Abstract
Background
Growing urbanisation and population requiring enhanced electricity generation as well as the increasing numbers of fossil fuel in Thailand pose important challenges to air quality management which impacts on the health of the population. Mortality attributed to ambient air pollution is one of the sustainable development goals (SDGs). We estimated the spatial pattern of mortality burden attributable to selected ambient air pollution in 2009 based on the empirical evidence in Thailand.
Methods
We estimated the burden of disease attributable to ambient air pollution based on the comparative risk assessment (CRA) framework developed by the World Health Organization (WHO) and the Global Burden of Disease study (GBD). We integrated geographical information systems (GIS)-based exposure assessments into spatial interpolation models to estimate ambient air pollutant concentrations, the population distribution of exposure and the concentration-response (CR) relationship to quantify ambient air pollution exposure and associated mortality. We obtained air quality data from the Pollution Control Department (PCD) of Thailand surface air pollution monitoring network sources and estimated the CR relationship between relative risk (RR) and concentration of air pollutants from the epidemiological literature.
Results
We estimated 650–38,410 ambient air pollution-related fatalities and 160–5,982 fatalities that could have been avoided with a 20 reduction in ambient air pollutant concentrations. The summation of population-attributable fraction (PAF) of the disease burden for all-causes mortality in adults due to NO2 and PM2.5 were the highest among all air pollutants at 10% and 7.5%, respectively. The PAF summation of PM2.5 for lung cancer and cardiovascular disease were 16.8% and 14.6% respectively and the PAF summations of mortality attributable to PM10 was 3.4% for all-causes mortality, 1.7% for respiratory and 3.8% for cardiovascular mortality, while the PAF summation of mortality attributable to NO2 was 7.8% for respiratory mortality in Thailand.
Conclusion
Mortality due to ambient air pollution in Thailand varies across the country. Geographical distribution estimates can identify high exposure areas for planners and policy-makers. Our results suggest that the benefits of a 20% reduction in ambient air pollution concentration could prevent up to 25% of avoidable fatalities each year in all-causes, respiratory and cardiovascular categories. Furthermore, our findings can provide guidelines for future epidemiological investigations and policy decisions to achieve the SDGs.
Introduction
Air pollution is a major global concern. Epidemiological studies have shown that exposure to ambient air pollution leads to adverse health effects, including increases in mortality and morbidity from cardiovascular and respiratory diseases [1–3]. Mortality attributed to ambient air pollution is identified as an indicator of the sustainable development goals (SDGs) [4]. Globally, ambient particulate matter pollution accounted for 4.2 million deaths and 103 million healthy life-years lost in 2015, representing 7.6% of total global mortality and making it the fifth-ranked global risk factor in the Global Burden of Diseases Study 2015 (GBD 2015) [5, 6].
Quantitative analyses of how different risk factors contribute to the overall disease burden provide critical information for health policymaking and priority-setting. The comparative risk assessment (CRA) approach developed by the World Health Organisation (WHO) and the GBD provides a framework for population risk assessment and comparison across risks at both global and national levels [6, 7].
Increasing urbanisation, industrialised area, traffic congestion, forest fires and agricultural burning contribute to escalating air pollution in Thailand [8, 9]. The pattern of exposure has differed across various areas with the specific characteristics of pollutant sources; furthermore, the effects from air pollution may vary at the subnational level, especially in urban area[10]. GBD [6, 7, 11] and previous CRA study in Thailand [12] estimated the disease burden attributable to ambient air pollution at national and regional scale but did not provide distribution at sub-national levels across the country. Other pollutants; such as, coarser particle matter (PM10) and nitrogen dioxide (NO2), might also be important to quantify the public health impact as per the GBD recommendation [5].
Over several decades, Thailand has developed an extensive air quality monitoring network with the aim of providing up-to-date empirical information on ambient air pollutants (i.e. PM2.5, PM10 and NO2) [13]. Spatial variability of air pollution concentrations from local air quality network provides country-specific information to investigate the magnitude and distribution of the public health impact for PM2.5, PM10 and NO2. Geographical information systems (GIS) and spatial analysis have been used to estimate the distribution of ambient air pollution exposure at unknown locations based on empirical data in many environmental epidemiology studies [14–16]. This approach can improve estimated exposure distribution at the national, sub-national and/or specific levels [17], and also provide valuable information for policy-makers to improve air quality and health benefits in specific locations.
This study aimed to quantify the magnitude and geographical distribution of disease burden in terms of mortality attributable to ambient air-pollutant exposure based on available and observed data from air monitoring measurements. We utilised GIS to explore the spatial variability of air pollution exposure, and adopted the CRA method developed by the WHO, the GBD [18] and others [19, 20] to quantify mortality attributable to ambient air pollution.
Methods
Overall approach to estimate the burden attributable to ambient air pollution
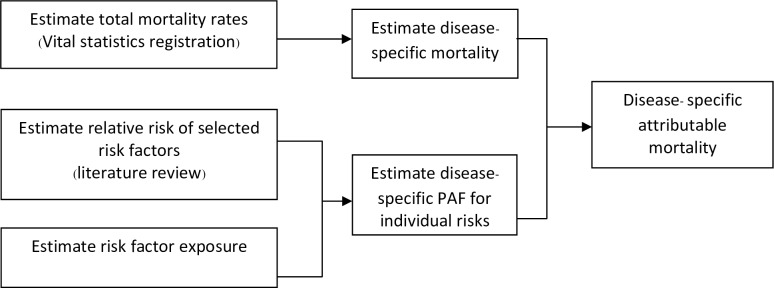
We employed the CRA framework which is defined as the systematic evaluation of the changes in the population health and ranking the different factors that contribute to the specific outcome to quantify the burden of disease attributable to ambient air pollution [21]. The general framework and its components are presented in Fig 1. Each component of the estimation is described as follows:
Fig 1. Methodology scheme of the comparative risk assessment.
Ambient air pollution exposure
Population distribution of exposure (Pe)
Relative risks and concentration–response relationships
Attributable mortality due to ambient air pollution
Ambient air pollutants exposure
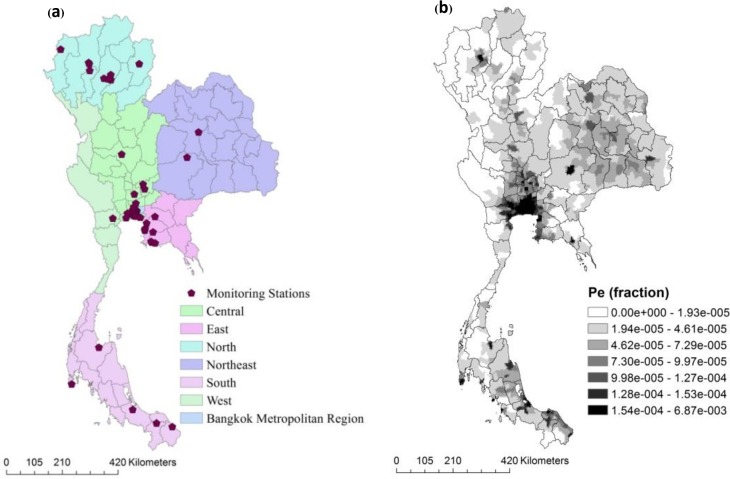
We estimated the exposure to three main ambient air pollutants, i.e. PM2.5, PM10, and NO2. We obtained air pollution data from the annual report on the state of air quality in Thailand under the enhancement and conservation of the National Environmental Quality Act of 1992[22], which falls under the responsibility of the Pollution Control Department, Ministry of Natural Resources and Environment. This department has 54 monitoring stations located in all six geographical regions of Thailand as (10, 2, 8, 1, 28 and 3 stations in Northern, Northeastern, Eastern, Western, Central, and Southern regions, respectively) and extensively monitors ambient air pollutants (Fig 2). All PM was reported in micrograms per cubic metre (ugm-3). The NO2 concentrations was measured in parts per billion (PPB) and converted into micrograms per cubic metre using a conversion factor of 1.88 (at 25°C and 1013 millibars) for the calculated concentration response coefficients.
