Abstract
Objective
Sex differences in risk factors of aortic valve calcification (AVC) by echocardiography have not been reported from a large prospective study in aortic stenosis (AS).
Methods
AVC was assessed using a prognostically validated visual score and grouped into none/mild or moderate/severe AVC in 1725 men and women with asymptomatic AS in the Simvastatin Ezetimibe in Aortic Stenosis study. The severity of AS was assessed by the energy loss index (ELI) taking pressure recovery in the aortic root into account.
Results
More men than women had moderate/severe AVC at baseline despite less severe AS by ELI (p<0.01). Moderate/severe AVC at baseline was independently associated with lower aortic compliance and more severe AS in both sexes, and with increased high-sensitive C reactive protein (hs-CRP) only in men (all p<0.01). In Cox regression analyses, moderate/severe AVC at baseline was associated with a 2.5-fold (95% CI 1.64 to 3.80) higher hazard rate of major cardiovascular events in women, and a 2.2-fold higher hazard rate in men (95% CI 1.54 to 3.17) (both p<0.001), after adjustment for age, hypertension, study treatment, aortic compliance, left ventricular (LV) mass and systolic function, AS severity and hs-CRP. Moderate/severe AVC at baseline also predicted a 1.8-fold higher hazard rate of all-cause mortality in men (95% CI 1.04 to 3.06, p<0.05) independent of age, AS severity, LV mass and aortic compliance, but not in women.
Conclusion
In conclusion, AVC scored by echocardiography has sex-specific characteristics in AS. Moderate/severe AVC is associated with higher cardiovascular morbidity in both sexes, and with higher all-cause mortality in men.
Trial registration number
ClinicalTrials.gov identifier: NCT00092677
Keywords: aortic valve calcification, aortic valve stenosis, sex, prognosis, echocardiography
Introduction
Sex differences in aortic stenosis (AS) have been pointed out both at the valvular and left ventricular (LV) level.1 From the large Simvastatin Ezetimibe in Aortic Stenosis (SEAS) study, sex differences in LV adaptation during progression of AS were recently reported, despite similar rate of AS progression in both sexes.2 By multidetector CT, it was demonstrated by Aggarwal et al that women have significantly lower aortic valve calcification (AVC) load than men independent of the severity of AS.3 From this, sex-specific cut-off values for Agatston score indicating severe AS were developed and validated.3 4 Although cardiac CT more accurately measures AVC in AS and may help identify severe AS in asymptomatic patients with discordantly graded AS by conventional echocardiographic measures, it is not recommended by current guidelines as a routine test in patients with AS.5 While CT primarily quantifies areas of valvular macrocalcification, recent studies by positron emission tomography have revealed that AVC also includes inflammation and microcalcification.6 7 Since different processes involved in AVC are reflected by echocardiography and CT, AVC by echocardiography is not synonymous with AVC assessed by CT.8 The association of AVC scored by echocardiography with higher rates of combined aortic valve replacement and death has previously been documented in two studies by Rosenhek et al. 9 10 However, sex-specific risk factors and prognostic implications of AVC scored by echocardiography have not been published from a large, prospective study. This was the aim of the present study.
Methods
Study population
The present analysis of the SEAS study included the 1725 men and women (92% of the total study population) that had images available for AVC scoring on the baseline echocardiogram. Compared with ineligible patients, the patients selected for the present analysis did not differ in age, sex, prevalence of hypertension or severity of AS (all p<0.05). The SEAS study protocol, baseline characteristics and outcome have been previously published.11 12 In short, 1873 asymptomatic patients with mostly moderate AS and without known diabetes, cardiovascular or renal disease were randomised to double-blind, placebo-controlled treatment with combined ezetimibe 10 mg and simvastatin 40 mg daily for ≥4 years.12 Hypertension was defined as history of hypertension, use of antihypertensive drug treatment or blood pressure ≥140/90 mm Hg at the clinic baseline visit.
