Abstract
Objectives
This article considers the potential of ‘theories of practice’ for studying and understanding varied (dis)engagement with HIV care and treatment services and begins to unpack the assemblage of elements and practices that shape the nature and duration of individuals’ interactions with HIV services.
Methods
We obtained data from a multicountry qualitative study that explores the use of HIV care and treatment services, with a focus on examining the social organisation of engagement with care as a practice and as manifested in the lives of people living with HIV in sub-Saharan Africa. The dataset comprised of 356 interviews with participants from six countries.
Results
We noted fluctuating interactions with HIV services in all countries. In line with theories of practice, we found that such varied engagement can be explained by (1) the availability, absence and connections between requisite ‘materialities’ (eg, health infrastructure, medicines), ‘competencies’ (eg, knowing how to live with HIV) and ‘meanings’ (eg, trust in HIV services, stigma, normalisation of HIV) and (2) a host of other life practices, such as working or parenting. These dynamics either facilitated or inhibited engagement with HIV services and were intrinsically linked to the discursive, cultural, political and economic fabric of the participating countries.
Conclusion
Practice theory provides HIV researchers and practitioners with a useful vocabulary and analytical tools to understand and steer people’s differentiated HIV service (dis)engagement. Our application of practice theory to engagement in HIV care, as experienced by HIV service users and providers in six sub-Saharan African countries, highlights the need for a practice-based approach in the delivery of differentiated and patient-centred HIV services.
Keywords: Social Theory, Patient Engagement, HIV, Health Services Research, Highly Active Antiretroviral Therapy, Africa
Introduction
Although varied engagement with HIV services has always been an issue in the HIV response, it has risen to the top of the list of policy priorities in the era of treatment as prevention. The HIV care continuum (also known as the HIV cascade) was initiated as a guiding model for delivering and measuring HIV services, allowing us to understand better barriers and facilitators in the progression from initial diagnosis of HIV to viral suppression.1 2 The continuum has helped to standardise measures of care and delivery of HIV treatment at an unprecedented scale, prolonging the lives of millions of people.3 4 Yet, a large proportion of unexpected deaths still occur throughout the HIV care continuum,5 6 and studies reveal how people living with HIV (PLHIV) are affected by social structures and step in and out of HIV services in multiple ways, leading to different forms of disruption to progression along the care continuum.7–10 There is a long history of scholars calling for recognition of how contextual factors, beyond biomedical interventions, shape HIV treatment experiences, engagement and outcomes.11–15 However, Blue and colleagues16 argue it can be difficult to pinpoint exactly how contextual factors interact with and affect health and how recognition of such factors can be translated into practical actions. They suggest that by shifting our attention to the organisation of social practices — the elements that shape our perceptions, interpretations and actions in daily life17 — we will be able to know exactly how structure and context intersect with agency. In this article, we consider the potential of ‘theories of practice’ for the study of (dis)engagement with HIV services and begin to unpack the assemblage of elements and life practices that shape HIV care and treatment (dis)engagement in sub-Saharan Africa.
Theories of practice and (dis)engagement with HIV services
There is a no single and unified theory of practice.18 Instead, there is a dynamic and collegial tradition of applying and building on the work of past and contemporary theorists. Common to them all, however, is that they treat practices as the primary units of enquiry and provide conceptual tools to unpack how and why certain practices emerge, persist and disappear. Practice theorists like Nicolini,19 Reckwitz,20 Schatzki21 and Shove18 have all offered their take on the background arrangements, or elements, that condition and shape the dynamic nature of a practice, such as engaging with HIV services. Shove et al 18 and Blue et al 16 have usefully condensed these background arrangements into three elements—namely, materialities, competencies and meanings—and draw our attention to the configurations and connections between these elements and other life practices (figure 1). Here, they argue, is the potential to understand what it takes for people to join, maintain or defect from a practice.
Figure 1.
Summary of theoretical framework.
These elements, their content, configurations and constellations, provide insight to the arrangements that exist in different spatial and temporal localities and may be able to explain differences in practice between people and settings. This encourages us to examine how dynamically integrated elements shape the way HIV service engagements are enacted. It further helps us understand how changes in the way HIV services are delivered, reconfigure and shape engagement with HIV care and treatment services. For instance, reducing or extending the opening hours of a health clinic (materiality, infrastructure) may change the ability of PLHIV to engage with HIV services.