Fig 2. Data sources for ambient air pollution exposure.
(a) Area of the study and location of surface monitoring stations network and (b) geographic pattern of population distribution of exposure (Pe).
Many studies have indicated that the health effects of PM2.5 are more harmful than PM10[23–27] with their concentrations highly correlated[28]. The PM10 correlation was also very high with NO2 but low with SO2[29]. PM10 was reported by all existing air quality network in Thailand whereas only a few stations reported PM2.5 concentrations, making analysis at the country level very difficult. Since PM2.5 is a component of PM10, it is possible to estimate PM2.5 from PM10 data based on the typical relationships between pollutants, as PM2.5 can be treated as a fixed weight fraction of PM10. We decided to convert PM10 to PM2.5 for stations without PM2.5 readings using the ratio of PM2.5 to PM10 based on the literature review. Many studies [30–32] have reported PM2.5 and PM10 ratios in the range of 0.35–0.7, and a local study in Thailand reported ratios of about 0.5 in Bangkok[33] and clearly stated that daily PM2.5 and PM10 concentrations were highly correlated (r ≥ 0.85)[33]. From the literature review, we decided to use the ratios for PM2.5 and PM10 from the local study in Thailand[33] which was similar to the WHO global analysis of disease burden due to outdoor air pollution in developing countries [19].
To estimate the exposure level of air pollutants across geographical area, we used inverse distance weighted (IDW) interpolation method [34–39] to estimate the spatiotemporal distribution of ambient air concentrations based on empirical data from the air quality monitoring stations across Thailand and a grid consisting of 40767 cells (3×3 km2 resolution). For cross-validation evaluation, comparisons of predicted values to observed values were essential information about the quality of the model [40, 41] using all existing data to estimate the trend and model autocorrelation. This removed one or more data locations and predicted their associated data using information from the other locations. We then assessed the accuracy of the model using the square root of the mean for the squared prediction errors (RMSE) based on the predicted and actual values at the existing point. In addition, we obtained cross-validation correlations using the squared Pearson correlation between the measured values at known-point observations and the spatial model predictions.
To quantify the different levels of exposure to ambient air pollution for estimation of avoidable disease burden, we used a 20% reduction in ambient air pollutant as a reasonable proportion of reduction in air pollutant level from the Mexico City Air Quality Management Team [42] for possible suggestion scenarios.
Population distribution of exposure (Pe)
To quantify the Pe, we acquired population data from the 2000 Gridded Population of the World, Version 3 (GPWv3), generated by the SEDAC (Socioeconomic Data and Applications Center) project at Columbia University [43]. This dataset was estimated from the human population from national and subnational input sources (usually administrative sources) of varying resolutions into regular latitude-longitude grids at a resolution of 2.5 arc-minute grid cells (or ~5 km at the equator). Pe was estimated as the proportion of the population by selected age groups counted in the grid divided by the total population in Thailand. We used population fractions from the Department of Provincial Administration, Thailand [44]. The total population of Thailand in 2009 was about 63 million, spread over an area of 514,000 square kilometres. We assumed the proportions of population located within each grid to have been exposed to the same pollutant concentrations in the grid cells. Pe value are presented in Fig 2. The minimum Pe per grid was zero, while the maximum was 6.9 × 10−3. The Pe(s) for grids in Bangkok and the vicinity were relatively high compared to other regions defined in Fig 2, reflecting higher population densities.
Relative risks and concentration–response relationships
Air pollutants such as PM10, PM2.5, and NO2 can cause a variety of detrimental public health effects including cardiovascular disease (CVD), respiratory disease, and lung cancer [3, 33, 45, 46]. Relative risk (RR) is commonly used to represent the results of exposure–response functions. This study estimated health impact associated with ambient air pollution using exposure to the risk of mortality based on the relationship between RR, concentration–response coefficient and ambient air pollution concentrations [19–21, 47]. The health impact function was defined as follows:
| (1) |
where β is the concentration–response coefficient (CR), as the slope of the log-linear relationship between ambient air pollution concentrations and mortality, and x–x0 or ΔX is the concentration change from baseline conditions or natural background concentration. We assumed natural background concentrations of 10 μgm-3 and 3 μgm-3 for PM10 and PM2.5, respectively, based on the WHO environmental burden of disease (EBD) study[19]. For NO2, we assumed no background concentrations (zero concentrations) for Thailand. Table 1 summarises estimations on the RRs based on the health impact function.
Table 1. Summary of relative risks selected to estimate the PAF of ambient air pollution in Thailand.
| Pollutant | Health end-point | Types | Relative risk | CRa | Age group (years) | References |
|---|---|---|---|---|---|---|
| PM10 | All-cause mortalityb | Short-term | 1.004 per 10 μgm-3 | 0.0004 | All ages | [9] |
| Respiratory mortalityc | Short-term | 1.004 per 10 μgm-3 | 0.0004 | All ages | [9] | |
| Cardiovascular mortalityd | Short-term | 1.002 per 10 μgm-3 | 0.0002 | All ages | [9] | |
| PM 2.5 | All-cause mortalityb | Long-term | 1.06 per 10 μgm-3 | 0.006 | Age >30 | [3] |
| Lung cancer mortalitye | Long-term | 1.14 per 10 μgm-3 | 0.013 | Age >30 | ||
| Cardiovascular mortalityd | Long-term | 1.12 per 10 μgm-3 | 0.011 | Age >30 | [49] | |
| NO2 | All-cause mortalityb | Short-term | 1.04 per 10 μgm-3 | 0.007 | All ages | [50] |
| Respiratory mortalityc | Short-term | 1.03 per 10 μgm-3 | 0.005 | All ages |
a Concentration–response coefficient.
b All-cause mortality excluded deaths attributed to external causes (ICD-10 codes V01–Y89).
c Respiratory mortality refers to ICD-10 code: J00-99.
d Cardiovascular mortality refers to ICD-10 code: I00-99
e Lung cancer mortality refers to ICD-10 codes C34.
We selected RR for short- and long-term effects based on available local study, systematic reviews and meta-analysis, as well as recommendations from previous EBD studies of ambient air pollutants. The chosen health outcomes were grouped following ICD-10 classification for all-causes mortality, except for deaths attributed to external causes (ICD-10: V01–Y89), cardiovascular disease (ICD-10: I00–I99), and respiratory disease (ICD-10: J00–J99). For PM10, we selected RR for all-causes mortality, respiratory and cardiovascular outcome based on available local study in Thailand [9]. The health outcomes of PM2.5 included all-causes mortality, cardiopulmonary mortality and lung cancer mortality in the population aged > 30 years, all due to long-term exposure to PM2.5, using annual average concentration as the exposure indicator [3]. Due to a lack of RR information in Thailand, this study used RR information for NO2 based on the systematic review and meta-analysis of 23 long-term studies on a global scale, published from 2004 to 2013, evaluating the relationship between NO2 and mortality outcome [48].