Echocardiographic measurements
Echocardiography was performed using a standardised protocol in 173 study centres in seven European countries.13 14 All echocardiograms were analysed at the echocardiographic core laboratory at Haukeland University Hospital, Bergen, Norway, and 94% were proofread by the same experienced reader. Quantitative echocardiography for assessment of AS and LV structure and function was performed following current guidelines.5 15 16 Previous analyses from the SEAS trial have shown excellent reproducibility for measurements of LV dimensions.17 Aortic valve area adjusted for pressure recovery in the aortic root (energy loss index (ELI)) was used as the primary measure of AS severity, given the superior prognostic value previously demonstrated.18 Aortic and mitral regurgitation were graded by colour Doppler. AVC was graded as none (no calcification), mild (isolated small spots), moderate (multiple bigger spots) and severe (extensive calcification of all cusps).9 LV mass was calculated using an autopsy validated formula.19 LV hypertrophy was considered present if LV mass/height2.7 was ≥49.2 g/m2.7 in men and 46.7 g/m2.7 in women.20 LV systolic function was assessed by biplane Simpson’s ejection fraction and by midwall shortening adjusted for circumferential end-systolic stress taking the mean transaortic valve gradient into account (stress-corrected midwall shortening (scMWS)).21 22 Aortic compliance was assessed from LV stroke volume/pulse pressure ratio.23
Study outcomes
The primary outcome of the SEAS study was major cardiovascular events, a composite endpoint consisting of aortic valve-related events (combined aortic valve replacement, congestive heart failure due to AS and cardiovascular death) and ischaemic cardiovascular events (combined non-fatal myocardial infarction, non-haemorrhagic stroke, coronary revascularisation, hospitalisation for unstable angina pectoris and cardiovascular death).12 Secondary outcomes included aortic valve events and ischaemic cardiovascular events analysed separately. All-cause mortality was a tertiary endpoint. All outcomes were classified by an independent endpoint classification committee blinded to study-group assignment.11
Ethics approval
The SEAS study was approved by ethics committees in all participating study centres, and all patients provided written informed consent.
Statistical analyses
Statistical analyses were performed using IBM SPSS 23.0. Patients were grouped according to sex and the baseline severity of AVC (none/mild vs moderate/severe AVC). Continuous variables are given as mean±SD and categorical variables as percentages. Differences between groups were tested by analysis of variance with Scheffe’s post hoc test and Bonferroni adjustment.
The intraclass correlation coefficient was used to assess both intraobserver and interobserver variability for the AVC score. Intraobserver variability was assessed in 500 randomly selected patients scored twice by one experienced sonographer (GC), several weeks apart. Interobserver variability was assessed in 150 randomly selected patients analysed twice by an experienced and less experienced reader (DC), respectively. Agreement between AVC scores was assessed by the Bland-Altman method using the 95% limits of agreement and the variation coefficient of the mean difference.
Binary logistic regression analyses reported as OR and 95% CIs were used to identify risk factors of AVC in women and men. Based on the logistic regression analyses, a propensity score for AVC was derived both in women and in men using the AVC score as dependent variable and age, aortic compliance, ELI, high-sensitive C reactive protein (hs-CRP) and scMWS as independent variables. The propensity scores reflect the likelihood that an individual patient would develop moderate/severe AVC versus none/mild AVC given all the other known variables other than the outcome variable. The association of AVC severity with study outcomes was tested in Kaplan-Meier plots with log-rank test and in multivariable Cox regression analyses with results reported as HR and 95% CI. We determined the interaction between gender and AVC by introducing the cross product of the two variables in the Cox regression analysis for major cardiovascular events. Further on, we run the multivariate Cox model separately in women and men and with adjustment for age, hypertension, LV mass index, ELI, aortic compliance, hs-CRP, scMWS and study treatment allocation. The respective covariates were selected based on a stepwise backward procedure. Additionally, the propensity scores for AVC were forced into the Cox models to account for gender differences in the distribution of the respective covariates. We examined the validity of the proportionality assumption by testing the significance of the covariate–time interaction terms. The variables included in the Cox analyses did not violate the proportionality assumption.
A p value <0.05 was considered statistical significant in all analyses.
