ABSTRACT
Checkpoint inhibitors have improved survival for patients with melanoma, non-small-cell lung cancer (NSCLC), bladder, head and neck and other cancers. Antibodies against PD-L1, including atezolizumab, avelumab and durvalumab, are also being developed and have been approved for various cancers. Compared with anti-CTLA-4 drugs, studies with anti-PD-1/PD-L1 agents have suggested higher response rates and improved survival. Targeting PD-L1 rather than PD-1 may also theoretically offer further benefit, with the potential for improved efficacy and reduced toxicity, although this has not been clearly shown by clinical experience to date. Anti-PD-L1 agents have shown good efficacy and manageable toxicity in several tumor types.
KEYWORDS: Atezolizumab, avelumab, bladder cancer, durvalumab, immunotherapy, lung cancer, melanoma, Merkel cell carcinoma, PD-L1
Introduction
In recent years, immunotherapy has revolutionized the treatment of many advanced stage cancers. Initial studies were primarily conducted in patients with metastatic melanoma, in whom anti-cytotoxic T-lymphocyte-associated antigen (CTLA)-4 and anti-programmed cell death (PD)-1 agents have dramatically improved survival. Inhibition of CTLA-4 and PD-1, resulting in increased activation of the immune system, has since shown efficacy in patients with non-small-cell lung cancer (NSCLC), bladder, head and neck, and other cancers.1
CTLA-4 and PD-1 immune checkpoints are negative regulators of T-cell immune function. Although the exact mechanisms of action of CTLA-4 and PD-1 therapy are still to be fully elucidated, it is believed these proteins operate at different stages of an immune response, with CTLA-4 regulating T-cell proliferation at the priming phase, primarily in lymph nodes, with PD-1, which is more broadly expressed on several immune cells, including activated T cells, regulatory T cells, B cells, monocytes, natural killer cells, and dendritic cells, thought to limit T-cell activity in the peripheral tissues during the effector phase.2
The PD-1 ligands, PD-L1 (B7-H1, CD274) and PD-L2 (B7-DC, CD273), are generally expressed in multiple tissues. However, while PD-L1 is widely expressed in a variety of haematopoietic and non-haematopoietic cells, PD-L2 expression is limited to antigen-presenting cells, macrophages, TH2 cells, and non-haematopoietic cells in the lung. PD-L1 is also frequently found to be highly expressed in many human cancer types. PD-L2 expression across tumor types is less prevalent than PD-L1 and its role seems to be linked to the regulation of T-cell priming and polarization. PD-1/PD-L1 interaction ensures that the immune system is activated only at the appropriate time to minimize the possibility of chronic autoimmune inflammation. When PD-L1 expressed on tumor cells binds to PD-1, an inhibitory signal is transmitted to the T cell, which reduces cytokine production and suppresses T-cell proliferation (Fig. 1). Tumor cells exploit this immune-checkpoint pathway as a mechanism by which to evade detection and inhibit the immune response. These deactivated T cells remain inhibited in the tumor microenvironment.3
Figure 1.

Blockade of PD1 and PD-L1/PD-L2 results in a restoration of antitumor immune response. Programmed death-ligand 1 (PD-L1) interacts with both CD80 and PD-1. PD-L1/CD80 interaction delivers inhibitory signals in T cells (peripheral T-cell tolerance).
Targeting PD-L1 instead of PD-1 offers a potential advantage in that PD-L2 remains uninhibited. It has been postulated that inhibiting PD-L2 may be less critical in the immune response to cancer since it is rarely overexpressed in tumor cells and also because it interacts with a potentially stimulatory receptor, repulsive guidance molecule b (RGMb). This interaction may be required for respiratory tolerance (Chen & Han 2015),4 which means that avoiding PD-L2 inhibition may thus theoretically reduce the likelihood of developing severe inflammatory lung toxicity.3 Targeting PD-L1 may also provide a means to preferentially enhance TH1 responses while allowing suppression of tumor-promoting TH2 responses.3 Moreover, targeting PD-L1 rather than PD-1 provides the advantage of inhibiting additional PD-ligand interactions, such as that of PD-L1/B7.1, which appears to uniquely function to inhibit T-cell responses.5,6 PD-L1 has also been reported to interact with CD80 on activated T cells to mediate an inhibitory signal in murine models and it has been proosed that this PD-L1/CD80 pathway has a crucial role in the induction and maintenance of T-cell tolerance (Butte, 2007; Park 2010).7,8 Further studies are needed to elucidate the relative contributions of these different interactions of PD-1, PD-L1 and PD-L2 during T cell activation or suppression.
It has also been postulated that some anti-PD-LI agents may also exert an anti-tumor effect independent of their PD-L1 inhibiting action. Antibody-dependent cell-mediated cytotoxicity (ADCC) has been shown to play a major role in immune-mediated antitumor responses in many preclinical studies and has been implicated as an important mechanism of action for several highly effective and widely used monoclonal antibodies used as cancer therapies. A fully human anti-PD-L1 could potentially mediate the ADCC lysis of tumor cells in addition to blocking the interaction of PD-1 with PD-L1 on tumor cells. For example, a recent study demonstrated the ability of avelumab, an antibody against PD-L1, to mediate ADCC lysis of human tumor targets while very low levels of avelumab-mediated lysis were seen with whole peripheral blood mononuclear cell (PBMCs),9 while other studies have shown avelumab-mediated ADCC lysis of chordoma cells and mesothelioma cell lines.10,11 However, the extent of the proposed additional mode of action has not been clearly shown in clinical studies. Meanwhile, other studies have suggested that PD-L1 can act independently of the adaptive immune system through modulating tumor cell metabolism and enhancing tumor cell glycolysis, thereby depleting glucose from immune cells in the tumor microenvironment.12
However, to date clinical experience has not shown a clear benefit of targeting PD-L1 over PD-1 in terms of improved efficacy or reduced toxicity. Moreover, it has also been suggested that PD-L2 may have a role in establishing immunity. In mice models of malaria, PD-L2 was necessary for establishing effective CD4T cell immunity against malaria, not only because it inhibited PD-L1 to PD-1 activity but because it also increased CD3 and inducible co-stimulator (ICOS) expression on T cells.13 Whether this model is relevant to patients with cancer is, however, unknown.
Anti-PD-L1 antibodies
Monoclonal antibodies against both PD-1 and PD-L1 have shown encouraging results, including the anti-PD-1 drug nivolumab (Opdivo®, Bristol-Myers Squibb), which is approved by the US Food & Drug Administration (FDA) for the treatment of metastatic melanoma, NSCLC, advanced renal cell carcinoma (RCC), Hodgkin's lymphoma, and advanced squamous cell carcinoma of the head and neck (SCCHN). The combination of nivolumab with the anti-CTLA-4 antibody ipilimumab has been been approved for patients with metastatic or unresectable melanoma. Another anti-PD-1 drug, pembrolizumab (Keytruda®, Merck & Co.), is approved for the treatment of metastatic melanoma, NSCLC, and SCCHN.
In addition to these anti-PD-1 agents, several antibodies that act against PD-L1 have received approval for various tumor types. Atezolizumab (MPDL3280A, Tecentriq®, Roche) is a fully humanized engineered IgG1 monoclonal antibody against PD-L1 that contains a modified Fc receptor designed to eliminate ADCC and complement-dependent cytotoxicity (CDC). Cancers with a high rate of somatic mutations, including NSCLC, melanoma and urothelial bladder cancer (UBC) appear to respond well to atezolizumab, because of an increase in tumor-specific antigens. Atezolizumab has been approved by the FDA for patients with locally advanced or metastatic UBC with disease progression after being previously treated or who are ineligible for platinum-based chemotherapy, as well as for patients with metastatic NSCLC whose disease has progressed during or following platinum-containing chemotherapy. Durvalumab (MEDI4736, Imfinzi®, AstraZeneca), an Fc optimized monoclonal IgG1 directed against PD-L1 has also been granted breakthrough therapy designation by the FDA for the treatment of patients with PD-L1 positive UBC. The Fc region of durvalumab is modified in such a way that it does not induce either ADCC or CDC. A third anti-PD-1 agent, avelumab (MSB0010718C, Bavencio®, Merck KGaA/Pfizer), a fully human monoclonal IgG1 antibody, has also received accelerated approval for previously treated locally advanced or metastatic UBC, as well as being the first anti-PD-L1 to receive approval for the treatment of metastatic Merkel cell carcinoma. Unlike atezolizumab or durvalumab, avelumab has been designed to mediate ADCC lysis of tumor cells.6 Key clinical trials leading to approval of these antibodies are summarized in Table 1.