Another important practice dynamic pertains to the connections and interactions between practices. People participate in a countless number of interwoven social practices, many of which coexist in harmony, while others codepend and may compete or collaborate with the practice(s) under investigation.18 22 Schatzki22 and Shove et al 18 speak of bundles of practices to describe such an assemblage of codependent practices and highlight the importance of understanding how a bundle of practices, through their connections, coevolve, share and compete for resources. This encourages us to identify the range of life practices that facilitate or inhibit engagement with HIV services and to use this insight to form or break and strengthen or weaken the links between them, with the aim of supporting HIV service engagement.
Against this background, and in support of a call from Blue et al 16 for more practice-oriented public health policy, we investigate (1) how engagements with HIV services are constituted and enacted by multiple elements and not just people alone and (2) how engagements with HIV services relate to other everyday practices.
Methods
In this study, the data were obtained from a qualitative multicountry study that examines how PLHIV, in the context of their social worlds, interact with HIV services. Ethical approval was granted by the London School of Hygiene and Tropical Medicine and the relevant ethics boards at each of the study settings. Informed and written consent was obtained from all participants on the agreement that confidentiality would be assured. Pseudonyms are therefore used throughout.
Study locations and participants
The study was conducted in Karonga (Malawi), Rakai and Kyamulibwa (Uganda), Kisesa (Tanzania), Kisumu (Kenya), Manicaland (Zimbabwe) and uMkhanyakude (South Africa)—settings that are hard hit by the HIV epidemic. We purposefully recruited a mix of healthcare workers (n=53), PLHIV (n=255) and family members of people who have died from HIV (n=48). Participants were recruited via health and demographic surveillance databases, health clinics or following verbal autopsy23 interviews with family members of recently deceased PLHIV to represent a broad distribution of sex, age and diagnosis and care histories (table 1).
Table 1.
Study participants shown by sampling category and country
| Country | Demographic surveillance site | Healthcare worker | People living with HIV | Family member of a deceased | ||
| Diagnosed, not on ART | On ART* | LTFU† | ||||
| Uganda | Rakai | 6 | 15 | 15 | 6 | 8 |
| Uganda | Kyamulibwa | 5 | 8 | 8 | 4 | 5 |
| Kenya | Kisumu | 8 | 10 | 15 | 6 | 11 |
| Tanzania | Kisesa | 7 | 13 | 20 | 4 | 6 |
| Malawi | Karonga | 5 | 9 | 20 | 4 | 6 |
| Zimbabwe | Manicaland | 4 | 16 | 35 | 8 | 6 |
| South Africa | uMkhanyakude | 18 | 1616 | 17 | 6 | 6 |
| Total | 53 | 87 | 130 | 38 | 48 | |
*ART: the PLHIV could have been taking ART for variable periods of time; countries had varying cut-off points but generally captured recently initiated and then longer term (over 5 years).
†LTFU: PLHIV had not collected ART from their registered clinic for a country-specific predetermined period of time.
ART, antiretroviral therapy; LTFU, lost to follow-up.
Data collection and analysis
Topics guides for PLHIV sought to elicit differentiated experiences of (dis)engagement with HIV testing, care and treatment services and place these in the context of their lived realities. Healthcare workers were invited to reflect on their experiences of engaging PLHIV and offering HIV services. Interviews with family members of the deceased sought to understand the circumstances that led to their death.
Interviews were conducted in the local language of each setting between October 2015 and May 2016, either in the participant’s own homes or in a health clinic. Interviews were audio-recorded and lasted between 45 and 90 min. All interviews were anonymised and either summarised (Kyamulibwa) or transcribed (Karonga, Kisesa, Rakai, Kisumu, Manicaland, uMkhanyakude) into English. Summaries were done in Kyamulibwa due to researchers at this site being historically trained in this method. Using NVivo 10, we drew on the thematic network analysis method by Attride-Stirling24 to cluster codes into basic and organising themes, which formed the development of a broad analytical coding framework.24 25 Emerging findings and comparisons between groups and countries were discussed and contrasted in an analysis workshop attended by study coordinators from each setting. This study draws on data coded, and thematically organised, by site coordinators under the heading 'HIV service engagement'. This subset of data was subsequently subject to a second round of thematic organising, driven by the two practice dynamics outlined above. The analysis is thus focused on engagement with HIV services as practice, rather than on characteristics of individual users of HIV services.