Results
Patient demographics
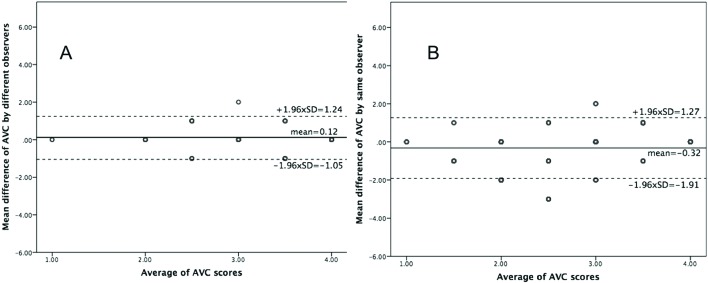
Reproducibility of the AVC visual scoring was excellent with an intraobserver agreement of 0.95 and interobserver agreement of 0.92. There was good interobserver (variation coefficient 4.7%, 95% limits of agreement −1.05 to 1.24) and intraobserver (variation coefficient 2.5%, 95% limits of agreement −1.91 to 1.27) agreement for scoring of AVC (figure 1). At baseline, moderate/severe AVC was found in 63% of women and 70% of men (p<0.01) (table 1). Irrespective of AVC severity, women were older, had higher scMWS and lower LV mass index, aortic valve area index and aortic compliance, and included more subjects with hypertension compared with men (all p<0.05) (table 1). Antihypertensive treatment did not differ between sexes except for higher use of diuretics among women in patients with none/mild AVC (p<0.05). Both men and women with moderate/severe AVC at baseline had higher LV mass index and lower scMWS compared with their none/mild AVC counterparts (all p<0.05). In the same AVC category, AS assessed by ELI was consistently more severe in women than in men (figure 2). Among men, moderate/severe AVC had higher hs-CRP compared with the none/mild AVC group (p<0.05) (table 1).
Figure 1.
Bland-Altman analyses of the aortic valve calcification (AVC) visual score measured by two different readers (A) and twice by the same reader (B) with the mean value of the difference between measurements (the solid reference line in each panel) and 95% limits of agreement (the dotted lines in each panel).
Table 1.
Clinical, biochemical and echocardiographical characteristics of women and men grouped according to aortic valve calcification (AVC) severity at baseline
| Women (N=664) | Men (N=1061) | |||
| None/mild AVC(n=247) | Moderate/severe AVC(n=417) | None/mild AVC(n=322) | Moderate/severe AVC(n=739) | |
|---|---|---|---|---|
| Age (years) | 68.2±9.4* | 69.9±9.3*† | 65.3±9.8 | 66.6±9.5† |
| Body mass index (kg/m²) | 27.5±5.4 | 26.5±5.1† | 27.1±3.6 | 26.8±3.9 |
| Hypertension | 89%‡ | 88%‡ | 79% | 81% |
| Antihypertensive treatment (%) | ||||
| Calcium antagonists | 36 | 31 | 36 | 32 |
| ACE inhibitors | 32 | 27 | 35 | 32 |
| Angiotensin receptor blockers | 21 | 21 | 19 | 18 |
| Diuretics | 52§ | 52 | 35 | 46 |
| Beta-blockers | 43 | 42 | 37 | 39 |
| No of antihypertensive agents | 1.4§ | 1.3§ | 1.1 | 1.2 |
| Systolic blood pressure (mm Hg) | 150±20‡ | 151±21* | 145±19 | 143±19 |
| Diastolic blood pressure (mm Hg) | 81±10 | 82±10 | 82±10 | 82±10 |
| Heart rate (beats/min) | 69±11* | 68±11* | 65±12 | 64±11 |
| Total cholesterol (mmol/L) | 6.0±1.0* | 6.0±1.1* | 5.6±0.9 | 5.5±1.0 |
| LDL cholesterol (mmol/L) | 3.7±0.9 | 3.7±1.0* | 3.6±0.8 | 3.5±0.9 |
| Creatine (µmol/L) | 84±13* | 85±14* | 98±14 | 99±15 |
| hs-CRP (mg/dL) | 0.26 (0.10–0.49) | 0.24 (0.10–0.48) | 0.18 (0.08–0.31) | 0.20 (0.08–0.44)§ |
| Peak aortic jet velocity (m/s) | 2.64±0.34 | 3.33±0.46† | 2.62±0.36 | 3.32±0.49† |
| Mean transvalvular gradient (mm Hg) | 16±4 | 27±8† | 16±5 | 26±9† |
| Aortic valve area (cm²) | 1.28±0.38* | 1.02±0.32*¶ | 1.64±0.55 | 1.27±0.43† |
| Aortic valve area index (cm²/m²) | 0.72±0.21* | 0.59±0.19** | 0.82±0.28 | 0.64±0.21† |
| Energy loss index (cm²/m²) | 1.06±0.54 | 0.77±0.33‡¶ | 1.13±0.57 | 0.83±0.42† |
| Ejection fraction (%) | 67±6 | 66±7 | 67±7 | 66±7† |
| scMWS (%) | 102±19§ | 99±20†‡ | 98±19 | 94±19** |
| LV mass index (g/m2.7) | 41.9±12.4‡ | 44.2±14.0§* | 45.3±15.7 | 47.9±14.7† |
| Stroke volume/pulse pressure (mL/mm Hg) | 3.25±1.51* | 3.07±1.34* | 4.54±2.17 | 4.21±2.00† |
| Mitral regurgitation | 64% | 70%* | 55% | 54% |
| Aortic regurgitation | 65%‡ | 72%‡ | 82% | 84% |
*p<0.001, **p<0.01, †p<0.05 between groups with none/mild versus moderate/severe AVC within same sex, ‡p<0.01, §p<0.05 between sexes, ¶p<0.001.
