ABSTRACT
TRAF2 dependent K63-polyubiquitinations have been recently shown to connect CD137 (4–1BB) stimulation to NF-κB activation. In a search of deubiquitinase enzymes (DUBs) that could regulate such a signaling route, A20 and CYLD were found to coimmunoprecipitate with CD137 and TRAF2 complexes. Indeed, overexpression of A20 or CYLD downregulated CD137-elicited ubiquitination of TRAF2 and TAK1 upon stimulation with agonist monoclonal antibodies. Moreover, overexpression of A20 or CYLD downregulated CD137-induced NF-κB activation in cultured cells and in gene-transferred hepatocytes in vivo, while silencing these deubiquitinases enhanced CD137 costimulation of primary human CD8 T cells. Therefore A20 and CYLD directly downregulate the signaling from a T and NK-cell costimulatory receptor under exploitation for cancer immunotherapy in clinical trials.
KEYWORDS: Deubiquitinases, A20, CYLD, CD137(41BB), TRAF2
Introduction
The family of the TNF receptor lacks cytoplasmic enzymatic domains and relies on partnering with adaptors, such as members of the TRAF family, for signal transduction.1-3 While some members of the TNFR family recruit a caspase activating machinery, others lack death domains and primarily activate inflammatory functions.4 This is the case, for instance, of CD137, CD27, OX40, GITR that are well known T-cell costimulators.5 In fact, upon ligation these surface receptors enhanced antitumor immune responses and agonist mAb are currently being tested across multiple clinical trials.6
TNFRSF9 (CD137 or 4–1BB) is a TNFR family member that is able to costimulate T and NK lymphocytes upon ligation with its natural ligand (4–1BBL) or with agonist monoclonal antibodies (mAbs).7 Its intracytoplasmic domain has been found to physically interact with the adaptors TRAF1 and TRAF2.8 While TRAF1 biochemical activity largely remains a mystery,9 TRAF2 encompasses a RING domain predicted to act as an E3 ubiquitin ligase generating K63 polyubiquitin chains. K63 polyubiquitination of protein substrates or free K63 polyubiquitin chains10 act as docking sites to sustain downstream signaling to the NF-κB pathway ubiquitinating several substrates such as TAK1, NEMO, IKKα and IKKβ.10 In the case of CD137, we have recently reported that receptor stimulation leads to K63 polyubiquitination of TRAF2 itself and that this pathway is necessary for canonical NF-κB signaling and for receptor internalization.11 Since the catalytic domain of TRAF2 is structurally doubtful to accommodate the E2 enzyme Ubc13,12 it is possible that this activity could be mediated by cIAPs, other E3 enzymes that are known to physically associate with TRAF2.13,14
E3 K63 polyubiquitin reactions are controlled by proteases that degrade these complexes and conform the so called Deubiquitinase (DUB) family.15-17 Among the DUBs able to degrade K63 polyubiquitins 2 enzymes stand out for their known regulatory activity on the NF-κB pathway and on immune and inflammatory responses, namely A20 and CYLD (Cylindromatosis Turban Tumor Syndrome).10,15,17
A2018 is encoded by the TNFα-inducible gene 3 (TNFAIP3) and is a complex enzyme since it encompasses an N-terminal OTU domain with K63 deubiquitinase activity and its C-terminal region a Zinc finger domain (ZF4 domain) with E3 K48-ubiquitin ligase activity that targets proteins for proteasomal degradation.18-20 A20 is considered a tumor suppressor gene in lymphoma.21,22 Gene-targeting experiments conclusively demonstrate an anti-inflammatory role for A20 that prevents autoimmunity and excessive inflammation.23-26 Selective gene deletion in the skin and immune cells including T and B cells23,26 strongly supports this critical regulatory role. In humans, A20 polymorphisms have been statistically linked to autoimmune conditions.27
CYLD28 is another DUB that removes and degrades K63-linked polyubiquitin chains from substrates,29,30 including those attached to TRAF217,31 and other molecular targets in the activation of the canonical NF-κB pathway.17,32 Its ability to downregulate the NF-κB is favored by its strong physical association with NEMO (IKKγ subunit). Deficient function of CYLD was first ascribed to cause predisposition to cylindromatosis, a condition causing multiple benign skin tumors.28 Knock-out mouse models have shown involvement of CYLD not only in immune cell function,33 but also in osteoclast function34 and spermatogenesis.35 Deubiquitination of mediators in NF-κB MAPK and Wnt signaling routes have been found to be involved in such processes.
Suspecting that E3 K63 polyubiquitin-removing enzymes could downregulate CD137-TRAF2 signals, we have found that both A20 and CYLD coimmunoprecipitate with this multiprotein complex. Furthermore, a marked inhibition of CD137-dependent NF-κB activation was observed when A20 or CYLD were overexpressed, whereas silencing of such genes upregulates the intensity of CD137 signaling. These results are consistent with a role of A20 and CYLD in downregulating CD137 costimulation.
Results
A20 and CYLD coimmunoprecipitate with CD137-TRAF2 complexes regulating ubiquitination reactions
In a previous report, we showed that CD137 ligation results in K63-polyubiquitination of TRAF2.11 Indeed TRAF2 is well known to physically interact with the cytoplasmic domain of CD137 as a signaling mediator and we mechanistically proposed that TRAF2, under CD137 stimulation, K63-polyubiquitinates sister TRAF2 moieties.7,16 Fig. 1A shows coimmunoprecipitation experiments confirming CD137-TRAF2 interaction.8 In these immunoprecipitates, we searched for the presence of deubiquitinases that would be candidates to downregulate such CD137 signaling pathway. As can be seen in Fig. 1A, when 293 T cells were cotransfected with CD137 and either Flag-tagged A20 and/or CYLD, both DUBs were found to associate with the CD137 complex. In these experiments TRAF2 was not transfected since 293 T constitutively express this adaptor protein. Furthermore, as shown in Fig. S1, an anti-CD137 mAb pulled down both TRAF2 and 2 different deletion mutants of A20: an N-terminal deletion missing the OTU deubiquitinase domain and a C-terminal deletion mutant lacking the ZF4 domain which mediates K48 E3 ligase activity.36 These data are interpreted in the sense that binding of A20 to the complex is independent of these enzymatic functions.17,37
Figure 1.
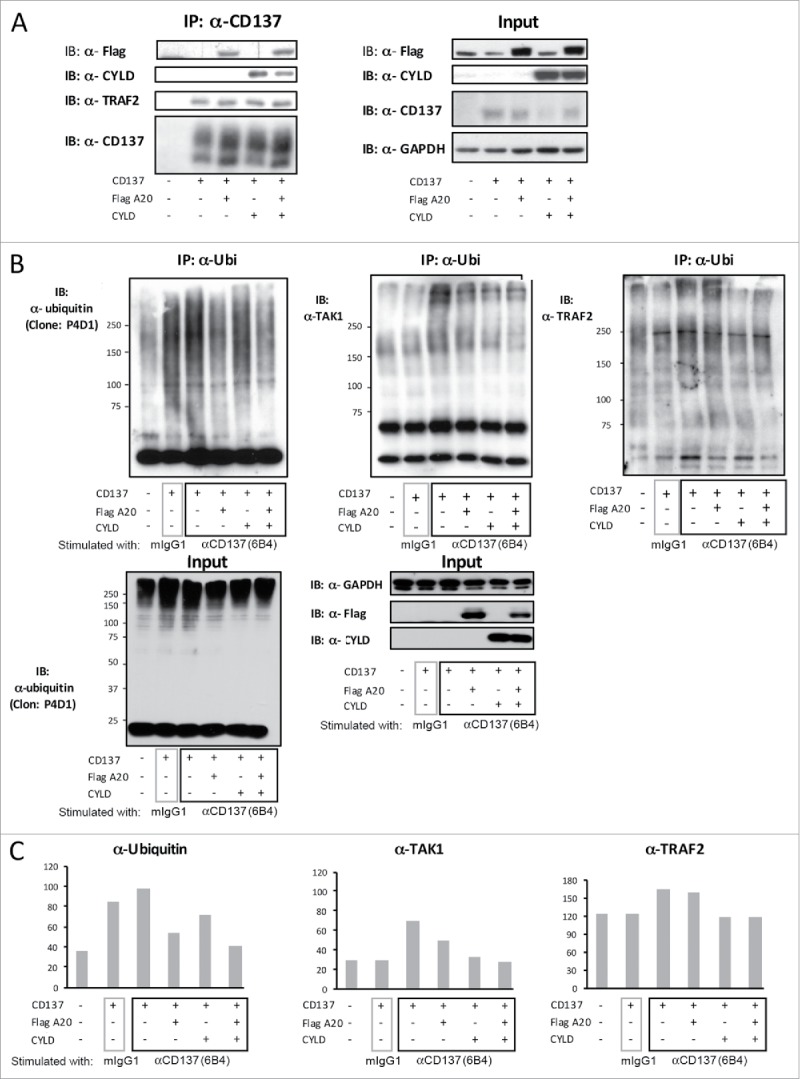
A20 and CYLD coprecipitate with CD137-TRAF2 and downregulate ubiquitination of TAK1 and TRAF2. (A) Lysates from 293 T cells transiently co-transfected with expression plasmids containing the indicated expression cassettes encoding human CD137, human A20-flag and CYLD were immunoprecipitated with Protein G sepharose-coupled anti-CD137 mAb (6B4). SDS-PAGE blots were probed with antibodies to detect flag, CYLD, TRAF2 and CD137. GAPDH was used as a loading control. (B, C and D) 293 T cells were cotransfected with plasmids encoding the indicated expression cassettes and 24 h following transfection, cells were incubated with the agonist anti-CD137 mAb 6B4 or control mouse IgG1. Lysates were immunoprecipitated with the anti-ubiquitin antibody FK2 coupled to protein G sepharose beads and blots were probed with the indicated detection antibodies anti-Ub, anti-TAK1 and anti-TRAF2. Content of expressed proteins in the input cell lysates are shown in each panel. (C) Maximal intensity band regions were quantified by densitometry and relative values presented in the bar diagrams. A representative case out of at least 4 experiments similarly performed is shown.
