Abstract
Introduction
Despite evidence of the high prevalence of antibiotic resistant infections in developing countries, studies on the clinical and economic impact of antibiotic resistance (ABR) to inform interventions to contain its emergence and spread are limited. The aim of this study was to analyze the published literature on the clinical and economic implications of ABR in developing countries.
Methods
A systematic search was carried out in Medline via PubMed and Web of Sciences and included studies published from January 01, 2000 to December 09, 2016. All papers were considered and a quality assessment was performed using the Newcastle-Ottawa quality assessment scale (NOS).
Results
Of 27 033 papers identified, 40 studies met the strict inclusion and exclusion criteria and were finally included in the qualitative and quantitative analysis. Mortality was associated with resistant bacteria, and statistical significance was evident with an odds ratio (OR) 2.828 (95%CI, 2.231–3.584; p = 0.000). ESKAPE pathogens was associated with the highest risk of mortality and with high statistical significance (OR 3.217; 95%CIs; 2.395–4.321; p = 0.001). Eight studies showed that ABR, and especially antibiotic-resistant ESKAPE bacteria significantly increased health care costs.
Conclusion
ABR is associated with a high mortality risk and increased economic costs with ESKAPE pathogens implicated as the main cause of increased mortality. Patients with non-communicable disease co-morbidities were identified as high-risk populations.
Introduction
Antimicrobial resistance (AMR) is the ability of bacteria, parasites, viruses and fungi to grow and spread in the presence of antimicrobial medicines that are normally active against them. AMR occurs via a range of resistance mechanisms, such as a modified antimicrobial target, enzymatic hydrolysis/degradation, efflux and impermeability. This resistance is mediated by diverse resistance genes that evolve as a result of antimicrobial selection pressure exerted by the appropriate and/or inappropriate use of antimicrobial medicines, and is aggravated by the void of new antimicrobial agents in the current therapeutic pipeline [1, 2]. AMR increases health-care costs, length of stay in hospitals, morbidity and mortality in both developed and developing countries [3]. A recent report estimated that 10 million deaths will be attributed to AMR by 2050, and 100 trillion USD of the world’s economic outputs will be lost if substantive efforts are not made to contain this threat [1, 4, 5].
The World Health Organization (WHO) published the first global surveillance report on antibiotic resistance (ABR) in 2014 to show the clinical impact of resistant bacteria in WHO regions across the world. This reported shown that five out of the six WHO regions had more than 50% resistance to third generation cephalosporins and fluoroquinolones in Escherichia coli and methicillin resistance in Staphylococcus aureus in hospital settings. Similarly, more than 50% resistance to third generation cephalosporins and carbapenems was reported in Klebsiella pneumoniae. The report attributed 45% of deaths in both Africa and South-East Asia to multi-drug resistant (MDR) bacteria. It further revealed that K. pneumoniae resistant to third generation cephalosporins was associated with elevated deaths in Africa (77%), the Eastern Mediterranean region (50%), South East Asia (81%) and Western Pacific region (72%) [2].
Several resistant bacteria have been increasingly involved in infectious diseases in humans, specifically, Enterococcus spp, S. aureus, K. pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa and Enterobacter spp. They are collectively termed ESKAPE and recently gained further global attention by being listed by the WHO as priority antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics [5]. The particularity of these bacteria is their ability to develop high level resistance to multiple drugs, thereby limiting therapeutic options and increasing morbidity and mortality. Numerous studies have confirmed that ESKAPE bacteria and their resistant clones, are actively transmitted in hospitals and communities in both developed and developing countries. The threat posed by these resistant bacteria is however exacerbated in developing countries due to sub-optimal hygiene conditions, poor infection, prevention and control measures, lack of surveillance and the dearth antimicrobial stewardship programs [6, 7]. Reports have shown high isolation rates of methicillin resistant S. aureus (MRSA) in healthcare settings in Cameroon (72%), South Africa (52%), Ethiopia (42.8%), Nigeria (29.6%), Kenya (27.7%), Ivory Cost (16.8%) and Morocco (14.4%) [2, 8–10]. In 2008, the prevalence of nosocomial acquired and MDR infections due to Enterobacteriaceae isolated from blood cultures were 57.1% and 15.4% respectively, in South Africa [11]. Likewise, rapid increases in the rates of infections due to carbapenemase-producing K. pneumonia, metallo-beta-lactamase-producing A. baumannii (MBL-AB), metallo-beta-lactamase-producing P. aeruginosa (MBL-PA), and extended-spectrum beta-lactamase (ESBL) producing Enterobacter spp. have been reported across the world [12–14]. In Saudi Arabia, the rate of P. aeruginosa producing carbapenemase was 33%, of which 27% were MBL-producers [15], while in India, a 22.4% prevalence of P. aeruginosa producing MBLs was reported in tertiary care hospitals [16].
MDR-ESKAPE bacteria have been reported in hospital acquired infections (HAI), particularly in intensive care units (ICUs) where immune-compromised patients suffering from some non-communicable diseases (NCDs) including diabetes, cancers, chronic lung, cardiovascular and kidney diseases were highly affected [6, 17–22]. The emergence and spread of these highly resistant bacteria in hospital care settings could thus have negative health repercussions and be an obstacle for the treatment of infections of patients with these NCDs [18, 23].
Despite the evidenced threat posed by ABR, information on its clinical and economic impact is limited in developing countries, and thus impede appropriate interventions for its containment [24, 25]. Heightened awareness of policy-makers, health care workers, and the general population about the risks associated with ABR is essential to preserve antibiotics for future generations [26, 27]. Hence, the aim of this study was to analyze the published literature on the clinical and economic impact of ABR in developing countries, in order to inform containment strategies such as antimicrobial stewardship programs and infection prevention and control measures in these nations.
Methods
The Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines were followed [28, 29].
Ethical consideration
This systematic review and meta-analysis was based on published reports, and was therefore exempt from ethical approval.
Systematic review of the literature
A systematic search was carried out independently by RF and LF, in Medline via PubMed and Web of Sciences from January 2000 to December 09, 2016, using a combination of boolean operators (AND/OR), Medical Subject Heading (MeSH) and pre-defined keywords. Only published after 2000 were considered to ensure that the analysis focuses on contextual literature that depict current resistance patterns, infection rates, prevention measures, and clinical practice guidelines. Peer-reviewed papers in English and French on the clinical and/or economic impacts of ABR in developing countries were retrieved and independently evaluated for eligibility by RF and LF based on titles and abstracts (Table 1). Thereafter, the full-texts of eligible papers were assessed according to pre-defined inclusion and exclusion criteria (Table 1), with inconsistencies and disagreements being resolved by consensus. Efforts were made to contact the authors when data was missing and full-texts could not be retrieved, and a hand search was conducted in the reference list of all selected papers.
Table 1. Eligibility criteria.
| Inclusion criteria |
| - Original research - Minimum of 20 patients - Studies conducted in developing countries as defined by World Bank criteria - Report on association between resistant bacteria and clinical outcome and/or financial impact - Antimicrobial susceptibility testing done by either disk diffusion, broth micro-dilution, agar dilution, E-test or VITEK using - CLSI/EUCAST/SFM guidelines - Papers published in French and English - Studies published from January 1, 2000 |
| Exclusion criteria |
| - Reports of antibiotic resistance unrelated to clinical outcome nor economic impact - Reports on parasites, viruses and fungi - Reports on treatment comparisons - Studies conducted in developed countries as defined by World Bank criteria - Reports published in languages other than French and English - Antibiotic resistance in wildlife, companion and aquatic animals - Grey literature, conference abstracts, reviews, meta-analysis, letters to editor, correspondence, editorials, comments and case reports. - Studies published before January 1, 2000 |
Screening and data extraction process
Papers were managed using EndNote (version X7.7.1, Thomson Reuters) and the data from eligible papers was abstracted independently by two authors (RF and LF) using a standardized data extraction spreadsheet in Excel® (Microsoft® Office Excel 2016). Relevant data from papers included countries, WHO regions, World Bank classification, publication year, type of study, participant characteristics (number of participant, diseases, age), hospital’ ward, bacteria, follow-up period, length of stay in hospital, mortality related to resistant bacteria, and, costs as described in Table 2.