Additional methodological details concerning the study are given in an online-only supplement included in the editorial paper26 at http://dx.doi.org/10.1136/ sextrans-2017-053172.
Results
In our findings, we present both thematic and person-centred quotations (figures 2 and 3) and refer to other papers published in this special issue that report on this dataset.
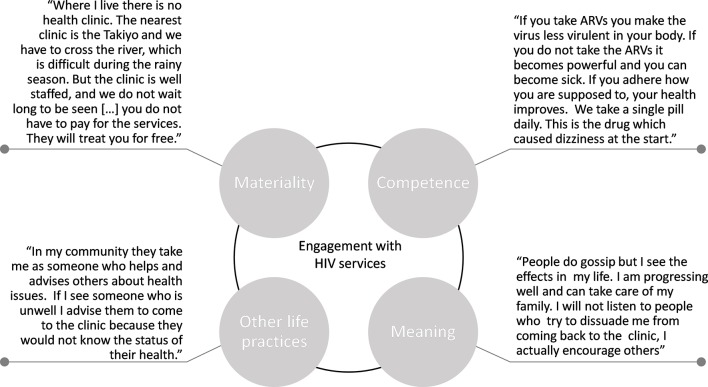
Figure 2.
Quotations from a male on ART, Southern Africa. ARV, antiretroviral drug.
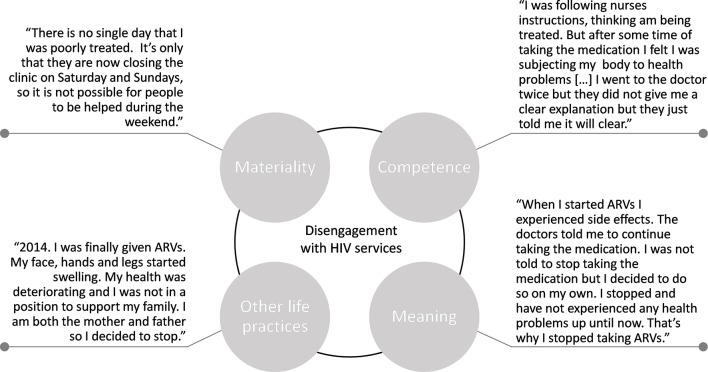
Figure 3.
Quotations from a female lost to follow-up, Southern Africa. ARV, antiretroviral drug.
Elements of ‘engagement’ with HIV services
The ‘materiality’ of engagement with HIV services: In all settings, participants observed that HIV services had improved from ‘the past’, underlining the temporal and dynamic nature of HIV infrastructures. Decentralisation of HIV services, as experienced by participants in most settings, meant that some people reported having to walk shorter distances and experiencing reduced waiting times:
In the past we had problems because people had to travel long distances to access their medication but right now it can be accessed near. […] They appreciate the assistance that they get from local clinics. (Female, on ART, Southern Africa)
The growing availability of free drugs was also noted (see figure 2). Nonetheless, in all sites, we observed variances in engagement with HIV services because of material constraints. These included supply barriers, such as poorly resourced health infrastructures, including occasional antiretroviral therapy (ART) stock-outs, reduced opening hours (see figure 3) and distance to the clinic. A materiality quote in figure 2 alludes to how distance barriers can vary seasonally, with swarming rivers requiring people to find alternative routes to go to the clinic to collect antiretroviral drugs during the rainy season.
Furthermore, household-level poverty continues to obstruct engagement with HIV services. Poverty restricts access to nutritious foods, public transport and over-the-counter medications to cope with side effects (see Bukenya et al 27). In South Africa, for instance, a number of healthcare providers talked about the importance of ensuring access to food parcels or disability grants to poor and vulnerable patients. This speaks to the poverty experienced by many PLHIV and the few social welfare services available in the South African setting to support engagement with HIV services.