hs-CRP, high-sensitive C reactive protein; LDL, low-density lipid; LV, left ventricular; scMWS, stress-corrected midwall shortening.
Figure 2.
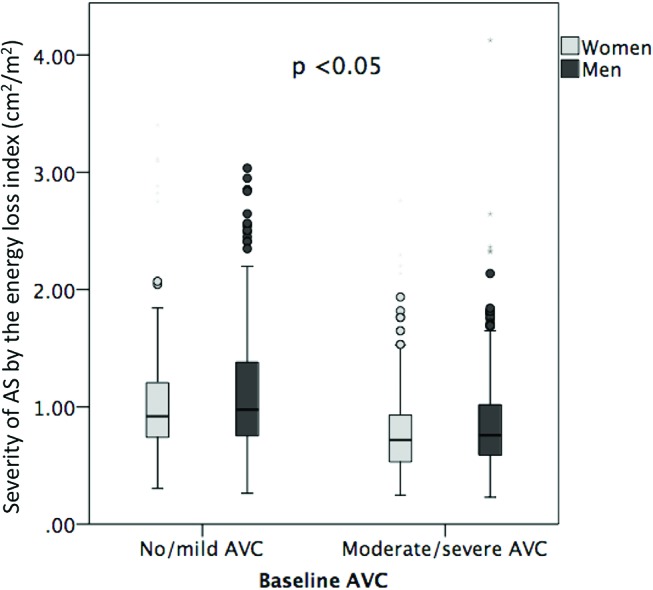
Severity of aortic stenosis by the energy loss index assessed in women and men and in the two aortic valve calcification (AVC) subgroups: no/mild AVC and moderate/severe AVC. p value <0.05 between women and men in each AVC category.
In logistic regression analyses, moderate/severe AVC was associated with lower aortic compliance and more severe AS in both sexes (both p<0.01) and with higher serum hs-CRP in men (p<0.01) (table 2). In subsequent regression analyses, no association between AVC and serum cholesterol or mitral and aortic valve regurgitation were found when they were added to the covariates (all p>0.05).
Table 2.
Independent risk factors of moderate/severe AVC in women and men
| Women | Men | |||
| Covariate | OR (95% CI) | p Value | OR (95% CI) | p Value |
|---|---|---|---|---|
| Age (years) | 1.02 (1.00 to 1.04) | 0.310 | 1.01 (0.99 to 1.03) | 0.176 |
| Aortic compliance (mL/mm Hg) | 0.77 (0.66 to 0.91) | 0.002 | 0.85 (0.77 to 0.93) | <0.001 |
| ELI (cm²/m²) | 0.11 (0.06 to 0.20) | <0.001 | 0.23 (0.15 to 0.34) | <0.001 |
| Hs-CRP (mg/dL) | 1.26 (0.92 to 1.72) | 0.146 | 1.84 (1.23 to 2.77) | 0.003 |
| scMWS (%) | 0.99 (0.98 to 1.00) | 0.234 | 0.99 (0.99 to 1.00) | 0.116 |
ELI, energy loss index; hs-CRP, high-sensitive C reactive protein; scMWS, stress-corrected midwall shortening.