To ascertain if these proteins were actually involved in the regulation of the ubiquitination reactions, HEK 293 T cells expressing CD137 were engaged with anti-CD137 mAb during 5 minutes. Then, ubiquitinated proteins were immunoprecipitated with antibodies against ubiquitin. The total amount of ubiquitinated proteins, TAK1 and TRAF2 (Fig. 1B) contained in these immunoprecipitates was analyzed by SDS-PAGE and Western blotting. These experiments, and their respective quantitative densitometries (Fig. 1C), indicate that both A20 and CYLD downregulated the level of ubiquitination of the analyzed proteins induced by CD137 stimulation (Fig. 1C). However, such an effect was clearer for both DUBs in the case of TAK1 as a substrate, while in the case of TRAF2, CYLD but not A20 overexpression downregulated the conjugated polyubiquitin content, a finding consistent with previous reports.17
A20 and CYLD inhibit NF-κB activation elicited by CD137 stimulation
We next assessed whether overexpression of A20 and CYLD down-regulates the NF-κB transcriptional activity triggered by the stimulation of CD137 with agonist mAb. In a previous study,11 we showed that CD137-mediated NF-κB activation was dependent on TRAF2 and required K63 ubiquitination, since overexpression of a dominant negative mutant form of ubiquitin (K63R) abrogated NF-κB activation.
Experiments using a NF-κB luciferase reporter construction indicated that enforced expression of either A20 or CYLD caused the suppression of NF-κB activation in response to the agonist anti-CD137 mAb 6B4 in transiently transfected 293 T cells (Fig. 2A).11 The extent of the inhibition was comparable to that exerted by mutant ubiquitin K63R through a negative dominant effect. Consistent with these findings attenuation in IκB degradation was observed upon overexpression of A20 and CYLD under similar experimental conditions (Fig. 2B). In addition, the enforced expression of A20 N-terminal and C-terminal deletion mutants showed that both truncated proteins retained the inhibitory activity on NF-κB activation (Fig. S2), even if, as described in the literature, the C-terminal part of the protein has a stronger effect on NF-κB inhibition.37,38
Figure 2.
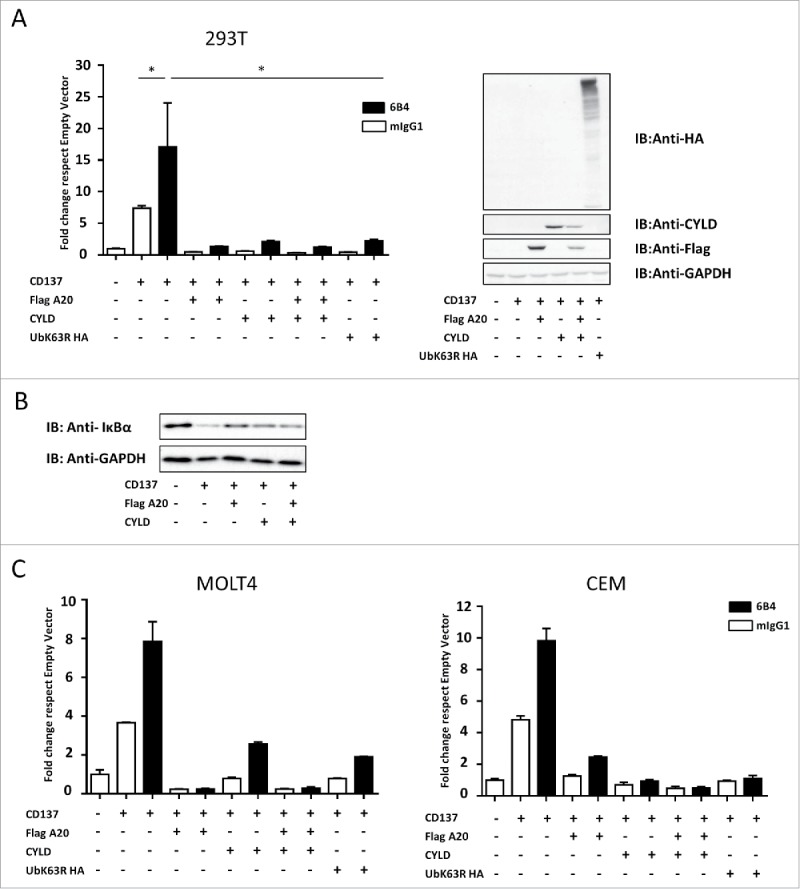
Overexpression of A20 and/or CYLD downregulates NF-κB activation elicited by CD137 ligation. (A) 293 T cells transiently transfected with the indicated expression plasmids for 18 hours were stimulated for 5 hours with anti-CD137 (6B4) or control mIgG1. Experiments were read out by means of cotransfection with the NF-κB luciferase reporter construction and subsequent incubation with luciferin. Results are shown as relative luminescence units between firefly luciferase activity and renilla luciferase as an internal control (mean ± SEM) given as fold change with respect to the empty vector (left panel). Immunoblots in lysates of the indicated transfectants confirm expression of the corresponding proteins (right panel). (B) Immunoblots for IκBα and anti-GAPDH (loading control) in lysates from 293 T cells transfected as in A with the indicated constructs. (C and D) Experiments performed as in A in MOLT4 and CEM T cells electroporated with the plasmids encoding the indicated expression cassettes. A representative set of experiments out of at least 6 performed is shown. In this case, cells were electroporated 18 hours before the experiment and cells were exposed to antibodies for 24 hours before NF-κB activity measurement in lysates. Quantitative RT-PCRs confirming mRNA expression of the plasmid were performed and CD137 surface expression was checked by flow cytometry (not shown). Shown experiments were consistent with at least 5 similarly performed.
The inhibition of CD137-mediated NF-κB activation by A20 and CYLD was also observed in transient transfections of transformed T lymphocytes of the MOLT4 and CEM cell lines (Fig. 2C), thus indicating that A20 and CYLD could regulate CD137 activity in the cell type where CD137 exerts its physiologic costimulatory functions.
Silencing A20 and/or CYLD up-regulates NF-κB activation following CD137 stimulation
We hypothesize that if A20 and CYLD immunoprecipitate with the CD137 proximal signaling complex and regulate the intensity of the signal, their reduced expression should result in NF-κB enhanced transcriptional activity.
To explore this point, we used magnetically sorted human CD8 T lymphocytes that were stimulated for 24 hours with anti-CD3/CD28 coated beads. Lymphocytes were then transduced with lentivirus encoding EGFP with or without 2 distinct shRNAs targeting the A20 transcript. Lymphocytes were then cultured on plates coated with anti-CD3 + anti-CD137 (6B4) mAbs or anti-CD3 mAb + control mIgG1 antibody (Fig. 3A). Three days later, GFP+ (transduced) and GFP− (non-transduced) lymphocytes (Fig. 3B) were analyzed for Bcl-xL expression by intracellular immunofluorescence as a parameter to measure CD137 costimulatory activity.7 Fig. 3 C (top) and D show an increase in Bcl-xL in CD8 T cells blasts transduced with the shRNAs targeting A20 compared with the non-transduced cells and control virus transduced cells (Fig. 3C and D).