Table 2. Description of eligible papers included in the systematic review.
| Country | Year | Type of study | Study population | Infection type | Hospital’ ward | Bacteria | Sample size cases/ controls |
Length of stay2 (%) |
Mortality3 n/N (%) |
References | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Case group | Control group | Case group | Control group | |||||||||
| STUDIES REPORTING IMPACT OF ABR ON THE MORBIDITY ONLY | ||||||||||||
| Turkey | 2015 | Retrospective cohort | NR | Nosocomial BSI | ICU | A. baumannii | 41/45 | 25.49 days (%NR) | 22.80 days (%NR) | NR | NR | [3] |
| Turkey | 2008 | Prospective case—control | Adults>16 years old | Nosocomial Infections | ICU and others | A. baumannii | 66/57 | 20.8 days (65.2%) | 15.4 days (40.4%) | NR | NR | [30] |
| STUDIES REPORTING IMPACT OF ABR ON THE MORTALITY ONLY | ||||||||||||
| Brazil | 2009 | Retrospective case-control | Adults >14 years old | Nosocomial infections | Medical-surgical ICU | P. aeruginosa | 63/182 | NR | NR | 31/63 (49%) | 61/182 (33%) | [31] |
| Brazil | 2009 | Case-control | Adults > 18 years old | BSI | NR | E. coli and K. pneumoniae | 30/64 | NR | NR | 7/30 (23.3%) |
12/64 (18.8%) |
[32] |
| China | 2004 | Case-control and Retrospective cohort | All ages | MDR-HAI | Various wards1 | P. aeruginosa | 44/68 | NR | NR | 24/44 (54.5%) | 11/68 (16.2%) | [33] |
| China | 2012 | Retrospective | Children < 15 years old | Pneumonia | Pediatric ICU | A. baumannii | 115/45 | NR | NR | 21/115 (18.26%) | 2/45 (4.44%) | [34] |
| China | 2015 | Retrospective Case-Control | NR | MRSA infections | Various | S. aureus | 57/116 | NR | NR | 12/57 (21%) | 9/116 (8%) |
[35] |
| Colombia | 2014 | Case-Control | All ages | CR-KP Infection | ICU | K. pneumoniae | 61/122 | NR | NR | 31/61 (50.8%) | 25/122 (20.4%) | [36] |
| India | 2014 | NR | Neonates | BSI | Neonatal ICU | A. baumannii | 33/32 | NR | NR | 9/33 (27.3%) | 3/32 (9.4%) |
[37] |
| Malaysia | 2009 | Case-control | NR | Nosocomial AB BSI | NR | A. baumannii | 53/56 | NR | NR | 25/53 (47.2%) | 14/56 (25%) | [38] |
| Malaysia | 2011 | Cross-sectional descriptive and case-control | NR | IR-A. baumannii BSI | NR | A. baumannii | 15/41 | NR | NR | 9/15 (64.3%) | 15/41 (40.5%) | [39] |
| Mexico | 2000 | Case-control | Children | Pneumoniae | NR | S. pneumoniae | 25/24 | NR | NR | 11/25 (44%) |
7/24 (29%) |
[40] |
| Thailand | 2011 | Case-control | Adults >15 years old | MDR-A. baumannii bacteremia | In and out-patient departments | A. baumannii | 24/25 | NR | NR | 22/24 (91.7%) | 12/25 (48%) | [41] |
| Thailand | 2012 | Case-control | Adults >15 years old | ESBL-producing bacteria in septicemia |
In and out-patient departments | E. coli | 32/113 | NR | NR | 9/32 (29%) | 13/113 (11.5%) | [42] |
| Thailand | 2015 | Case-control | Adults>18 years old | HAI | ICU and general wards | A. baumannii | 139/132 | NR | NR | 79/139 (57%) |
3/132 (2%) |
[43] |
| Thailand | 2015 | Retrospective cohort | Adults | Ventilator Associated Pneumoniae | ICU | A. baumannii | 220/33 | NR | NR | 125/220 (56.8%) | 7/33 (21.2%) |
[44] |
| STUDIES REPORTING IMPACT OF ABR ON THE MORBIDITY AND MORTALITY | ||||||||||||
| Brazil | 2015 | Case-control | Cancer children <18 years old | MDR-GNB Infection | Oncology pediatric ICU | Gram Negative Bacteria | 47/54 | 8 days (63.8%) | 2 days (37%) | 12/47 (25.5%) | 9/54 (16.7%) | [17] |
| Brazil | 2006 | Retrospective cohort | >1-year-old | BSI | Various wards1 | S. aureus | 61/50 | >10 days (65.9%) | >10 days (34.1%) | 33/61 (54.9%) | 12/50 (24.7%) | [45] |
| Brazil | 2006 | Retrospective cohort | All ages | BSI | Various wards1 | K. pneumoniae | 56/52 | >10 days (56.2%) |
>10 days (43.8%) | 18/56 (69.2%) | 8/52 (30.8%) |
[46] |
| Brazil | 2008 | Case-control | Adults | VAP | ICU | S. aureus | 29/32 | >8 days (89.7%) | >8 days (90.6%) | 11/29 (37.9%) | 8/32 (25%) |
[47] |
| Brazil | 2012 | Case-control | Adults > 18 years old | Bacteremia | ICU | P. aeruginosa | 29/48 | 43 days (NR) | 43.1 days (NR) | 13/29 (44.8%) | 26/48 (54.2%) |
[22] |
| China | 2012 | Retrospective cohort | > 1 year old | BSI | Various wards1 | S. aureus | 75/43 | 55.3 days (NR) | 38.7 days (NR) | 25/75 (33.3%) | 8/43 (18.6%) | [48] |
| China | 2015 | Retrospective | Geriatric inpatients | Bacteremia | Various wards1 | A. baumannii | 39/86 | 36.7 days (NR) |
36.1 days (NR) | 31/39 (79.5%) |
38/86 (44.2%) |
[49] |
| China | 2015 | Retrospective case-control | NR | Enterococci infections | Various wards1 | Enterococci | 44/176 | 37 days (NR) | 17 days (NR) | 3/44 (6.8%) | 3/176 (1.7%) | [50] |
| Colombia | 2014 | Prospective cohort | Adult | CR-A. baumannii Infections | ICU | A. baumannii | 104/61 | 19 days (NR) | 16.2 days (NR) | 42/104 (40%) |
13/61 (21%) | [51] |
| India | 2014 | Observational | Adults | Septicemia | Various wards | GNB and GPB | 133/87 | 14 days (NR) | 11 days (NR) | 16/133 (12%) | 2/87 (2%) |
[52] |
| Jordan | 2015 | Matched case-control | Cancer patients | Nosocomial A. baumannii infections | Medical-surgical ICU | A. baumannii | 161/262 | 12 days (NR) |
3 days (NR) |
118/161 (73.3%) | 142/232 (61.2%) | [53] |
| Palestine | 2009 | Prospective case—control | Neonates | Nosocomial septicemia |
Neonatal ICU | A. baumannii | 40/100 | 20 days (62.5%) |
20 days (35%) |
15/40 (37.5%) | 12/100 (13.2%) | [54] |
| Senegal | 2016 | Classic retrospective cohort and retrospective parallel cohort | All ages | ESBL- producing Enterobacteriaceae | Various wards1 |
K. pneumoniae Enterobacter E. coli |
110/76 | 22.6 days (NR) |
14 days (NR) |
52/110 (47.3%) | 17/76 (22.4%) |
[55] |
| Thailand | 2007 | Prospective case—control | Adults | HAI | Various wards1 | E. coli and K. pneumoniae | 74/74 | 22.5 days (NR) |
17.5 days (NR) |
26/74 (35.1%) | 12/74 (16.2%) | [56] |
| Thailand | 2008 | Cohort | Adults | Community-onset BSI | Various wards1 | E. coli and K. pneumoniae | 36/108 | 8 days (NR) | 6 days (NR) | 13/36 (36%) | 16/108 (15%) | [57] |
| Thailand | 2014 | Retrospective cohort | Adults>18 years old | HAI | Various wards1 | A. nosocomialis and A. pittii | 25/58 | 9 days (NR) | 4 days (NR) | 3/25 (12%) | 20/58 (35%) | [58] |
| Thailand | 2009 | Retrospective cohort | Adult> 15 years old | Nosocomial BSI | Various wards1 | A. baumannii | 67/131 | 37 days (NR) | 27 days (NR) | 35/67 (52.2%) | 26/131 (19.9%) | [59] |
| Thailand | 2006 | Cross-sectional | All ages | Community-acquired pneumoniae | NR | S. pneumoniae | 22/42 | 12.2 days (NR) | 15.5 days (NR) | 2/22 (9.1%) |
4/42 (9.5%) |
[60] |
| Thailand | 2009 | Case-control | Adult>18 years old | Nosocomial BSI | Various wards1 | E. coli and K. pneumoniae | 51/94 | 26 days (NR) | 16 days (NR) | 26/51 (51.0%) | 28/94 (29.8%) | [61] |
| Thailand | 2013 | Retrospective Case-control | Neonates | CR- A. baumannii Bacteremia | Neonatal ICU | A. baumannii | 14/44 | 34 days (NR) |
24.5 days (NR) | 6/14 (42.9%) | 3/44 (5.9%) |
[62] |
| Thailand | 2016 | Retrospective Case-control | Neonates | VAP | Neonatal ICU | A. baumannii | 63/25 | 51 days (NR) | 41 days (NR) |
10/63 (15.9%) |
0/25 (0%) |
[19] |
| Turkey | 2015 | Observational retrospective cohort | All ages | HAI | ICU | K. pneumoniae | 47/51 | 19 days (37.3%) | 11 days (29.94%) | 21/47 (44.7%) |
26/51 (51%) | [63] |
| Turkey | 2000 | Retrospective | Adults | Bacteremia | ICU | S. aureus | 46/55 | 50.3 days (NR) | 32.7 days (NR) | 15/46 (32.6%) |
7/55 (12.7%) |
[21] |
| Turkey | 2015 | NR | NR | Nosocomial infections | Emergency ICU and Pediatric ICU | P. aeruginosa | 32/8 | 20.58 days (NR) | 6.33 days (NR) | 14/32 (43.8%) | 2/8 (25%) | [64] |
LOS: Length of stay; NR: Not reported; BSI: Bloodstream infection, HAI: Hospital-acquired infection, VAP: Ventilator-Associated Pneumoniae; CR: Carbapenem-resistant; GNB: Gram negative bacteria; GPB: Gram positive bacteria
1 various wards
2 LOS attributed to the specific bacteria responsible of the infections
3: Overall mortality attributed to the specific bacteria responsible of the infections, ICU: Intensive Care Unit.