Attributable mortality due to ambient air pollution
To investigate the magnitude of the disease burden attributable to ambient air pollution and mortality associated with ambient air pollution, exposure is expressed as the fraction of disease or death attributable to the risk factor in a population and referred to as the population-attributable fraction (PAF) [18, 21]. The PAF has long been used to estimate the proportion reduction of burden that can be attributed to specified risk factors [51, 52]. The exposed population may be divided into multiple categories based on the level or length of exposure, each with its own RR. With multiple (n) exposure categories, the PAF is given by the following generalised equation [18]:
| (2) |
PAF = proportion of disease burden attributable to ambient air pollution
Pei = proportion estimates of the population in exposure category i, including the unexposed
RRi = relative risk (magnitude of the association between ambient air pollution and disease) in exposure category “i”, compared to the reference level
We calculated PAF using Eq 3 and performed GIS raster algebra analysis using the raster resampling technique for different resolutions. The coarse grid (Pe) served as the basis for our estimate of the total burden of disease across Thailand. To calculate the expected number of mortality cases due to ambient air pollution exposure (E), we applied PAF to the number of mortalities as the following equation;
| (3) |
E = expected number of deaths due to ambient air pollution
N = baseline number of deaths for each disease outcome
The number of disease specific deaths was obtained from the Thai Burden of Disease (BOD) study [53] conducted every five year to provide burden of disease information to setting national health planning priorities. The BOD study estimated age-, sex-, and cause-specific mortality by verifying cause of death from the national vital registration with a nation-wide verbal autopsy (VA) study [53–55]. The VA study was conducted in 2005 based on a sample of 3,316 in-hospital and 6,328 outside-hospital deaths from 28 selected districts in nine provinces [56]. Completeness adjustment of the vital registration was based on the mid census Survey of Population Change (SPC) conducted by the National Statistical Office [57, 58].
Results
Ambient air pollution concentrations in Thailand
Table 2 shows the statistics of average change in concentration (ΔX) values and statistical summary of model performance (best fit) corresponding to a spatial interpolation model for PM10, PM2.5, and NO2 from spatial interpolation based on surface monitoring measurements across Thailand. Fig 3 indicates the cross validation between the measured values at the monitoring stations and the model predictions. This study determined the average ΔX to be 41.7 μgm-3 (95%CI: 41.6–-41.75), 22.8 μgm-3 (95%CI: 22.81–22.87), 12.05 ppb (95%CI: 12–12.1), and 2.95 ppb (95%CI: 2.93–2.96), and the maximum concentrations were about 84.1 μgm-3, 44.1 μgm-3 and 48.03 ppb for PM10, PM2.5 and NO2, respectively (Table 2).
Table 2. Summary of statistical significance of the air quality data and spatial interpolation models.
| Statistics measured | Pollutants | |||||
|---|---|---|---|---|---|---|
| PM10 a | PM10 model | PM2.5 a, b | PM2.5 model | NO2 a | NO2 model | |
| Concentrations | 54.5 | 41.7 | 27.2 | 22.8 | 17.1 | 12.1 |
| (95% CI) | (49.6–59.3) | (41.6–41.8) | (24.8–29.7) | (22.7–22.9) | (14.1–20.1) | (12–12.1) |
| Minimum | 23.5 | 16.9 | 11.7 | 10.4 | 3 | 3 |
| Maximum | 100.7 | 84.1 | 50.4 | 44.1 | 50.6 | 48.03 |
| SD | 18.4 | 6.97 | 9.2 | 3.5 | 11.3 | 5.2 |
| Correlation c (r) |
*0.44 16.7 |
*0.44 8.41 |
*0.75 7.8 |
|||
| RMSE | ||||||
Note.
a Unit: μgm-3 for PM, ppb for NO2.
b PM2.5/PM10 ratio = 0.5.
c Correlation coefficients refers to measured vs. modelled concentrations.
* Correlation is significant at the 0.01 level (two-tailed).
SD: standard deviation; RMSE: root-mean-square error.
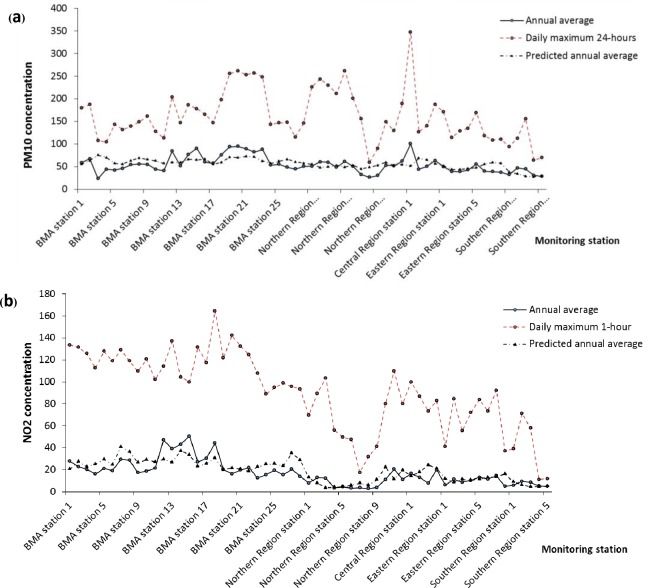
Fig 3. Annual average and predicted concentrations (1997–2009) by monitoring stations.
(a) PM10 and (b) NO2.
We calculated coefficients of correlation between the model predictions and the measured values at the monitoring stations. The Pearson correlation coefficients between best-fit models and actual concentrations for PM10, PM2.5 and NO2 were 0.44 (95%CI: 0.2–0.7), 0.75 (95%CI: 0.7–0.8), and 0.63 (95%CI: 0.5–0.75), respectively, which were statistically significant for measured ambient air concentrations (p-value <0.01).
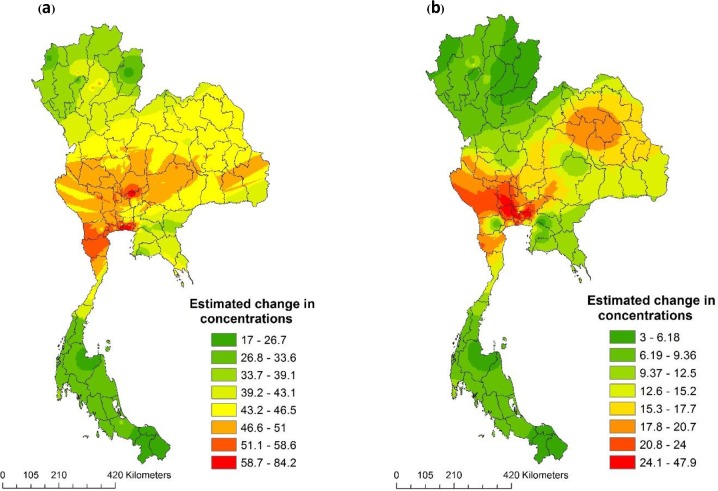
Fig 4 visualises the geographical distribution of annual mean estimate for exposure to ambient air pollutants across the study period. The exposure estimated for PM10, PM2.5, NO2 concentrations appeared in a range from 16.9 μgm-3 to 84.1 μgm-3, 10.4 μgm-3 to 44.1 μgm-3 and 3 ppb to 48 ppb, respectively, indicating that the Bangkok Metropolitan Region was more polluted than other regions in Thailand.
Fig 4. Spatial interpolation of change in concentrations (ΔX) of Thailand 2009.
(a) PM10 (μgm-3), (b) NO2 (ppb) and (c) PM2.5 (μgm-3).
Health impact and the population attributable fraction (PAF)
The health impact of ambient air pollutants was based on the relationship between change in concentrations (ΔX) and RR as described in Eq 1. Table 2 presented the estimation of RR in each pollutant and health outcome. The spatial distribution of RR across the country indicated a range from 1.01 to 1.35, depending on air pollutants and health outcome. Subsequently, this study determined the summation of PAF grids based on Eq 2 for each pollutant in Thailand in 2009, as shown in Table 3.