Figure 3.
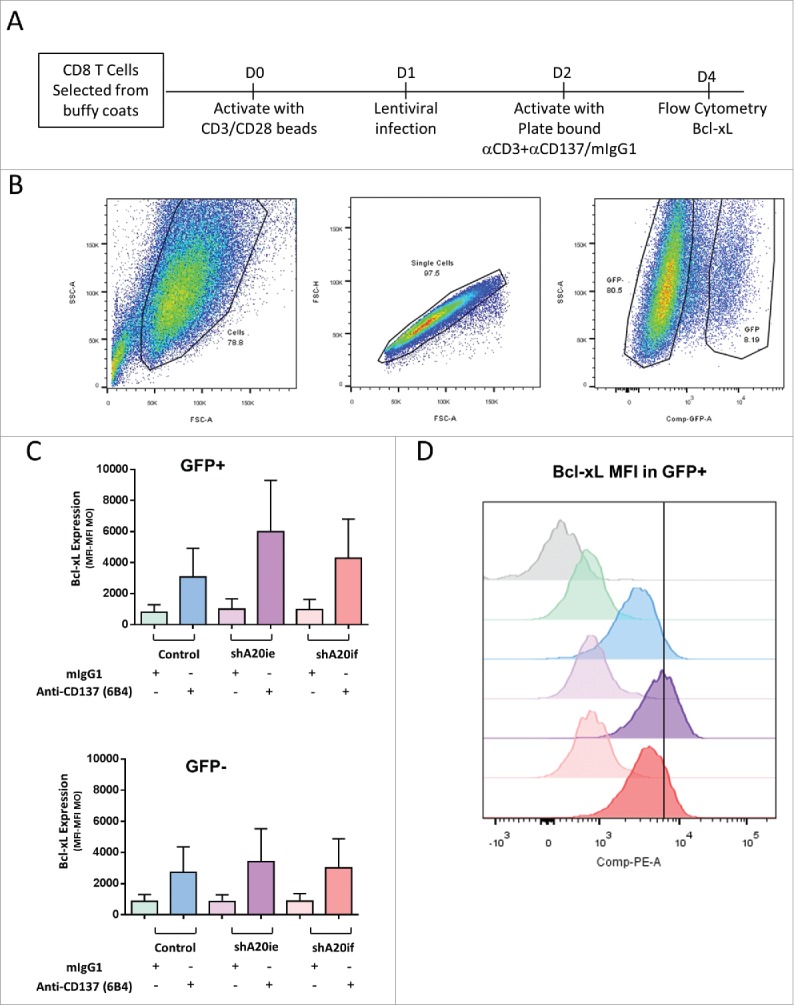
Silencing of A20 in primary human T cells with a lentivirus encoding a specific shA20 enhances Bcl-xL expression following CD137 stimulation. CD3-CD28 activated human CD8 T cells were transfected with pLVTHM/A20ie, pLVTHM/A20if viruses or a control pLVTHM lentivirus. A scheme of the experiments is provided in (A). EGFP expression permits FACS gating of the successfully transfected cell population as shown in (B). In (C) Bcl-xL intracellular immunostaining intensity (MFI) is shown on gated GFP-positive and GFP-negative cells following stimulation with plate bound anti-CD137 mAb or control antibody during 72 hours in culture. In (D) a representative case is presented with the intensity of fluorescence of the corresponding color-coded histograms for each experimental condition. A representative experiment out of 2 independently performed is shown.
Furthermore, we electroporated siRNA oligonucleotides into human primary CD8 T cells to assess the effect of A20 and CYLD on the costimulating effects of CD137 (Fig. 4A). Fig. 4B shows a representative experiment indicating that upon A20 or CYLD transient silencing, CD137 costimulation triggered higher expression of CD25 and Bcl-xL, which are well known effects of CD137 costimulation.7
Figure 4.
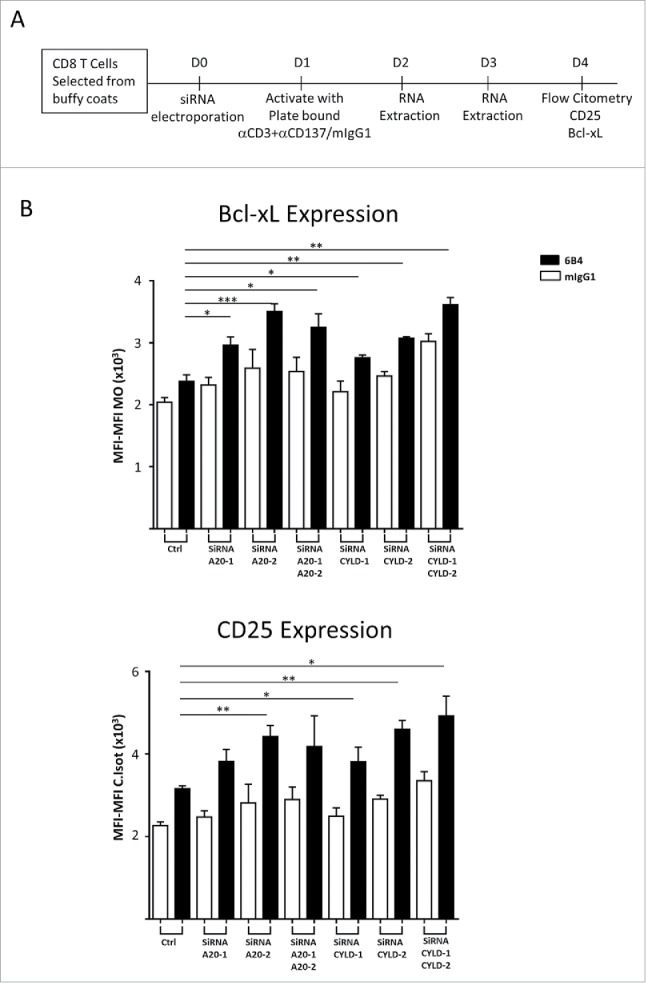
siRNA-silencing of A20 and CYLD augments costimulation triggered by CD137 stimulation on human primary CD8 T cells. (A) Schematic representation of the experiments. (B) Human CD8 T cells were electroporated with the indicated siRNAs and underwent stimulation with anti-CD3 plus anti-CD137 mAb. Four days later CD25 and Bcl-xL expression were monitored by immunofluorescence and flow cytometry. Data are shown as mean intensity of fluorescence (mean ± SEM). Downregulation of the corresponding mRNAs was confirmed by qRT-PCR (not shown). A representative experiment out of at least 3 independently performed is shown. Statistical comparisons were performed using students t tests (* p < 0.05, **p < 0.01, ***p < 0.001).
A20 and CYLD increased in vivo the NF-κB activation triggered by treatment with agonist anti CD137 mAb
Using hydrodynamic gene transfer technology, cDNA-encoding vectors can be forced to enter into hepatocytes allowing the expression of the encoded genes in vivo.39 Using this approach, we have previously shown that hydrodynamic gene transfer of plasmids encoding CD137 and a NF-κB luciferase reporter system were efficiently expressed in hepatocytes in vivo and could respond to systemic administration of agonist anti-CD137 mAb with increased liver luciferase biosynthesis.11
In the experiments shown in Fig. 5, using hydrodynamic gene transfer, we assessed the effect of enforced expression of A20 or/and CYLD on CD137-mediated NF-κB activity (Fig. 5A). Both the anti-CD137 mAb 6B4 and the clinical grade anti-CD137 mAb Urelumab enhanced the luciferase-dependent light emission in the liver area upon CD137 engagement by agonist antibodies (Fig. 5B and C). However, enforced coexpression of either A20 or CYLD hindered luciferase expression. Fig. 5D shows the light emission pattern in representative mice. These data indicate that both A20 and CYLD can efficiently repress ligand-activated CD137-signaling, an outcome that could be exploited to improve the T-cell costimulatory activity of CD137 agonists.
Figure 5.
