Abstract
Objective
Neutrophils are prominent components of solid tumours and exhibit distinct phenotypes in different tumour microenvironments. However, the nature, regulation, function and clinical relevance of neutrophils in human gastric cancer (GC) are presently unknown.
Design
Flow cytometry analyses were performed to examine levels and phenotype of neutrophils in samples from 105 patients with GC. Kaplan-Meier plots for overall survival were performed using the log-rank test. Neutrophils and T cells were isolated, stimulated and/or cultured for in vitro and in vivo regulation and function assays.
Results
Patients with GC showed a significantly higher neutrophil infiltration in tumours. These tumour-infiltrating neutrophils showed an activated CD54+ phenotype and expressed high level immunosuppressive molecule programmed death-ligand 1 (PD-L1). Neutrophils activated by tumours prolonged their lifespan and strongly expressed PD-L1 proteins with similar phenotype to their status in GC, and significant correlations were found between the levels of PD-L1 and CD54 on tumour-infiltrating neutrophils. Moreover, these PD-L1+ neutrophils in tumours were associated with disease progression and reduced GC patient survival. Tumour-derived GM-CSF activated neutrophils and induced neutrophil PD-L1 expression via Janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) signalling pathway. The activated PD-L1+ neutrophils effectively suppressed normal T-cell immunity in vitro and contributed to the growth and progression of human GC in vivo; the effect could be reversed by blocking PD-L1 on these neutrophils.
Conclusions
Our results illuminate a novel mechanism of PD-L1 expression on tumour-activated neutrophils in GC, and also provide functional evidence for these novel GM-CSF-PD-L1 pathways to prevent, and to treat this immune tolerance feature of GC.
Keywords: GASTRIC CANCER
Significance of this study.
What is already known on this subject?
Neutrophils represent as one of the most abundant immune cells infiltrated in solid tumours.
Neutrophils have been confirmed to be increased in gastric cancer (GC).
Neutrophils exert either pathogenic or protective properties in different types and stages of tumours.
What are the new findings?
Neutrophils with an activated/immunosuppressive phenotype are highly enriched within GC and are associated with disease progression and are negatively correlated with patient survival.
GCs prolonged neutrophil lifespan, and tumour-derived GM-CSF efficiently activated neutrophils and induced programmed death-ligand 1 (PD-L1) expression on neutrophils by activating Janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) signalling pathways.
Tumour-associated neutrophils suppress T-cell's function in a PD-L1-dependent fashion, and, in doing so, contribute to the GC progression in vitro and in vivo.
How might it impact on clinical practice in the foreseeable future?
Our in vitro and in vivo data together provide a model of immunosuppression involving GM-CSF-PD-L1 on neutrophils in GC. Our findings further suggest several possible therapeutic targets, including GM-CSF, neutrophils and PD-L1. Given the apparent relationship between neutrophils levels and the advanced progression observed in the studied patients with GC, thought should be given to the use of these PD-L1+ neutrophils as novel diagnostic biomarkers for GC.
Introduction
Gastric cancer (GC) is one of the leading causes of cancer death in low/middle-income countries.1 The prognosis of GC is very poor, with a 5-year survival less than 30%. Currently, the pathogenesis of GC is unclear. However, its occurrence, development, and prognosis are closely related to the cross-talks between different immune cells in the GC microenvironment.2
Besides tumour cells, various immune cell types are the main components of the GC environment.3 Among them, neutrophils, as the most abundant leucocytes, are also one type of mostly infiltrated immune cells in GC.4 Currently, neutrophil researches associated with GC are largely focused on their peripheral number and function in patients with GC. It is reported that elevated neutrophil/lymphocyte ratio in peripheral blood of patients with GC predicts poor overall survival following resection for gastric adenocarcinoma.5 As to neutrophils in tumours, only several studies showed relationships between the tumour-infiltrating neutrophils and the prognosis of patients with GC assessed by immunohistochemistry,6 suggesting that neutrophils may be potential therapeutic targets for GC. Notably, in humans, virtually nothing is known about the phenotype, function and associated clinical relevance of neutrophils in GC.