Table 3. Relative risk and population-attributable fractions (PAFs) based on level of ambient air pollutants in Thailand, 2009.
| Pollutants | Health end-point (mortality) |
RR | PAF (fraction) | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Max | Min | Summation | Mean (per grid) | 95%CI for mean | ||
| PM10 | All-cause | 1.03 | 0.005 | 1.05 | 1.00 | 0.017 | 6.96E-07 | 6.7E-07–7.3E-07 |
| Respiratory | 1.03 | 0.005 | 1.05 | 1.00 | 0.017 | 6.96E-07 | 6.7E-07–7.3E-07 | |
| Cardiovascular | 1.04 | 0.006 | 1.07 | 1.01 | 0.038 | 1.6E-06 | 1.5E-06–1.7E-06 | |
| PM2.5 | All-cause | 1.14 | 0.02 | 1.29 | 1.06 | 0.076 | 3.31E-06 | 3.17E-06–3.45E-06 |
| Lung cancer | 1.35 | 0.06 | 1.80 | 1.15 | 0.169 | 8.16E-06 | 7.8E-06–8.5E-06 | |
| Cardiovascular | 1.3 | 0.05 | 1.65 | 1.13 | 0.146 | 6.9E-06 | 6.6E-06–7.2E-06 | |
| NO2 | All-cause | 1.01 | 0.004 | 1.03 | 1.00 | 0.010 | 4E-07 | 3.7E-07–4.2E-07 |
| Respiratory | 1.037 | 0.02 | 1.16 | 1.00 | 0.025 | 1.03E-06 | 0.95E-06–1.1E-06 | |
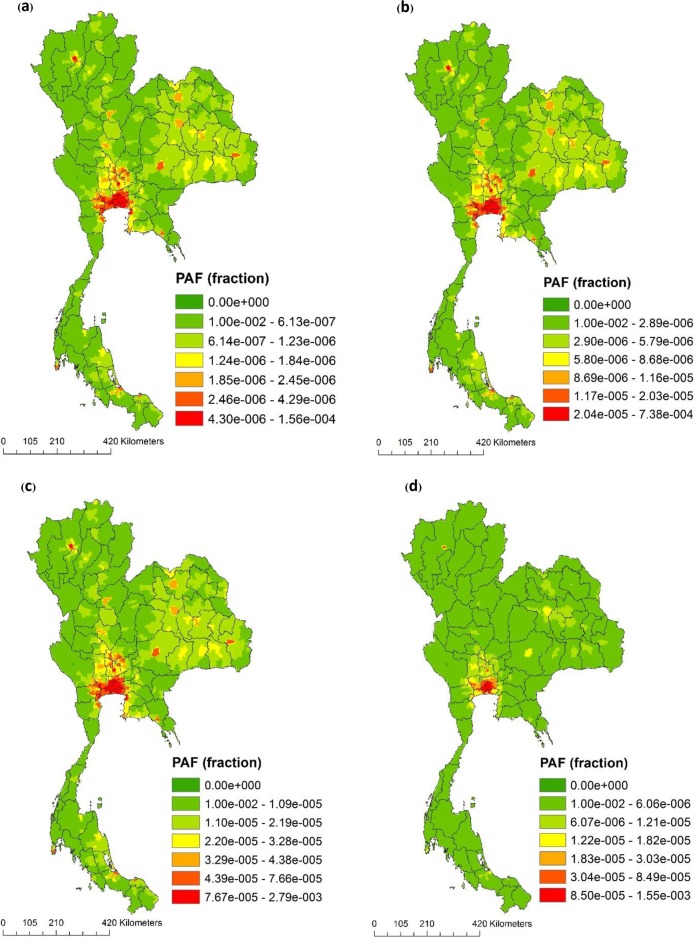
This study estimated the average PAF grid for all-causes mortality due to PM10, PM2.5, NO2 at approximately 1.41 x 10−6 (95% CI: 1.35 x 10−6–1.47 x 10−6), 3.31 x 10−6 (95% CI: 3.17 x 10−6–3.45 x 10−6), and 4.6 x 10−6 (95% CI: 4.2 x 10−7–4.8 x 10−6), respectively. The average PAF for lung cancer caused by PM2.5 was approximately 8.16 x 10−6 (95% CI: 7.8 x 10−6–8.5 x 10−6). Fig 5 illustrates that the spatial variability of PAF due to long-term ambient air pollution exposure varied across Thailand. The results of this study indicated that the Bangkok Metropolitan Area had the largest percentage of total mortality attributable to PM2.5 across all ages (level ranged widely from 2.93 x 10−4 to 7.4 x 10−4 depending on the risk estimate used), which was the highest among the three air pollutants. The largest percentage of mortality attributable to NO2 was also the highest in the Bangkok Metropolitan Area (PAF ranged between 1.67 x 10−4 to 4.51 x 10−4 depending on the selected health end-point).
Fig 5. Spatial variations of population attributable fractions (PAF) in Thailand 2009.
(a) All-cause mortality due to PM10, (b) all-cause mortality due to long- term effect of PM2.5, (c) cardiovascular mortality due to long- term effect of PM2.5 and, (d) respiratory mortality due to NO2.
The summation of the PAFs for all grids in the category of air pollutants and disease outcomes based on Eq 2 and Table 1 are represented in Table 3. The PAFs for all-causes mortality for PM10, PM2.5, and NO2 were approximately 0.02, 0.1 and 0.1, respectively. The PAFs for respiratory mortality caused by PM10 and NO2 were approximately 0.02 and 0.07, respectively; PAFs for cardiovascular mortality caused by PM10 and PM2.5 were approximately 0.04 and 0.15, respectively, while PAF for lung cancer caused by PM2.5 was approximately 0.17. PM2.5 had the highest model estimated PAF at 17% of lung cancer burden.
Table 4 indicates the results for mortality caused by ambient air pollutants. The best estimate demonstrated ambient air pollution-related mortality, which included pollution from PM2.5, PM10 and NO2. Annually, there are about 3652–38410, 653–934 and 4024–15361 cases of all-causes, respiratory and cardiovascular mortality, respectively, from long-term exposure to PM and NO2, reflecting the highest CR from PM and the underlying cause-specific mortality for each health outcome.
Table 4. Avoidable mortality and the percentage of deaths estimated to be caused by level of air pollutant in Thailand (2009).
| Pollutants Health outcome |
Mortality, in hundreds | ||||
|---|---|---|---|---|---|
| Current estimate (1) | Decrease 20% of pollutants concentration (2) |
Avoided mortality (1–2) |
Percentage of current estimate | ||
| PM10 | All-cause | 126.9 | 96.2 | 30.8 | 24.3 |
| Respiratorya | 6.52 | 4.9 | 1.6 | 24.4 | |
| Cardiovasculara | 40.24 | 30.5 | 9.8 | 24.2 | |
| PM2.5 | All-cause | 269.9 | 210.1 | 59.8 | 22.2 |
| Cardiovasculara | 153.6 | 11.9 | 34.0 | 22.1 | |
| Lung-cancera | 24.6 | 19.1 | 5.4 | 22.0 | |
| NO2 | All-cause | 36.5 | 29.2 | 7.3 | 19.9 |
| Respiratorya | 9.3 | 7.5 | 1.9 | 19.8 | |
a The mortality numbers are not additive because these health outcomes are subsets of all-cause mortality.
The results in this study indicated that, if PM and NO2 were reduced by 20% from current levels, the health burden could be reduced in about 5982 cases for all-causes mortality, 160–581 and 146–3401 cases for respiratory and cardiovascular mortality, respectively, depending on each pollutant in Thailand. Similarly, the health burden would have been reduced annually to about 3081 cases for all-causes mortality per year if the highest concentrations for ambient particulate matter (PM10) across the country, which was about 84.6 μg/m3, had been reduced to 65 μg/m3. Respiratory mortality attributed to PM10 could be reduced annually to about 160 cases per year, or about 24.4% of the current estimate due to respiratory mortality. Other health outcomes of particulate matter, such as cardiovascular and lung cancer, could also be reduced annually to about 146 and 542 cases per year, respectively.