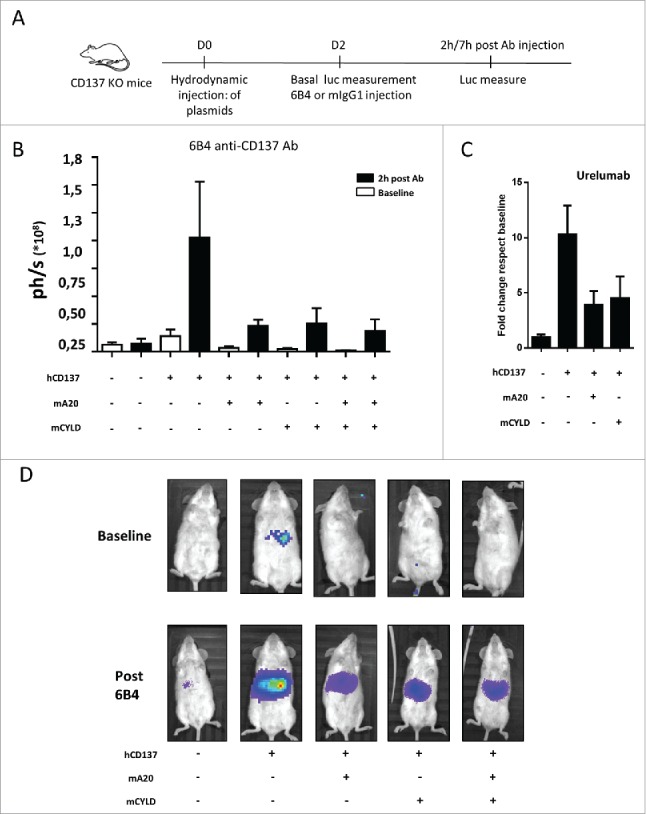
A20 and CYLD decrease CD137-elicited NF-κB activation in vivo. (A) Mice (6 per group) were hydrodinamically gene transferred to the liver with expression plasmids encoding the NF-κB luciferase reporter construct and the indicated genes (A). 48 hours later mice were dosed intraperitoneally with the anti-CD137 mAb 6B4 (B) or urelumab (C) and bioluminiscence was monitored by light emission from the upper abdomen (photons/sec in B and fold change over baseline in C). (D) Shows images of representative mice in B. Baseline was measured immediately before intraperitoneal mAb injection. Results represent mean ± SEM of an experiment out of at least 4 performed under similar conditions and rendering similar results.
Discussion
Our study shows that CD137 signaling toward canonical NF-κB activation is regulated by A20 and CYLD. This study herein extends our previous results showing that CD137 stimulation induced TRAF2-dependent K63 polyubiquitination events resulting in NF-κB activation,11 further supporting the key importance of K63-ubiquitination in CD137 signaling.
The signaling events downstream of CD137 are an important issue due to the critical roles of this TNFR-family member in cancer immunotherapy.40 On the one hand, agonist anti-CD137 mAb potently induce CD8-mediated anti-tumor immunity in mice.41 On the other hand, the CD137 cytoplasmic tail is critical for the functions of chimeric antigen receptors targeting CD19 with successful application in patients against B-cell lymphomas and leukemias.42-44 Therefore elucidating the mechanisms and regulation of the CD137 signaling pathway is relevant for understanding its immunotherapeutic functions and to gain insights into how to modulate those to improve its therapeutic efficacy.
There is evidence for both TRAF2 and TRAF1 functioning as adaptors for CD137 signaling.45-47 There is ample published evidence for TRAF2 involvement in ubiquitination.10 A recent report has also linked TRAF1 to the function of the linear ubiquitin binding complex (LUBAC) although it is unknown if this applies to TNFR members in addition to TLR members.48
TRAF2 is a RING domain containing protein reportedly endowed with E3 ubiquitin ligase activity, generating K63 polyubiquitin chains in conjunction with Ubc13 as the necessary E2 enzyme.49 A number of reports have shown a K63 ubiquitin ligase reaction attributed to TRAF2,49,50 although questions have been raised on the ability of TRAF2 to actually accommodate the transubiquitination reaction based on the structural characteristics of the TRAF2 E3 domain.12 Therefore, it cannot be ruled out that another E3 enzyme associated with the complex might be responsible for the K63 ubiquitination reactions. Interestingly, TRAF2 itself is an important substrate of the polyubiquitination reaction and we propose that CD137 crosslinking causes TRAF2-mediated polyubiquitination of neighboring sister TRAF2 molecules in the CD137 activation complex, which could be one of the earliest signaling events,7 in addition to TRAF2 phosphorylation51 and a cofactor requirement for sphingosine 1 phosphate.52
The function of K63 ubiquitination is to create docking sites for protein-protein interactions allowing the signaling cascade to proceed downstream toward NF-κB activation.53 Several proteins, such as TAB1/2, TAK1, NEMO and IKKβ are polyubiquitinated.53 Lymphocyte activation pathways need elements to regulate the function by feed-back quenching signals. Following the example of kinases and phosphatases, K63 polyubiquitin reactions do not go unchecked, since a broad family of K63 deubiquitinases control the extent of K63-ubiquitination10,17
Indeed, we have shown that A20 and CYLD bind to the CD137-TRAF2 complex thereby permitting coimmunoprecipitation of these proteins. TRAF2 has been shown to interact with other members of the TNFR family such as OX40, CD40, GITR and CD27 and hence binding to these DUB enzymes is unlikely to be exclusive of CD137. A20 is well known to regulate signaling toward NF-κB activation through TNFR1 and CD40.54,55 In turn, CYLD has been recently discovered to regulate ubiquitination elicited by TNFR1 by means of the SPATA2 adaptor that links CYLD to the signaling receptor machinery.56
Consistent with the presence of CYLD and A20 in the complex, polyubiquitination of proteins in the CD137 activation complex was reduced and, more importantly, overexpression of A20 or/and CYLD decreased NF-κB activation. The effects were prominent on TAK1 which is a hub kinase in the pathway.57 We cannot rule out that A20 and CYLD might also be exerting an additional role downstream in the NF-κB pathway, but overexpression and silencing data speak of a direct regulation on proximal CD137 signaling activity. Figure 6 shows a sketch of the proposed functional and protein-to-protein interaction toward NF-κB activation and its regulation by CYLD and A20.
Figure 6.
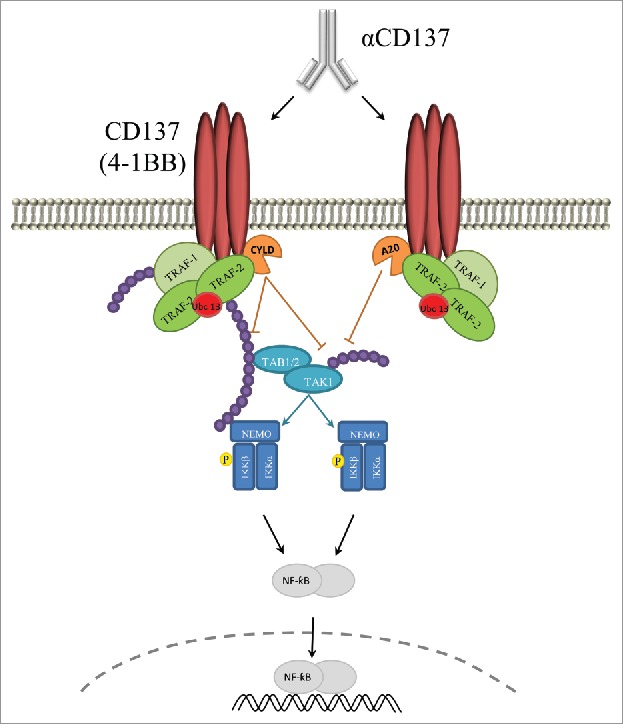
Schematic representation of the proposed regulation of CD137 signaling by A20 an CYLD. Graphical abstract of the reported physical and functional signaling interactions of CD137 shown to be regulated by A20 and CYLD in such a way that attenuate NF-κB activation. Purple beads indicate K63 polyubiquitin chains that can be degraded by A20 and CYLD.