Table 1.
Key studies of anti-PD-L1 antibodies.
| Reference | Patients | Treatment | Efficacy | Safety |
|---|---|---|---|---|
| Urothelial bladder cancer | ||||
| Rosenberg et al 2016 [14] | Previously treated locally advanced or metastatic UBC, n = 310 | IV atezolizumab 1200 mg, Q3W | ORR: All patients 15% [11–20], p = 0·0058). IC 2/3: 27% [95% CI 19–37], p < 0·0001; IC1/2/3: 18% [13–24], p = 0·0004 vs. historical control ORR of 10% |
Grade 3–4 TRAEs, n = 50 (16%); fatigue most common, n = 5 |
| Grade 3–4 immune-mediated AEs, n = 15 (5%); pneumonitis, increased AST/ALT, rash and dyspnoea the most common | ||||
| Balar et al 2017 [18] | Previously untreated (cisplatin ineligible) locally advanced or metastatic UBC, n = 119 | IV atezolizumab 1200 mg, Q3W | At 17.2 months median follow-up, ORR 23% (95% CI 16–31), CRR 9%; 19/27 responses ongoing. | TRAEs in ≥ 10% of patients were fatigue (30%), diarrhea (12%), and pruritus (11%) patients). |
| Responses occurred across all PD-L1 and poor prognostic factor subgroups. | ||||
| Eight% had an AE leading to treatment discontinuation. | ||||
| Immune-mediated AEs, n = 14 (12%). | ||||
| Median PFS 2.7 months (2.1–4.2). | ||||
| Median OS 15.9 months (1.4 - not estimable). | ||||
| Tumor mutation load was associated with response. | ||||
| Patel et al 2017 [16] |
Previously treated or cisplatin ineligible metastatic UBC, n = 153 | IV avelumab 10 mg/kg Q2W | Preliminary analysis: | Grade ≥ 3 TRAE 7.5% TRAEs of any grade in ≥ 10% of pts were infusion-related reaction (22.8%) and fatigue (12.0%). |
| ORR 17.6% (95% CI 12.0–24.6), 9 CR, 18 PR; 24/27 (88.9%) ongoing. | ||||
| SD, n = 36 pts (DCR 41.2%) | ||||
| Median PFS 6.4 weeks (95% CI 6.1–11.4); Median OS 7.0 months (95% CI 5.6–11.1). ORR 25.0% (95% CI 14.4–38.4) in patients with PD-L1 ≥ 5% vs 14.7% (95% CI 7.6–24.7) in patients with PD-L1 < 5% (p = 0.178). |
Immune-related TRAE, n = 28 (11.6%). | |||
| Non-small-cell lung cancer | ||||
| Fehrenbacher et al 2016 [22] | Previously treated NSCLC, n = 277 | IV atezolizumab 1200 mg (n = 142) or docetaxel 75 mg/m2 (n = 135) Q3W | OS:12.6 months (95% CI 9.7–16.4) with atezolizumab vs 9.7 months (8.6–12.0) with docetaxel (HR 0.73 [95% CI 0.53–0.99]; p = 0·04). Increasing improvement in OS was associated with increasing PD-L1 expression | Grade 3/4 TRAEs, atezolizumab n = 16 (11%), docetaxel n = 52 (39%) |
| AEs leading to discontinuation: atezolizumab n = 11 (8%), docetaxel n = 30 (22%). | ||||
| One (< 1%) patient in the atezolizumab group vs 3 (2%) patients in the docetaxel group died from a TRAE. | ||||
| Barlesi et al [23] | Previously treated NSCLC, n = 850 | IV atezolizumab 1200 mg (n = 425) or docetaxel 75 mg/m2 (n = 425) Q3W | Preliminary analysis: | Grade 3/4 TRAEs, atezolizumab 15%, docetaxel 43%AEs leading to discontinuation: atezolizumab 8%, docetaxel 19% |
| ORR: 52% with atezolizumab vs 18% with docetaxel | ||||
| OS: 13.8 months (95% CI: 11.8–15.7) with atezolizumab vs 9.6 months (95% CI: 8.6–11.2) with docetaxel (HR 0.73 [95% CI, 0.62–0.87]; p = 0.0003). | ||||
| Gulley et al 2017 [24] | Progressive or platinum-resistant metastatic or recurrent NSCLC, n = 184 | IV avelumab 10 mg/kg Q2W | At 8·8 months median follow-up duration: | Grade ≥ 3 TRAEs, n = 23 (13%); most common were infusion-related reactions and increased lipase level (both 2%). |
| ORR: 12% (95% CI 8–18), one CR, 21 PR.SD, n = 70 (38%).DCR, n = 92 (50%) (confirmed response or SD as their best overall response). | ||||
| Serious TRAEs, n = 16 (9%) | ||||
| Most frequent TRAEs of any grade: fatigue (25%), infusion-related reaction (21%), and nausea (13%). | ||||
| Garassino et al 2016 [25] | Previously treated NSCLC, n = 307 | IV durvalumab 10 mg/kg Q2W | ORR: | Grade ≥ 3 TRAEs, 10.2% |
| PD-L1 ≥ 25%, 16.4% (95% CI: 10.8–23.5) | TRAEs leading to discontinuation, 2.7% | |||
| PD-L1 <25%, 7.5% (95% CI: 3.1–14.9) | ||||
| PD-L1 ≥ 90%, 30.9% (95% CI: 20.2–43.3) | ||||
| Merkel cell carcinoma | ||||
| Kaufman et al [33] | Stage IV chemotherapy-refractory Merkel cell carcinoma, n = 88 | IV avelumab 10 mg/kg Q2W | ORR: (31.8% (95.9% CI: 21.9–43.1), 8 CR, 20 PR; responses ongoing in 23/28. | Grade 3 TRAEs, n = 4 (5%): lymphopenia in 2 patients, blood creatine phosphokinase increase in one patient, aminotransferase increase in one patient, and blood cholesterol increase in one patient; no grade 4 TRAEs |
| Serious TRAEs, n = 5 (6%). | ||||
Other anti-PD-L1 antibodies are at earlier stages of development, including LY3300054 (Lilly), which is being assesses in a phase 1a/b study alone or in combination with other agents in advanced refractory solid tumors. Another anti-PD-L1 antibody, BMS-936559, produced durable tumor regression in patients with metastatic NSCLC, melanoma, renal-cell cancer, and ovarian cancer but is no longer under development.14
Urothelial bladder cancer
Metastatic UBC is an aggressive malignancy with poor prognosis. The current standard of care involves various platinum-based chemotherapy regimens but the majority of patients do not respond to treatment and second-line options are limited. Evidence for effective immunotherapy with bacillus Calmette-Guérin (BCG) in the treatment of non-invasive bladder cancer, PD-L1 tumor expression in patients with high-risk bladder cancer, and the high mutational load of UBC led to anti-PD-L1 agents being assessed in these patients.
In a phase I trial, 68 patients with UBC were treated with atezolizumab with 67 evaluable for efficacy.15 Many patients had poor prognostic factors at baseline, 93% were pre-treated with cisplatin or carboplatin-based chemotherapy, with 72% receiving ≥ 2 previous systemic treatments. Tumors with PD-L1-positive tumor-infiltrating immune cells had particularly high response rates; objective response rate (ORR) was 43% for those with immunohistochemistry (IHC) stained tumors scored as 2/3 (i.e. ≥ 5% tumor immune cells positive for PD-L1), with 2 (7%) complete responses, and 11% for those with IHC 0/1 (< 5% expression) tumors. Responses were rapid and occurred at a median of 42 d after starting treatment. This study also indicated that atezolizumab is well tolerated, with lower adverse event (AE) rates than many of the standard second-line treatment options for metastatic UBC.