Statistical analysis
Meta-analyses were undertaken using Comprehensive Meta-analysis software (Biostat, Inc., New Jersey, USA) version 3 for Windows, to determine overall mortality risk associated with resistance. Sub-group analyses for mortality were conducted for the data by WHO region, World Bank classification, countries, group of bacteria, and bacterial species where there were three or more studies that could be combined. Forest plots were performed to assess the significance of the results and generated using 95% confidence intervals (CIs). Analyses were undertaken across sub-groups for the selected outcome and the results presented as odds ratios. Studies were weighted in favor of those with narrower confidence intervals (more precise results), and the random-effects method was used to provide more confident data considering heterogeneity within and between reports. The I-square (I2) statistic with cut-off values of 25, 50 and 75% was used to assess low, moderate and high heterogeneity respectively, and a p-value of <0.05 was considered statistically significant. Publication bias was evaluated using the funnel plot and statistical egger’s test.
Quality assessment
Quality assessment was performed independently by RF and LF using the Newcastle-Ottawa quality assessment scale (NOS) for each study included in the systematic review and meta-analysis [65]. NOS assesses methodological quality, based on three-dimensional criteria and included (i) selected population, (ii) comparability of groups, and (iii) outcome/exposure of interest. Studies were scored using a scale with a possible maximum of eight points where a score ≥ 6 indicated high-quality studies, a score between 3–6 as moderate and a score ≤ 3 as low quality.
Results
Literature search and study selection
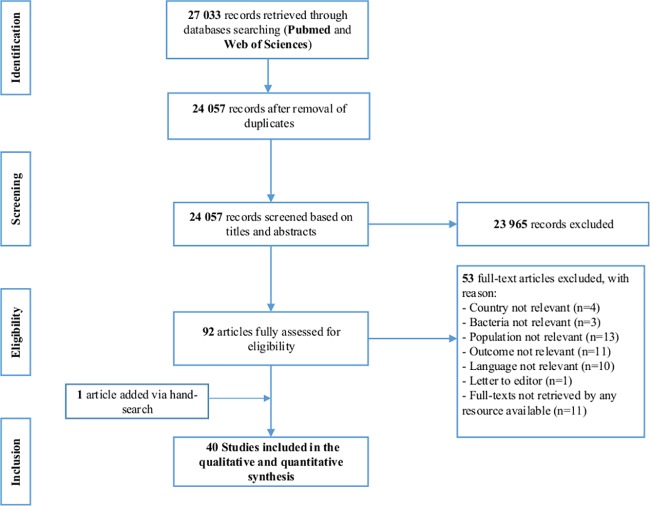
The systematic search conducted in the two electronic databases generated 27 033 papers. A total of 24 057 papers were screened for probable inclusion according to titles and abstracts after de-duplication. Of these, the full texts of 92 eligible papers were fully evaluated based on predefined inclusion and exclusion criteria. One article was added following a hand-search in the reference lists of included papers. Forty studies were finally eligible for the qualitative and quantitative analysis (Fig 1), of which 18 were of high quality, while 15 and seven were moderate and low quality respectively.
Fig 1. Prisma Flow-chart illustrating the study selection process.
Description and characteristics of studies included in systematic review
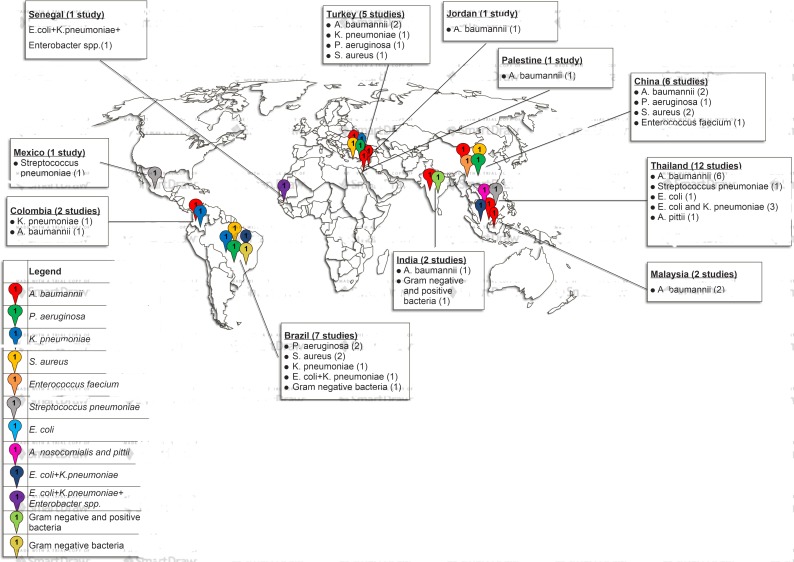
The majority of data analyzed were obtained from single center studies conducted in 11 countries. Thirty percent (n = 12) of the observational studies on ABR were conducted in hospitals and communities in Thailand, the rest were performed in 10 different-countries namely Brazil (n = 7; 17.5%), China (n = 6; 15%), Turkey (n = 5; 12.5%), Colombia (n = 2; 5%), Malaysia (n = 2; 5%), India (n = 2; 5%), Mexico (n = 1; 2.5%), Jordan (n = 1; 2.5%), Palestine (n = 1; 2.5%), and Senegal (n = 1; 2.5%) (Table 2 and Fig 2).
Fig 2. Graphical representation of AMR in developing countries included in the study.
Fourteen studies investigated the impact of ABR on mortality, two reported its impact on morbidity only (Table 2) while 24 considered both morbidity and mortality concomitantly. Eight studies reported on the economic consequences of ABR (Table 3). A. baumannii (n = 14; 35%), K. pneumoniae (n = 6; 15%), S. aureus (n = 5; 12.5%), P. aeruginosa (n = 4; 10%) represented the main pathogens reported with ICUs being the principal hospital ward concerned (Tables 2 and 3).