A20 is a double-edge enzyme with K63 deubiquitylation activity in its N-terminal OTU domain and a K48 ubiquitin ligase E3 function on the ZF4 domain. Both functions are important for TNFR-family signaling, deactivating proteins and eliminating docking sites in the case of K63 deubiquitinase reactions, and targeting inhibitors for degradation by means of K48-ubiquitylation.54, 58 Our data with deletion variants do not provide a definitive answer, since both mutants retain the ability to coprecipitate with the CD137-TRAF2 complex and inhibit CD137-mediated NF-κB activation. A possible interpretation is that both K63 deubiquitination and K48 polyubiquitination give rise to similar functional results but this intriguing finding warrants further investigation. In this line, A20 knock-in mice with loss of function mutations either in the OTU domain or in the ZF4 domain do not recapitulate the severe inflammatory phenotype of A20 knockout mice.59 Indeed our results are consistent with recent findings in A20-deficient MEFs which show that transfection of A20 mutants of either domain do not rescue the inhibition effect on NF-κB activation as elicited by TNFα.58 Further complexity to fully understand A20 regulation on CD137 is expected to come from posttranscriptional modifications of the protein by serine phosphorylations,60 dimerization58 and oxidation.61
We propose that CD137 signaling below a certain threshold, for instance, due to stochastic receptor associations or in the absence of strong CD137L interactions, would be rapidly quenched by A20 or CYLD deubiquitinase activities. However, in response to a strong stimulus CD137 signaling would be able to proceed once sufficient E3 polyubiquitin ligase activity is in progress. In addition A20 and CYLD may destroy K63-polyubiquitin chains to terminate signaling once the agonist mAb or CD137L are no longer available. At this point, it is unclear if A20 and CYLD are redundant for this function or not, as shown for B-cell function and development.62 In our experiments TRAF2 itself seems to be mainly a substrate for CYLD but not for A20 in agreement with previous literature.17
Moreover, A20 and CYLD relative levels of expression are known to change with cell activation17 and hence are both DUBs are not likely to be redundant even if in our experiments both attain similar levels of inhibition on CD137-elicited NF-κB activation. It is noteworthy that A20 is the most induced transcriptional target regulated by the canonical NF-κB pathway.18 This can be interpreted as a negative feedback loop acting to curtail excessive signaling through CD137 and other NF-κB activating receptors. This interpretation, if correct, provides translational relevance to our findings and is consistent with the observation that A20 and CYLD overexpression mitigate CD137 signaling in hepatocytes in vivo.
It is of note that TRAF6 and TRAF5 are also endowed with E3 K63-ubiquitin ligase activity3 and it is not surprising that CYLD, A20 (or perhaps other DUBs) control these signaling functions as well.63-66 In this way A20 and CYLD would regulate signaling from other T-cell costimulatory members of the TNFR family. By contrast, it is unlikely that A20 or CYLD would affect CD28-mediated co-stimulatory signaling offering another difference between CD137 and CD28 costimulatory signaling, that for instance show different time courses in the induction of Bcl-xL and CD25 expression (data not shown).
Another interesting area of research will be opened up if the LUBAC machinery is connected to TRAF1 signal transduction. How deubiquitinases regulate linear polyubiquitins might become important to understand CD137 regulation.48 Interestingly A20 and CYLD have recently been shown to regulate this pathway67 and A20 is known to bind linear M1-polyubiquitins through its C-terminal ZF7 domain.17,68
From a translational point of view, A20 and CYLD could be critical modulators of immunotherapy with anti-CD137 mAbs. We can, for instance, assess the activity of the anti-CD137 mAb Urelumab administered to mice in an experimental setting that could recapitulate events that are also taking place in patients undergoing urelumab clinical trials.69,70 In this context, the interplay of CD137 with TRAF2, A20 and/or CYLD offers possibilities to devise biomarkers, enhance costimulatory activity and might be conducive to strategies supporting the critical activity of the CD137 cytoplasmic domain in clinically successful chimeric antigen receptors for adoptive T-cell therapy (CARs).42
Materials and methods
Transfections and expression cassettes
HEK293 T cells were obtained from ATCC in 2008. Cells were cultured in complete RPMI medium (RPMI 1640 with Glutamax [Gibco, Invitrogen] containing 10% heat-inactivated FBS [Sigma-Aldrich], 100 IU/ml penicillin and 100 μg/ml streptomycin [Gibco]). Cells were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's instructions. For overexpression of CD137, A20, CYLD, we used the following plasmids: pCMV6-hCD137(GeneCopoeia), pCMV6-mA20 (Origen), pCEFL-FLAG-hA20 WT, N-ter (1–390), C-ter (390–790) and pCMV6-mCYLD (Origene).
Immunoprecipitation and immunoblotting
HEK293 T cells were transfected as described above with plasmids encoding CD137, FLAG-A20 and/or CYLD. Eighteen hours after transfection, stimulation with anti-CD137 mAb for 5 min was accomplished with 5 µg/ml anti-CD137 (clone 6B4) or mIgG1 (Biolegend) as a control. Then cells were lysed at 4°C in lysis buffer (50 mM Tris-HCl [pH 8.5], 150 mM NaCl, 1% Nonidet P-40, 5 mM EDTA, and EDTA free complete protease inhibitor [Roche]). Soluble lysate was immunoprecipitated with anti-ubiquitin mAb (clone FK2, ENZO Life sciences) coupled to protein G-Sepharose 4 Fast Flow beads (GE Healthcare). Proteins were eluted in Laemmli buffer, separated by SDS-PAGE, and transferred to polyvinylidene difluoride membranes (Millipore). Membranes were blocked in TBS containing 0.05% Tween-20 and 5% non-fat milk for 1 h. Immunoblots were performed with the following antibodies against ubiquitin (clone P4D1, Mouse monoclonal; Santa Cruz), TAK1 (rabbit polyclonal; Invitrogen), TRAF2 (C-20) (rabbit polyclonal; Santa Cruz), CYLD (rabbit monoclonal; Cell signaling), IκBα (mouse monoclonal; Cell signaling), FLAG (mouse monoclonal; Sigma-Aldrich) and HA (Rabbit polyclonal;Abcam). GAPDH (mouse monoclonal; Sigma-Aldrich) was used as a loading control. Blots were developed with the SuperSignal West Dura Extended Duration Substrate (Pierce). Bands were quantified by densitometry using ImageJ (NIH) software.
NF-κB luciferase reporter assay
Transient transfection of HEK293 T cells was performed using Lipofectamine 2000 according to the manufacturer's instructions. Luciferase reporter assays were performed with pκB-Luc plasmid which encodes a firefly luciferase reporter gene under the control of a NF-κB-responsive promoter71 and Renilla luciferase reporter vector pRL-TK (Promega). Briefly, cells were co-transfected with 200 ng pκB-Luc plasmid, 200 ng constitutive expression plasmid Renilla luciferase reporter vector pRL-TK (Promega), and 5 ng pCMV6-hCD137 (GeneCopoeia), 500 ng pcDNA 3.1+HA-ubiquitin K63R (a kind gift from Gijs Versteeg, Mount Sinai School of Medicine), 500 ng pCMV6-mCYLD (Origene) and/or 500 ng pCMV6-mA20 (Origen) as indicated. Five hours after transfection cells were stimulated for 18 hours with 10 μg/ml anti-CD137 mAb (clone 6B4) or mAb control (mIgG1, Biolegend) and lysed in dual reporter lysis buffer (Promega). Firefly luciferase signal were normalized using Renilla luciferase values.
For luciferase reporter experiments in lymphoid cell lines, 40 × 106 CEM or MOLT4 cells per condition were electroporated using the Gene Pulser MX System (300 V, 950 μF Bio-Rad, Hercules, CA) with plasmids encoding human CD137 (40 μg), K63R (80 μg), A20 (80 µg), CYLD (80 µg), NF-κB-firefly luciferase reporter (40 μg), pRL-TK (40 μg), or empty vector (20–30 μg) as indicated. After 18 h, live cells were isolated using Ficoll density gradient separation, washed, and stimulated during 24 h with 10 μg/ml anti-CD137 mAb (clone 6B4) or control Ab (Mouse IgG1; Biolegend).
siRNA and shRNA gene silencing
CD8 T cells were isolated by negative immunomagnetic selection (Miltenyi) from a buffy coat (Navarra Blood and Tissue Bank. Navarrabiomed Biobank. Navarra Health Department) and purity verified to be above 90%.
Lentivirus encoding shRNA to silence endogenous A20 expression and EGFP were produced by the Viral Vector Platform at Inbiomed Foundation as described previously.72,73 Purified human CD8 T cells were preactivated 24 hours with CD3/CD28 magnetics beads prior lentiviral infection, which was performed at a multiplicity of infection of 10 overnight. After, cells were incubated with plated bound anti-CD3 (OKT3) and anti-CD137 (6B4) or mIgG1 mAbs for 48 hours and Bcl-xL expression levels were monitored by flow cytometry. These lentiviruses had been previously validated to silence A20.73
Silencing of endogenous A20 or CYLD expression in human CD8 T cells was achieved by RNA interference. The following siRNAs were purchased from Dharmacom Thermo Fisher: human A20 1(GCAAAAGAAUCAAAACAAA), human A20 2 (GCUUUGUAUUUGAGCAAUA), human CYLD 1 (CGCUGUAACUCUUUAGCAU), human CYLD 2 (GGAAGAAGGUCGUGGUCAA). Purified human CD8 T cells were electroporated with 200pmol siRNA per 107 cells using the Gene Pulser MX System (Bio-Rad, Hercules, CA.) 24 hours following electroporation cells were stimulated with plate bound anti-CD3 (OKT3) coated at 1 µg/ml and anti-CD137 (6B4) or mIgG1 at 5 µg/ml. 72 hours later Bcl-xL and surface CD25 expression was assessed by flow cytometry.