Discussion
We presented a combination of GIS spatial analysis and empirical information on CRA to quantify the geographical distribution of PAFs and 2009 mortality due to various ambient air pollutants (PM2.5, PM10 and NO2) across Thailand, based on available empirical data. We predicted mortality attributable to short- and long-term ambient air pollution exposure ranging between 933 and 27 thousands persons depending on air pollutants across the country. PAFs varied across the country, as expected; PAF and exposure to air pollutants were relatively concentrated in the Bangkok metropolitan area, which had the largest number of monitoring stations, population density and air pollutant concentrations in Thailand, especially for PM and NO2.
Our estimate of all causes mortality attributable to PM2.5, was about 38,410 deaths or 6% of total deaths in Thailand. This proportion was not much different in terms of proportion compared to the GBD 2015[5], which had estimated 7.6% of total mortality for long-term exposure to PM2.5 globally. GBD used existing surface monitoring data to assemble a georeferenced global PM2.5 measurement database of 2005 annual average concentrations from available national/regional/local air quality monitoring reports and excluded PM10 and NO2 from their estimation [59]. The surface monitoring measurements dataset for the Asia region was based primarily on measured PM2.5 and appeared in the annual ambient air quality monitoring report from Australia and New Zealand [60]. The surface monitoring measurement datasets for other Asian countries (e.g. Thailand) were obtained from the Clean Air Initiative Asia (CAI-Asia) [59], which was generated from available datasets in 2005. All air quality stations were available for monitored important pollutants such as PM10 and NO2 in Thailand since the enhancement and conservation of the National Environmental Quality Act of 1992[61]. PM stations for the GBD study were about 16 stations for representing entire areas in the Southeast Asia region [59], reflecting significant evidence of air pollutant concentrations and long distance correlation (i.e. regional scale).
PAF is an estimation of the proportion of cases in the entire study population that can be attributed to air pollution. It can illustrate the health impact gained if the exposure to the counterfactual level can be reduced. The PAFs in this study were between 7.6% and 16.9% (Table 3) depending on health outcomes. In another approach to assess exposure based on the same RR information [3], Fann et al.[62] estimated that the largest percentage (between 7% and 17% depending on the health outcomes) of mortality attributable to PM2.5 was in southern California in the United States, using the Community Multiscale Air Quality (CMAQ) Modelling System[63] and health impact function. Anenberg et al.[20] also estimated the global burden of mortality due to PM2.5 to be about 2% for cardiopulmonary and lung cancer mortality, and 7% for all-causes mortality using the global atmospheric chemical transport model [64]. In another related study based on the same CR[3, 65], Ying Li et al. 2010[66] estimated the disease burden attributed to particulate matter exposure in the United Arab Emirates (UAE) from surface air monitoring station data and the spatial interpolated modelling technique. Their estimates of attributable fractions for PM were represented spatially and ranged from 12% to 28% of the total all-causes mortality in the UAE in adults aged >30 years in 2007, or at approximately 545 excess deaths annually[66]. The all-causes mortality due to PAF of PM10 and PM2.5 in our study were approximately 3 and 8%and lower compared to Ying Li et al. The means of PM10 concentration (μgm-3) for the UAE (90–665 μgm-3) were higher than those in Thailand (ranging between 20 and 84 μgm-3) because the UAE is situated in a desert region and severe dust storms occur in the Arabian Gulf region[66]. Moreover, the dispersion of pollutants from other continents may be another factor producing a high natural background of pollutants (e.g. PM10 90 μgm-3 and PM2.5, 45 μgm-3).
According to a previous study on the mortality risk estimation due to air pollution in Thailand, the pollution mix, seasonality and demographics may be different from developed countries in Europe and North America[31]. We attempted to use available RR information from local epidemiological studies to reduce the bias caused by extrapolation of findings to another location[66]. Therefore, we used the RR information for PM10 all-cause and respiratory outcome from a local study that investigated the association between effects of exposure to air pollution on mortality risks in Thailand[9]. For NO2, we estimated all-causes mortality and respiratory mortality based on evidences from a systematic review and meta-analysis on a global scale due to the lack of RR information at the local level. However, the burden of NO2 showed the largest mortality contribution, and the high correlation between NO2 and PM2.5 (around 0.7–0.8) of meta-analysis still suggests the possibility that NO2 effects could be due in part to confounding from particulate matter. Hence, future epidemiological studies about information on the RR for Thailand should be conducted to reduce bias and improve PAF estimation.
Our results may be underestimated, since GBD recommended O3 as one of the indicators to quantify air pollution exposure associated with adverse health outcomes similar to those induced by PM (i.e. respiratory, cardiopulmonary diseases) [46, 66]. Several studies[67, 68] indicated that NO2 contributed O3 formation as a precursor with heavy traffic load, large population density and meteorological factors [69–71]. Moreover, mixtures of O3 and NO2 might react to form dinitrogen pentoxide (N2O5), that could create a greater risk than either O3 or NO2. Further studies should consider the analysis with the role of O3 as a possible important effect on the health outcomes.
For the quality of information on the levels of mortality and causes of death, several studies stated that mortality statistics in Thailand were low quality, with 20–40% of deaths are registered with unknown or nonspecific causes in the past decade [56, 72, 73]. However, we used the best available mortality information from the study that had been initiated to verify cause of deaths (COD) reported by vital registration from the nation-wide VA study[53, 56, 74], and adjusted the completeness of the vital registration was based on the mid census SPC conducted by the National Statistical Office [57, 58].
Our study might have some limitations and uncertainties. For the exposure assessment based on air quality monitoring station may depend on the location, density and distance of the monitoring network to nearby emission sources. In particular, the low number of measuring sites displayed in some regions (e.g. two and five stations in the north-eastern (about 160,000 km2) and southern (about 70,713 km2) regions, which may have some limitations in simulating the uniformly distributed annual ambient air pollution exposure on a large scale (e.g. national or regional scale. We recommend that empirical-based models at the national level are required to identify the priority sites of where new monitoring stations should be located to increase the air monitoring stations in a large population area [75], to improve the empirical-based estimation in future research.
Furthermore, monitoring stations with a measurement capacity for PM2.5 remained limited at the national level at the time of this study [76]. Therefore, we recommend the use of a PM2.5 /PM10 ratio based on available local study and empirical information in WHO’s EBD study[19] to estimate the exposure to PM2.5. As the remote sensing technique was used for estimating the surface PM2.5 concentrations from satellite observations, remote sensing-derived PM2.5 measures have been found to be well correlated with actual ground-level PM2.5 measurements [77, 78]. Therefore, future research may consider remote sensing data combined with ground monitoring station data in Thailand for greater precision in assessing PM2.5 exposure [47].
Another limitation was regarding the PAF estimation on a spatial scale based on two different grid resolutions of Pe and air pollutant concentration layer. Although, we used the resampling and interpolation technique in the raster algebra process[79], this may have produced some variation of grid and uncertainty from the estimation[66]. Further studies should employ a multi-spatial resolution approach [80, 81] and/or consider the consistency of spatial resolution on air concentrations and the population of exposure distribution [48].
Finally, our findings indicate a significant health impact due to air pollution problems in Thailand and that a 20% reduction in air pollutants could reduce the number of annual deaths by about 160–7,425 per year. Therefore, the government should increase its effort and investment into controlling air pollution to achieve the SDGs. As previously stated, air pollution problems and their burden of disease are geographically specific. Thus, autonomy and the capacities of local authorities in managing their own problems are certainly required, as well as a national healthy public policy framework [82] to effectively deal with these problems.