In a phase II trial involving 310 patients with cisplatin-resistant metastatic bladder cancer, ORR with atezolizumab was 15%.16 The response rate varied based upon the expression of tissue PD-L1, with a 27% response in those patients with the highest level (≥ 5%) of PD-L1 expression. With longer follow-up, ORR by independent review was 26% (95% CI 18–36) in the PD-L1 ≥ 5% group and 18% (95% CI 13–24) in the PD-L1 <5% group. Other important variables that were found to be associated with response were The Cancer Genome Atlas (TCGA) subtype and mutational burden. Atezolizumab response was observed in all TCGA subtypes, with a highest response rate of 34% for the luminal cluster subtype tumors. With a median follow-up of 11.7 months, ongoing responses were recorded in 38 (84%) of 45 responders, with the median duration of response (DOR) not yet reached. Nearly 85% of responders had a continuous disease-free status during the follow-up. Immune and non-immune system-related toxicities were reported in 22% of patients, leading to a dose interruption in 30% and dose discontinuation in 4% of patients.
Atezolizumab was given accelerated approval from the FDA for previously treated UBC on the basis of these results. However, it was recently announced that atezolizumab failed to achieve its primary end point of overall survival (OS) compared with chemotherapy in the phase III IMvigor211 study in 931 previously treated patients with locally advanced or metastatic UBC (http://www.roche.com/media/store/releases/med-cor-2017–05–10.htm). Although data have not yet been reported, this has raised the question of whether the FDA approval of atezolizumab for treatment of metastatic UBC might be revoked, since approval was contingent upon results from this confirmatory phase III trial.
Avelumab and durvalumab have also been investigated in patients with pretreated UBC. In a phase Ib expansion cohort, 44 patients with urothelial carcinoma progressing after platinum-based chemotherapy and unselected for PD-L1 expression received avelumab 10 mg/kg every 2 weeks.17 Confirmed ORR was 18.2% (95% CI 8.2–32.7), including 5 complete responses and 3 partial responses. Median DOR was not reached and responses were ongoing in 6 patients. Seven of the 8 patients who responded had PD-L1-positive tumors. Median progression-free survival (PFS) was 11.6 weeks and median OS was 13.7 months, with a one-year OS rate of 54.3%. The most frequent treatment-related AEs were fatigue/asthenia, infusion-related reaction and nausea. Grade 3/4 AEs occurred in 3 patients and included asthenia, aspartate transaminase (AST) elevation, creatine phosphokinase (CPK) elevation, and decreased appetite. In an updated analysis of this study, confirmed ORR was 17.6% (95% CI 12.0–24.6) with 9 complete responses and 18 partial responses in 153 patients with ≥ 6 months follow-up, 88.9% of these responses were ongoing.18 ORR was 25.0% (95% CI: 14.4–38.4) in patients with PD-L1-positive tumors (≥ 5%) and 14.7% (95% CI: 7.6–24.7; p = 0.178) in patients with PD-L1-negative tumors. Median PFS was 6.4 weeks, and median OS was 7.0 months. Avelumab was well tolerated, with the most frequent AEs infusion-related reaction and fatigue.
Similarly, in a phase I/II multicenter open-label study, durvalumab demonstrated a manageable safety profile and evidence of meaningful clinical activity in patients with bladder cancer who were PD-L1-positive.19 A total of 61 patients, 40 of whom were PD-L1-positive (≥ 25% of tumor cells or tumor-infiltrating immune cells) and most of whom were heavily pretreated, had an ORR of 31.0% (46.4% in the PD-L1-positive subgroup, none in the PD-L1-negative subgroup) at a median follow-up duration of 4.3 months. Responses were ongoing in 12 of 13 responding patients. The most frequent AEs were fatigue (13.1%), diarrhea (9.8%), and decreased appetite (8.2%). Three patients (4.9%) experienced grade 3 treatment-related AEs, with no grade 4/5 AEs. One patient discontinued treatment because of acute kidney injury.
Anti-PD-L1 antibodies may also have role as first-line treatment of locally advanced or metastatic urothelial cancer.20 In a single-arm, phase II study, 123 previously untreated patients who were cisplatin ineligible received atezolizumab every 21 d until disease progression. At 17.2 months' median follow-up, the ORR was 23% (95% CI 16–31) and the complete response rate was 9%. Responses occurred across all PD-L1 and poor prognostic factor subgroups. Median PFS was 2.7 months and median OS was 15.9 months. The most frequent treatment-related AEs were fatigue, diarrhea, and pruritus. Eight percent of patients had an AE leading to treatment discontinuation.
Renal cell carcinoma
Inhibition of vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR) represent the standard treatment of metastatic renal cell carcinoma (RCC). However, even in patients with an initial response, resistance often develops within the first year, usually with significant toxicities. Because of this, new approaches with therapies that produce durable responses are needed. The activity and generally good safety profile of anti-PD-1/PD-L1 inhibitors provided a rationale to investigate their use in metastatic RCC.
In a phase Ia trial of atezolizumab in 70 patients with pretreated advanced or metastatic RCC, 63 of whom had clear-cell histology, ORR was 15%.21 A higher ORR was seen in the subgroup with higher PD-L1 expression (18% IHC 1–3 patients compared with 9% in IHC 0 patients). The highest response rates were observed in patients with a high Fuhrman grade or sarcomatoid features (22%), grade 4 tumors (25%), or sarcomatoid histology (33%). Median OS was 28.9 months; 20.6 months for patients with prior anti-VEGF inhibitor therapy and 29.1 months for patients not previously treated. In patients with IHC 0 tumors, median OS was 28.8 months and was not yet reached in the IHC 1–3 subgroup. Immune-mediated AEs occurred in 43% of patients, most commonly grade 1 rash (20%) and grade 2 hypothyroidism (10%). Grade 3 AEs occurred in 17% of patients.
The median OS of 20.6 months achieved with atezolizumab compares with a median OS of 25 months observed with nivolumab in a comparative study versus everolimus in previously treated RCC patients.22 Indeed, the median OS of atezolizumab is closer to that which was achieved in the everolimus arm in this previous trial (19.6 months). The ORR with atezolizumab was also below expectations when compared with that achieved with nivolumab (15% vs. 25%). Future studies will investigate biomarkers on high-grade tumors.
Ongoing clinical trials are investigating the interaction between anti-angiogenesis and cancer immunotherapy with atezolizumab being assessed in combination with other therapies in patients with previously untreated metastatic RCC. In a phase II trial (NCT01984242), patients will receive atezolizumab alone or in combination with bevacizumab or sunitinib, while atezolizumab with bevacizumab is also being compared with sunitinib in a phase III trial (NCT02420821).
Gastric cancer
In the phase Ib JAVELIN study, patients with gastric or gastroesophageal junction (GEJ) cancer who had progressed after ≥ 2 lines of prior therapy (2 L) or who had received first-line chemotherapy but had not yet progressed (switch-maintenance, Mn) were treated with avelumab.23 In a preliminary analysis of 75 patients (2 L, n = 20: Mn, n = 55), responses were observed in 7 patients (2 L, n = 3, all partial responses; Mn, n = 4, one complete response, 3 partial responses). PD-L1 expression was evaluable in 55 patients including 3 with a response. Median PFS was longer in PD-L1-positive patients; in the 2 L group, median PFS was 36.0 weeks (95% CI: 6.0, 36.0) for PD-L1-positive patients and 11.6 weeks (2.1, 21.9) for PD-L1-negative patients (using a ≥ 1% cutoff), while in the Mn group median PFS for PD-L1-positive and PD-L1-negative patients was 17.6 weeks (5.9, 18.0) and 11.6 weeks (5.7, 17.7), respectively. Avelumab showed an acceptable safety profile. Phase III trials in first- and third-line gastric/GEJ cancer are underway.
NSCLC
The open-label, phase II POPLAR trial of atezolizumab in NSCLC was the first randomized study to show that PD-L1 expression on tumor cells and tumor-infiltrating immune cells has an important role in regulation of antitumor immunity and predicting response to therapy.24 A total of 285 patients who progressed on post-platinum chemotherapy were randomized to receive atezolizumab 1200 mg or docetaxel 75 mg/m2 once every 3 weeks. Baseline PD-L1 expression was scored by IHC in tumor cells and tumor-infiltrating immune cells. Atezolizumab showed a significant improvement in OS compared with docetaxel (12.6 vs. 9.7 months). This was most marked in patients with higher PD-L1 expression, with patients with the lowest (< 1%) PD-L1 levels having a similar OS in both the atezolizumab and docetaxel groups. The strong improvement in OS without an improvement in PFS or ORR in these patients, together with the observation that OS improved with atezolizumab in both responding and non-responding patients, implies that standard radiographic endpoints (RECIST criteria) might underestimate the treatment benefit of atezolizumab. Atezolizumab was well tolerated with a safety profile consistent with previous studies.