Antibodies and flow cytometry
For flow cytometry analysis, the single-cell suspensions were treated with Beriglobine (CSL Behring) in a phosphate buffered saline–based buffer (Gibco/Life Technologies) containing 10% fetal bovine serum (Sigma-Aldrich) to avoid unspecific staining and were stained with the following fluorochrome-conjugated antibodies: anti–CD8- BV510 (SK1 [Biolegend]), anti–Bcl-xL-PE (7 B2.5 [Southern Biotech]), anti–CD25-APC (BC96 [Biolegend]) and Zombie NIR ([Biolegend]). Fluorescence signals were acquired with a BD Biosciences FACSCanto II flow cytometer. Data were analyzed using FlowJo software (Tree Star, Inc.). Stainings were controlled by isotype fluorochrome-matched mAbs and Minus One.
In vivo hydrodynamic gene transfer and determination of luciferase activity
6–8 week old CD137 KO mice (BALB/c) were injected with a total amount of 42 μg plasmid DNA by hydrodynamic injection, consisting of the administration of 1.8–2.0 ml DNA solution via tail vein in 5 seconds.39 Animals were injected with plasmids encoding a NF-κB-firefly luciferase reporter (6 μg), CD137 (12 μg), A20 (12 μg), CYLD (12 μg), or with an empty plasmid (empty vector) as required. 48 h after hydrodynamic injection, mice were i.p. inoculated with 100 μg human anti-CD137 mAb (clone 6B4 or Urelumab) or control mouse IgG1. Bioluminescence imaging was performed before mAb treatment (baseline) and 2–7 hours after mAb treatment.
Statistics
Descriptive statistics were calculated with PRISMA software and comparisons were performed by U-Mann-whitney tests or student's t tests. Statistical significance (p< 0.05) are indicated by * in the figures.
Supplementary Material
Disclosure of potential conflicts of interest
MJ-K is a full time employee in AstraZeneca Medimmune and a former employee of Bristol-Myers Squibb; IM serves as a consultant for Bristol Myers Squibb, AstraZeneca, Lilly, Bayer, Roche-Genentech, Bioncotech, Merck-Serono, Incyte, Servier, Alligator and Tusk Therapeutics.
Acknowledgments
We are grateful to helpful scientific discussions with Drs. Estanislao Nistal, Iván Martínez Forero, Sandra Hervas and David Sancho. Technical support by Diego Alignani, Eneko Elizalde and Carmen Molina are greatly appreciated.
Financial support comes from MINECO (SAF2011–22831 and SAF2014–52361-R) (I.M), Departamento de Salud del Gobierno de Navarra, Redes temáticas de investigación cooperativa RETICC, European Commission VII Framework and Horizon 2020 programs (AICR and PROCROP), Fundación de la Asociación Española Contra el Cáncer (AECC), Fundación BBVA. MER-R receives support for a Rio Ortega Contract and EB is supported by CIBERONC. The cost of this publication was paid in part by FEDER funds.
References
- 1.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3(8):609-20. doi: 10.1038/nri1148. PMID:12974476 [DOI] [PubMed] [Google Scholar]
- 2.Zapata JM, Lefebvre S, Reed JC. Targeting TRAfs for therapeutic intervention. Adv Exp Med Biol. 2007;597:188-201. doi: 10.1007/978-0-387-70630-6_15. PMID:17633027 [DOI] [PubMed] [Google Scholar]
- 3.Chung JY, Park YC, Ye H, Wu H. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J Cell Sci. 2002;115(Pt 4):679-88. PMID:11865024 [DOI] [PubMed] [Google Scholar]
- 4.Brenner D, Blaser H, Mak TW. Regulation of tumour necrosis factor signalling: live or let die. Nat Rev Immunol. 2015;15(6):362-74. doi: 10.1038/nri3834. PMID:26008591 [DOI] [PubMed] [Google Scholar]
- 5.Croft M. The TNF family in T cell differentiation and function–unanswered questions and future directions. Semin Immunol. 2014;26(3):183-90. doi: 10.1016/j.smim.2014.02.005. PMID:24613728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanmamed MF, Pastor F, Rodriguez A, Perez-Gracia JL, Rodriguez-Ruiz ME, Jure-Kunkel M, Melero I. Agonists of Co-stimulation in Cancer Immunotherapy Directed Against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015;42(4):640-55. doi: 10.1053/j.seminoncol.2015.05.014. PMID:26320067 [DOI] [PubMed] [Google Scholar]
- 7.Sanchez-Paulete AR, Labiano S, Rodriguez-Ruiz ME, Azpilikueta A, Etxeberria I, Bolanos E, Lang V, Rodriguez M, Aznar MA, Jure-Kunkel M, et al.. Deciphering CD137 (4-1BB) signaling in T-cell costimulation for translation into successful cancer immunotherapy. Eur J Immunol. 2016;46(3):513-22. doi: 10.1002/eji.201445388. PMID:26773716 [DOI] [PubMed] [Google Scholar]
- 8.Saoulli K, Lee SY, Cannons JL, Yeh WC, Santana A, Goldstein MD, Bangia N, DeBenedette MA, Mak TW, Choi Y, et al.. CD28-independent, TRAF2-dependent costimulation of resting T cells by 4-1BB ligand. J Exp Med. 1998;187(11):1849-62. PMID:9607925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sabbagh L, Andreeva D, Laramee GD, Oussa NA, Lew D, Bisson N, Soumounou Y, Pawson T, Watts TH. Leukocyte-specific protein 1 links TNF receptor-associated factor 1 to survival signaling downstream of 4-1BB in T cells. J Leukoc Biol. 2013;93(5):713-21. doi: 10.1189/jlb.1112579. PMID:23446150 [DOI] [PubMed] [Google Scholar]
- 10.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458(7237):430-7. doi: 10.1038/nature07959. PMID:19325622 [DOI] [PubMed] [Google Scholar]
- 11.Martinez-Forero I, Azpilikueta A, Bolanos-Mateo E, Nistal-Villan E, Palazon A, Teijeira A, Perez-Chacon G, Morales-Kastresana A, Murillo O, Jure-Kunkel M, et al.. T cell costimulation with anti-CD137 monoclonal antibodies is mediated by K63-polyubiquitin-dependent signals from endosomes. J Immunol. 2013;190(12):6694-706. doi: 10.4049/jimmunol.1203010. PMID:23690480 [DOI] [PubMed] [Google Scholar]
- 12.Yin Q, Lamothe B, Darnay BG, Wu H. Structural basis for the lack of E2 interaction in the RING domain of TRAF2. Biochemistry. 2009;48(44):10558-67. doi: 10.1021/bi901462e. PMID:19810754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uren AG, Pakusch M, Hawkins CJ, Puls KL, Vaux DL. Cloning and expression of apoptosis inhibitory protein homologs that function to inhibit apoptosis and/or bind tumor necrosis factor receptor-associated factors. Proc Natl Acad Sci U S A. 1996;93(10):4974-8. PMID:8643514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mace PD, Smits C, Vaux DL, Silke J, Day CL. Asymmetric recruitment of cIAPs by TRAF2. J Mol Biol. 2010;400(1):8-15. doi: 10.1016/j.jmb.2010.04.055. PMID:20447407 [DOI] [PubMed] [Google Scholar]
- 15.Harhaj EW, Dixit VM. Regulation of NF-kappaB by deubiquitinases. Immunol Rev. 2012;246(1):107-24. doi: 10.1111/j.1600-065X.2012.01100.x. PMID:22435550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Forero I, Rouzaut A, Palazon A, Dubrot J, Melero I. Lysine 63 polyubiquitination in immunotherapy and in cancer-promoting inflammation. Clin Cancer Res. 2009;15(22):6751-7. doi: 10.1158/1078-0432.CCR-09-1225. PMID:19887490 [DOI] [PubMed] [Google Scholar]
- 17.Lork M, Verhelst K, Beyaert R. CYLD, A20 and OTULIN deubiquitinases in NF-kappaB signaling and cell death: so similar, yet so different. Cell Death Differ. 2017;24(7):1172-83. doi: 10.1038/cdd.2017.46. PMID:28362430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma A, Malynn BA. A20: linking a complex regulator of ubiquitylation to immunity and human disease. Nat Rev Immunol. 2012;12(11):774-85. doi: 10.1038/nri3313. PMID:23059429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verstrepen L, Verhelst K, van Loo G, Carpentier I, Ley SC, Beyaert R. Expression, biological activities and mechanisms of action of A20 (TNFAIP3). Biochem Pharmacol. 2010;80(12):2009-20. doi: 10.1016/j.bcp.2010.06.044. PMID:20599425 [DOI] [PubMed] [Google Scholar]