Conclusions
This study aimed to quantify the magnitude and distribution of disease burden caused by ambient air pollution for policy-makers and planner by presenting an integrated exposure assessment, using a spatial interpolation model from empirical data, population distribution exposure and health impact function to estimate the national disease burden attributable to ambient air pollution. In addition, the GIS-based population exposure assessments for PAFs and the estimation of the number of deaths due to ambient air pollution exposure are useful for prioritising policy to reduce and prevent adverse health effects in Thailand. We hope that our findings offer a national estimate and benefit decision-making by stakeholders and policy-makers to promote and develop air quality management and health co-benefit strategies to achieve the SDGs in the future.
Acknowledgments
We are grateful to the Air Quality and Noise Management Bureau, Pollution Control Department, Thailand for providing the ambient air pollution data from the air quality monitoring stations network in Thailand.
Data Availability
Air quality data that support the findings of this study are owned by the Division of Air Quality Data, Air Quality and Noise Management Bureau, Pollution Control Department. For further information about data on Thailand's air and noise pollution please visit http://aqnis.pcd.go.th/en, or http://air4thai.pcd.go.th/web/index.php (in Thai). For permission to use the data, please contact the air monitoring division, Air quality and noise management bureau, Pollution Control Department. Tel: (+66) 2 298-2346 e-mail: e-petition@pcd.go.th. The proportion of the population by selected age groups are collected from Official Statistics Registration Systems from the Department of Provincial Administration, Thailand at http://stat.dopa.go.th/stat/statnew/statMenu/newStat/home.php (in Thai) or http://stat.bora.dopa.go.th/new_stat/webPage/statByAge.php (in Thai). For permission to use the data, please contact the Bureau of Registration Administration (BORA), Thailand via e-mail: m03095061@bora.dopa.go.th. The high-resolution population dataset (number of persons per grid cell) used in this study can be obtained from Socioeconomic Data and Applications Center (SEDAC) website (http://sedac.ciesin.columbia.edu/data/set/gpw-v3-population-count).
Funding Statement
This work was supported by the Thai Health Promotion Foundation (ThaiHealth 58-03229).
References
- 1.WHO: Air quality guidelines. Global update 2005. Particulate matter, ozone, nitrogen dioxide and sulfur dioxide. In.; 2006. [PubMed]
- 2.Dockery DW, Pope CA, 3rd, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG Jr., Speizer FE: An association between air pollution and mortality in six U.S. cities. N Engl J Med 1993, 329(24):1753–1759. doi: 10.1056/NEJM199312093292401 [DOI] [PubMed] [Google Scholar]
- 3.Pope CA 3rd, Burnett RT, Thun MJ, Calle EE, Krewski D, Ito K, Thurston GD: Lung cancer, cardiopulmonary mortality, and long-term exposure to fine particulate air pollution. Jama 2002, 287(9):1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization. Department of Public Health E, Health SDo: Ambient Air Pollution: A Global Assessment of Exposure and Burden of Disease: World Health Organization; 2016. [Google Scholar]
- 5.Forouzanfar MH, Afshin A, Alexander LT, Anderson HR, Bhutta ZA, Biryukov S, Brauer M, Burnett R, Cercy K, Charlson FJ et al. : Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. The Lancet , 388(10053):1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen AJ, Brauer M, Burnett R, Anderson HR, Frostad J, Estep K, Balakrishnan K, Brunekreef B, Dandona L, Dandona R et al. : Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H: A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012, 380(9859):2224–2260. doi: 10.1016/S0140-6736(12)61766-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vichit-Vadakan N, Vajanapoom N: Health Impact from Air Pollution in Thailand: Current and Future Challenges. Environmental Health Perspectives 2011, 119(5):A197–A198. doi: 10.1289/ehp.1103728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo Y, Li S, Tawatsupa B, Punnasiri K, Jaakkola JJK, Williams G: The association between air pollution and mortality in Thailand. Scientific Reports 2014, 4:5509 doi: 10.1038/srep05509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vichit-Vadakan N, Vajanapoom N, Ostro B: Part 3. Estimating the effects of air pollution on mortality in Bangkok, Thailand. Research report (Health Effects Institute) 2010(154):231–268. [PubMed] [Google Scholar]
- 11.Forouzanfar MH, Alexander L, Anderson HR, Bachman VF, Biryukov S, Brauer M, Burnett R, Casey D, Coates MM, Cohen A et al. : Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. The Lancet , 386(10010):2287–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thailand BoD: Comparative risk assessment in Thai population 2009 (in Thai). In.: International Health Policy Program; Thailand; 2009. [Google Scholar]
- 13.Bank W: Thailand—Environment monitor 2002: air quality. In. Washington, DC: World Bank; 2002. [Google Scholar]
- 14.Nuckols JR, Ward MH, Jarup L: Using geographic information systems for exposure assessment in environmental epidemiology studies. Environ Health Perspect 2004, 112(9):1007–1015. doi: 10.1289/ehp.6738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gorai AK, Tuluri F, Tchounwou PB: A GIS based approach for assessing the association between air pollution and asthma in New York State, USA. International journal of environmental research and public health 2014, 11(5):4845–4869. doi: 10.3390/ijerph110504845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang W, Ying Y, Wu Q, Zhang H, Ma D, Xiao W: A GIS-based spatial correlation analysis for ambient air pollution and AECOPD hospitalizations in Jinan, China. Respiratory Medicine 2015, 109(3):372–378. doi: 10.1016/j.rmed.2015.01.006 [DOI] [PubMed] [Google Scholar]
- 17.Valari M, Menut L, Chatignoux E: Using a chemistry transport model to account for the spatial variability of exposure concentrations in epidemiologic air pollution studies. Journal of the Air & Waste Management Association (1995) 2011, 61(2):164–179. [DOI] [PubMed] [Google Scholar]
- 18.Murray CJ, Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S: Comparative quantification of health risks conceptual framework and methodological issues. Popul Health Metr 2003, 1(1):1 doi: 10.1186/1478-7954-1-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ostro B: Outdoor air pollution: assessing the environmental burden of disease at national and local levels. WHO Environmental Burden of Disease Series, No. 5. 2004.