AVC and outcome
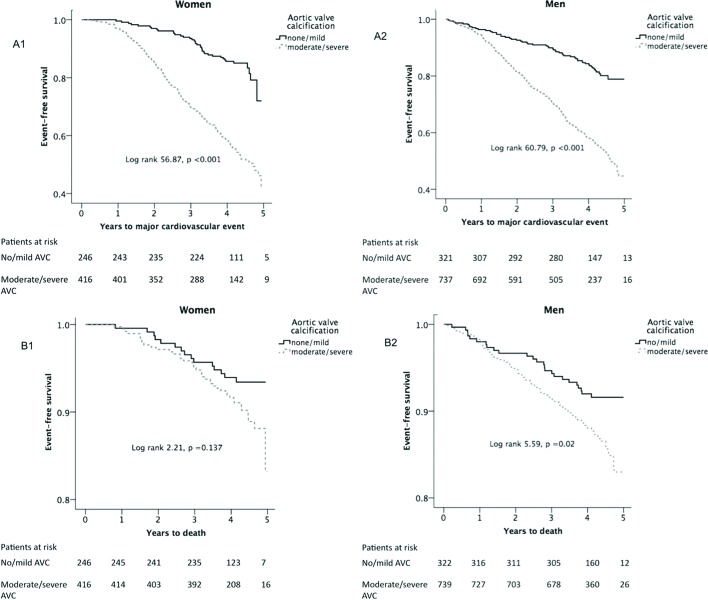
In Kaplan-Meier tests, moderate/severe AVC at baseline predicted higher rates of major cardiovascular events during a median of 4.3 years follow-up in both sexes and higher all-cause mortality in men (all p<0.05) (figure 3).
Figure 3.
Kaplan-Meier curves reporting survival free of major cardiovascular events (A), and survival (B) in women (left panels) and men (right panels). AVC, aortic valve calcification.
In multivariate Cox analyses, adjusting for differences in baseline characteristics between groups and known prognosticators in AS including age, hypertension, scMWS, LV mass index, aortic compliance, ELI, hs-CRP, randomised study treatment allocation, as well as the propensity scores for AVC, presence of baseline moderate/severe AVC was associated with higher HR of combined major cardiovascular events and aortic valve events in both sexes (table 3). In further Cox analyses, the results remained unchanged when total cholesterol, mitral or aortic regurgitation and type of antihypertensive medication were added to the covariates. In subsequent Cox analyses, presence of moderate/severe AVC at baseline was associated with increased all-cause mortality only in men and increased hazard of ischaemic cardiovascular events only in women (table 3).
Table 3.
The association of baseline moderate/severe aortic valve calcification with study outcomes in women and men
| Women | Men | |||||
| n | HR (95% CI) | p Value | n | HR (95% CI) | p Value | |
| Major cardiovascular events | 172 | 2.50 (1.64 to 3.80) | <0.001 | 270 | 2.21 (1.54 to 3.17) | <0.001 |
| Aortic valve events | 162 | 2.44 (1.58 to 3.76) | <0.001 | 246 | 2.96 (1.94 to 4.49) | <0.001 |
| Ischaemic cardiovascular events | 70 | 1.86 (1.01 to 3.44) | 0.047 | 141 | 1.51 (0.98 to 2.35) | 0.065 |
| All-cause mortality* | 47 | 1.40 (0.70 to 2.79) | 0.338 | 96 | 1.78 (1.04 to 3.06) | 0.036 |
| Aortic valve replacement or death | 170 | 2.19 (1.46 to 3.30) | <0.001 | 279 | 2.77 (1.90 to 4.05) | <0.001 |
*Model adjusted for age, energy loss index, aortic compliance and left ventricular mass index.
n, number of events.
Discussion
This post hoc analysis within the large SEAS study is the first to demonstrate that AVC in AS scored by echocardiography has sex-specific risk factors and that presence of moderate/severe AVC at baseline predicts higher rates of major cardiovascular events and combined aortic valve replacement and death both in women and men. Our sex-specific findings add to previous reports by Rosenhek et al. 9 10 Furthermore, in this study, an independent association with higher all-cause mortality was found only in men and with higher risk of ischaemic cardiovascular events only in women. Of note these associations persisted after adjustment in multivariate models for important confounders including age,9 hypertension,24 AS severity,9 10 aortic compliance, LV structure and systolic function,20 hs-CRP and randomised study treatment.