- 20.Wertz I, Dixit V. A20–a bipartite ubiquitin editing enzyme with immunoregulatory potential. Adv Exp Med Biol. 2014;809:1-12. PMID:25302362 [DOI] [PubMed] [Google Scholar]
- 21.Schmitz R, Hansmann ML, Bohle V, Martin-Subero JI, Hartmann S, Mechtersheimer G, Klapper W, Vater I, Giefing M, Gesk S, et al.. TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lymphoma and primary mediastinal B cell lymphoma. J Exp Med. 2009;206(5):981-9. doi: 10.1084/jem.20090528. PMID:19380639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Honma K, Tsuzuki S, Nakagawa M, Tagawa H, Nakamura S, Morishima Y, Seto M. TNFAIP3/A20 functions as a novel tumor suppressor gene in several subtypes of non-Hodgkin lymphomas. Blood. 2009;114(12):2467-75. doi: 10.1182/blood-2008-12-194852. PMID:19608751 [DOI] [PubMed] [Google Scholar]
- 23.Matmati M, Jacques P, Maelfait J, Verheugen E, Kool M, Sze M, Geboes L, Louagie E, Mc Guire C, Vereecke L, et al.. A20 (TNFAIP3) deficiency in myeloid cells triggers erosive polyarthritis resembling rheumatoid arthritis. Nat Genet. 2011;43(9):908-12. doi: 10.1038/ng.874. PMID:21841782 [DOI] [PubMed] [Google Scholar]
- 24.Hammer GE, Turer EE, Taylor KE, Fang CJ, Advincula R, Oshima S, Barrera J, Huang EJ, Hou B, Malynn BA, et al.. Expression of A20 by dendritic cells preserves immune homeostasis and prevents colitis and spondyloarthritis. Nat Immunol. 2011;12(12):1184-93. doi: 10.1038/ni.2135. PMID:22019834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagamachi A, Nakata Y, Ueda T, Yamasaki N, Ebihara Y, Tsuji K, Honda Z, Takubo K, Suda T, Oda H, et al.. Acquired deficiency of A20 results in rapid apoptosis, systemic inflammation, and abnormal hematopoietic stem cell function. PLoS One. 2014;9(1):e87425. doi: 10.1371/journal.pone.0087425. PMID:24498102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giordano M, Roncagalli R, Bourdely P, Chasson L, Buferne M, Yamasaki S, Beyaert R, van Loo G, Auphan-Anezin N, Schmitt-Verhulst AM, et al.. The tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20) imposes a brake on antitumor activity of CD8 T cells. Proc Natl Acad Sci U S A. 2014;111(30):11115-20. doi: 10.1073/pnas.1406259111. PMID:25024217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vereecke L, Beyaert R, van Loo G. The ubiquitin-editing enzyme A20 (TNFAIP3) is a central regulator of immunopathology. Trends Immunol. 2009;30(8):383-91. doi: 10.1016/j.it.2009.05.007. PMID:19643665 [DOI] [PubMed] [Google Scholar]
- 28.Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, Green H, Brown C, Biggs PJ, Lakhani SR, et al.. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25(2):160-5. doi: 10.1038/76006. PMID:10835629 [DOI] [PubMed] [Google Scholar]
- 29.Sun SC. CYLD: a tumor suppressor deubiquitinase regulating NF-kappaB activation and diverse biological processes. Cell Death Differ. 2010;17(1):25-34. doi: 10.1038/cdd.2009.43. PMID:19373246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424(6950):801-5. doi: 10.1038/nature01802. PMID:12917691 [DOI] [PubMed] [Google Scholar]
- 31.Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424(6950):797-801. doi: 10.1038/nature01811. PMID:12917690 [DOI] [PubMed] [Google Scholar]
- 32.Clark K, Nanda S, Cohen P. Molecular control of the NEMO family of ubiquitin-binding proteins. Nat Rev Mol Cell Biol. 2013;14(10):673-85. doi: 10.1038/nrm3644. PMID:23989959 [DOI] [PubMed] [Google Scholar]
- 33.Tsagaratou A, Grammenoudi S, Mosialos G. Differential requirement of IKK2 for CYLD-dependent representation of thymic and peripheral T-cell populations. Eur J Immunol. 2011;41(10):3054-62. doi: 10.1002/eji.201041160. PMID:21728169 [DOI] [PubMed] [Google Scholar]
- 34.Jin W, Chang M, Paul EM, Babu G, Lee AJ, Reiley W, Wright A, Zhang M, You J, Sun SC. Deubiquitinating enzyme CYLD negatively regulates RANK signaling and osteoclastogenesis in mice. J Clin Invest. 2008;118(5):1858-66. doi: 10.1172/JCI34257. PMID:18382763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wright A, Reiley WW, Chang M, Jin W, Lee AJ, Zhang M, Sun SC. Regulation of early wave of germ cell apoptosis and spermatogenesis by deubiquitinating enzyme CYLD. Dev Cell. 2007;13(5):705-16. doi: 10.1016/j.devcel.2007.09.007. PMID:17981138 [DOI] [PubMed] [Google Scholar]
- 36.Rodriguez MS, Egana I, Lopitz-Otsoa F, Aillet F, Lopez-Mato MP, Dorronsoro A, Lobato-Gil S, Sutherland JD, Barrio R, Trigueros C, et al.. The RING ubiquitin E3 RNF114 interacts with A20 and modulates NF-kappaB activity and T-cell activation. Cell Death Dis. 2014;5:e1399. doi: 10.1038/cddis.2014.366. PMID:25165885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klinkenberg M, Van Huffel S, Heyninck K, Beyaert R. Functional redundancy of the zinc fingers of A20 for inhibition of NF-kappaB activation and protein-protein interactions. FEBS Lett. 2001;498(1):93-7. PMID:11389905 [DOI] [PubMed] [Google Scholar]
- 38.Song HY, Rothe M, Goeddel DV. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-kappaB activation. Proc Natl Acad Sci U S A. 1996;93(13):6721-5. PMID:8692885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6(7):1258-66. doi: 10.1038/sj.gt.3300947. PMID:10455434 [DOI] [PubMed] [Google Scholar]
- 40.Melero I, Hirschhorn-Cymerman D, Morales-Kastresana A, Sanmamed MF, Wolchok JD. Agonist antibodies to TNFR molecules that costimulate T and NK cells. Clin Cancer Res. 2013;19(5):1044-53. doi: 10.1158/1078-0432.CCR-12-2065. PMID:23460535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Melero I, Shuford WW, Newby SA, Aruffo A, Ledbetter JA, Hellstrom KE, Mittler RS, Chen L. Monoclonal antibodies against the 4-1BB T-cell activation molecule eradicate established tumors. Nat Med. 1997;3(6):682-5. PMID:9176498 [DOI] [PubMed] [Google Scholar]
- 42.Kawalekar OU, O'Connor RS, Fraietta JA, Guo L, McGettigan SE, Posey AD Jr, Patel PR, Guedan S, Scholler J, Keith B, et al.. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T Cells. Immunity. 2016;44(2):380-90. doi: 10.1016/j.immuni.2016.01.021. PMID:26885860 [DOI] [PubMed] [Google Scholar]
- 43.Fesnak AD, June CH, Levine BL. Engineered T cells: the promise and challenges of cancer immunotherapy. Nat Rev Cancer. 2016;16(9):566-81. doi: 10.1038/nrc.2016.97. PMID:27550819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Long AH, Haso WM, Shern JF, Wanhainen KM, Murgai M, Ingaramo M, Smith JP, Walker AJ, Kohler ME, Venkateshwara VR, et al.. 4-1BB costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med. 2015;21(6):581-90. doi: 10.1038/nm.3838. PMID:25939063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cannons JL, Choi Y, Watts TH. Role of TNF receptor-associated factor 2 and p38 mitogen-activated protein kinase activation during 4-1BB-dependent immune response. J Immunol. 2000;165(11):6193-204. PMID:11086053 [DOI] [PubMed] [Google Scholar]
- 46.McPherson AJ, Snell LM, Mak TW, Watts TH. Opposing roles for TRAF1 in the alternative versus classical NF-kappaB pathway in T cells. J Biol Chem. 2012;287(27):23010-9. doi: 10.1074/jbc.M112.350538. PMID:22570473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sabbagh L, Pulle G, Liu Y, Tsitsikov EN, Watts TH. ERK-dependent Bim modulation downstream of the 4-1BB-TRAF1 signaling axis is a critical mediator of CD8 T cell survival in vivo. J Immunol. 2008;180(12):8093-101. PMID:18523273 [DOI] [PubMed] [Google Scholar]