- 20.Anenberg SC, Horowitz LW, Tong DQ, West JJ: An estimate of the global burden of anthropogenic ozone and fine particulate matter on premature human mortality using atmospheric modeling. Environ Health Perspect 2010, 118(9):1189–1195. doi: 10.1289/ehp.0901220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pinichka C, Bundhamcharoen K, Shibuya K: Diseases Burden of Chronic Obstructive Pulmonary Disease (COPD) Attributable to Ground-Level Ozone in Thailand: Estimates Based on Surface Monitoring Measurements Data. Global journal of health science 2016, 8(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.The Enhancement and Conservation of National Environmental Quality Act, B.E. 2535
- 23.Chen CC, Tsai SS, Yang CY: Association between Fine Particulate Air Pollution and Daily Clinic Visits for Migraine in a Subtropical City: Taipei, Taiwan. International journal of environmental research and public health 2015, 12(5):4697–4708. doi: 10.3390/ijerph120504697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pope CA, 3rd, Ezzati M, Dockery DW: Fine-particulate air pollution and life expectancy in the United States. N Engl J Med 2009, 360(4):376–386. doi: 10.1056/NEJMsa0805646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dockery DW: Health Effects of Particulate Air Pollution. Annals of Epidemiology 2009, 19(4):257–263. doi: 10.1016/j.annepidem.2009.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kloog I, Ridgway B, Koutrakis P, Coull BA, Schwartz JD: Long- and Short-Term Exposure to PM(2.5) and Mortality: Using Novel Exposure Models. Epidemiology (Cambridge, Mass) 2013, 24(4):555–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xing Y-F, Xu Y-H, Shi M-H, Lian Y-X: The impact of PM2.5 on the human respiratory system. Journal of Thoracic Disease 2016, 8(1):E69–E74. doi: 10.3978/j.issn.2072-1439.2016.01.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wilson WE, Suh HH: Fine particles and coarse particles: concentration relationships relevant to epidemiologic studies. Journal of the Air & Waste Management Association (1995) 1997, 47(12):1238–1249. [DOI] [PubMed] [Google Scholar]
- 29.Wanida Jinsart KT, Samarnchai Loetkamonwit, Sarawut Thepanondh, Kanae Karita & Eiji Yano: Roadside Particulate Air Pollution in Bangkok. Journal of the Air & Waste Management Association 2002, 52(9):1102–1110. [DOI] [PubMed] [Google Scholar]
- 30.Ostro BD, Broadwin R, Lipsett MJ: Coarse and fine particles and daily mortality in the Coachella Valley, California: a follow-up study. Journal of exposure analysis and environmental epidemiology 2000, 10(5):412–419. [DOI] [PubMed] [Google Scholar]
- 31.Vichit-Vadakan N, Vajanapoom N, Ostro B: The Public Health and Air Pollution in Asia (PAPA) Project: Estimating the Mortality Effects of Particulate Matter in Bangkok, Thailand. Environmental Health Perspectives 2008, 116(9):1179–1182. doi: 10.1289/ehp.10849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Qian Z, Zhang J, Wei F, Wilson WE, Chapman RS: Long-term ambient air pollution levels in four Chinese cities: inter-city and intra-city concentration gradients for epidemiological studies. Journal of exposure analysis and environmental epidemiology 2001, 11(5):341–351. doi: 10.1038/sj.jea.7500170 [DOI] [PubMed] [Google Scholar]
- 33.Vichit-Vadakan N, Ostro BD, Chestnut LG, Mills DM, Aekplakorn W, Wangwongwatana S, Panich N: Air pollution and respiratory symptoms: results from three panel studies in Bangkok, Thailand. Environmental Health Perspectives 2001, 109(Suppl 3):381–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoek G, Brunekreef B, Goldbohm S, Fischer P, van den Brandt PA: Association between mortality and indicators of traffic-related air pollution in the Netherlands: a cohort study. The Lancet 2002, 360(9341):1203–1209. [DOI] [PubMed] [Google Scholar]
- 35.Dilip Kumar Jha MS, Anup Das, Vinithkumar N.V. and Kirubagaran R.: Evaluation of Interpolation Technique for Air Quality Parameters in Port Blair, India. Universal Journal of Environmental Research and Technology 2011, 1(3):301–310. [Google Scholar]
- 36.Gong G, Mattevada S, O’Bryant SE: Comparison of the accuracy of kriging and IDW interpolations in estimating groundwater arsenic concentrations in Texas. Environmental Research 2014, 130(0):59–69. [DOI] [PubMed] [Google Scholar]
- 37.Bartier PM, Keller CP: Multivariate interpolation to incorporate thematic surface data using inverse distance weighting (IDW). Computers & Geosciences 1996, 22(7):795–799. [Google Scholar]
- 38.Weber D, Englund E: Evaluation and comparison of spatial interpolators. Math Geol 1992, 24(4):381–391. [Google Scholar]
- 39.Kim E, Park H, Hong Y-C, Ha M, Kim Y, Kim B-N, Kim Y, Roh Y-M, Lee B-E, Ryu J-M et al. : Prenatal exposure to PM10 and NO2 and children's neurodevelopment from birth to 24 months of age: Mothers and Children's Environmental Health (MOCEH) study. Science of The Total Environment 2014, 481(0):439–445. [DOI] [PubMed] [Google Scholar]
- 40.Moradi Dashtpagerdi M, Sadatinejad SJ, Zare Bidaki R, Khorsandi E: Evaluation of Air Pollution Trend Using GIS and RS Applications in South West of Iran. Journal of the Indian Society of Remote Sensing 2014, 42(1):179–186. [Google Scholar]
- 41.Ivina O, Nouretdinov I, Gammerman A: Valid predictions with confidence estimation in an air pollution problem. Progress in Artificial Intelligence 2012, 1(3):235–243. [Google Scholar]
- 42.Cesar H, Aburto VHB, Cicero-Fernandez P, Dorland K, Cruz RM, Brander L, Cropper M, Martinez ACG, Olaiz-Fernandez G, Bolivar APM et al. : Improving Air Quality in Metropolitan Mexico City: An Economic Valuation; 2002. [Google Scholar]
- 43.CIESIN: Gridded Population of the World, Version 3 (GPWv3): Population Density Grid. In.: NASA Socioeconomic Data and Applications Center (SEDAC); 2005. [Google Scholar]
- 44.Department of Provincial Administration T: Official Statistics Registration Systems. In.; 2009.
- 45.Anderson HR, Spix C, Medina S, Schouten JP, Castellsague J, Rossi G, Zmirou D, Touloumi G, Wojtyniak B, Ponka A et al. : Air pollution and daily admissions for chronic obstructive pulmonary disease in 6 European cities: results from the APHEA project. Eur Respir J 1997, 10(5):1064–1071. [DOI] [PubMed] [Google Scholar]
- 46.Barnett AG, Williams GM, Schwartz J, Best TL, Neller AH, Petroeschevsky AL, Simpson RW: The effects of air pollution on hospitalizations for cardiovascular disease in elderly people in Australian and New Zealand cities. Environ Health Perspect 2006, 114(7):1018–1023. doi: 10.1289/ehp.8674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Evans J, van Donkelaar A, Martin RV, Burnett R, Rainham DG, Birkett NJ, Krewski D: Estimates of global mortality attributable to particulate air pollution using satellite imagery. Environ Res 2013, 120:33–42. doi: 10.1016/j.envres.2012.08.005 [DOI] [PubMed] [Google Scholar]
- 48.Kim YM, Kim JW, Lee HJ: Burden of disease attributable to air pollutants from municipal solid waste incinerators in Seoul, Korea: a source-specific approach for environmental burden of disease. Sci Total Environ 2011, 409(11):2019–2028. doi: 10.1016/j.scitotenv.2011.02.032 [DOI] [PubMed] [Google Scholar]
- 49.Pope CA, 3rd, Burnett RT, Turner MC, Cohen A, Krewski D, Jerrett M, Gapstur SM, Thun MJ: Lung cancer and cardiovascular disease mortality associated with ambient air pollution and cigarette smoke: shape of the exposure-response relationships. Environ Health Perspect 2011, 119(11):1616–1621. doi: 10.1289/ehp.1103639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faustini A, Rapp R, Forastiere F: Nitrogen dioxide and mortality: review and meta-analysis of long-term studies. Eur Respir J 2014, 44(3):744–753. doi: 10.1183/09031936.00114713 [DOI] [PubMed] [Google Scholar]
- 51.Levin ML: The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum 1953, 9(3):531–541. [PubMed] [Google Scholar]
- 52.Rockhill B, Newman B, Weinberg C: Use and misuse of population attributable fractions. Am J Public Health 1998, 88(1):15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bundhamcharoen K, Odton P, Phulkerd S, Tangcharoensathien V: Burden of disease in Thailand: changes in health gap between 1999 and 2004. BMC Public Health 2011, 11:53–53. doi: 10.1186/1471-2458-11-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hill K: Estimating census and death registration completeness. Asian and Pacific population forum 1987, 1(3):8–13, 23–14. [PubMed] [Google Scholar]
- 55.SH P: Use of direct and indirect techniques for estimating the completeness of death registration systems. Data bases for mortality measurement Papers of the Meeting of the United Nations/World Health Organization Working Group on Data Bases for Measurement of Levels, Trends and Differentials in Mortality, Bangkok, 20–23 October 1981 New York, NY United Nations (Population Studies, No 84) 1984, 84:66–76.