Table 3. Studies describing mortality rate associated with resistant and MDR ESKAPE bacteria.
| Authors | Hospital Wards | Bacteria | Mortality rate | P-value | References |
|---|---|---|---|---|---|
| Al Jarousha et al. (2009) | Neonatal ICU | MDR-A. baumannii (15/40) | 37.5% | 0.001 | [54] |
| Susceptible A. baumannii (12/100) | 12% | ||||
| Anunnatsiri et al. (2011) |
ICU | MDR-A. baumannii (22/24) | 91.7% | 0.001 | [41] |
| Susceptible A. baumannii (12/25) | 48% | ||||
| Amer et al. (2015) | Emergency ICU /Pediatric ICU |
CR-MBLP-P. aeruginosa (14/32) | 43,8% | 0.2 | [64] |
| CR-MBLN-P. aeruginosa (2/8) | 25% | ||||
| Furtado et al. (2009) | ICU | Imipenem-resistant P. aeruginosa (31/63) | 49% | 0.02 | [31] |
| Imipenem-susceptible P. aeruginosa (61/182) | 33% | ||||
| Marra et al. (2006) | ICU | ESBL-producing K. pneumoniae (18/56) | 32.14% | 0.042 | [46] |
| Non-ESBL K. pneumoniae (8/52) | 15.38% | ||||
| Moreira et al. (2008) | ICU | ORSA (11/29) | 37.9% | 0.41 | [47] |
| OSSA (8/32) | 25% | ||||
| Serefhanoglu et al. (2009) | ICU | MDR-ESBL-producing-E. coli and K. pneumoniae (7/30) | 23.3% | 0.606 | [32] |
| Non-MDR-ESBL-producing-E. coli and K. pneumoniae (12/64) | 18.8% | ||||
| Tuon et al. (2012) | ICU | Carbapenem-resistant P. aeruginosa (13/29) | 54.2% | 0.043 | [22] |
| Carbapenem-susceptible P. aeruginosa (26/48) | 44.8% | ||||
| Chen et al. (2012) | ICU | MRSA (25/75) | 33% | 0.01 | [48] |
| MSSA (8/43) | 18.6% | ||||
| Fu et al. (2015) | ICU | XDR A. baumannii (31/39) | 79.5% | 0.1 | [49] |
| Non-XDR A. baumannii (38/86) | 44.2% | ||||
| Jia et al. (2015) | ICU | Linezolid non-susceptible Enterococci (3/44) | 6.8% | 0.521 | [50] |
| Linezolid-susceptible Enterococci (2/44) | 4.5% | ||||
| Un-infected Control patients (3/176) | 1.7% | ||||
| Yao et al. (2015) | ICU | MRSA (12/57) | 21% | 0.002 | [35] |
| MSSA (9/116) | 8% | ||||
| Gomez Rueda et al. (2014) | ICU | Carbapenem resistant K. pneumoniae (31/61) | 50.8% | 0.042 | [36] |
| Carbapenem-susceptible K. pneumoniae (20/61) | 32.7% | ||||
| Un-infected control patients (25/122) | 20.4% | ||||
| Kumar et al. (2014) | ICU | Carbapenem-resistant A. baumannii (9/33) | 27.3% | 0.074 | [37] |
| Carbapenem-susceptible A. baumannii (3/32) | 9.4% | ||||
| Nazer et al. (2015) | ICU | MDR-A. baumannii (118/161) | 73.3% | 0.015 | [53] |
| Non-MDR-A. baumannii (142/232) | 61.2% | ||||
| Deris et al. (2011) | ICU | Imipenem-resistant -A. baumannii (6/15) | 42.9% | 0.201 | [39] |
| Imipenem-susceptible A. baumannii (9/41) | 24.3% | ||||
| Inchai et al. (2015) | ICU | MDR-A. baumannii (10/72) | 13.9% | 0.001 | [44] |
| XDR- A. baumannii (88/220) | 40% | ||||
| PDR-A. baumannii (7/12) | 58.3% | ||||
| Jamulitrat et al. (2009) | ICU | Imipenem-resistant-A. baumannii (35/67) | 52.2% | 0.001 | [59] |
| Imipenem-susceptible A. baumannii (26/131) | 19.9%% | ||||
| Thatrimontrichai et al. (2016) | ICU | Carbapenem-resistant A. baumannii (10/63) | 15.9% | 0.01 | [19] |
| Carbapenem-susceptible A. baumannii (1/13) | 7.7% | ||||
| Un-infected control patients (0/25) | 0% | ||||
| Topeli et al. (2000) | ICU | MRSA (15/46) | 32.6% | 0.02 | [21] |
| MSSA (7/55) | 12.7% |
CR: Carbapenem-resistant; CS: Carbapenem susceptible; MBL: Metallo-beta-lactamase; IS: imipenem sensitive; IR: imipenem resistant; ICU: Intensive Care Unit; OSSA: Oxacillin-sensitive-S. aureus; ORSA: Oxacillin-resistant-S. aureus; PDR: Pan drug resistant; XDR: Extensive drug resistant
Statistical analysis
Primary analyses
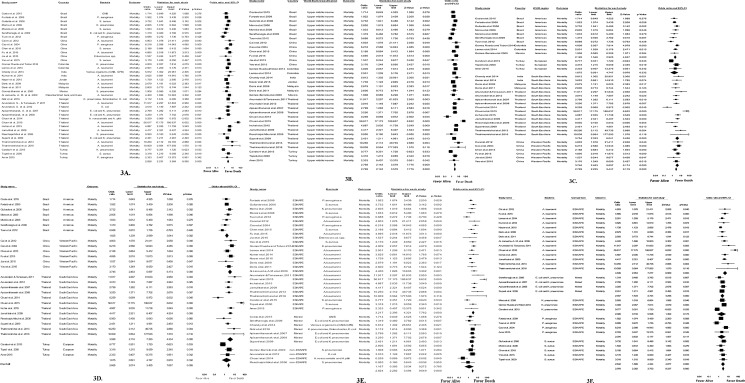
Pooled estimates revealed 90% prevalence (95%CI, 2.852–3.557; p = 0.000) of mortality attributable to infections in developing countries with greater mortality associated with ABR at an odds ratio (OR) 2.828 (95%CI, 2.231–3.584; p = 0.000) (Fig 3A).
Fig 3. Forest plot of impact of ABR on mortality and sub-group analyses per World Bank classification, WHO regions, countries, group of bacteria and bacteria species.
3A. Forest plot of overall impact of antibiotic-resistance on mortality in included studies. 3B. Forest plot of impact of ABR on mortality analyzed per World Bank Classification. 3C. Forest plot of impact of ABR on mortality analyzed per WHO regions. 3D. Forest plot of impact of ABR on mortality analyzed per countries. 3E. Forest plot of impact of AMR on mortality analyzed per group of bacteria. 3F. Forest plot of impact of ABR on mortality analyzed per bacterial species.
Subgroup analyses
The subgroup analyses were performed by World Bank classification, WHO region, country, group of bacteria and bacterial species. Fig 3B presents a forest plot of mortality due to AMR categorized per World Bank classification. The risk of mortality due to resistant bacteria was high in upper middle-income countries (OR 2.769, 95% CIs, 2.142–3.579; p = 0.000), with studies from lower-middle and low-income nations not being evaluated due to insufficient data.
Four out of the six WHO regions were included in the analysis, with three showing a high risk of mortality (Fig 3C). High statistical significance was observed in the Americas (OR 2.126, 95% CIs; 1.546–2.925; p = 0.000), South East Asia (OR 3.754, 95% CIs; 2.333–6.041; p = 0.000) and the Western Pacific (OR 3.746, 95% CIs; 2.463–5.697; p = 0.000) (Fig 3C). Results from Europe were not statistically significant and insufficient reports precluded analysis in Africa.
Subgroup analyses per country showed high statistical significance (OR 2.665, 95%CIs; 2.074–3.425, p = 0.000) (Fig 3D) in favor of mortality. Brazil, China and Thailand, had statistically significant risk of mortality with OR being 1.825 (95%CIs; 1.239–2.689; p = 0.002), 3.746 (95%CIs; 2.463–5.697; p = 0.000), 3.928 (95%CIs; 2.116–7.293; p = 0.000) respectively, in contrast to Turkey, which was not statistically significant (Fig 3D). In other countries, the number of reports was insufficient (less than three) to perform the meta-analysis.