Herein, we show that activated neutrophils with an immunosuppressive phenotype are highly enriched within GC and that they are associated with disease progression and are negatively correlated with patient survival following surgery. Moreover, we demonstrate that GCs prolonged neutrophil lifespan, and that tumour-derived GM-CSF efficiently activated neutrophils and induced programmed death-ligand 1 (PD-L1) expression on neutrophils by activating Janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) signalling pathways. In turn, these neutrophils suppress T-cell's normal function in a PD-L1-dependent fashion, and, in doing so, contribute to the suppression of antitumour immunity and the progression of GC.
Materials and methods
Patients and specimens
Fresh gastric tumour, peritumorous and non-tumour (non-tumour tissues, at least 5 cm distant from the tumour site) tissues and autologous peripheral blood were obtained from patients with GC who underwent surgical resection at the Southwest Hospital of Third Military Medical University. None of these patients had received chemotherapy or radiotherapy before surgery. Patients with infectious diseases, autoimmune disease or multi-primary cancers were excluded. Peripheral blood from 18 healthy donors was used as control. The clinical stages of tumours were determined according to the tumour, node, metastases (TNM) classification system of the International Union Against Cancer (7th edition). The study was approved by the Ethics Committee of the Southwest Hospital of Third Military Medical University. Written informed consent was obtained from each subject. Antibodies and other reagents are listed in online supplementary table S1.
gutjnl-2016-313075supp001.pdf (202.7KB, pdf)
Isolation of single cells from GC tissues
Isolation of neutrophils and T cells
Tumour and non-tumour tissues were processed into single cell suspension as described above (see online supplementary methods). Then the single cell suspension was stained with antihuman CD45 and antihuman CD66b antibodies, and neutrophils from autologous tumour and non-tumour tissues were sorted by fluorescence activating cell sorter (FACS) (FACSAria II; BD Biosciences). Peripheral blood mononuclear cells (PBMCs) from healthy donors and autologous GC patients were isolated by density gradient centrifugation using Ficoll-Paque Plus. Blood neutrophils were harvested after lyses of red blood cells with lyses solution. CD3+ T cells, CD4+ T cells and CD8+ T cells from PBMCs were purified by using anti-CD3 (Milteniy Biotec), anti-CD4 and anti-CD8 (StemCell Technologies) magnetic beads. The sorted cells were used unless their viability was determined >90% and their purity was determined >95%.
gutjnl-2016-313075supp002.pdf (105.3KB, pdf)
Preparation of TTCS and NTCS and supernatant-conditioned neutrophils
Tumour tissue culture supernatants (TTCS) or non-tumour tissue culture supernatants (NTCS) were prepared by plating autologous tumour or non-tumour gastric tissues in 1 mL RPMI-1640 medium for 24 hours. The supernatant was then centrifuged and harvested. To generate supernatant-conditioned neutrophils, neutrophils from healthy donors were first harvested and cultured with 50% TTCS or NTCS for 12 hours, and then washed with RPMI-1640 medium for three times. Neutrophils cultured with RPMI-1640 medium were used as controls.
Neutrophil stimulation
Neutrophils from healthy donors were stimulated with 50% TTCS or 50% autologous NTCS, or 50% TTCS with a neutralising antibody against human GM-CSF (10 μg/mL), or 50% autologous NTCS with human recombinant (hr) GM-CSF (100 ng/mL) for 12 hours, or were stimulated with 50% TTCS or hr GM-CSF (100 ng/mL) for 3, 6 or 12 hours. After stimulation, the cells were harvested for flow cytometric analysis and western blot. For the signalling pathway inhibition experiments, these neutrophils were pretreated with 5 μL (20 μM) AG490 (a JAK inhibitor), FLLL32 (an STAT3 inhibitor), BAY 11-7082 (an IκBα inhibitor), SP600125 (a c-Jun N-terminal kinase (JNK) inhibitor), SB203580 (a mitogen-activated protein kinase (MAPK) inhibitor) or Wortmannin (a PI3K inhibitor) for 1 hours, then the cells were stimulated with 50% TTCS or hr GM-CSF (100 ng/mL) for 12 hours and harvested as above. Since the inhibitor was dissolved in dimethyl sulfoxide (DMSO), parallel cell groups were treated with DMSO (5 μL) or culture media as controls.
