Neutrophils are the predominant leucocytes in the blood and act as the first line of host defence against invading pathogens. Neutrophils have also been shown to play important roles in the other pathological conditions, including cancer. In the past decade, many efforts have been made to clarify the roles of neutrophils in cancer development and progression. It appears that neutrophils have both antitumour and protumour functions.1 On one hand, neutrophils can directly kill tumour cells by releasing antimicrobial and cytotoxic contents that are prestored in their granules. Neutrophils can induce apoptosis in tumour cells and reduce tumour growth when administrated into tumour-bearing animals. Neutrophils are regarded as important effector cells for monoclonal antibody (mAb)-mediated immunotherapy, where they interact with mAb through the Fc receptor, leading to antibody-dependent cell cytotoxicity. Neutrophils also have antimetastatic activity. In mouse models of cancer metastasis, neutrophils at the premetastatic site produce cytotoxic substances to eliminate tumour cells and limit their metastatic spread. In addition, neutrophils are able to regulate the activation of T cells and other immune cells to elicit antitumour immune responses. On the other hand, emerging evidence suggests that neutrophils possess protumour properties including the induction of malignant transformation, enhancement of tumour growth, establishment of premetastatic niche, stimulation of angiogenesis and promotion of immune evasion by suppression of innate and adaptive immune cells (eg, T cells and NK cells). The dual roles of neutrophils in cancer might be explained by their plasticity and the existence of distinct neutrophil subsets with differing properties within the tumours, which could be driven by signals from the tumours.2 3 In particular, neutrophils seem to have both stimulatory and suppressive roles in T-cell immunity. During the early phase of tumour growth, neutrophils tend to inhibit primary tumour growth by recruiting and activating CD8+ T cells.4 5 On the contrary, during the late stage of cancer, tumour-derived factors polarise neutrophils to an immunosuppressive phenotype that suppresses antitumour T-cell responses and promotes immune evasion.6 However, the detailed mechanisms responsible for the modulation of neutrophil function in T-cell immunity in human cancer remain largely unknown.
In this issue of Gut, Wang et al7 identified a new mechanism by which neutrophils suppressed T-cell function and promoted the growth of gastric cancer. By analysing clinical samples, they generated data to show that the number of neutrophils was increased in the tumour tissues of patients with gastric cancer. The neutrophils accumulated in gastric cancer tissues had prolonged survival, displayed an activated phenotype and expressed higher levels of programmed death-ligand 1 (PD-L1), an important co-inhibitory molecule that interacts with programmed death 1 (PD-1) on T cells to block their proliferation and activity. They further demonstrated that the increased infiltration of PD-L1+ neutrophils in tumour tissues was associated with disease progression and poor patient survival. In line with the findings observed in tumour tissues, neutrophils isolated from the peripheral blood of healthy donors strongly expressed PD-L1 when primed with tumour tissue-derived culture supernatants. Finally, the authors demonstrated that the activated PD-L1+ neutrophils effectively inhibited the proliferation and activity of PD-1+ T cells (but not PD-1− T cells) in vitro and dampened T-cell-mediated antitumour immune responses to promote gastric cancer growth ex vivo; however, blocking PD-L1/PD-1 interaction by an anti-PD-L1 antibody reversed these effects, indicating that neutrophils promote gastric cancer growth via suppression of T-cell function in a PD-L1/PD-1 interaction-dependent manner. While looking for the mechanism by which tumours induce the immunosuppressive phenotype in neutrophils, the authors found that tumour cell-derived granulocyte-monocyte colony-stimulating factor (GM-CSF) efficiently induced the expression of PD-L1 on neutrophils through activation of the Janus kinase/signal transducer and activator of transcription 3 signalling pathway.
The work by Wang and colleagues adds new evidence to support the protumour function of neutrophils. These cells seem to promote tumour growth through both direct and indirect mechanisms (figure 1). Neutrophils can produce a wide spectrum of proteinases and inflammatory factors that directly promote tumour cell proliferation, such as neutrophil elastase, prostaglandin E2 and interleukin-1 β (IL-1β).8 9 Neutrophils can also promote tumour growth by regulating the tumour microenvironment.10 Neutrophils are suggested to share similarities with granulocytic myeloid-derived suppressor cells, which exhibit potent immunosuppressive activities in inflammatory diseases and cancer.11 Neutrophils-mediated immune suppression involves multiple mechanisms, including release of inducible nitric oxide synthase (iNOS) (in mouse), production of arginase 1 (ARG1) (in human) and recruitment of regulatory T cells (Treg) (in human and mouse). In this study, the authors found that neutrophils could suppress T-cell function though an increased PD-L1/PD-1 interaction, which adds a new layer of complexity to the immunosuppressive roles of neutrophils in cancer. They also demonstrated that PD-L1 blockade in neutrophils reversed the inhibition of T-cell function to a greater extent than blocking iNOS and ARG1, suggesting that PD-L1 is the main factor that mediates the immunosuppressive roles of neutrophils in T cells in human gastric cancer. Intriguingly, neutrophils have been reported to inhibit T-cell activity to promote lung metastasis without affecting primary tumour growth in a mouse breast cancer model.12 Wang et al7 showed that the neutrophil percentage within human gastric cancer was associated with advanced lymphatic invasion; however, no significant correlation was observed between the neutrophil percentage and distant metastasis. The effect of PD-L1+ neutrophils on gastric cancer metastasis was not tested. Thus, whether PD-L1+ neutrophils may influence gastric cancer metastasis is a question waiting to be explored.
