthesis of bacillomycin D by Bacillus amyloliquefaciens strain FZB42. Appl Environ Microbiol. 2007; 73: 6953–6964. doi: 10.1128/AEM.00565-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lanes: 1, genomic DNA from wild-type B. subtilis subsp. krictiensis digested with ClaI; 2, 3, 21, 22, 23, and 31, genomic DNAs from various transformants digested with ClaI; pJJ121E2-1, the spectinomycin resistance gene from the mini-Tn10 of pIC333 digested with XbaI and BamHI.
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
(DOCX)
Data Availability Statement
The nucleotide sequences of the iturin and surfactin biosynthesis genes from B. subtilis subsp. krictiensis have been deposited in GenBank (accession numbers KU170613 and KC454625, respectively).