Neutrophil survival assay
Neutrophils from healthy donors were stimulated with 50% TTCS or 50% autologous NTCS, or 20%, 40% or 80% TTCS for 16 hours, and then were harvested. Neutrophil survival was quantified using Annexin V Apoptosis Detection Kit I (BD Biosciences) or APO-Direct Apoptosis Detection (eBioscience) according to the manufacturer's instructions.
In vitro neutrophil-T cell co-culture system
In a 5-day incubation, bead-purified peripheral CD3+ T cells (1×105 cells/well in 96-well plates) were labelled with carboxyfluorescein succinimidyl ester (CFSE) and co-cultured with autologous neutrophils isolated from tumour or non-tumour tissues at a 2:1 ratio in 200 μL RPMI-1640 medium containing rhIL-2 (20 IU/mL), anti-CD3 (2 μg/mL), and anti-CD28 (1 μg/mL) antibodies, with or without a human PD-L1 neutralising antibody (20 μg/mL). In another co-culture system, TTCS-conditioned or NTCS-conditioned blood neutrophils were co-cultured with autologous or allogeneic CFSE-labelled bead-purified blood CD3+ T cells, CD4+ T cells, CD8+ T cells or CFSE-labelled FACS-sorted blood PD-1+ or PD-1− CD3+ T cells at a 1:1 ratio in a similar condition as above, in the presence or absence of a neutralising antibody against human PD-L1 (20 μg/mL), an arginase-1 inhibitor nor-NOHA (250 μM), or an induced nitric oxide synthase (iNOS) inhibitor 1400 W (10 μM). After 5-day incubation, the cells were harvested for intracellular cytokine staining.
In vivo tumour inhibition assay
All animal experiments were undertaken with review and approval from the Animal Ethical and Experimental Committee of Third Military Medical University. 106 GC cells (SGC-7901) in 100 μL of buffered saline were subcutaneously injected into the axillary tissues of female nonobese diabetic/severe combined immunodeficient (NOD/SCID) mice (5–7 week, one tumour per mouse). Normal peripheral neutrophils from healthy donors were stimulated with 50% TTCS for 12 hours. Then, 5×106 anti-CD3-stimulated and anti-CD28-stimulated (2 µg/mL anti-CD3 and 1 µg/mL anti-CD28) polyclonal T cells were co-cultured with autologous peripheral neutrophils, or TTCS-conditioned neutrophils (TCN) at a 1:1 ratio in the presence or absence of a neutralising antibody against human PD-L1 (5 μg/mL) or a control IgG (5 μg/mL) for 24 hours, and were subsequently injected into the peritoneum in 100 μL of buffered saline on day 10 after inoculation. Tumour size was measured every 2 days by two independent observers using callipers fitted with a vernier scale. Tumour volume was calculated based on three perpendicular measurements. Once the mice were sacrificed, the tumours were photographed, and were further fixed for immunohistochemical staining, real-time PCR and ELISA, and the spleens were dissociated into single cells for flow cytometry.
Immunohistochemistry, immunofluorescence, flow cytometry, real-time PCR, ELISA, western blot analysis and microarray experiments are described in online supplementary methods.