In the OAK study, 1225 patients with previously treated NSCLC were stratified according to PD-L1 status, number of prior chemotherapy regimens and histology before being randomized to atezolizumab 1200 mg or docetaxel 75 mg/m2 every 3 weeks. In a preliminary analysis of data from 850 patients, there was a 27% improvement in OS in the atezolizumab group compared with docetaxel (p = 0.0003), regardless of PD-L1 expression levels and including patients with PD-L1 expression of < 1%.25 When patients were stratified according to their PD-L1 expression level, OS was 59% greater among patients in the highest tertile of PD-L1 expression with atezolizumab compared with docetaxel (p < 0.0001). However, even in patients with no PD-L1 expression, there was a significant 25% improvement in OS with atezolizumab compared with docetaxel. Improvements in OS were similar in patients with squamous and non-squamous histology. Atezolizumab was well tolerated with a favorable safety profile.
Both avelumab and durvalumab has also been investigated in NSCLC. In a dose-expansion cohort of an open-label, phase 1 study, 184 patients with progressive or platinum-resistant metastatic or recurrent NSCLC with squamous or non-squamous histology and not selected based on PD-L1 expression were treated with avelumab for a median of follow-up of 8.8 months.26 A total of 22 (95% CI 8–18) patients (12%) achieved a confirmed ORR, including one complete response and 21 partial responses, and 92 (50%) achieved disease control. The most common treatment-related AEs were fatigue (25%), infusion-related reaction (21%), and nausea (13%). Grade 3/4 AEs occurred in 13% of patients, with the most common being infusion-related reaction and increased lipase level. In the phase II ATLANTIC study in heavily pretreated patients with locally advanced or metastatic NSCLC, ORR with durvalumab was 7.5% (95% CI, 3.1–14.5) in patients with PD-L1 expression <25% and 16.4% (95% CI, 10.8–23.5) in patients with PD-L1 expression ≥ 25%.27 An ORR of 30.9% (95% CI, 20.2–43.3) was observed in patients with PD-L1 expression ≥ 90%. Durvalumab showed a manageable safety and tolerability profile, with most AEs low grade and resolved with treatment delay and/or immunosuppressive interventions.
Although anti-PD-1 and anti-PD-L1 monotherapies have shown clinical activity in advanced NSCLC, responses in patients with PD-L1-expressing tumors have generally been limited. Phase I/II data have shown that combining the anti-PD-L1 antibody durvalumab and the anti-CTLA-4 antibody tremelimumab may provide greater benefit to patients with PD-L1 tumors. ARCTIC (NCT02352948) is a global, phase III, randomized, open-label multicenter study in patients with advanced pre-treated NSCLC assessing the safety and clinical activity of durvalumab plus tremelimumab or either agent as monotherapy vs. standard of care (erlotinib, gemcitabine, or vinorelbine) in patients with PD-L1-positive tumors.28 Recruitment started in January 2015 and the study is ongoing.
Mesothelioma
In a phase Ib trial, 53 patients with unresectable pleural or peritoneal mesothelioma were treated with avelumab.29 All patients had progressed after a platinum/pemetrexed regimen and, of 39 evaluable patients, 14 (35.9%) were found to be PD-L1-positive using a cut-off of ≥ 5%. ORR was 9.4% with 5 partial responses. Response was ongoing in 4 of these patients, and the median duration of response was not reached. Twenty-five patients had stable disease (47.2%), and the disease control rate (DCR) was 56.6%. ORR was 14.3% in patients who were PD-L1-positive compared with 8% in PD-L1-negative patients. The median PFS was 17.1 weeks in PD-L1-positive patients and 7.4 weeks in PD-L1-negative patients. Treatment-related AEs occurred in 41 patients, with the most frequent including infusion-related reactions (37.7%), fatigue (15.1%), chills (15.1%), and pyrexia (11.3%). Grade ≥ 3 AEs occurred in 4 patients; these included colitis, decreased lymphocytes, and increased gamma-glutamyl transpeptidase (GGT) and CPK levels.
Melanoma
A study of 35 patients with metastatic melanoma who were treated with atezolizumab at doses of 1–20 mg/kg showed an ORR of 26% (9/35) with some patients experiencing tumor shrinkage within days of initiating treatment.30 PFS at 24 weeks was 35%. Analysis of archival tumors showed a correlation between PD-L1 status and efficacy. The incidence of all grade 3/4 AEs was 33%, including hyperglycemia (7%), elevated alanine transaminase (ALT) (7%) and AST (4%). No treatment-related deaths occurred during the study.
In a phase Ib study, patients with BRAF-mutant unresectable melanoma were treated with atezolizumab in combination with the MEK inhibitor cobimetinib and the BRAF inhibitor vemurafenib and induced an impressive response rate.31 In 30 patients who had received ≥ 1 dose of atezolizumab, the response rate with the triplet was 83%, including 3 complete responses (10%) and 21 partial responses (70%), with progressive disease in just one patient.The most frequent AEs were arthralgia, nausea, fatigue, flu-like symptoms, elevated liver enzymes, maculopapular rash, and photosensitivity. Grade 3/4 AEs were observed in 40% of patients. There were 3 treatment-related serious AEs, all resolved with dose interruptions and/or dose reductions. AEs observed with the triple combination were similar to those with atezolizumab and vemurafenib. No deaths were reported. On the basis of these data, a phase III study has been designed to explore cobimetinib, vemurafenib and atezolizumab in combination vs. cobimetinib plus vemurafenib (with placebo) for patients with previously untreated BRAF-mutant metastatic melanoma (NCT02908672).
In an earlier cohort of this phase Ib study, atezolizumab was combined with vemurafenib alone (i.e., without cobimetinib) in patients with BRAF-mutant metastatic melanoma.32 Among the 17 patients treated with the combination, ORR was 76% with 3 complete responses. Median duration of response was 20.9 months and median PFS was 10.9 months. In another earlier study, atezolizumab was also evaluated in combination with cobimetinib (i.e., without vemurafenib).33 Patients received various dose regimens, with cobimetinib escalated from 20 to 60 mg daily for the first 21 d of a 28-day cycle and atezolizumab given as 800 mg every 2 weeks. Excluding 2 patients with ocular melanoma, ORR was 45% and DCR was 75% in the remaining 20 patients. Median PFS was 12 months. After a median follow-up of 18.9 months, the median OS had not yet been reached. Ten patients were BRAF-mutant and 10 were BRAF wild-type and response rates were similar irrespective of BRAF mutation status. However, median PFS was 15.7 months in the wild-type BRAF group, compared with 11.9 months in BRAF-mutated patients. Based on these findings, a phase III study will investigate the combination of atezolizumab plus cobimetinib compared with atezolizumab alone in patients with untreated BRAF wild-type unresectable melanoma.
Durvalumab has also been assessed as a treatment of melanoma. A phase I, open-label study evaluated the safety and efficacy of IV durvalumab at 3 or 10 mg/kg every 2 weeks in combination with dabrafenib 150 mg twice daily plus trametinib 2 mg daily, or trametinib alone in patients with stage IIIc/IV melanoma.34 Patients were enrolled according to BRAF status into dose escalation cohorts followed by dose expansion: BRAF mutant in cohort A (durvalumab plus dabrafenib plus trametinib); BRAF wild-type in cohort B (durvalumab plus trametinib) or cohort C (sequential trametinib then durvalumab). In a preliminary analysis of 50 patients, dose-limiting toxicities were observed in single patients in cohort A (reversible grade 3 thrombocytopenia) and cohort B (reversible G3 choroidal effusion). Durvalumab 10 mg/kg was selected as the dose expansion in all cohorts. The most frequent treatment-related AEs by cohort were pyrexia (63%) and fatigue (54%) in cohort A, diarrhea (30%) and rash (25%) in cohort B, and vomiting (67%) in cohort C. Two patients discontinued due to treatment-related AEs. ORR for cohorts A, B, and C were 69%, 21%, and 13%, respectively, and the corresponding DCR, including complete responses, partial responses and stable disease, was 100%, 79% and 80%, respectively. Moreover, some patients experienced stable disease for 12 weeks or more (15%, 53%, and 40%, respectively). Most responses were ongoing (range of duration: 0.1+ to 32+ weeks). In cohort A, 16 of 18 patients (89%) continued to respond to therapy, with up to 50 weeks of follow-up.