- 56.Rao C, Porapakkham Y, Pattaraarchachai J, Polprasert W, Swampunyalert N, Lopez AD: Verifying causes of death in Thailand: rationale and methods for empirical investigation. Population Health Metrics 2010, 8(1):11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Office NS: Report on the 2005–2006 National Survey of Population Change. In. Bangkok: Royal Government of Thailand; 2006. [Google Scholar]
- 58.Prasartkul P, Vapattanawong P: The completeness of death registration in Thailand: Evidence from demographic surveillance system of the Kanchanaburi Project. World health & population 2006, 8(3):43–51. [DOI] [PubMed] [Google Scholar]
- 59.Brauer M, Amann M, Burnett RT, Cohen A, Dentener F, Ezzati M, Henderson SB, Krzyzanowski M, Martin RV, Van Dingenen R et al. : Exposure assessment for estimation of the global burden of disease attributable to outdoor air pollution. Environ Sci Technol 2012, 46(2):652–660. doi: 10.1021/es2025752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Environment Canterbury NZ: Annual ambient air quality monitoring report. In.; 2005.
- 61.Thailand Go: Enhancement and Conservation of National Environmental Quality Act, B.E. 2535. In. Edited by Thailand Go; 1992.
- 62.Fann N, Lamson AD, Anenberg SC, Wesson K, Risley D, Hubbell BJ: Estimating the national public health burden associated with exposure to ambient PM2.5 and ozone. Risk Anal 2012, 32(1):81–95. doi: 10.1111/j.1539-6924.2011.01630.x [DOI] [PubMed] [Google Scholar]
- 63.Dennis RL, Byun DW, Novak JH, Galluppi KJ, Coats CJ, Vouk MA: The next generation of integrated air quality modeling: EPA's models-3. Atmospheric Environment 1996, 30(12):1925–1938. [Google Scholar]
- 64.Horowitz LW: A global simulation of tropospheric ozone and related tracers: Description and evaluation of MOZART, version 2. Journal of Geophysical Research 2003, 108(D24). [Google Scholar]
- 65.Pope CA, 3rd, Burnett RT, Thurston GD, Thun MJ, Calle EE, Krewski D, Godleski JJ: Cardiovascular mortality and long-term exposure to particulate air pollution: epidemiological evidence of general pathophysiological pathways of disease. Circulation 2004, 109(1):71–77. doi: 10.1161/01.CIR.0000108927.80044.7F [DOI] [PubMed] [Google Scholar]
- 66.Li Y, Gibson JM, Jat P, Puggioni G, Hasan M, West JJ, Vizuete W, Sexton K, Serre M: Burden of disease attributed to anthropogenic air pollution in the United Arab Emirates: estimates based on observed air quality data. Sci Total Environ 2010, 408(23):5784–5793. doi: 10.1016/j.scitotenv.2010.08.017 [DOI] [PubMed] [Google Scholar]
- 67.Zhang K, Batterman S: Air pollution and health risks due to vehicle traffic. The Science of the total environment 2013, 0:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dijkema MB, Mallant SF, Gehring U, van den Hurk K, Alssema M, van Strien RT, Fischer PH, Nijpels G, Stehouwer CD, Hoek G et al. : Long-term Exposure to Traffic-related Air Pollution and Type 2 Diabetes Prevalence in a Cross-sectional Screening-study in the Netherlands. Environmental Health 2011, 10(1):76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho H-S, Choi M: Effects of Compact Urban Development on Air Pollution: Empirical Evidence from Korea. Sustainability 2014, 6(9):5968. [Google Scholar]
- 70.Zhao H, Wang S, Wang W, Liu R, Zhou B: Investigation of Ground-Level Ozone and High-Pollution Episodes in a Megacity of Eastern China. PLoS ONE 2015, 10(6):e0131878 doi: 10.1371/journal.pone.0131878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.An JL, Wang YS, Li X, Sun Y, Shen SH, Shi LQ: [Analysis of the relationship between NO, NO2 and O3 concentrations in Beijing]. Huan jing ke xue = Huanjing kexue 2007, 28(4):706–711. [PubMed] [Google Scholar]
- 72.Mathers CD, Ma Fat D, Inoue M, Rao C, Lopez AD: Counting the dead and what they died from: an assessment of the global status of cause of death data. Bulletin of the World Health Organization 2005, March 2005. [PMC free article] [PubMed] [Google Scholar]
- 73.Tangcharoensathien V, Faramnuayphol P, Teokul W, Bundhamcharoen K, Wibulpholprasert S: A critical assessment of mortality statistics in Thailand: potential for improvements. Bulletin of the World Health Organization 2006, 84(3):233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Makka N, Kusreesakul K, Amornvisaisordej C, Bundhamcharoen K: Regional Differences in Burden of Disease in Thailand, Year 2009. Journal of Health Science 2016, 25(2). [Google Scholar]
- 75.Puangthongthub S, Wangwongwatana S, Kamens RM, Serre ML: Modeling the space/time distribution of particulate matter in Thailand and optimizing its monitoring network. Atmospheric Environment 2007, 41(36):7788–7805. [Google Scholar]
- 76.Simachaya W: Overview of Air Quality Management in Thailand. In. Edited by Department PC; 2007. [Google Scholar]
- 77.van Donkelaar A, Martin RV, Park RJ: Estimating ground-level PM2.5 using aerosol optical depth determined from satellite remote sensing. Journal of Geophysical Research: Atmospheres 2006, 111(D21):n/a-n/a. [Google Scholar]
- 78.Liu Y, Sarnat JA, Kilaru V, Jacob DJ, Koutrakis P: Estimating Ground-Level PM2.5 in the Eastern United States Using Satellite Remote Sensing. Environmental Science & Technology 2005, 39(9):3269–3278. [DOI] [PubMed] [Google Scholar]
- 79.Teegavarapu RSV, Meskele T, Pathak CS: Geo-spatial grid-based transformations of precipitation estimates using spatial interpolation methods. Computers & Geosciences 2012, 40(Supplement C):28–39. [Google Scholar]
- 80.Wickramasinghe C, Jones S, Reinke K, Wallace L: Development of a Multi-Spatial Resolution Approach to the Surveillance of Active Fire Lines Using Himawari-8. Remote Sensing 2016, 8(11):932. [Google Scholar]
- 81.García-Llamas P, Calvo L, Álvarez-Martínez JM, Suárez-Seoane S: Using remote sensing products to classify landscape. A multi-spatial resolution approach. International Journal of Applied Earth Observation and Geoinformation 2016, 50(Supplement C):95–105. [Google Scholar]
- 82.Services MoHaS: National Health Policy Framework 2010–2020: towards quality health and social welfare services. In. Edited by Services MoHaS; 2010. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Air quality data that support the findings of this study are owned by the Division of Air Quality Data, Air Quality and Noise Management Bureau, Pollution Control Department. For further information about data on Thailand's air and noise pollution please visit http://aqnis.pcd.go.th/en, or http://air4thai.pcd.go.th/web/index.php (in Thai). For permission to use the data, please contact the air monitoring division, Air quality and noise management bureau, Pollution Control Department. Tel: (+66) 2 298-2346 e-mail: e-petition@pcd.go.th. The proportion of the population by selected age groups are collected from Official Statistics Registration Systems from the Department of Provincial Administration, Thailand at http://stat.dopa.go.th/stat/statnew/statMenu/newStat/home.php (in Thai) or http://stat.bora.dopa.go.th/new_stat/webPage/statByAge.php (in Thai). For permission to use the data, please contact the Bureau of Registration Administration (BORA), Thailand via e-mail: m03095061@bora.dopa.go.th. The high-resolution population dataset (number of persons per grid cell) used in this study can be obtained from Socioeconomic Data and Applications Center (SEDAC) website (http://sedac.ciesin.columbia.edu/data/set/gpw-v3-population-count).