Statistical analysis
Results are expressed as mean±SEM. Student's t-test was generally used to analyse the differences between two groups, but when the variances differed, the Mann-Whitney U test was used. Correlations between parameters were assessed using the Pearson correlation analysis and linear regression analysis as appropriate. Overall survival was defined as the interval between surgery and death. The known tumour-unrelated deaths (eg, accidental death) were excluded from the death record for this study. Cumulative survival time was calculated by the Kaplan-Meier method, and survival was measured in months; the log-rank test was applied to compare between two groups. SPSS statistical software V.13.0 was used for all statistical analysis. All data were analysed using two-tailed tests, and p<0.05 was considered statistically significant.
Results
Neutrophils are enriched in GC environment with tumour progression and associated with poor patient survival
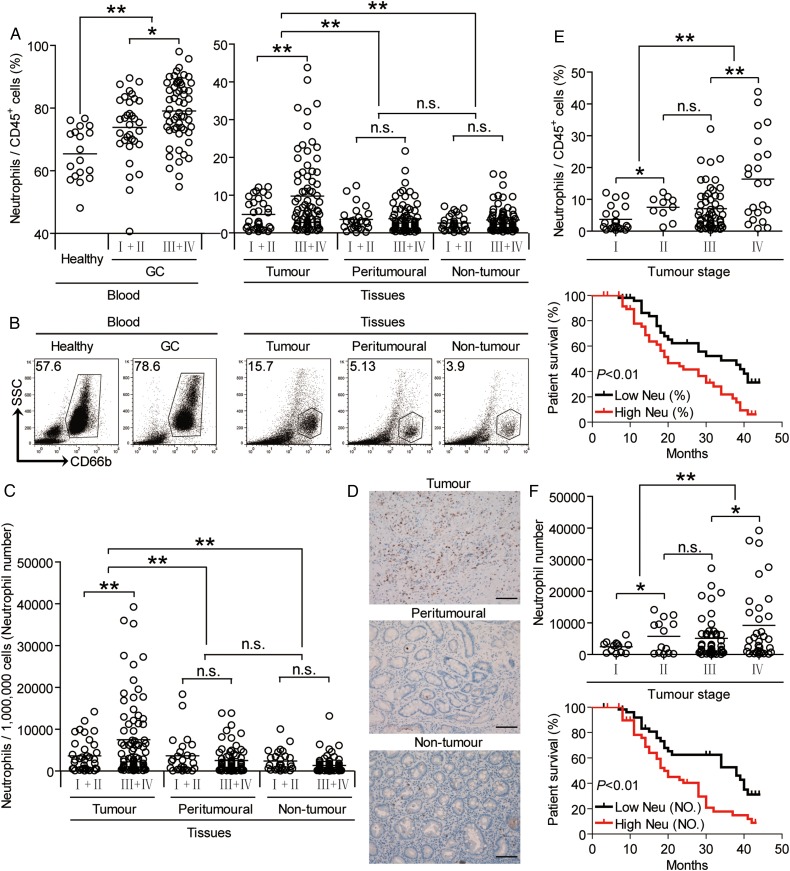
To evaluate the potential role of neutrophils in human GC, we analysed neutrophil percentage within the total CD45+ leucocytes in different samples at various stages. Peripheral blood samples from healthy donors were used as controls. Notably, patients with GC showed a higher neutrophil percentage in peripheral blood than healthy donors. Within the patient cohort, tumours contained a significantly higher neutrophil percentage than peritumorous and non-tumour tissues (figure 1A, B). Moreover, as the cancer progressed, we found that the percentage of neutrophils significantly increased in each of the tested samples (figure 1A). Similar observations were made when analysing the total number of neutrophils per million total cells in each tissue (figure 1C). Furthermore, immunohistochemical staining also showed that neutrophils were accumulated in tumours (figure 1D), indicating a potential role for neutrophils in the GC microenvironment. In keeping with this finding, an increased neutrophil percentage was correlated with increased tumour size and advanced lymphatic invasion (see online supplementary figure S1).
Figure 1.