This study also reported pharmacodynamic parameters, including the impact of treatment on immune activation, in a subset of patients that had biopsies at baseline and day 15 that were stained for CD8+ cell infiltration into the tumor. Patients in all cohorts, but most notably in cohort A, had evidence of increased CD8+ infiltration after treatment. There was also evidence of increased levels of IFN-γ in peripheral blood, which was again most pronounced and rapid in cohort A, but also showed smaller and more protracted increases in B and C cohorts. Patients with a BRAF mutation who were treated with BRAF/MEK inhibitor had the greatest detectable immune activation and the greatest clinical activity. This trial showed that durvalumab can be combined with trametinib with or without dabrafenib at full doses with a manageable safety profile, and evidence of clinical activity regardless of BRAF mutation status.
A phase Ib/II combination trial evaluating IMCgp100 (an immune-mobilizing monoclonal T cell receptor against cancer) in combination with durvalumab and tremelimumab (a fully human monoclonal IgG2 anti-CTLA-4 antibody) for the treatment of metastatic cutaneous melanoma has been initiated (NCT02535078).
Merkel cell carcinoma
Merkel cell carcinoma is an aggressive cutaneous malignancy associated with poor survival. Chemotherapy has been shown to produce responses but they are seldom durable. In a single-arm, open-label, phase II trial, 88 patients with stage IV chemotherapy-refractory, histologically confirmed Merkel cell carcinoma were treated with IV avelumab 10 mg/kg every 2 weeks.35 Overall, 28 of 88 patients (31.8%) achieved an objective response, including 8 complete responses and 20 partial responses. Responses were ongoing in 23 patients (82%) at the time of analysis. Better responses were observed in the subgroup of patients who had received fewer lines of previous therapy, probably because these patients had a more functional immune system. Avelumab was well tolerated, with 5 grade 3 treatment-related AEs. The most common AEs were fatigue, diarrhea, nausea, asthenia and infusion-related reactions. Three patients permanently discontinued treatment because of an AE. These findings indicate that checkpoint inhibitors could become the standard of care as first-line treatment of advanced Merkel cell carcinoma.
SCCHN
Outcomes for patients with recurrent and metastatic SCCHN are generally poor and new treatments are needed. PD-L1 is expressed in SCCHN tumors and is associated with response to anti-PD-L1 treatment. An ongoing phase I/II, multicenter, open-label study (NCT01693562) is evaluating the safety and efficacy of durvalumab in multiple solid tumor types including SCCHN. As of 29 April 2016, 62 patients who had received a median of 3 prior systemic treatments (range 1–13) had been treated.36 Median duration of follow-up was 25.0 months (range 1.4–31.6). The most frequent treatment-related AEs were fatigue (18%), diarrhea, (8%), and nausea (8%). Five patients (8%) had grade ≥ 3 AEs and there were no treatment-related AEs leading to death. Among 7 responders, 6 patients had a duration of response ≥ 12 months with the longest being 19.8 months. Six-month OS was 62% and 12-month OS was 42%. Preliminary analysis revealed no clear difference in OS by PD-L1 status. These results show durable responses and a safety profile of durvalumab consistent with previous reports. Ongoing clinical trials of durvalumab with and without tremelimumab in SCCHN are listed in Table 2.
Table 2.
Ongoing clinical trials of durvalumab ± tremelimumab in SCCHN.
| Ongoing clinical trials of durvalumab ± tremelimumab in SCCHN | |
|---|---|
| Trial |
Design |
| Study 1108 (NCT01693562) | Phase 1/2 study of durvalumab monotherapy in patients with advanced solid tumors |
| Study 11 (NCT02262741) | Phase 1 dose-exploration/dose-expansion study of durvalumab ± tremelimumab in patients with R/M HNSCC |
| SCORES (NCT02499328) | Phase 1b/2 dose-exploration/dose-expansion study of durvalumab in combination with AZD9150 or AZD5069 in patients with advanced solid tumors and HNSCC |
| HAWK (NCT02207530) | Phase 2 study of durvalumab monotherapy as 2nd line therapy in patients with PD-L1-positive HNSCC |
| CONDOR (NCT02319044) | Phase 2 study of durvalumab monotherapy as 2nd line therapy in patients with PD-L1-nagative HNSCC |
| EAGLE (NCT02369874) | Phase 3 study of durvalumab ± tremelimumab vs standard of care in 2nd line HNCSS |
| KESTREL (NCT02551159) | Phase 3 study of durvalumab ± tremelimumab vs standard of care in 1st line HNCSS |
Hodgkin's lymphoma
Hodgkin's lymphoma is usually treated with chemotherapy, radiation therapy, or a combination of both. A promising approach in lymphoma is the use of immune checkpoint inhibitors and, in 2016, nivolumab was approved to treat Hodgkin's lymphoma that had relapsed or progressed after autologous stem cell transplantation and brentuximab vedotin.
Preliminary data have indicated that the combination of atezolizumab and obinutuzumab appears well tolerated with early evidence of activity in patients with heavily pretreated relapsed or refractory diffuse large B-cell lymphoma (DLBCL) or follicular lymphoma (FL).37 Ongoing clinical trials of atezolizumab in patients with lymphoma, as well as those with other anti PD-L1 antibodies, are shown in Table 3.
Table 3.
Ongoing clinical trials with anti-PD-L1 antibodies in lymphoma.
| Ongoing clinical trials with Anti PD-L1 antibodies in Lymphoma: | |
|---|---|
| Trial |
Design |
| NCT02220842 | A phase Ib study of atezolizumab in combination with obinutuzumab, an anti-CD20 antibody, in patients with relapsed or refractory follicular lymphoma or diffuse large B cell lymphoma |
| NCT02596971 | A phase Ib study of atezolizumab combined with obinutuzumab plus chemotherapy in patients with follicular or diffuse large B cell lymphoma |
| NCT01375842 | A phase I study of atezolizumab in patients with locally advanced or metastatic solid tumors |
| NCT02729896 | A phase I study of atezolizumab combined with obinutuzumab and polatuzumab vedotin, a CD79B antibody-drug conjugate, in patients with relapsed or refractory follicular or diffuse large B cell lymphoma |
| NCT02631577 | A phase I study of atezolizumab combined with obinutuzumab plus chemotherapy in patients with relapsed or refractory follicular lymphoma |
| NCT02643303 | A phase I/II study of durvalumab combined with tremelimumab and Poly-ICLC, a Toll-like receptor 3 agonist, in patients with advanced and biopsy-accessible cancers, including cutaneous T cell lymphoma |
| NCT02733042 | A phase I/II study of durvalumab in patients with lymphoma |
| NCT02401048 | A phase I/II study of durvalumab combined with ibrutinib, a targeted therapy, in patients with relapsed or refractory lymphoma |
| NCT02549651 | A phase I study of durvalumab +/− tremelimumab or AZD9150, a STAT3 inhibitor, in patients with diffuse large B cell lymphoma |
| NCT02603419 | A phase I study of avelumab, an anti-PD-L1 antibody, in patients with Hodgkin lymphoma |
| NCT02793466 | A phase I study of durvalumab in pediatric and adolescent patients with lymphoma |
Discussion
The development of immuno-modulating monoclonal antibodies (anti-CTLA-4, anti-PD-1 and anti-PD-L1) has significantly improved the prognosis for many patients with cancer. Clinical trials with these agents have shown objective clinical activity in several malignancies, including melanoma, NSCLC, bladder, head and neck, and other cancers. Compared with anti-CTLA-4 drugs, studies with anti PD-1/PD-L1 agents have suggested higher response rates as well as improved PFS and OS. The difference in toxicity between anti-CTLA-4 and anti-PD-1/PD-Ll treatment is not surprising considering that PD-L1/PD-1 inhibition primarily results in disinhibiting existing chronic immune responses, rather than generating new, autoreactive T cells, as is the case with anti-CTLA-4 therapy.3
Targeting PD-L1 rather than PD-1 may also theoretically offer further benefits, with the potential for improved efficacy and reduced toxicity. However, clinical experience has not clearly evidenced this and further studies are needed to investigate whether anti-PD-L1 antibodies offer any benefit over anti-PD-1 agents across different tumor types. To date, no clear differences in efficacy between anti-PD-1 and anti-PD-L1 antibodies has been observed and immune-related toxicity appears broadly similar, although the incidence of treatment-related grade ≥ 3 AEs seems to be slightly lower with PD-L1 inhibitors than with anti-PD-1 s (Fig. 2).16,19,25,35,38-45 Selected immune-related AEs reported in clinical trials of anti-PD-L1 agents are shown in Table 4. Standard treatment algorithms for immune-related AEs originally developed for ipilimumab, that utilize immune-modulating medications, including corticosteroids, antihistamines, anti-tumor necrosis factor medications and calcineurin inhibitors, also appear to be relevant for anti-PD-1 and anti-PD-L1 agents.46,47
Figure 2.