The value of AVC assessment by echocardiography has been debated after development of a quantitative method to assess valvular macrocalcification by CT.8 The main question about the usefulness of echocardiography in assessment of AVC has been related to reproducibility. As demonstrated by this study, both intraobserver and interobserver reproducibility was excellent. It has been demonstrated that AVC by echocardiography, despite grossly correlated with CT findings, reveals a higher calcification degree than computer tomography, and that the 4-point classification system classifies patients with AS in four classes that partly overlap in AVC score by CT.8 These differences are, in our view, inherent to the physics of ultrasound and X-ray that by their nature reveal partially different types of tissue lesion. While CT allows a quantitative measurement of macrocalcifications, the echocardiographical AVC scoring assesses semiquantitatively the presence of hyperechoic zones that probably also include areas of microcalcification and inflammation.6 7 While we acknowledge the diagnostic value CT can have in certain groups of patients with AS, as those with low-flow, low-grade AS with normal ejection fraction,25 we believe echocardiography to be the only feasible method to be applied in regular follow-up of the majority of patients with AS outside specialised heart valve centres. As demonstrated in the present analysis, assessment of AVC by echocardiography is reliable and of prognostic value both in women and men, independent of traditional prognostic markers in AS. Our findings add to previous documentation from an international registry showing that AVC by CT was associated with increased mortality in both sexes.4
Our finding that moderate/severe AVC was less prevalent in women than men despite comparable AS severity adds to previous studies on AVC by echocardiography9 10 and confirms previous observations by CT using the Agatston score.3 Differences in body surface area, aortic valve weight and aortic annulus size documented by previous studies may contribute to explain these sex differences.26 The association of AVC with lower aortic compliance underlines the systemic arterial involvement in degenerative AS.27
Hs-CRP and elevated serum creatine are well-known factors associated with atherosclerosis. This study found that higher AVC was associated with more severe AS in both sexes, and with serum hs-CRP particularly in men. In contrast, the Olmsted County study did not find any relation between hs-CRP levels and AS severity or the degree of valve calcification.28 The Chronic Renal Insufficiency Cohort study reported an inversed relation between AVC and estimated glomerular filtration rate in patients with non-end-stage chronic kidney disease.29 However, when adjusted for cardiovascular risk factors such as CRP and homocysteine, the association became non-significant. Recently published research demonstrates proinflammatory and procoagulation changes in the plasma of patients with AS.30 Our study additionally reveals an independent association between the systemic inflammatory marker hs-CRP and AVC only in men, while no association between AVC and serum creatine or estimated glomerular filtration rate was found.
Study limitations
The SEAS study excluded patients with known coronary heart disease, heart failure, diabetes mellitus, renal failure, history of stroke, peripheral vascular disease and patients with indication for lipid-lowering therapy. Thus, projection of results to less selective AS patients should be done with caution. AVC is assessed semiquantitatively by echocardiography. We acknowledge the value of CT in quantitative assessment of valvular macrocalcifications. However, as demonstrated, echocardiographic assessment of AVC was highly reproducible both for experienced and less experienced sonographers and may be more available and feasible in clinical cardiology practice. However, further studies are needed to directly compare performance and prognostic value of AVC scored by echocardiography and CT.
Conclusion
In conclusion, in patients with AS and without diabetes mellitus or known renal or cardiovascular disease participating in the SEAS study, presence of moderate/severe AVC by echocardiography at baseline was associated with higher hazard rates of major cardiovascular events and combined aortic valve replacement and death in both sexes, with higher hazard rates of ischaemic cardiovascular events in women and with higher all-cause mortality in men. These associations were independent of important confounders including older age, hypertension, aortic compliance, AS severity and LV structure and function.
Key messages.
What is already known on this subject?
Sex-specific risk factors of aortic valve calcification (AVC) scored by echocardiography and its prognostic relevance in women and men with aortic stenosis (AS) have not been previously reported from a large, prospective study.
What might this study add?
This post hoc analysis within the Simvastatin Ezetimibe in Aortic Stenosis study is the first to demonstrate sex-specific risk factors of AVC scored by echocardiography. Moderate/severe AVC at study baseline independently predicted higher rates of major cardiovascular events and combined death and aortic valve replacement in both sexes, higher hazard rate of ischaemic cardiovascular events only in women, and higher all-cause mortality only in men.
How might this impact on clinical practice?
Assessment of AVC by echocardiography is highly reproducible and of prognostic value in the follow-up of women and men with AS, independent of traditional prognostic markers.
Footnotes
Contributors: HKT, GC, EG and DC were involved in the conception and design, as well as in the analysis and interpretation of data. All coauthors have revised the manuscript critically and approved it for submission.
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The SEAS study was approved by ethics committees in all participating study centers, and all patients provided written informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Correction notice: Since this paper was first published online the author name Constantino Mancusi has been updated to Costantino Mancusi.