Neutrophils accumulate in gastric cancer (GC) with disease progression and poor patient survival. (A) Neutrophil percentage in CD45+ cells or (C) the total number of neutrophils per million total cells in each tissue of patients with GC by gating on CD45+CD66b+ cells or counting. Cumulative results from 105 patients with GC and 18 healthy donors are shown. (B) Dot plots of surface molecule staining for neutrophils gating on CD45+ cells. (D) Representative analysis of CD15+ (brown) neutrophil distributions in tissues of patients with GC by immunohistochemical staining. Scale bars: 100 micrometers. (E and F) Neutrophil percentage (E) or neutrophil number (F) among tumour, node, metastases (TNM) stages was compared. Kaplan-Meier plots for overall survival by median neutrophil percentage (5.24%) (E) or median neutrophil number (3008 per million) (F). The horizontal bars in panels A, C, E and F represent mean values. Each ring in panels A, C, E and F represents one patient. *p<0.05, **p<0.01, n.s. p>0.05 for groups connected by horizontal lines. Neu (%), neutrophil percentage; Neu (NO.), neutrophil number.
gutjnl-2016-313075supp003.pdf (2.1MB, pdf)
Next, we evaluated the clinical relevance of intratumorous neutrophils in GC. Comparing patients with high (≥5.24% median level) versus low (<5.24%) neutrophil percentage level, the 44-month overall survival rates were significantly lower for those within the higher neutrophil percentage group (figure 1E). Similar results were obtained when the patient cohort was stratified based on intratumorous neutrophil number (figure 1F). Taken together, these findings suggest that increased intratumorous neutrophils are associated with tumour progression and poor survival for patients with GC.
PD-L1 expression and activation of neutrophils are correlated in human GC environment
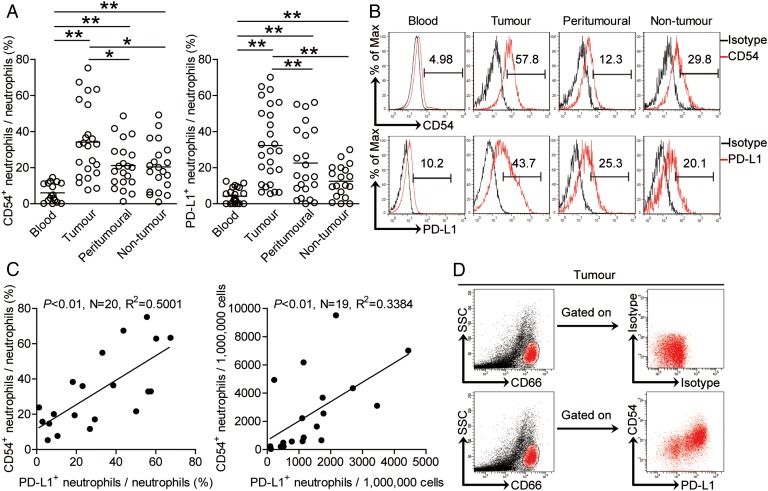
Next, we found that intratumorous neutrophils expressed significantly higher level of neutrophil activation marker CD54 than those expressed on peritumorous and non-tumour tissue neutrophils, whereas peripheral neutrophils expressed little CD54 (figure 2A,B) Interestingly, intratumorous neutrophils expressed significantly higher level of immunosuppressive molecule PD-L1 than those expressed on peritumorous, non-tumour or peripheral neutrophils (figure 2A, B). Moreover, significant correlations were found between the levels of CD54 and PD-L1 expression on neutrophils in tumours analysed (figure 2C). Finally, the co-existence of CD54 and PD-L1 on neutrophils was further confirmed by examining expression of these proteins in tumour samples (figure 2D). Taken together, the above data indicate that tumour-infiltrating neutrophils exhibit an activated immunosuppressive phenotype.
Figure 2.