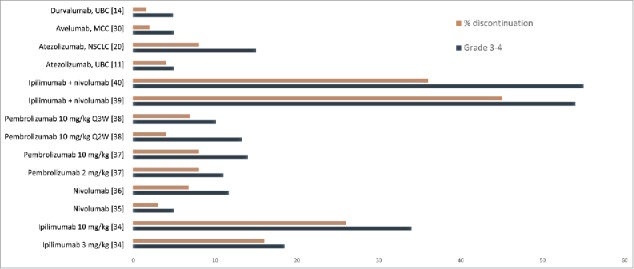
Grade 3–4 adverse events and discontinuations in clinical trials of anti-PD-L1 and anti PD-1 antibodies.
Table 4.
Anti PD-L1 safety profile: selected immune-related adverse events experienced in clinical trials; (a) most frequent toxicities; (b) less frequent toxicities.(a)
| Most frequent toxicities | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Atezolizumab |
Avelumab |
Durvalumab |
|||||||||||||||||
| Bladder [14] |
NSCLC [22] |
Kidney [19] |
Gastric [21] |
Mesothelioma [27] |
Merkel [33] |
Melanoma [32] |
Head and neck [34] |
Bladder [17] |
|||||||||||
| Selected AE Organ category % | Any | G3-4 | Any | G3/4 | Any | G3/4 | Any | G3/4 | Any | G3/4 | Any | G3/4 | Any | G3/4 | Any | G3/4 | Any | G3/4 | |
| Skin | 30 | 2 | 9 | 1 | 29 | 4 | 3 | 0 | 15 | 0 | 24 | 0 | 26 | 0 | 18 | nr | 3 | 0 | |
| Gastrointestinal | 8 | <1 | 16 | 1 | 11 | 0 | nr | nr | nr | nr | 9 | 9 | 32 | 5 | 8 | nr | 16 | 0 | |
| Endocrine | nr | nr | 6 | 1 | nr | nr | nr | nr | 4 | 0 | 3 | 0 | 0 | 0 | nr | nr | nr | nr | |
| Hepatic | 3 | 1 | nr | nr | nr | nr | nr | nr | nr | nr | 1 | 1 | 11 | 0 | nr | 3 | nr | nr | |
| Pulmunary | 2 | 1 | 10 | 7 | 3 | 0 | nr | nr | 2 | 0 | 1 | 1 | 5 | 0 | nr | nr | nr | nr | |
| Infusion reaction | 2 | 1 | nr | nr | nr | nr | 16 | 0 | 38 | 0 | nr | nr | nr | nr | 0 | 0 | 3 | 2 | |
nr: not reported(b)
(b).
| Less frequent toxicities | |||||||||||||||||||
| Atezolizumab |
Avelumab |
Durvalumab |
|||||||||||||||||
| Bladder [14] |
NSCLC [22] |
Kidney [19] |
Gastric [21] |
Mesothelioma [27] |
Merkel [33] |
Melanoma [32] |
Head and neck [34] |
Bladder [17] |
|||||||||||
| Selected AE Organ category % |
All |
G3-4 |
All |
G3/4 |
All |
G3/4 |
All |
G3/4 |
All |
G3/4 |
All |
G3/4 |
All |
G3/4 |
All |
G3/4 |
All |
G3/4 |
|
| Bone marrow | 3 | 1 | 2 | 0 | 11 | 4 | 5 | 0 | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | |
| myalgia | nr | nr | 5 | 1 | 4 | 0 | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | |
| Diabetes | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | 1 | 1 | nr | nr | nr | nr | nr | nr | |
| Neurologic | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | |
| Uveitis | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | |
| Renal | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | nr | 1 | 1 | |
nr: not reported
The success of PD-1 and PD-L1-directed immunotherapy in several different malignancies has highlighted the absence of effective biomarkers available to predict patient response to treatment, important given that these treatments are expensive and can be associated with significant toxicities. Although expression of PD-L1 in the tumor appears to be crucial for therapeutic activity, and initial studies indicating that expression of PD-L1 in tumors was associated with higher response rates, subsequent research has questioned the usefulness of PD-L1 expression status as a biomarker for patient selection, especially since many patients considered PD-L1-negative experience a benefit from treatment.48
There are several major limitations with the use of PD-L1 as a biomarker. PD-L1 is an immunological rather than a molecular marker and, as such, is dynamic and inducible and can vary over time and by anatomic site. To reliably assess the status of the immune response against the tumor at time of therapy onset, PD-L1 expression needs to be evaluated as close as possible to the beginning of treatment. PD-L1 expression in tumor biopsies collected months or years earlier may not be an accurate indication of PD-L1 status at the time of treatment initiation; therapies given after biopsy but before administration of anti-PD-1/PD-L1 treatment (e.g. radiation, chemotherapy) may alter PD-L1 expression.
In addition, PD-L1 is not broadly expressed in a tumor, but is only expressed in certain areas. This means that the correct choice of sample type to be analyzed is essential for accurate evaluation of PD-L1 expression. Ample samples are preferred since PD-L1 presence follows a geographical pattern and accumulates in lymphocyte-rich areas. PD-L1-positive tumor cells are usually localized adjacent to the tumor-infiltrating lymphocytes. The expression of PD-L1 on tumor cells is induced by the presence of cytokines produced by the cells of the immune system and increased concentration of cytokines has also been highlighted along this border. This location can affect the ability of a biopsy to detect PD-L1 expression and could be a problem in some kinds of cancer where large tumor samples may not be available. PD-L1 expression in some tumors may be missed in small biopsy specimens, such as needle biopsies
Another important limitation is that there are different antibodies, with different analysis systems and different cut-off values for expression. This makes evaluation of the results complex. The analytical performance and dynamic range of 3 of the 4 PD-L1 diagnostic assays (22C3, 28–8, and SP263) appear to be very similar in terms of identifying PD-L1 tumor positive cells and immune cells. The fourth one SP142 consistently stained fewer tumor cells, although it appeared to be similar in performance to the other 3 vis-à-vis immune cells. However, even if these diagnostic assays are shown to be equivalent in terms of identifying the same PD-L1 positive patients, whether an assay that is not matched to a particular drug is equivalent to the approved matched companion assay (e.g., 22C3 with pembrolizumab) with regard to predicting similar clinical outcomes remains an outstanding issue in the absence of data from controlled clinical trials.
At present, the use of PD-L1 expression as predictive biomarker is still controversial. Indeed, other than for the first-line treatment of NCSLC with pembrolizumab, PD-L1 status cannot generally be considered a useful marker for selecting patients given the clinical benefit seen in many patients even in the absence of PD-L1 overexpression. However, a companion diagnostic PD-L1 (SP142) assay has been FDA approved for use with atezolizumab, although PD-L1 testing is not required in the atezolizumab label.
The total tumor mutational burden (i.e., total number of mutations present in a tumor) has also been indicated as a potential biomarker. This mutational load has been shown to correlate with patient response to checkpoint inhibition and was more significantly associated with response rate than expression of PD-L1 in a trial of atezolizumab in metastatic UBC.16 It has been suggested that highly mutated tumors are more likely to harbor neoantigens, thereby making them targets of activated immune cells.
Other biomarkers or histopathological features (i.e., inflammatory cells in the tumor microenvironment) should be prospectively investigated for selecting patients who can most benefit from immunotherapy. For example, emerging data suggest that biomarkers based on immunoprofiling and mismatch repair deficiency may be more useful predictors of treatment response than PD-L1 and is likely be a major field of interest in the future.49,50 In the future, it is likely that a combination of biomarkers will be needed to determine whether a particular patient will benefit from treatment with anti-PD-1/PD-L1 agents as well as future checkpoint inhibitor drugs.