Studies were categorized into three groups of bacteria namely ESKAPE, non-ESKAPE, and mixed (both ESKAPE and non-ESKAPE). The ESKAPE group was associated with the highest risk of mortality with a high statistical significance (OR 3.217; 95%CIs; 2.395–4.321; p = 0.001) (Fig 3E). Although, the non-ESKAPE group was not associated with the risk of mortality (OR 1.167; 95%CIs; 0.385–3.534; p = 0.000), when combined with ESKAPE within a study, it became statistically significant (OR 2.634; 95%CIs; 1.858–3.734; p = 0.000) (Fig 3E).
High risk of mortality due to antibiotic-resistant A. baumannii was observed with high statistical significance (OR 4.636; 95%CIs; 2.954–7.277; p = 0.000), followed by S. aureus (OR 2.842; 95%CIs; 1.868–4.324; p = 0.000). P. aeruginosa (OR 2.076; 95%CIs; 0.833–5.177; p = 0.117) and K. pneumoniae (OR 2.026; 95%CIs; 0.733–5.598; p = 0.173) were not significantly associated with mortality (Fig 3F).
Discussion
AMR is a global public health threat that affects human health, particularly hospitalized patients, and has substantive health and financial consequences. This study analyzed the published literature on the clinical and economic implications of ABR in developing countries from 40 eligible studies. Antibiotic-resistant bacteria were associated with increased mortality (OR 2.8341, 95%CIs; 2.2180–3.6213; P = 0.000), consistent with several reports in both developed and developing countries [66–69]. The main ward involved was the ICU, possibly due to the heavy use of antibiotics and hence the selection pressure for ABR development and prevalence in these units [4, 23, 70, 71]). This concurred with studies from Mexico, Brazil, China, Thailand, France and Serbia, that reported high mortality due to antibiotic-resistant bacteria in ICUs [17, 49, 67, 71–73]. The study further showed that ABR research is neglected in developing countries with only one report from low-income (Senegal), two from lower-income (Palestine and Jordan), and 37 from upper-middle income nations (Table 1 and Fig 2). Developing countries are thus far behind high resource settings in the fight against AMR and that requiring considerable efforts to reduce its consequences [74]. Three WHO regions, i.e., the Americas, South East Asia and the Western Pacific region showed the highest risk of mortality associated with MRSA and K. pneumoniae resistant to third generation cephalosporins. Our results concurred with the 2014’s WHO report, which showed a significant increase of mortality due to antibiotic-resistant K. pneumoniae and S. aureus in hospitals particularly in ICU across WHO regions [2]. Resistance levels could be explained by the practices of self-medication and the purchase of antibiotics over-the-counter common in these settings. Policies and regulations promoting rational antibiotic use are also minimal or non-existent. Additionally, limitations in managing nosocomial infections, sub-optimal infection control measures, unsafe water, poor hygienic conditions, lack of knowledge and inadequately trained personnel might also be associated with the prevailing resistance in these regions. Comprehensive studies are needed to provide accurate and reliable data to inform decision-makers about the danger of ABR in developing countries and suggest a way forward for the alleviation of the resulting implications.
Resistant ESKAPE bacteria including carbapenem-resistant A. baumannii, MBL- producing P. aeruginosa, ESBL-producing K. pneumoniae, and MRSA represented the most common resistant bacteria associated with increased mortality. These bacteria were the main cause of morbidity and mortality in bloodstream infections in hospital settings, with a high statistical significance (OR 2.978, 95%CIs; 2.362–3.753; p = 0.000) (Fig 3F). This concurred with the WHO Global Antimicrobial Surveillance System (GLASS), which recognized A. baumannii, K. pneumoniae, and S. aureus, as priority pathogens in blood specimens and list them together with P. aeruginosa as priority antibiotic resistant-bacteria for research and development in 2017 [4, 5].
According to the meta-analysis, MDR-ESKAPE were associated with a greater risk of mortality than mono-drug (including imipenem, methicillin, and linezolid) resistant bacteria, with a high statistical significance (OR 2.846, 95% CIs; 1.744–4.643; p = 0.000; versus OR 2.301; 95%CIs; 1.718–3.082; p = 0.000; Table 3). Moreover, when comparing the mortality risk between resistant- and susceptible-ESKAPE pathogens (Table 3), results showed that carbapenem-resistant A. baumannii (CRAB) were associated with higher mortality risk than susceptible strains with a high statistical significance [2, 5]. The pooled estimate of mortality rate ranged from 15.9 to 91.7% (p = 0.001), consistent with a report from Taiwan, where a significant increase of mortality from 14% to 46% (p = 0.0001) was associated with carbapenem-resistant-A. baumannii implicated in HAIs during 2003–2008 [75].
Although the mortality attributable to ESKAPE pathogens is indisputable compared to non-ESKAPE pathogens, we observed that when these two groups infected patients concomitantly, they were associated with a long length of hospital stay (LOS) and a higher mortality. This concurred with studies from Senegal [55], Turkey [3] and China [35, 50] which have reported high LOS and death due to MDR-A. baumannii, ESBL-producing Enterobacteriaceae and MRSA, respectively.
Eight studies reported that ABR increased health care costs with resistant ESKAPE bacteria being the main causative agents associated with high hospital costs (Table 4). Four out of the eight revealed that length of stay had an impact on hospital costs. LOS was also a risk factor for acquisition of nosocomial infections, and thereby increased mortality. Overall, health-care costs in all studies for case and control groups were 8,107.375 USD versus 5,469.487 USD respectively. Two studies indicated health care costs >10 000 USD in Thailand and Colombia [19, 51] while one report showed cost ≥ 35 000 USD in Turkey [3]. In contrast, three studies reported overall hospital costs ≤ 1000 USD [55–57], with one below 250 USD in Senegal [55]. These differences are attributed to the diverse socio-economic characteristics of the countries concerned.
Table 4. Summary of data on health care costs associated with resistant infections.
| Country | WHO Region | World Bank classification | Settings | Follow-up period | Overall Health care costs | References | ||
|---|---|---|---|---|---|---|---|---|
| Case group | Control group | p-value | ||||||
| Colombia | Americas (PAHO) | Upper Middle Income | Tertiary hospital | 30 days | 11 822 USD | 7 178 USD | < 0.001 | [51] |
| India | South East Asia (SEARO) | Upper middle income | Tertiary hospital | NR | 1 478 USD | 790 USD | < 0.001 | [52] |
| Senegal | Africa (AFRO) | Low income | Hospital | NR | 228 USD | 122 USD | < 0.0001 | [55] |
| Thailand | South East Asia (SEARO) | Upper middle income | University Hospital | 34 days | 935 USD | 122 USD | < 0.05 | [56] |
| Thailand | South East Asia (SEARO) | Upper middle income | University Hospital | 43 days | 615 USD | 214 USD | < 0.05 | [57] |
| Thailand | South East Asia (SEARO) | Upper middle income | University Hospital | NR | 2731 USD | 1 199 USD | < 0.001 | [58] |
| Thailand | South East Asia (SEARO) | Upper middle income | University Hospital | NR | 11 773 USD | 7 797.9 USD | < 0.05 | [19] |
| Turkey | Europe(EURO) | Upper middle income | University Hospital | 28 days | 35 277 USD | 26 333 USD | < 0.282 | [3] |
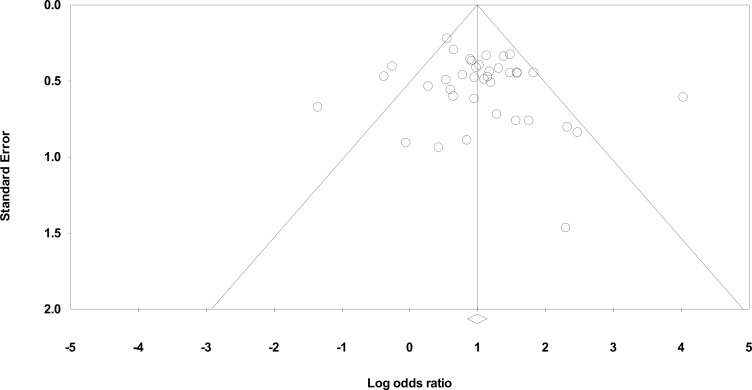
In terms of the limitations of the study, several papers were not included in the meta-analysis because they did not provide sufficient information regarding clinical and/or economic impact of ABR in developing countries. We were unable to present the genomic characteristics of antibiotic-resistant bacteria due to the scarcity of data. In addition, we did not focus on antibiotic classes and resistance patterns due to the lack of standard methods for identification and interpretation in developing countries. Moderate heterogeneity (I2 = 58.88%, p = 0.000) was reported, which could be due to various external factors, such as different type of studies (retrospective, retrospective cohort, retrospective case-control, prospective cohort, prospective case-control, etc.), diverse populations (adult, children, neonates), infection prevention and control measures and antimicrobial stewardship practices. Moreover, minor publication bias was observed in the funnel plot (Fig 4) which could possibly be attributed to the lack of reports from lower-middle and low-income countries. We tried to limit the influence of heterogeneity and publication bias in our statistical analysis by using the random effects model that considers differences within and between studies, as well as by including articles in different languages (English and French).