References
- 1. Shames S, Gillam LD. Sex differences in aortic valve calcification. Circ Cardiovasc Imaging 2013;6:8–10. 10.1161/CIRCIMAGING.112.983288 [DOI] [PubMed] [Google Scholar]
- 2. Cramariuc D, Rogge BP, Lønnebakken MT, et al. . Sex differences in cardiovascular outcome during progression of aortic valve stenosis. Heart 2015;101:209–14. 10.1136/heartjnl-2014-306078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Aggarwal SR, Clavel MA, Messika-Zeitoun D, et al. . Sex differences in aortic valve calcification measured by multidetector computed tomography in aortic stenosis. Circ Cardiovasc Imaging 2013;6:40–7. 10.1161/CIRCIMAGING.112.980052 [DOI] [PubMed] [Google Scholar]
- 4. Clavel MA, Pibarot P, Messika-Zeitoun D, et al. . Impact of aortic valve calcification, as measured by MDCT, on survival in patients with aortic stenosis: results of an international registry study. J Am Coll Cardiol 2014;64:1202–13. 10.1016/j.jacc.2014.05.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vahanian A, Alfieri O, Andreotti F, et al. . Joint Task Force on the management of Valvular Heart Disease of the European Society of Cardiology (ESC) European Association for Cardio-Thoracic Surgery (EACTS). Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451–96. 10.1093/eurheartj/ehs109 [DOI] [PubMed] [Google Scholar]
- 6. Pawade TA, Cartlidge TR, Jenkins WS, et al. . Optimization and reproducibility of aortic valve 18F-Fluoride positron emission tomography in patients with aortic Stenosis. Circ Cardiovasc Imaging 2016;9(10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dweck MR, Jones C, Joshi NV, et al. . Assessment of valvular calcification and inflammation by positron emission tomography in patients with aortic stenosis. Circulation 2012;125:76–86. 10.1161/CIRCULATIONAHA.111.051052 [DOI] [PubMed] [Google Scholar]
- 8. Messika-Zeitoun D, Aubry MC, Detaint D, et al. . Evaluation and clinical implications of aortic valve calcification measured by electron-beam computed tomography. Circulation 2004;110:356–62. 10.1161/01.CIR.0000135469.82545.D0 [DOI] [PubMed] [Google Scholar]
- 9. Rosenhek R, Klaar U, Schemper M, et al. . Mild and moderate aortic stenosis. natural history and risk stratification by echocardiography. Eur Heart J 2004;25:199–205. 10.1016/j.ehj.2003.12.002 [DOI] [PubMed] [Google Scholar]
- 10. Rosenhek R, Binder T, Porenta G, et al. . Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000;343:611–7. 10.1056/NEJM200008313430903 [DOI] [PubMed] [Google Scholar]
- 11. Rossebø AB, Pedersen TR, Boman K, et al. . Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med 2008;359:1343–56. 10.1056/NEJMoa0804602 [DOI] [PubMed] [Google Scholar]
- 12. Rossebø AB, Pedersen TR, Allen C, et al. . Design and baseline characteristics of the simvastatin and ezetimibe in aortic stenosis (SEAS) study. Am J Cardiol 2007;99:970–3. 10.1016/j.amjcard.2006.10.064 [DOI] [PubMed] [Google Scholar]
- 13. Cramariuc D, Rieck AE, Staal EM, et al. . Factors influencing left ventricular structure and stress-corrected systolic function in men and women with asymptomatic aortic valve stenosis (a SEAS Substudy). Am J Cardiol 2008;101:510–5. 10.1016/j.amjcard.2007.09.100 [DOI] [PubMed] [Google Scholar]
- 14. Rieck AE, Cramariuc D, Staal EM, et al. . Impact of hypertension on left ventricular structure in patients with asymptomatic aortic valve stenosis (a SEAS substudy). J Hypertens 2010;28:377–83. 10.1097/HJH.0b013e328332fa44 [DOI] [PubMed] [Google Scholar]
- 15. Nishimura RA, Otto CM, Bonow RO, et al. . 2014 AHA/ACC Guideline for the Management of Patients With Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129:e521–e643. 10.1161/CIR.0000000000000031 [DOI] [PubMed] [Google Scholar]