The ‘competence’ of engagement with HIV services: Healthcare providers in all settings worked under the principle that they needed to recruit PLHIV into HIV care and treatment, towards the teleological aim of ‘staying alive’. This involved equipping PLHIV with the practical knowledge required to successfully engage with HIV treatment services and manage HIV stigma. This included instructions on what to eat and drink, safe sexual practices, to whom to disclose HIV status, how to relate to a spouse and how to access and adhere to antiretroviral drugs (see Ondenge et al 28). A number of participants attributed the state of their current health to this practical knowledge.
What they did well for me to be where I am today is the fact that they counselled and educated me […] They continue to counsel me against negative things said about HIV positive people. (Female, on ART, Southern Africa)
We found that the ability of healthcare staff to impart this knowledge, and for PLHIV to take on board their knowledge and advice, was intrinsically linked to the staffing and resourcefulness of health clinics (cf. things) and whether PLHIV hold staff in high regards (cf. meanings) (see Ondenge et al28). In all sites, we also identified PLHIV who actively encouraged friends and family to go and get tested or discursively imparted treatment knowledge to peers who were at risk of defaulting:
I give advice if the person is on treatment. She is supposed to follow the procedures which she is given at the hospital and not stop taking the medicine. (Female, on ART, Southern Africa)
In general, family and community members appeared central to the project of building ‘HIV literacy’ and creating an enabling environment where HIV-related knowledge could be shared and encourage engagement with HIV services.
The ‘meanings’ of engagement with HIV services: A perceived and experienced closeness to HIV, having directly witnessed or experienced the impact of HIV and trust in the value of treatment and care, emerged as central to accepting treatment (see Renju et al and Wringe et al 29 30).
I accepted treatment because I can see they [ART] give life. In the past you would see people wasting away and die. (Male, on ART, Southern Africa)
Ideologies, norms and fears circulating in the communities were also found to affect people’s different abilities to engage with HIV services. In all settings, persistent stigma made it difficult for some PLHIV to accept their HIV status, affecting their ART initiation (see Bonnington et al 31). PLHIV on ART who experienced improved health appeared to find positive meaning from their engagement (eg, able to care for the family), helping them manage and resist stigma (see figure 2). There were also numerous accounts conveying what can be referred to as a ‘normalisation’ of HIV (see Bonnington et al 31). In Tanzania, for example, a woman recently diagnosed with HIV exemplifies how this normalisation of HIV makes living with, and disclosing HIV, easier for her:
When I informed him [husband] about the test, he told me not to be sad because that was a normal issue because many people are infected … when you follow up on taking your medicine you will eventually feel like others. (Female, pre-ART, Eastern Africa)
These brief examples begin to unpack the material things, competencies and meanings that either enable or hinder engagement with HIV services. Rather than seeing them as separate contextual factors, practice theory encourage us to explore how health services, ART and poverty (things), and the HIV know-how of healthcare workers, PLHIV and community members (competence), converge to influence how PLHIV perceive, experience (meaning) and ultimately practice (dis)engagement with HIV services (see figures 2 and 3). Our findings allude to links between various key elements, for example, how the improved availability of ART (things)—in some settings—has transformed HIV from being a fatal disease to a chronic illness (meaning) or how the resource availability of health facilities (things) influence the know-how of healthcare staff and PLHIV (competence). Recognition of these links encourages us to explore how engagement with HIV services is either amplified or attenuated by the presence or absence of particular elements. The elements are also not distributed evenly across sites and changed over time and conditioned by the discursive, cultural, political and economic fabric of a given context. Negative references to ‘the past’ and positive statements about the ‘now’ suggest that some elements have been reconfigured over time, impacting people’s experiences of, and commitment to, engagement with HIV services.