Fig 4. Funnel plot of standard error by log odds ratio.
Conclusion and recommendations
The key findings of this study confirm that ABR, particularly antibiotic-resistant ESKAPE pathogens are associated with a high risk of mortality and greater economic costs. Developing countries need to optimize their management of communicable and non-communicable diseases, implement infection, prevention and control (IPC) measures, as well as antimicrobial stewardship programs (ASP) in both hospital and community settings to reduce morbidity, mortality and the costs associated with ABR. Furthermore, optimization of rational antibiotic use at regional and national levels, is essential to ensure a high quality and effective of therapeutic options [76]. Substantial and sustainable efforts to develop rapid diagnostics, new antibiotics and vaccines are required. An international platform for global real-time surveillance and monitoring of antimicrobial resistance could advance containment of this threat.
Supporting information
(DOCX)
(DOCX)
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by the Antimicrobial Research Unit and the College of Health Sciences of the University of KwaZulu-Natal through a scholarship awarded to R.C. Founou and L.L. Founou as well as the National Research Foundation South African Research Chair in Antibiotic Resistance and One Health Grant No: 98342 awarded to SY Essack. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.O’Neill J. Tackling Drug-Resistant Infections Globally: Final Report And Recommendations. Review On Antimicrobial Resistance 2016.
- 2.World Health Organization. Antimicrobial Resistance Global Report on Surveillance. Geneva: World Health Organization; 2014. [Google Scholar]
- 3.Gulen TA, Guner R, Celikbilek N, Keske S, Tasyaran M. Clinical importance and cost of bacteremia caused by nosocomial multi drug resistant acinetobacter baumannii. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2015;38:32–5. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Global Action Plan on Antimicrobial resistance. Geneva: WHO; 2015. [DOI] [PubMed] [Google Scholar]
- 5.World Health Organization. Global Priority List of Antibiotic-Resistance Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Geneva: World Health Organization; 2017. [Google Scholar]
- 6.Årdal C, Outterson K, Hoffman SJ, Ghafur A, Sharland M, Ranganathan N, et al. International cooperation to improve access to and sustain effectiveness of antimicrobials. Lancet. 2016;387: 296–307. doi: 10.1016/S0140-6736(15)00470-5 [DOI] [PubMed] [Google Scholar]
- 7.Founou LL, Founou RC, Essack SY. Antibiotic Resistance in the Food Chain: A Developing Country-Perspective. Front Microbiol. 2016;7:1881 doi: 10.3389/fmicb.2016.01881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kejela T, Bacha K. Prevalence and antibiotic susceptibility pattern of methicillin-resistant Staphylococcus aureus (MRSA) among primary school children and prisoners in Jimma Town, Southwest Ethiopia. Annals of Clinical Microbiology and Antimicrobials 2013. 2013;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Njoungang LL, Nwobegahay JM, Ayangma CR, Njukeng AP, Kengne M, Abeng EM, et al. Prevalence and antibiotic resistance patterns of strains of Staphylococcus aureus isolated at the Yaounde Military Hospital, Cameroon. Microbiol Res Int. 2015;3(4):56–63. [Google Scholar]
- 10.Perovic O, Iyaloo S, Kularatne R, Lowman W, Bosman N, Wadula J, et al. Prevalence and Trends of Staphylococcus aureus Bacteraemia in Hospitalized Patients in South Africa, 2010 to 2012: Laboratory-Based Surveillance Mapping of Antimicrobial Resistance and Molecular Epidemiology. PLoS One. 2015;10(12):e0145429 doi: 10.1371/journal.pone.0145429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perovic O, Koornhof HJ, Crewe-Brown HH, Duse AG, van Nierop W, Galpin JS. Pseudomonas aeruginosa bacteraemia in an academic hospital in South Africa. South African medical journal Suid-Afrikaanse tydskrif vir geneeskunde. 2008;98(8):626–32. [PubMed] [Google Scholar]
- 12.Perovic O, Britz E, Chetty V, Singh-Moodley A. Molecular detection of carbapenemase-producing genes in referral Enterobacteriaceae in South Africa: A short report. South African medical journal Suid-Afrikaanse tydskrif vir geneeskunde. 2016;106(10):975–7. doi: 10.7196/SAMJ.2016.v106i10.11300 [DOI] [PubMed] [Google Scholar]
- 13.Perovic O, Singh-Moodley A, Duse A, Bamford C, Elliott G, Swe-Han KS, et al. National sentinel site surveillance for antimicrobial resistance in Klebsiella pneumoniae isolates in South Africa, 2010–2012. Samj South African Medical Journal. 2014;104(8):563 doi: 10.7196/samj.7617 [DOI] [PubMed] [Google Scholar]
- 14.Rajagunalan S, Chakraborty S, Dhama K, SV S. Antibiotic resistance -an emerging health problem: causes, worries, challenges and solutions–A review. antibiotic resistance—an emerging health problem: causes, worries, challenges and solutions. Inter J Curr Res. 2013;5(7):1880–92. [Google Scholar]
- 15.Slavcovici A, Maier C, Radulescu A. Antimicrobial Resistance Of ESKAPE-pathogens in Culture-Positive Pneumonia. FARMACIA. 2015;63(2):201–5. [Google Scholar]
- 16.Kali A, Srirangaraj S, Kumar S, Divya HA, Kalyani A, Sivaraman U. Detection of metallo-beta-lactamase producing Pseudomonas aeruginosa in intensive care units. AMJ 2013;6(12):686–93. doi: 10.4066/AMJ.2013.1824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa PO, Atta EH, Silva AR. Infection with multidrug-resistant gram-negative bacteria in a pediatric oncology intensive care unit: risk factors and outcomes. J Pediatr (Rio J). 2015;91(5):435–41. [DOI] [PubMed] [Google Scholar]
- 18.Jasovsky D, Littmann J, Zorzet A, Otto C. Antimicrobial resistance—a threat to the world’s sustainable development. UPSALA JOURNAL OF MEDICAL SCIENCES. 2016;121:159–64. doi: 10.1080/03009734.2016.1195900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thatrimontrichai A, Techato C, Dissaneevate S, Janjindamai W, Gunlawadee M, Supika K, et al. Risk factors and outcomes of carbapenem-resistant Acinetobacter baumannii ventilator-associated pneumonia in the neonate: A case-case-control study. Journal of Infection and Chemotherapy. 2016;22(7–8):444–9. [DOI] [PubMed] [Google Scholar]
- 20.Tomson G, Vlad L. The need to look at antibiotic resistance from a health systems perspective. Upsala Journal of Medical Sciences. 2014;119(2):117–24. doi: 10.3109/03009734.2014.902879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topeli A, Unal S, Akalin HE. Risk factors influencing clinical outcome in Staphylococcus aureus bacteraemia in a Turkish University Hospital. Int J Antimicrob Agents. 2000;14(1):57–63. [DOI] [PubMed] [Google Scholar]
- 22.Tuon FF, Gortz LW, Rocha JL. Risk factors for pan-resistant Pseudomonas aeruginosa bacteremia and the adequacy of antibiotic therapy. The Brazilian journal of infectious diseases: an official publication of the Brazilian Society of Infectious Diseases. 2012;16(4):351–6. [DOI] [PubMed] [Google Scholar]
- 23.Kelly BG, Vespermann A, Bolton DJ. The role of horizontal gene transfer in the evolution of selected foodborne bacterial pathogen. Food and Chemical Toxicology. 2009;47 951–68. doi: 10.1016/j.fct.2008.02.006 [DOI] [PubMed] [Google Scholar]