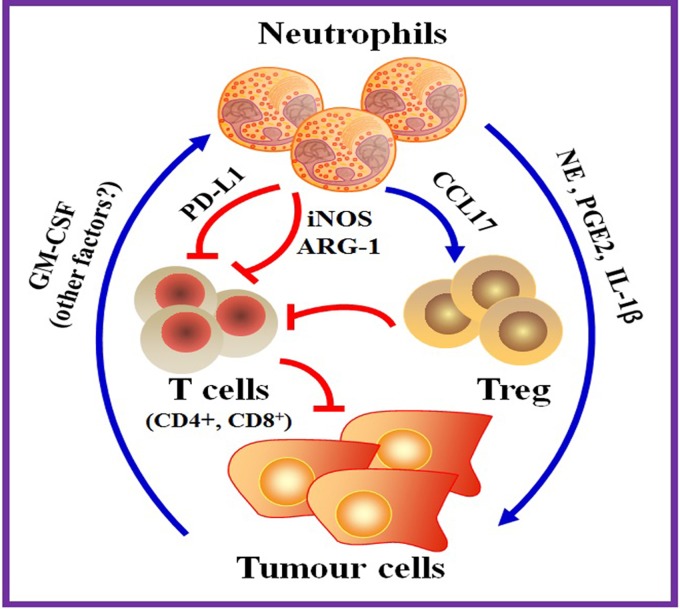
Figure 1.
Neutrophils promote tumour growth through direct and indirect mechanisms. Neutrophils can secret proteinases and inflammatory factors that directly promote the proliferation of tumour cells, such as neutrophil elastase (NE), prostaglandin E2 (PGE2) and interleukin-1 β (IL-1β). Neutrophils can also suppress T-cell immunity to promote tumour growth. Neutrophils inhibit antitumour T-cell responses through cell contact-dependent and independent mechanisms. Neutrophils have previously been shown to produce inducible nitric oxide synthase (iNOS) and arginase 1 (ARG1) to inhibit T-cell function. Neutrophils have also been reported to recruit regulatory T cells (Treg) by releasing chemokine C-C motif ligand 17 (CCL17). Wang et al demonstrated that tumour-derived granulocyte-monocyte colony-stimulating factor (GM-CSF) induced the expression of programmed death-ligand 1 (PD-L1) on neutrophils, which in turn diminished T-cell immunity via its interaction with PD-1 on T cells, ultimately leading to increased tumour growth.
In this study, the authors demonstrated that the induced expression of PD-L1 on neutrophils was specific to GM-CSF but not to the other factors such as granulocyte colony-stimulating factor (G-CSF), IL-17A and IL-10. Indeed, G-CSF and IL-17A have previously been shown to induce an immunosuppressive phenotype in neutrophils in mouse models of breast cancer.12 13 IL-10 has been reported to induce PD-L1 expression on macrophages in human hepatocellular carcinoma (HCC).14 The reasons for the discrepancies in these findings are likely to be different species, cancer types and experimental systems used in these studies. GM-CSF has been suggested to cooperate with tumour necrosis factor α to induce PD-L1 expression on neutrophils in human HCC.15 Whether other factors in the gastric cancer milieu are also involved in the induction of PD-L1 expression on neutrophils warrants further investigation. In addition, further study is needed to understand whether GM-CSF has a dose-specific effect on neutrophils as has been observed for interferon-γ, with low doses showing a stimulatory role in T-cell function and higher doses inducing a PD-L1-expressing phenotype that profoundly suppresses T-cell responses.4 16 Moreover, the finding that tumour-activated neutrophils suppress T-cell immunity though PD-L1/PD-1 interaction and promote primary tumour growth needs to be confirmed in other human cancers or in a spontaneous mouse tumour model.