‘HIV service engagement’ and other life practices
Engagements with HIV services do not happen in a vacuum but are inextricably interwoven with other life practices and a bundle of supportive practices, such as disclosing HIV status, managing stigma, relationship building with health providers and participating in support groups. The practice of accepting one’s HIV status emerged as particularly influential in instigating supportive practices, such as participating in peer groups or developing health-enabling patient–provider relationships (see Ondenge et al28). Accepting or denying one’s HIV-positive status, thus, had knock-on effects on other practices associated with HIV service engagement. The configuration of this complex of supportive or conflicting practices appeared to be co-dependent on the elements described above, resulting in significant variation.
Other life practices also appeared to either compete or collaborate with engagement practices. Clients were not merely HIV service users; they were also parents, spouses, breadwinners and believers. A number of participants engaged with faith healing and alternative and complementary medical practices, and this influenced their engagement with HIV services (see Moshabela et al 32). Parenting could play a significant role in engagement, too. A woman from Zimbabwe made a conscious decision to stop treatment, explaining that the treatment and associated side effects prevented her from being a parent (see figure 3).
For other participants, to stay alive and to be a parent for their children served instead as a key motivator to engage with HIV services (see McLean et al 33). Working, too, could take precedence over going to HIV services. In Kenya, a man who was asked to come to the clinic regularly failed to show up out of fear he would lose his job:
If you are my boss you cannot allow me to leave work [to go to the clinic] every three days or once a week. (Male, lost to follow-up, Eastern Africa)
Discussion
Our findings shed light on some of the ways in which social structures and contextual factors shape engagement with HIV services. We applied theories of practice to shift attention away from the characteristics of PLHIV and lists of the social determinants or ecological factors that shape engagements with HIV services, to the social organisation of HIV service engagement as practice. This revealed how the availability, absence and connections between requisite materialities, competencies and meanings shape engagement with HIV services. It also revealed that HIV service engagement, as a practice, is influenced by a host of other social practices, characterising the lives of people in low-resource and high HIV prevalence communities in sub-Saharan Africa.
While many of our observations have been noted before, recognition of their connections, or of the significance of some elements and practices, offers important clues for how engagements with HIV services become easier in some households or communities than in others. For instance, we noted that well-managed, drug-filled and accessible HIV services (materiality that is considered meaningful), discursive and practical knowledge through counselling (competency) and embodied experiences of HIV (meaning), such as improved health, are ‘resources’ for engagement with HIV services. If one of these elements is removed, for example, if drugs are unavailable or if a client feels healthy and cannot comprehend seroconversion in absence of symptoms, engagement with HIV services may get disrupted. Meaning emerged as particularly noteworthy, with PLHIV projecting meaning to the ‘things’ or ‘discursive knowledge’ they encountered.
We noted temporal differences in some settings where recent improvements in HIV service delivery,4 coupled with a discursive normalisation of HIV, have led to a reconfiguration of elements, which enhances people’s experiences of, and enables engagement with, HIV services. This, however, is not sufficient to sustain engagement. We also noted that engagement is affected by a bundle of related practices, such as accepting and disclosing one’s HIV status, and recurrent enactments, such as being a parent or a spouse. Depending on the context, being a parent or a spouse can either sustain or disrupt engagement.34 This illustrates that HIV service engagement, as practice, must be considered in relation to other social practices. We believe these findings may offer some explanation to high rates of loss to follow-up7 9 35 36 and mortality5 6 along the HIV care continuum. Others who have offered related critique have focused on the therapeutic itineraries or illness trajectories of PLHIV, demonstrating how particular life circumstances impact their ability to manoeuvre HIV treatment and care engagement.37 38 However, by shifting the analytical focus from individuals to the elements and practices that shape individuals’ engagement with HIV services, theories of practice offer a new and innovative framework for understanding HIV service engagements, while simultaneously transcending the dualisms of individual agency and structural conditions18 that guide much current HIV treatment literature. Although we have alluded to some country differences here (eg, welfare services in South Africa), future research needs to disentangle how different cultural, socioeconomic and policy spaces within and between countries shape engagement with HIV services. Nonetheless, we have provided a brief snapshot of what theories of practice can offer the study of HIV services (dis)engagement. Although the theory is not without its critics,39 40 we encourage other researchers to use the analytical tools offered by this approach to study and improve engagement with HIV services and health services more generally.