Another important development in future years will be greater understanding of how to optimally use these agents in combination with each other as well as with other treatments. Combining immunotherapies that target distinct immune pathways has the potential to overcome the barriers that tumor cells use to evade the immune system and may provide a clinical benefit in more patients than achieved with single-agent treatments. In addition, combining immune-based approaches with other treatment modalities, such as targeted agents, chemotherapy or radiation, may offer a complementary or even synergistic effect. However, identifying the most effective and tolerable combination regimens (e.g., schedules, doses, sequences) will need to be answered across the various tumor types.
References
- 1.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol. 2016;39(1):98-106. doi: 10.1097/COC.0000000000000239. PMID:26558876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012;24(2):207-12. doi: 10.1016/j.coi.2011.12.009. PMID:22236695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen DS, Irving BA, Hodi FS. Molecular pathways: next-generation immunotherapy-inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res. 2012;18(24):6580-87. doi: 10.1158/1078-0432.CCR-12-1362. PMID:23087408 [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Han X. Anti–PD-1/PD-L1 therapy of human cancer: past, present, and future. J Clin Invest. 2015;125(9): 3384-91. doi: 10.1172/JCI80011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paterson AM, Brown KE, Keir ME, Vanguri VK, Riella LV, Chandraker A, Sayegh MH, Blazar BR, Freeman GJ, Sharpe AH. The programmed death-1 ligand 1:B7-1 pathway restrains diabetogenic effector T cells in vivo. J Immunol. 2011;187(3):1097-105. doi: 10.4049/jimmunol.1003496. PMID:21697456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang J, Riella LV, Chock S, Liu T, Zhao X, Yuan X, Paterson AM, Watanabe T, Vanguri V, Yagita H, et al.. The novel costimulatory programmed death ligand 1/B7.1 pathway is functional in inhibiting alloimmune responses in vivo. J Immunol. 2011;187(3):1113-19. doi: 10.4049/jimmunol.1100056. PMID:21697455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111-22. doi: 10.1016/j.immuni.2007.05.016. PMID:17629517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JJ, Omiya R, Matsumura Y, Sakoda Y, Kuramasu A, Augustine MM, Yao S, Tsushima F, Narazaki H, Anand S, et al.. B7-H1/CD80 interaction is required for the induction and maintenance of peripheral T-cell tolerance. Blood. 2010;116(8):1291-8. doi: 10.1182/blood-2010-01-265975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyerinas B, Jochems C, Fantini M, CR1 Heery, Gulley JL, Tsang KY, Schlom J. Antibody-dependent cellular cytotoxicity activity of a novel anti-PD-L1 antibody avelumab (MSB0010718C) on human tumor cells. Cancer Immunol Res. 2015;3(10):1148-1157. PMID:26014098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii R, Friedman ER, Richards J, Tsang KY, Heery CR, Schlom J, Hodge JW. Enhanced killing of chordoma cells by antibody-dependent cell-mediated cytotoxicity employing the novel anti-PD-L1 antibody avelumab. Oncotarget. 2016;7(23):33498-511. doi: 10.18632/oncotarget.9256. PMID:27172898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna S, Thomas A, Abate-Daga D, Zhang J, Morrow B, Steinberg SM, Orlandi A, Ferroni P, Schlom J, Guadagni F, et al.. Malignant mesothelioma effusions are infiltrated by CD3+ T cells highly expressing PD-L1 and the PD-L1+ tumor cells within these effusions are susceptible to ADCC by the anti-PD-L1 antibody avelumab. J Thorac Oncol. 2016;11(11):1993-2005. doi: 10.1016/j.jtho.2016.07.033. PMID:27544053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang CH, Qiu J, O'Sullivan D, Buck MD, Noguchi T, Curtis JD, Chen Q, Gindin M, Gubin MM, van der Windt GJ, et al.. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162(6):1229-41. doi: 10.1016/j.cell.2015.08.016. PMID:26321679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karunarathne DS, Horne-Debets JM, Huang JX, Faleiro R, Leow CY, Amante F, Watkins TS, Miles JJ, Dwyer PJ, Stacey KJ, et al.. Programmed death-1 ligand 2-mediated regulation of the PD-L1 to PD-1 axis is essential for establishing CD4(+) T cell immunity. Immunity. 2016;45(2):333-45. doi: 10.1016/j.immuni.2016.07.017. PMID:27533014 [DOI] [PubMed] [Google Scholar]
- 14.Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al.. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455-65. doi: 10.1056/NEJMoa1200694. PMID:22658128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Powles T, Eder JP, Fine GD, Braiteh FS, Loriot Y, Cruz C, Bellmunt J, Burris HA, Petrylak DP, Teng SL, et al.. MPDL3280A (anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515(7528):558-62. doi: 10.1038/nature13904. PMID:25428503 [DOI] [PubMed] [Google Scholar]
- 16.Rosenberg JE, Hoffman-Censits J, Powles T, van der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, et al.. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-20. doi: 10.1016/S0140-6736(16)00561-4. PMID:26952546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Apolo AB, Infante JR, Balmanoukian A, Patel MR, Wang D, Kelly K, Mega AE, Britten CD, Ravaud A, Mita AC, et al.. Avelumab, an Anti-Programmed Death-Ligand 1 Antibody, In Patients With Refractory Metastatic Urothelial Carcinoma: Results From a Multicenter, Phase Ib Study. J Clin Oncol. 2017;35(19):2117-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel MR, Ellerton JA, Infante JR, Agrawal M, Gordon MS, Aljumaliy R, Britten CD, Dirix L, Lee K-W, Taylor MH, et al.. Avelumab in patients with metastatic urothelial carcinoma: Pooled results from two cohorts of the phase 1b JAVELIN Solid Tumor trial. J Clin Oncol. 2017;35(suppl 6S) Abstract 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Massard C, Gordon MS, Sharma S, Rafii S, Wainberg ZA, Luke J, Curiel TJ, Colon-Otero G, Hamid O, Sanborn RE, et al.. Safety and efficacy of durvalumab (MEDI4736), an anti-programmed cell death ligand-1 immune checkpoint inhibitor, in patients with advanced urothelial bladder cancer. J Clin Oncol. 2016;34(26):3119-25. doi: 10.1200/JCO.2016.67.9761. PMID:27269937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balar AV, Galsky MD, Rosenberg JE, Powles T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J, Perez-Gracia JL, et al.. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017;389(10064):67-76. doi: 10.1016/S0140-6736(16)32455-2. PMID:27939400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDermott DF, Sosman JA, Sznol M, Massard C, Gordon MS, Hamid O, Powderly JD, Infante JR, Fassò M, Wang YV, et al.. Atezolizumab, an anti-programmed death-ligand 1 antibody, in metastatic renal cell carcinoma: long-term safety, clinical activity, and immune correlates from a phase Ia study. J Clin Oncol. 2016;34(8):833-42. doi: 10.1200/JCO.2015.63.7421. PMID:26755520 [DOI] [PubMed] [Google Scholar]
- 22.Motzer RJ, Escudier B, McDermott DF, George S, Hammers HJ, Srinivas S, Tykodi SS, Sosman JA, Procopio G, Plimack ER, et al.. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–13. doi: 10.1056/NEJMoa1510665. PMID:26406148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hyun Cheol Chung, Hendrik-Tobias Arkenau, Lucjan Wyrwicz, Do-Youn Oh, Keun-Wook Lee, Jeffrey R. Infante, Kevin M. Chin, Anja von Heydebreck, Yoon-Koo Kang, Howard Safran. Safety, PD-L1 expression, and clinical activity of avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with advanced gastric or gastroesophageal junction cancer. J Clin Oncol. 2016;34(Suppl 4S):abstract 167. [Google Scholar]
- 24.Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J, Mazieres J, Park K, Smith D, Artal-Cortes A, Lewanski C, et al.. Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicentre, open-label, phase 2 randomised controlled trial. Lancet. 2016;387(10030):1837-46. doi: 10.1016/S0140-6736(16)00587-0. PMID:26970723 [DOI] [PubMed] [Google Scholar]