Consistent with the previous findings that the increased presence of neutrophils in tumour tissues is associated with advanced disease progression and a poorer outcome, this study also suggests that there is a significant correlation between the percentage of PD-L1+ neutrophils within tumours and patient survival, which may provide a novel prognostic biomarker for gastric cancer. More importantly, this work also establishes a basis for the use of anti-PD-L1 antibodies for gastric cancer therapy. PD-L1 is expressed on tumour cells and tumour stromal cells in response to inflammatory stimuli. Blockade of the interaction between PD-L1 and PD-1 potentiates immune responses in vitro and mediates antitumour activity in both preclinical studies and clinical trials. The elevated expression of PD-L1 on neutrophils may be responsible, at least in part, for the immune evasion of gastric cancer. Thus, the use of anti-PD-L1 antibodies, either alone or in combination with other immunotherapies, may redirect the immunosuppressive properties of neutrophils and boost antitumour T-cell responses in patients with gastric cancer.
In conclusion, this study is the first demonstration of the interaction among tumour cells, neutrophils and T cells through a specific GM-CSF/PD-L1/PD-1 signalling pathway to favour gastric cancer growth. This new information will help us better understand the roles of neutrophils in cancer, and provide a novel approach for cancer therapy.
Footnotes
Contributors: The authors contributed equally to the writing of the commentary.
Funding: This work was supported by the National Natural Science Foundation of China (81672416, 81201660) and the Natural Science Foundation of Jiangsu Province (BK20141303).
Competing interests: None declared.
Provenance and peer review: Commissioned; externally peer reviewed.
References
- 1.Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer 2016;16:431–46. 10.1038/nrc.2016.52 [DOI] [PubMed] [Google Scholar]
- 2.Fridlender ZG, Sun J, Kim S, et al. . Polarization of tumor-associated neutrophil phenotype by TGF-beta: ‘N1′ versus ‘N2′ TAN. Cancer Cell 2009;16:183–94. 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sagiv JY, Michaeli J, Assi S, et al. . Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep 2015;10:562–73. 10.1016/j.celrep.2014.12.039 [DOI] [PubMed] [Google Scholar]
- 4.Singhal S, Bhojnagarwala PS, O'Brien S, et al. . Origin and role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell 2016;30:120–35. 10.1016/j.ccell.2016.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, et al. . Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest 2014;124:5466–80. 10.1172/JCI77053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mishalian I, Bayuh R, Levy L, et al. . Tumor-associated neutrophils (TAN) develop pro-tumorigenic properties during tumour progression. Cancer Immunol Immunother 2013;62:1745–56. 10.1007/s00262-013-1476-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TT, Zhao YL, Peng LS, et al. . Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut 2017. doi: 10.1136/gutjnl-2016-313075. [Epub ahead of print 8 Mar 2017] 10.1136/gutjnl-2016-313075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Houghton AM, Rzymkiewicz DM, Ji H, et al. . Neutrophil elastase-mediated degradation of IRS-1 accelerates lung tumor growth. Nat Med 2010;6:219–33. 10.1038/nm.2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonio N, Bønnelykke-Behrndtz ML, Ward LC, et al. . The wound inflammatory response exacerbates growth of pre-neoplastic cells and progression to cancer. EMBO J 2015;34:2219–36. 10.15252/embj.201490147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou SL, Zhou ZJ, Hu ZQ, et al. . Tumor-associated neutrophils recruit macrophages and T-regulatory cells to promote progression of hepatocellular carcinoma and resistance to sorafenib. Gastroenterology 2016;150:1646–58. 10.1053/j.gastro.2016.02.040 [DOI] [PubMed] [Google Scholar]
- 11.Kwak Y, Kim HE, Park SG. Insights into myeloid-derived suppressor cells in inflammatory diseases. Arch Immunol Ther Exp (Warsz) 2015;63:269–85. 10.1007/s00005-015-0342-1 [DOI] [PubMed] [Google Scholar]
- 12.Coffelt SB, Kersten K, Doornebal CW, et al. . IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015;522:345–8. 10.1038/nature14282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casbon AJ, Reynaud D, Park C, et al. . Invasive breast cancer reprograms early myeloid differentiation in the bone marrow to generate immunosuppressive neutrophils. Proc Natl Acad Sci USA 2015;112:E566–75. 10.1073/pnas.1424927112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuang DM, Zhao Q, Peng C, et al. . Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009;206:1327–37. 10.1084/jem.20082173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.He G, Zhang H, Zhou J, et al. . Peritumoural neutrophils negatively regulate adaptive immunity via the PD-L1/PD-1 signaling pathway in hepatocellular carcinoma. J Exp Clin Cancer Res 2015;34:141 10.1186/s13046-015-0256-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Kleijn S, Langereis JD, Leentjens J, et al. . IFN-γ-stimulated neutrophils suppress lymphocyte proliferation through expression of PD-L1. PLoS ONE 2013;8:e72249 10.1371/journal.pone.0072249 [DOI] [PMC free article] [PubMed] [Google Scholar]