Conclusion
HIV service engagements should be positioned within a constellation of practices at the local level, many of which will not ‘shift’ easily to accommodate what is needed to perform engagement. Standardised expectations of patient engagement with HIV services, if not negotiated among other practices locally and not only individually, can run counter to its actualisation. The often poor fit between HIV care and treatment services and the lived realities of PLHIV call for a broader practice-oriented HIV response. This could involve a mix of structural initiatives and more patient-centred and differentiated HIV care and treatment services, which, when combined, can tweak and address the elements and bundles of practice that shape (dis)engagement with HIV services.
Key messages.
People living with HIV are affected by numerous factors and step in and out of HIV services, leading to different forms of disruption to progression along the HIV care continuum.
It is difficult to pinpoint how social structures interact and translate into varied engagements with HIV services.
Theories of practice provide HIV researchers and practitioners with a useful vocabulary and analytical tools to understand and steer differentiated HIV service (dis)engagements.
Standardised expectations of patient engagement with HIV services, if not negotiated among other practices locally, and not only individually, can run counter to their actualisation.
Acknowledgments
We would like to thank all the participants and fieldworkers who contributed their time and effort to the study. We would also like to acknowledge the support of ALPHA Network (http://alpha.lshtm.ac.uk) representatives at each Health and Demographic Surveillance System (HDSS) site who facilitated the implementation of the fieldwork and many other colleagues within the ALPHA Network who made helpful suggestions throughout the design and conduct of the research.
Footnotes
Handling editor: Jackie A Cassell
Contributors: All authors contributed to the development of The Bottlenecks Study protocol under the leadership of AW and OB. CN, JW, MM, WD, KO supervised the data collection by trained research assistants and prepared detailed site reports. MS conducted the analysis and prepared the first draft of this manuscript. OB, AW, JR, JS, SP, SB, MM made significant contributions to the manuscript and revised it for intellectual content. All authors have read and commented on the manuscript. All authors have approved the final manuscript and act as guarantors of the paper.
Funding: The bottlenecks study was funded by the Bill & Melinda Gates Foundation (OPP1082114). This paper was also made possible with the support of The Wellcome Trust (085477/Z/08/Z) and the National Institutes of Health (NIH) through the IeDEA project. AW is funded by a Population Health Scientist award, jointly funded by the UK Medical Research Council (MRC) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement and is also part of the EDCTP2 programme supported by the European Union. Research (undertaken in Kisesa and) reported in this publication was supported by the National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health & Human Development, National Institute on Drug Abuse, National Cancer Institute and the National Institute of Mental Health, in accordance with the regulatory requirements of the NIH under award number U01AI069911 East Africa IeDEA Consortium. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Competing interests: None declared.
Ethics approval: Ethical approval was granted by the London School of Hygiene and Tropical Medicine and the relevant ethics boards at each of the study settings. These were the following: Malawi National Health Sciences Research Committee no. 15/5/1427 (Karonga); Medical Research Coordination Committee MR/53/100/370 (Kisesa); Uganda National Council for Science and technology (UNCST)- HS1857 (Kyamulibwa) and Office of the president ADM154/212/01 (Rakai); Kenya Medical Research Institute (KEMRI) Scientific and Ethics Review Unit (SERU) KEMRI/SERU/CGHR/018/3115 (Kisumu); Medical Research Council of Zimbabwe MRCZ/A/1990 (Manicaland) and University of KwaZulu Natal (UKZN) UKZN/BE338/15 (uMkhanyakude). Informed and written consent was obtained from all participants.
Provenance and peer review: Commissioned; externally peer reviewed.
Data sharing statement: The data corpus sits with the ALPHA Network at the LSHTM. The ALPHA Network and its members will continue to publish from the data. Access to the data may be provided on request from AW (Alison.Wringe@lshtm.ac.uk).