- 25.Barlesi F, Park K, Ciardiello F, von Pawel J, Gadgeel S, Hida T, Kowalski D, Dols MC, Cortinovis D, Leach J, et al.. Primary analysis from OAK, a randomized phase III study comparing atezolizumab with docetaxel in 2L/3L NSCLC. Ann Oncol. 2016;27(suppl 6): LBA44 PR. [Google Scholar]
- 26.Gulley JL, Rajan A, Spigel DR, Iannotti N, Chandler J, Wong DJL, Leach J, Edenfield WJ, Wang D, Grote HJ, et al.. Avelumab for patients with previously treated metastatic or recurrent non-small-cell lung cancer (JAVELIN Solid Tumor): dose-expansion cohort of a multicentre, open-label, phase 1b trial. Lancet Oncol. 2017;18(5):599-610. doi: 10.1016/S1470-2045(17)30240-1. PMID:28373005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garassino MC, Vansteenkiste JF, Kim J, Léna H, Mazières J, Powderly J, Dennis P, Huang Y, Wadsworth C, Rizvi N. Durvalumab in ≥ 3rd-line locally advanced or metastatic, EGFR/ALK wild-type NSCLC: results from the phase 2 ATLANTIC study. J Thoracic Oncol. 2017;12(1 Suppl):S10−11 Abstract PL04a.03. doi: 10.1016/j.jtho.2016.11.012 [DOI] [Google Scholar]
- 28.Planchard D, Yokoi T, McCleod MJ, Fischer JR, Kim YC, Ballas M, Shi K, Soria JC. A phase III study of durvalumab (MEDI4736) with or without tremelimumab for previously treated patients with advanced NSCLC: rationale and protocol design of the ARCTIC study. Clin Lung Cancer. 2016;17(3):232-236. doi: 10.1016/j.cllc.2016.03.003. PMID:27265743 [DOI] [PubMed] [Google Scholar]
- 29.Hassan R, Thomas A, Patel MR, Nemunaitis JJ, Bennouna J, Powderly JD, Taylor MH, Dowlati A, Chen F, Leach J, et al.. Avelumab (MSB0010718C; anti-PD-L1) in patients with advanced unresectable mesothelioma from the JAVELIN solid tumor phase Ib trial: Safety, clinical activity, and PD-L1 expression. J Clin Oncol. 2016;34(Suppl):abstract 8503. PMID:27863199.27863199 [Google Scholar]
- 30.Hamid O, Sosman JA, Lawrence DP, Sullivan RJ, Ibrahim N, Kluger HM, Boasberg PD, Flaherty K, Hwu P, Ballinger M, et al.. Clinical activity, safety, and biomarkers of MPDL3280A, an engineered PD-L1 antibody in patients with locally advanced or metastatic melanoma (mM). J Clin Oncol. 2013;31(Suppl):abstract 9010. PMID:23918947.23918947 [Google Scholar]
- 31.Sullivan RJ, Hamid O, Gonzalez R, et al.. Safety and clinical activity of atezolizumab + cobimetinib + vemurafenib in BRAFV600-mutant metastatic melanoma. Presented at: Society for Melanoma Research Annual Meeting; Boston, Massachusetts, November 6-9, 2016. [Google Scholar]
- 32.Hamid O, et al.. Preliminary clinical safety, tolerability and activity of atezolizumab (anti-PD-L1) combined with Zelboraf in BRAFv600 metastatic melanoma. Presented at the Society for Melanoma Research 2015 International Congress; November 18–21,2015; San Francisco, CA. [Google Scholar]
- 33.Infante J, Kim TM, Friedmann J, et al.. Safety and clinical activity of atezolizumab combined with cobimetinib in metastatic melanoma. Presented at: Society for Melanoma Research Annual Meeting; Boston, Massachusetts, November 6-9, 2016. [Google Scholar]
- 34.Ribas A, Butler M, Lutzky J, Lawrence DP, Robert C, Miller W, Linette GP, Ascierto PA, Kuzel T, Algazi AP, et al.. Phase I study combining anti-PD-L1 (MEDI4736) with BRAF (dabrafenib) and/or MEK (trametinib) inhibitors in advanced melanoma. J Clin Oncol. 2015;33(suppl):abstract 3003. PMID:25667273.25667273 [Google Scholar]
- 35.Kaufman HL, Russell J, Hamid O, Bhatia S, Terheyden P, D'Angelo SP, Shih KC, Lebbé C, Linette GP, Milella M, et al.. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: a multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016;17(10):1374-85. doi: 10.1016/S1470-2045(16)30364-3. PMID:27592805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segal NH, Ou S-HI, Balmanoukian AS, Massarelli E, Brahmer JR, Weiss J, Schoffski P, Antonia SJ, Massard C, Zandberg P, et al.. Updated safety and efficacy of durvalumab (MEDI4736), an anti-PD-L 1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort. Ann Oncol. 2016;27(suppl 6):949O. [Google Scholar]
- 37.Till BG, Park SL, Popplewell LL, Goy A, Penuel E, Venstrom JM, Liu B, Fingerle-Rowson G, Byon J, Woodard P, et al.. Safety and clinical activity of atezolizumab (Anti-PDL1) in combination with obinutuzumab in patients with relapsed or refractory non-Hodgkin lymphoma. Blood. 2015;126:5104. [Google Scholar]
- 38.Michot JM, Bigenwald C, Champiat S, Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A, Bahleda R, Hollebecque A, et al.. Immune-related adverse events with immune checkpoint blockade: a comprehensive review. Eur J Cancer. 2016;54:139–48. doi: 10.1016/j.ejca.2015.11.016. PMID:26765102 [DOI] [PubMed] [Google Scholar]
- 39.Ascierto PA, Del Vecchio M, Robert C, Mackiewicz A, Chiarion-Sileni V, Arance A, Lebbé C, Bastholt L, Hamid O, Rutkowski P, et al.. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18(5):611-22. doi: 10.1016/S1470-2045(17)30231-0. PMID:28359784 [DOI] [PubMed] [Google Scholar]
- 40.Weber JS, D'Angelo SP, Minor D, Hodi FS, Gutzmer R, Neyns B, Hoeller C, Khushalani NI, Jr Miller WH, Lao CD, et al.. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16(4):375-84. doi: 10.1016/S1470-2045(15)70076-8. PMID:25795410 [DOI] [PubMed] [Google Scholar]
- 41.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E, et al.. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320-30. doi: 10.1056/NEJMoa1412082. PMID:25399552 [DOI] [PubMed] [Google Scholar]
- 42.Ribas A, Puzanov I, Dummer R, Schadendorf D, Hamid O, Robert C, Hodi FS, Schachter J, Pavlick AC, Lewis KD, et al.. Pembrolizumab versus investigator-choice chemotherapy for ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol. 2015;16(8):908-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robert C, Schachter J, Long GV, et al.. Pembrolizumab versus ipilimumab in advanced melanoma. N Engl J Med. 2015;372:2521–32. doi: 10.1056/NEJMoa1503093. PMID:25891173 [DOI] [PubMed] [Google Scholar]
- 44.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, et al.. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–17. doi: 10.1056/NEJMoa1414428. PMID:25891304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P, et al.. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. PMID:26027431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber JS, Kähler KC, Hauschild A. Management of immune-related adverse events and knetics of response with ipilimumab. J Clin Oncol. 2012;30(21):2691-97. doi: 10.1200/JCO.2012.41.6750. PMID:22614989 [DOI] [PubMed] [Google Scholar]
- 47.Nishino M, Ramaiya NH, Chambers ES, Adeni AE, Hatabu H, Jänne PA, Hodi FS, Awad MM. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J Immunother Cancer. 2016;4:84. doi: 10.1186/s40425-016-0193-2. PMID:28018599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Festino L, Botti G, Lorigan P, Masucci GV, Hipp JD, Horak CE, Melero I, Ascierto PA. Cancer treatment with anti-PD-1/PD-L1 agents: is PD-L1 expression a biomarker for patient selection? Drugs. 2016;76(9):925-45. doi: 10.1007/s40265-016-0588-x. PMID:27229745 [DOI] [PubMed] [Google Scholar]
- 49.Le DT Uram JN, Wang H, Wang H, Bartlett BR, Aulakh LK, Lu S, Kemberling H, Wilt C, Luber BS, et al.. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372(26):2509–20. doi: 10.1056/NEJMoa1500596. PMID:26028255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Galon J, Fox BA, Bifulco CB, Masucci G, Rau T, Botti G, Marincola FM, Ciliberto G, Pages F, Ascierto PA, et al.. Immunoscore and Immunoprofiling in cancer: an update from the melanoma and immunotherapy Bridge 2015. J Transl Med. 2016;14:273. doi: 10.1186/s12967-016-1029-z. PMID:27650038 [DOI] [PMC free article] [PubMed] [Google Scholar]