- 24.Andriamanantena TS, Ratsima E, Rakotonirina HC, Randrianirina F, Ramparany L, Carod J. Dissemination of multidrug resistant acinetobacter baumannii in various hospitals of antananarivo madagascar. Ann Clin Microbiol Antimicrob. 2010;9(17):2–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joloba M, Bwanga F. Drug resistance in mycobacterium tuberculosis. in: Antimicrobial resistance in developing countries. BMC infectious diseases. 2010;2010:117. [Google Scholar]
- 26.Ndihokubwayo JB, Yahaya AA, Desta AT, Ki-Zerbo G, Odei EA, Keita B, et al. Antimicrobial resistance in the African Region: Issues, challenges and actions proposed. African health Monitor. 2013(16). [Google Scholar]
- 27.Tansarli GS, Poulikakos P, Kapaskelis A, E FM. Proportion of extended-spectrum b-lactamase (ESBL)-producing isolates among Enterobacteriaceae in Africa: evaluation of the evidence—systematic review. J Antimicrob Chemother. 2014. [DOI] [PubMed] [Google Scholar]
- 28.Moher D, Liberati A, Tetzlaff J, Altman DG, Group TP. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med. 2009;6(7):e1000097 doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of Observational Studies in Epidemiology: A Proposal for Reporting. JAMA. 2000;283(15). [DOI] [PubMed] [Google Scholar]
- 30.Baran G, Erbay A, Bodur H, Onguru P, Akinci E, Balaban N, et al. Risk factors for nosocomial imipenem-resistant Acinetobacter baumannii infections. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2008;12(1):16–21. [DOI] [PubMed] [Google Scholar]
- 31.Furtado GH, Bergamasco MD, Menezes FG, Marques D, Silva A, Perdiz LB, et al. Imipenem-resistant Pseudomonas aeruginosa infection at a medical-surgical intensive care unit: risk factors and mortality. J Crit Care. 2009;24(4):625.e9–14. [DOI] [PubMed] [Google Scholar]
- 32.Serefhanoglu K, Turan H, Timurkaynak FE, Arslan H. Bloodstream infections caused by ESBL-producing E. coli and K. pneumoniae: risk factors for multidrug-resistance. The Brazilian journal of infectious diseases: an official publication of the Brazilian Society of Infectious Diseases. 2009;13(6):403–7. [DOI] [PubMed] [Google Scholar]
- 33.Cao B, Wang H, Sun H, Zhu Y, Chen M. Risk factors and clinical outcomes of nosocomial multi-drug resistant Pseudomonas aeruginosa infections. The Journal of hospital infection. 2004;57(2):112–8. doi: 10.1016/j.jhin.2004.03.021 [DOI] [PubMed] [Google Scholar]
- 34.Cai XF, Sun JM, Bao LS, Li WB. Risk factors and antibiotic resistance of pneumonia caused by multidrug resistant Acinetobacter baumannii in pediatric intensive care unit. World journal of emergency medicine. 2012;3(3):202–7. doi: 10.5847/wjem.j.1920-8642.2012.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yao Z, Peng Y, Chen X, Bi J, Li Y, Ye X, et al. Healthcare Associated Infections of Methicillin-Resistant Staphylococcus aureus: A Case-Control-Control Study. PLoS One. 2015;10(10):e0140604 doi: 10.1371/journal.pone.0140604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gomez RV, Zuleta TJJ. Risk factors for infection with carbapenem-resistant Klebsiella pneumoniae: a case-case-control study. Colombia medica (Cali, Colombia). 2014;45(2):54–60. [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar A, Randhawa VS, Nirupam N, Rai Y, Saili A. Risk factors for carbapenem-resistant Acinetobacter baumanii blood stream infections in a neonatal intensive care unit, Delhi, India. J Infect Dev Ctries. 2014;8(8):1049–54. doi: 10.3855/jidc.4248 [DOI] [PubMed] [Google Scholar]
- 38.Deris ZZ, Harun A, Shafei MN, Rahman RA, Johari MR. Outcomes and appropriateness of management of nosocomial Acinetobacter bloodstream infections at a teaching hospital in northeastern Malaysia. The Southeast Asian journal of tropical medicine and public health. 2009;40(1):140–7. [PubMed] [Google Scholar]
- 39.Deris ZZ, Shafei MN, Harun A. Risk factors and outcomes of imipenem-resistant Acinetobacter bloodstream infection in North-Eastern Malaysia. Asian Pacific journal of tropical biomedicine. 2011;1(4):313–5. doi: 10.1016/S2221-1691(11)60050-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gomez-Barreto D, Calderon-Jaimes E, Rodriguez RS, Monteros DLE. Clinical outcome of invasive infections in children caused by highly penicillin-resistant Streptococcus pneumoniae compared with infections caused by penicillin-susceptible strains. Arch Med Res. 2000;31(6):592–8. [DOI] [PubMed] [Google Scholar]
- 41.Anunnatsiri S, Tonsawan P. Risk factors and clinical outcomes of multidrug-resistant Acinetobacter baumannii bacteremia at a university hospital in Thailand. The Southeast Asian journal of tropical medicine and public health. 2011;42(3):693–703. [PubMed] [Google Scholar]
- 42.Anunnatsiri S, Towiwat P, Chaimanee P. Risk factors and clinical outcomes of extended spectrum beta-lactamase (ESBL)-producing Escherichia coli septicemia at Srinagarind University Hospital, Thailand. The Southeast Asian journal of tropical medicine and public health. 2012;43(5):1169–77. [PubMed] [Google Scholar]
- 43.Chusri S, Silpapojakul K, McNeil E, Singkhamanan K, Chongsuvivatwong V. Impact of antibiotic exposure on occurrence of nosocomial carbapenem-resistant Acinetobacter baumannii infection: a case control study. Journal of infection and chemotherapy: official journal of the Japan Society of Chemotherapy. 2015;21(2):90–5. [DOI] [PubMed] [Google Scholar]
- 44.Inchai J, Pothirat C, Bumroongkit C, Limsukon A, Khositsakulchai W, Liwsrisaku C. Prognostic factors associated with mortality of drug-resistant Acinetobacter baumannii ventilator-associated pneumonia. Journal of Intensive Care. 2015;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guilarde AO, Turchi MD, Martelli CM, Primo MG. Staphylococcus aureus bacteraemia: incidence, risk factors and predictors for death in a Brazilian teaching hospital. The Journal of hospital infection. 2006;63(3):330–6. doi: 10.1016/j.jhin.2006.02.011 [DOI] [PubMed] [Google Scholar]
- 46.Marra AR, Wey SB, Castelo A, Gales AC, Cal RG, Filho JR, et al. Nosocomial bloodstream infections caused by Klebsiella pneumoniae: impact of extended-spectrum beta-lactamase (ESBL) production on clinical outcome in a hospital with high ESBL prevalence. BMC Infect Dis. 2006;6:24 doi: 10.1186/1471-2334-6-24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moreira MR, Cardoso RL, Almeida AB, Gontijo FPP. Risk factors and evolution of ventilator-associated pneumonia by Staphylococcus aureus sensitive or resistant to oxacillin in patients at the intensive care unit of a Brazilian university hospital. The Brazilian journal of infectious diseases: an official publication of the Brazilian Society of Infectious Diseases. 2008;12(6):499–503. [DOI] [PubMed] [Google Scholar]
- 48.Chen R, Yan ZQ, Feng D, Luo YP, Wang LL, Shen DX. Nosocomial bloodstream infection in patients caused by Staphylococcus aureus: drug susceptibility, outcome, and risk factors for hospital mortality. Chin Med J (Engl). 2012;125(2):226–9. [PubMed] [Google Scholar]
- 49.Fu Q, Ye H, Liu S. Risk factors for extensive drug-resistance and mortality in geriatric inpatients with bacteremia caused by Acinetobacter baumannii. American Journal of Infection Control. 2015;43(8):857–60. doi: 10.1016/j.ajic.2015.03.033 [DOI] [PubMed] [Google Scholar]
- 50.Jia X, Ma W, Xu X, Yang S, Zhang L. Retrospective analysis of hospital-acquired linezolid-nonsusceptible enterococci infection in Chongqing, China, 2011–2014. Am J Infect Control. 2015;43(12):e101–6. doi: 10.1016/j.ajic.2015.07.027 [DOI] [PubMed] [Google Scholar]