References
- 1. Hill A, Pozniak A. HIV treatment cascades: how can all countries reach the UNAIDS 90-90-90 target? AIDS 2015;29:2523–5. 10.1097/QAD.0000000000000864 [DOI] [PubMed] [Google Scholar]
- 2. Bärnighausen T. The HIV treatment cascade and antiretroviral impact in different populations. Curr Opin HIV AIDS 2015;10:391–4. 10.1097/COH.0000000000000205 [DOI] [PubMed] [Google Scholar]
- 3. UNAIDS. Global AIDS update 2016. Geneva, Switzerland: Joint United Nations Programme on HIV/AIDS, 2016. [Google Scholar]
- 4. Levi J, Raymond A, Pozniak A, et al. . Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Global Health 2016;1:e000010 10.1136/bmjgh-2015-000010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Todd J. Special Issue: Measuring HIV Associated Mortality in Africa. Global Health Action 2014;7 10.3402/gha.v7.25210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slaymaker E. The bad news - residual mortality on the HIV care continuum and insights on the experiences of people who died of HIV. In: HIV mortality trends in Africa in the treatment Era: new evidence from the ALPHA Network of Community-based HIV surveillance studies: 18 July 2016. 2016. International AIDS Conference, Durban.
- 7. Kranzer K, Govindasamy D, Ford N, et al. . Quantifying and addressing losses along the continuum of care for people living with HIV infection in sub-Saharan Africa: a systematic review. J Int AIDS Soc 2012;15:17383 10.7448/IAS.15.2.17383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Layer EH, Kennedy CE, Beckham SW, et al. . Multi-level factors affecting entry into and engagement in the HIV continuum of care in Iringa, Tanzania. PLoS One 2014;9:e104961 10.1371/journal.pone.0104961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosen S, Fox MP, Gill CJ. Patient retention in antiretroviral therapy programs in sub-Saharan Africa: a systematic review. PLoS Med 2007;4:e298 10.1371/journal.pmed.0040298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Skovdal M, Campbell C, Nhongo K, et al. . Contextual and psychosocial influences on antiretroviral therapy adherence in rural Zimbabwe: towards a systematic framework for programme planners. Int J Health Plann Manage 2011;26:296–318. 10.1002/hpm.1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Auerbach JD, Parkhurst JO, Cáceres CF. Addressing social drivers of HIV/AIDS for the long-term response: conceptual and methodological considerations. Glob Public Health 2011;6(Suppl 3):S293–309. 10.1080/17441692.2011.594451 [DOI] [PubMed] [Google Scholar]
- 12. Gardner EM, McLees MP, Steiner JF, et al. . The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis 2011;52:793–800. 10.1093/cid/ciq243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Campbell C. Letting them die: why HIV/AIDS intervention programmes fail. Health Educ Res 2005;20:266–7. [Google Scholar]
- 14. Marsland R. (Bio)sociality and HIV in Tanzania: finding a living to support a life. Med Anthropol Q 2012;26:470–85. 10.1111/maq.12002 [DOI] [PubMed] [Google Scholar]
- 15. Paparini S, Rhodes T. The biopolitics of engagement and the HIV cascade of care: a synthesis of the literature on patient citizenship and antiretroviral therapy. Crit Public Health 2016;26:501–17. 10.1080/09581596.2016.1140127 [DOI] [Google Scholar]
- 16. Blue S, Shove E, Carmona C, et al. . Theories of practice and public health: understanding (un)healthy practices. Crit Public Health 2016;26:36–50. 10.1080/09581596.2014.980396 [DOI] [Google Scholar]
- 17. Hargreaves T. Practice-ing behaviour change: applying social practice theory to pro-environmental behaviour change. J Consum Cult 2011;11:79–99. 10.1177/1469540510390500 [DOI] [Google Scholar]
- 18. Shove E, Pantzar M, Watson M. The dynamics of social practice: everyday life and how it changes. Sage Publications, 2012. [Google Scholar]
- 19. Nicolini D. Practice theory, work, and organization: an introduction. Oxford university press, 2012. [Google Scholar]
- 20. Reckwitz A. Toward a theory of social practices a development in culturalist theorizing. Eur J Soc Theory 2002;5:243–63. 10.1177/13684310222225432 [DOI] [Google Scholar]
- 21. Schatzki TR. Social practices: a wittgensteinian approach to human activity and the social. Cambridge Univ Press, 1996. [Google Scholar]
- 22. Schatzki TR. Site of the social: a philosophical account of the constitution of social life and change. Penn State Press, 2010. [Google Scholar]