- 16. Lang RM, Badano LP, Mor-Avi V, et al. . Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Ame Soc Echocard 2015;2839:1e14–39. [DOI] [PubMed] [Google Scholar]
- 17. Cramariuc D, Cioffi G, Rieck AE, et al. . Low-flow aortic stenosis in asymptomatic patients: valvular-arterial impedance and systolic function from the SEAS Substudy. JACC Cardiovasc Imaging 2009;2:390–9. 10.1016/j.jcmg.2008.12.021 [DOI] [PubMed] [Google Scholar]
- 18. Bahlmann E, Gerdts E, Cramariuc D, et al. . Prognostic value of energy loss index in asymptomatic aortic stenosis. Circulation 2013;127:1149–56. 10.1161/CIRCULATIONAHA.112.078857 [DOI] [PubMed] [Google Scholar]
- 19. Devereux RB, Alonso DR, Lutas EM, et al. . Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57:450–8. 10.1016/0002-9149(86)90771-X [DOI] [PubMed] [Google Scholar]
- 20. Gerdts E, Rossebø AB, Pedersen TR, et al. . Relation of left ventricular mass to prognosis in initially asymptomatic mild to moderate aortic valve stenosis. Circ Cardiovasc Imaging 2015;8:e003644 10.1161/CIRCIMAGING.115.003644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaasch WH, Zile MR, Hoshino PK, et al. . Stress-shortening relations and myocardial blood flow in compensated and failing canine hearts with pressure-overload hypertrophy. Circulation 1989;79:872–83. 10.1161/01.CIR.79.4.872 [DOI] [PubMed] [Google Scholar]
- 22. de Simone G, Devereux RB, Roman MJ, et al. . Assessment of left ventricular function by the midwall fractional shortening/end-systolic stress relation in human hypertension. J Am Coll Cardiol 1994;23:1444–51. 10.1016/0735-1097(94)90390-5 [DOI] [PubMed] [Google Scholar]
- 23. de Simone G, Roman MJ, Koren MJ, et al. . Stroke volume/pulse pressure ratio and cardiovascular risk in arterial hypertension. Hypertension 1999;33:800–5. 10.1161/01.HYP.33.3.800 [DOI] [PubMed] [Google Scholar]
- 24. Rieck ÅE, Cramariuc D, Boman K, et al. . Hypertension in aortic stenosis: implications for left ventricular structure and cardiovascular events. Hypertension 2012;60:90–7. 10.1161/HYPERTENSIONAHA.112.194878 [DOI] [PubMed] [Google Scholar]
- 25. Pibarot P, Clavel MA. Management of paradoxical low-flow, low-gradient aortic stenosis: need for an integrated approach, including assessment of symptoms, hypertension, and stenosis severity. J Am Coll Cardiol 2015;65:67–71. 10.1016/j.jacc.2014.10.030 [DOI] [PubMed] [Google Scholar]
- 26. Roberts WC, Ko JM. Weights of operatively-excised stenotic unicuspid, bicuspid, and tricuspid aortic valves and their relation to age, sex, body mass index, and presence or absence of concomitant coronary artery bypass grafting. Am J Cardiol 2003;92:1057–65. 10.1016/j.amjcard.2003.07.018 [DOI] [PubMed] [Google Scholar]
- 27. Rieck AE, Gerdts E, Lønnebakken MT, et al. . Global left ventricular load in asymptomatic aortic stenosis: covariates and prognostic implication (the SEAS trial). Cardiovasc Ultrasound 2012;10:43 10.1186/1476-7120-10-43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agmon Y, Khandheria BK, Jamil Tajik A, et al. . Inflammation, infection, and aortic valve sclerosis; Insights from the Olmsted County (Minnesota) population. Atherosclerosis 2004;174:337–42. 10.1016/j.atherosclerosis.2004.01.028 [DOI] [PubMed] [Google Scholar]
- 29. Guerraty MA, Chai B, Hsu JY, et al. . Relation of aortic valve calcium to chronic kidney disease (from the chronic renal insufficiency cohort study). Am J Cardiol 2015;115:1281–6. 10.1016/j.amjcard.2015.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mourino-Alvarez L, Baldan-Martin M, Gonzalez-Calero L, et al. . Patients with calcific aortic stenosis exhibit systemic molecular evidence of ischemia, enhanced coagulation, oxidative stress and impaired cholesterol transport. Int J Cardiol 2016;225:99–106. 10.1016/j.ijcard.2016.09.089 [DOI] [PubMed] [Google Scholar]