- 51.Lemos EV, Hoz FP, Alvis N, Einarson TR, Quevedo E, Castaneda C, et al. Impact of carbapenem resistance on clinical and economic outcomes among patients with Acinetobacter baumannii infection in Colombia. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2014;20(2):174–80. [DOI] [PubMed] [Google Scholar]
- 52.Chandy SJ, Naik GS, Balaji V, Jeyaseelan V, Thomas K, Lundborg CS. High cost burden and health consequences of antibiotic resistance: the price to pay. J Infect Dev Ctries. 2014;8(9):1096–102. doi: 10.3855/jidc.4745 [DOI] [PubMed] [Google Scholar]
- 53.Nazer LH, Kharabsheh A, Rimawi D, Mubarak S, Hawari F. Characteristics and Outcomes of Acinetobacter baumannii Infections in Critically Ill Patients with Cancer: A Matched Case-Control Study. Microbial drug resistance (Larchmont, NY). 2015;21(5):556–61. [DOI] [PubMed] [Google Scholar]
- 54.AlJarousha AM, ElJadba AH, AlAfifi AS, ElQouqa AI. Nosocomial multidrug-resistant Acinetobacter baumannii in the neonatal intensive care unit in Gaza City, Palestine. International journal of infectious diseases: IJID: official publication of the International Society for Infectious Diseases. 2009;13(5):623–8. [DOI] [PubMed] [Google Scholar]
- 55.Ndir A, Diop A, Ka R, Faye PM, Dia-Badiane NM, Ndoye B, et al. Infections caused by extended-spectrum beta-lactamases producing Enterobacteriaceae: clinical and economic impact in patients hospitalized in 2 teaching hospitals in Dakar, Senegal. Antimicrob Resist Infect Control. 2016;5:13 doi: 10.1186/s13756-016-0114-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Apisarnthanarak A, Kiratisin P, Saifon P, Kitphati R, Dejsirilert S, Mundy LM. Risk factors for and outcomes of healthcare-associated infection due to extended-spectrum beta-lactamase-producing Escherichia coli or Klebsiella pneumoniae in Thailand. Infect Control Hosp Epidemiol. 2007;28(7):873–6. doi: 10.1086/518725 [DOI] [PubMed] [Google Scholar]
- 57.Apisarnthanarak A, Kiratisin P, Saifon P, Kitphati R, Dejsirilert S, Mundy LM. Predictors of mortality among patients with community-onset infection due to extended-spectrum beta-lactamase-producing Escherichia coli in Thailand. Infect Control Hosp Epidemiol. 2008;29(1):80–2. doi: 10.1086/524321 [DOI] [PubMed] [Google Scholar]
- 58.Chusri S, Chongsuvivatwong V, Rivera JI, Silpapojakul K, Singkhamanan K, McNeil E, et al. Clinical outcomes of hospital-acquired infection with Acinetobacter nosocomialis and Acinetobacter pittii. Antimicrob Agents Chemother. 2014;58(7):4172–9. doi: 10.1128/AAC.02992-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jamulitrat S, Arunpan P, Phainuphong P. Attributable mortality of imipenem-resistant nosocomial Acinetobacter baumannii bloodstream infection. Journal of the Medical Association of Thailand = Chotmaihet thangphaet. 2009;92(3):413–9. [PubMed] [Google Scholar]
- 60.Reechaipichitkul W, Assawasanti K, Chaimanee P. Risk factors and clinical outcomes of penicillin resistant S. pneumoniae community-acquired pneumonia in Khon Kaen, Thailand. The Southeast Asian journal of tropical medicine and public health. 2006;37(2):320–6. [PubMed] [Google Scholar]
- 61.Superti SV, Augusti G, Zavascki AP. Risk factors for and mortality of extended-spectrum-beta-lactamase-producing Klebsiella pneumoniae and Escherichia coli nosocomial bloodstream infections. Rev Inst Med Trop Sao Paulo. 2009;51(4):211–6. [DOI] [PubMed] [Google Scholar]
- 62.Thatrimontrichai A, Apisarnthanarak A, Chanvitan P, Janjindamai W, Dissaneevate S, Maneenil G. Risk factors and outcomes of carbapenem-resistant Acinetobacter baumannii bacteremia in neonatal intensive care unit: a case-case-control study. The Pediatric infectious disease journal. 2013;32(2):140–5. doi: 10.1097/INF.0b013e318270b108 [DOI] [PubMed] [Google Scholar]
- 63.Candevir UA, Kurtaran B, Inal AS, Komur S, Kibar F, Cicekdemir HY, et al. Risk Factors of Carbapenem-Resistant Klebsiella pneumoniae Infection: A Serious Threat in ICUs. Medical Science Monitor. 2015;21:219–24. doi: 10.12659/MSM.892516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Amer WH. Effect of Carbapenem Resistant Metallo-Beta-Lactamase Positive Pseudomonas aeruginosa on Mortality and Morbidity of Intensive Care Unit Nosocomial Infections. Int J Curr Microbiol App Sci. 2015;4(11):167–76. [Google Scholar]
- 65.Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. 2011. [Google Scholar]
- 66.Combes A, Luyt CE, Fagon JY, Wolff M, Trouiellet JL, Chastre J. Impact of piperacillin resistance on the outocme of pseudomonas ventillator associated pneumonia. Intensive Care Med. 2006;32:1970–8. doi: 10.1007/s00134-006-0355-7 [DOI] [PubMed] [Google Scholar]
- 67.Cornejo J, Vilar CD, Pe´rez JC, Silva NSA, Herna´ndez SS, Fernandez VP. The impact of hospital-acquired infections with multidrug-resistant bacteria in an oncology intensive care unit. Inter J Infect Dis 2015;31:31–4. [DOI] [PubMed] [Google Scholar]
- 68.Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum b-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy 2007;60:913–20. doi: 10.1093/jac/dkm318 [DOI] [PubMed] [Google Scholar]
- 69.Tacconelli E, De Angelis G, Cataldo MA, Pozzi E, Cauda R. Does antibiotic exposure increase the risk of methicillin-resistant Staphylococcus aureus (MRSA) isolation? A systematic review and meta-analysis. Journal of Antimicrobial Chemotherapy. 2008;61:26–38. doi: 10.1093/jac/dkm416 [DOI] [PubMed] [Google Scholar]
- 70.Santajit S, Indrawattana N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed Research International. 2016:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bastug A, Kayaaslan B, Kazancioglu S, But A, Aslaner H, Akinci E, et al. Emergence of multidrug resistant isolates and mortality predictors in patients with solid tumors or hematological malignancies. J Infect Dev Ctries. 2015;9(10):1100–7. doi: 10.3855/jidc.6805 [DOI] [PubMed] [Google Scholar]
- 72.Djordjevic Z, Folic M, Zecevic DR, Ilic G, Jankovic S. Risk factors for carbapenem-resistant Pseudomonas aeruginosa infection in a tertiary care hospital in Serbia. Journal of Infection in Developing Countries. 2013;7(9):686–90. doi: 10.3855/jidc.3516 [DOI] [PubMed] [Google Scholar]
- 73.Inchai J, Liwsrisakun C, Theerakittikul T, Chaiwarith R, Khositsakulchai W, Pothirat C. Risk factors of multidrug-resistant, extensively drug-resistant and pandrug-resistant Acinetobacter baumannii ventilator-associated pneumonia in a Medical Intensive Care Unit of University Hospital in Thailand. Journal of infection and chemotherapy: official journal of the Japan Society of Chemotherapy. 2015;21(8):570–4. [DOI] [PubMed] [Google Scholar]
- 74.Laxminarayan R, Sridhar D, Blaser M, Wang M, Woolhouse M. Achieving global targets for antimicrobial resistance. Science (New York, NY). 2016. [DOI] [PubMed] [Google Scholar]
- 75.Su C-H, Wang J-T, Hsiung CA, Chien L-J, Chi C-L, Yu H-T, et al. Increase of Carbapenem-Resistant Acinetobacter baumannii Infection in Acute Care Hospitals in Taiwan: Association with Hospital Antimicrobial Usage. PLoS ONE 2012;7(5):e37788 doi: 10.1371/journal.pone.0037788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Doare KL, Bielicki J, Heath PT, Sharland M. Systematic Review of Antibiotic Resistance Rates Among Gram-Negative Bacteria in Children With Sepsis in Resource-Limited Countries. J Pediatric Infect Dis 2015;4(1):11–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.