- 23. Wringe A, Renju J, Seeley J, et al. . Bottlenecks to HIV care and treatment in sub-Saharan Africa: a multi-country qualitative study. Sex Transm Infect 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Attride-Stirling J. Thematic networks: an analytic tool for qualitative research. Qual Res 2001;1:385–405. 10.1177/146879410100100307 [DOI] [Google Scholar]
- 25. Skovdal M, Cornish F. Qualitative research for development: a guide for practitioners. Rugby: Practical Action Publishing, 2015. [Google Scholar]
- 26. Wringe A, Renju A, Seeley A. et al. . Bottlenecks to HIV care and treatment in sub-Saharan Africa: a multicountry qualitative study. Sex Transm Infect 2017. 10.1136/sextrans-2017-053172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bukenya D, Wringe A, Moshabela M, et al. . Where are we now? A multi country qualitative study on access to pre-ART care services, a precursor for ART initiation. Sex Trans Infect 2017. [Google Scholar]
- 28. Ondenge K, Renju J, Bonnington O, et al. . “I am treated well if I adhere to my HIV medication”: Putting patient-provider interactions in context through insights from qualitative research in five sub-Saharan African countries. Sex Trans Infect 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Renju J, Moshabela M, McLean E, et al. . “Side effects” are “central effects” that challenge retention on antiretroviral therapy in HIV treatment programmes in six sub-Saharan African countries: A multi-country qualitative study. Sex trans infect 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wringe A, Moshabela M, Nyamukapa C, et al. . HIV testing experiences and their implications for patient engagement with HIV care and treatment on the eve of “test and treat”: findings from a multi-country qualitative study. Sex Trans Infect 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bonnington O, Wamoyi J, Daaki W, et al. . Changing forms of HIV-related stigma along the HIV care and treatment continuum in sub-Saharan Africa: a temporal analysis. Sex Trans Infect 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Moshabela M, Bukenya D, Darong G, et al. . Traditional healers, faith healers and medical doctors: medical pluralism as a bottleneck along the cascade of care for HIV/AIDS in Eastern and Southern Africa. Sex trans infect 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McLean E, Renju J, Wamoyi J, et al. . “I wanted to safeguard the baby”: a qualitative study to understand the experiences of Option B+ for pregnant women and the potential implications for “test and treat” in four sub-Saharan African settings. Sex Trans Infect 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wamoyi J, Renju J, Moshabela M, et al. . Understanding the relationship between couple dynamics and engagement with HIV care services: insights from a qualitative study in Eastern and Southern Africa. Sex Trans Infect 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mugglin C, Estill J, Wandeler G, et al. . Loss to programme between HIV diagnosis and initiation of antiretroviral therapy in sub-Saharan Africa: systematic review and meta-analysis. Trop Med Int Health 2012;17:1509–20. 10.1111/j.1365-3156.2012.03089.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sibanda EL, Weller IV, Hakim JG, et al. . The magnitude of loss to follow-up of HIV-exposed infants along the prevention of mother-to-child HIV transmission continuum of care: a systematic review and meta-analysis. AIDS 2013;27:2787–97. 10.1097/QAD.0000000000000027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dlamini-Simelane TT, Moyer E. 'Lost to follow up': rethinking delayed and interrupted HIV treatment among married Swazi women. Health Policy Plan 2016:czw117 10.1093/heapol/czw117 [DOI] [PubMed] [Google Scholar]
- 38. Hsieh A, Rodrigues J, Skovdal M, et al. . Treatment of HIV infection in pregnant women M, Childr: From patient to person: the need for an ‘HIV trajectories’ perspective in the delivery of prevention of mother-to-child-transmission services. AIDS 2014;28 3:S399–409. 10.1097/QAD.0000000000000341 [DOI] [PubMed] [Google Scholar]
- 39. Bonnington O. The indispensability of reflexivity to practice: the case of home energy efficiency. J Crit Realism 2015;14:461–84. 10.1179/1572513815Y.0000000009 [DOI] [Google Scholar]
- 40. Turner S. Brains/practices/relativism: social theory after cognitive science. University of Chicago Press, 2002. [Google Scholar]