- 48.Abdul-Sater AA, Edilova MI, Clouthier DL, Mbanwi A, Kremmer E, Watts TH. The signaling adaptor TRAF1 negatively regulates Toll-like receptor signaling and this underlies its role in rheumatic disease. Nat Immunol. 2017;18(1):26-35. doi: 10.1038/ni.3618. PMID:27893701 [DOI] [PubMed] [Google Scholar]
- 49.Shi CS, Kehrl JH. Tumor necrosis factor (TNF)-induced germinal center kinase-related (GCKR) and stress-activated protein kinase (SAPK) activation depends upon the E2/E3 complex Ubc13-Uev1 A/TNF receptor-associated factor 2 (TRAF2). J Biol Chem. 2003;278(17):15429-34. doi: 10.1074/jbc.M211796200. PMID:12591926 [DOI] [PubMed] [Google Scholar]
- 50.Borghi A, Verstrepen L, Beyaert R. TRAF2 multitasking in TNF receptor-induced signaling to NF-kappaB, MAP kinases and cell death. Biochem Pharmacol. 2016;11:61-10. doi: 10.1016/j.bcp.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 51.Shen RR, Zhou AY, Kim E, Lim E, Habelhah H, Hahn WC. IkappaB kinase epsilon phosphorylates TRAF2 to promote mammary epithelial cell transformation. Mol Cell Biol. 2012;32(23):4756-68. doi: 10.1128/MCB.00468-12. PMID:23007157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, et al.. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature. 2010;465(7301):1084-8. doi: 10.1038/nature09128. PMID:20577214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7(8):758-65. doi: 10.1038/ncb0805-758. PMID:16056267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wertz IE, O'Rourke KM, Zhou H, Eby M, Aravind L, Seshagiri S, Wu P, Wiesmann C, Baker R, Boone DL, et al.. De-ubiquitination and ubiquitin ligase domains of A20 downregulate NF-kappaB signalling. Nature. 2004;430(7000):694-9. doi: 10.1038/nature02794. PMID:15258597 [DOI] [PubMed] [Google Scholar]
- 55.Tavares RM, Turer EE, Liu CL, Advincula R, Scapini P, Rhee L, Barrera J, Lowell CA, Utz PJ, Malynn BA, et al.. The ubiquitin modifying enzyme A20 restricts B cell survival and prevents autoimmunity. Immunity. 2010;33(2):181-91. doi: 10.1016/j.immuni.2010.07.017. PMID:20705491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kupka S, De Miguel D, Draber P, Martino L, Surinova S, Rittinger K, Walczak H. SPATA2-mediated binding of CYLD to HOIP enables CYLD recruitment to signaling complexes. Cell Rep. 2016;16(9):2271-80. doi: 10.1016/j.celrep.2016.07.086. PMID:27545878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen ZJ. Ubiquitination in signaling to and activation of IKK. Immunol Rev. 2012;246(1):95-106. doi: 10.1111/j.1600-065X.2012.01108.x. PMID:22435549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lu TT, Onizawa M, Hammer GE, Turer EE, Yin Q, Damko E, Agelidis A, Shifrin N, Advincula R, Barrera J, et al.. Dimerization and ubiquitin mediated recruitment of A20, a complex deubiquitinating enzyme. Immunity. 2013;38(5):896-905. doi: 10.1016/j.immuni.2013.03.008. PMID:23602765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De A, Dainichi T, Rathinam CV, Ghosh S. The deubiquitinase activity of A20 is dispensable for NF-kappaB signaling. EMBO Rep. 2014;15(7):775-83. doi: 10.15252/embr.201338305. PMID:24878851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wertz IE, Newton K, Seshasayee D, Kusam S, Lam C, Zhang J, Popovych N, Helgason E, Schoeffler A, Jeet S, et al.. Phosphorylation and linear ubiquitin direct A20 inhibition of inflammation. Nature. 2015;528(7582):370-5. doi: 10.1038/nature16165. PMID:26649818 [DOI] [PubMed] [Google Scholar]
- 61.Kulathu Y, Garcia FJ, Mevissen TE, Busch M, Arnaudo N, Carroll KS, Barford D, Komander D. Regulation of A20 and other OTU deubiquitinases by reversible oxidation. Nat Commun. 2013;4:1569. doi: 10.1038/ncomms2567. PMID:23463012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chu Y, Soberon V, Glockner L, Beyaert R, Massoumi R, van Loo G, Krappmann D, Schmidt-Supprian M. A20 and CYLD do not share significant overlapping functions during B cell development and activation. J Immunol. 2012;189(9):4437-43. doi: 10.4049/jimmunol.1200396. PMID:23002441 [DOI] [PubMed] [Google Scholar]
- 63.Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424(6950):793-6. doi: 10.1038/nature01803. PMID:12917689 [DOI] [PubMed] [Google Scholar]
- 64.Lin FT, Lin VY, Lin VT, Lin WC. TRIP6 antagonizes the recruitment of A20 and CYLD to TRAF6 to promote the LPA2 receptor-mediated TRAF6 activation. Cell Discov. 2016;2:15048. doi: 10.1038/celldisc.2015.48. PMID:27134758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shi CS, Kehrl JH. TRAF6 and A20 regulate lysine 63-linked ubiquitination of Beclin-1 to control TLR4-induced autophagy. Sci Signal. 2010;3(123):ra42. doi: 10.1126/scisignal.2000751. PMID:20501938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jung SM, Lee JH, Park J, Oh YS, Lee SK, Park JS, Lee YS, Kim JH, Lee JY, Bae YS, et al.. Smad6 inhibits non-canonical TGF-beta1 signalling by recruiting the deubiquitinase A20 to TRAF6. Nat Commun. 2013;4:2562. doi: 10.1038/ncomms3562. PMID:24096742 [DOI] [PubMed] [Google Scholar]
- 67.Draber P, Kupka S, Reichert M, Draberova H, Lafont E, de Miguel D, Spilgies L, Surinova S, Taraborrelli L, Hartwig T, et al.. LUBAC-recruited CYLD and A20 regulate gene activation and cell death by exerting opposing effects on linear ubiquitin in signaling complexes. Cell Rep. 2015;13(10):2258-72. doi: 10.1016/j.celrep.2015.11.009. PMID:26670046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tokunaga F, Nishimasu H, Ishitani R, Goto E, Noguchi T, Mio K, Kamei K, Ma A, Iwai K, Nureki O. Specific recognition of linear polyubiquitin by A20 zinc finger 7 is involved in NF-kappaB regulation. Embo J. 2012;31(19):3856-70. doi: 10.1038/emboj.2012.241. PMID:23032187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sanmamed MF, Rodriguez I, Schalper KA, Onate C, Azpilikueta A, Rodriguez-Ruiz ME, Morales-Kastresana A, Labiano S, Perez-Gracia JL, Martin-Algarra S, et al.. Nivolumab and Urelumab enhance antitumor activity of human T lymphocytes engrafted in Rag2-/-IL2Rgammanull immunodeficient mice. Cancer Res. 2015;75(17):3466-78. doi: 10.1158/0008-5472.CAN-14-3510. PMID:26113085 [DOI] [PubMed] [Google Scholar]
- 70.Segal NH, Logan TF, Hodi FS, McDermott D, Melero I, Hamid O, et al.. Results from an integrated safety analysis of Urelumab, an Agonist Anti-CD137 monoclonal antibody. Clinical Cancer Research: An official journal of the American Association for Cancer Research 2017;28(8):1929-36. [DOI] [PubMed] [Google Scholar]
- 71.Wang X, Li M, Zheng H, Muster T, Palese P, Beg AA, Garcia-Sastre A. Influenza A virus NS1 protein prevents activation of NF-kappaB and induction of alpha/beta interferon. J Virol. 2000;74(24):11566-73. PMID:11090154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Carcamo-Orive I, Gaztelumendi A, Delgado J, Tejados N, Dorronsoro A, Fernandez-Rueda J, Pennington DJ, Trigueros C. Regulation of human bone marrow stromal cell proliferation and differentiation capacity by glucocorticoid receptor and AP-1 crosstalk. J Bone Miner Res. 2010;25(10):2115-25. doi: 10.1002/jbmr.120. PMID:20499359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dorronsoro A, Lang V, Jakobsson E, Ferrin I, Salcedo JM, Fernandez-Rueda J, Fechter K, Rodriguez MS, Trigueros C. Identification of the NF-kappaB inhibitor A20 as a key regulator for human adipogenesis. Cell Death Dis. 2013;4:e972. doi: 10.1038/cddis.2013.494. PMID:24357803 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