Expression of programmed death-ligand 1 (PD-L1) on neutrophils is correlated with the activation pattern of neutrophils in gastric cancer (GC). (A) Statistics analysis of CD54+ neutrophil percentage or (B) PD-L1+ neutrophil percentage in total neutrophils in each samples of patients with GC (n=23). (B) Expression of molecules CD54 and PD-L1 on neutrophils. Colour histograms represent staining of neutrophil activation marker CD54 and immunosuppressive functional molecule PD-L1; black, isotype control. (C) The correlations between CD54+ neutrophils and PD-L1+ neutrophils in human tumours were analysed. Results are expressed as percentage of CD54+ neutrophils and PD-L1+ neutrophils in neutrophils or the number of CD54+ neutrophils and PD-L1+ neutrophils per million total cells in tumour tissues of patients with GC. (D) Co-expression of molecules CD54 and PD-L1 on tumour-infiltrating neutrophils. The horizontal bars in panels A represent mean values. Each ring or dot in panels A or C represents one patient. *p<0.05, **p<0.01 for groups connected by horizontal lines.
Human GC environments contribute to neutrophil survival and maintain neutrophil activated immunosuppressive phenotype
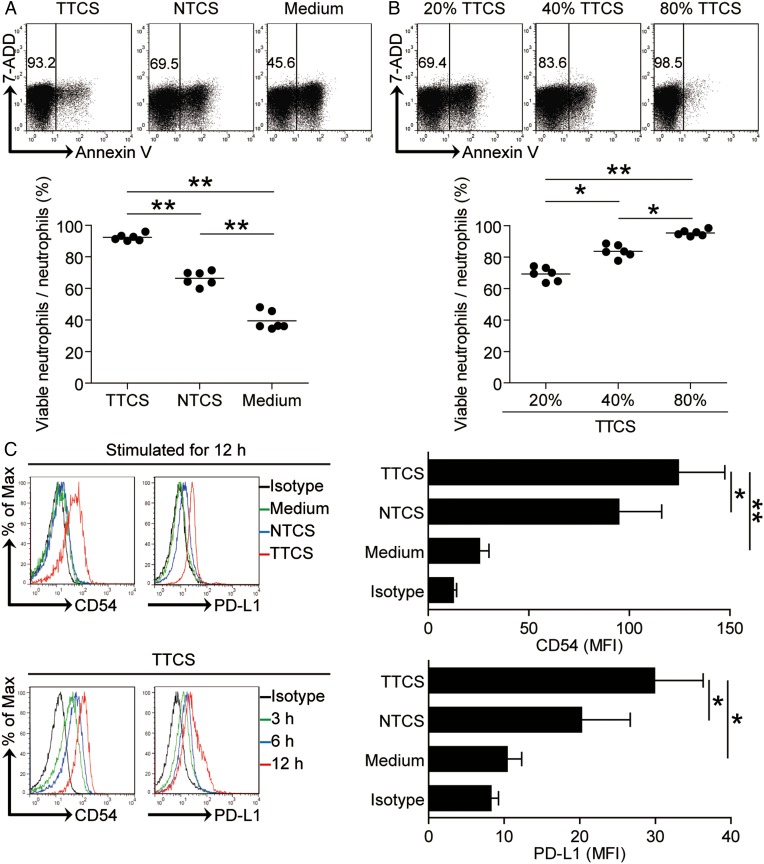
The results described above suggested that GC environments trigger the accumulation of neutrophils, so we wondered the reason for this accumulation. We hypothesised that GC environment sustained the survival of neutrophils. To test this hypothesis, we assessed the survival of neutrophils after exposure to TTCS, and the results showed that neutrophils exposed to TTCS exhibited a delayed onset of apoptosis in a dose-dependent manner when compared to those exposed to NTCS from autologous GC patients by annexin V (figure 3A, B) and deoxyuridine triphosphate nucleotides (dUTP) (see online supplementary figure S2A, B) detection. However, we detected no change of human leukocyte antigen-antigen D related (HLA-DR) expression on these neutrophils (see online supplementary figure S2C).
Figure 3.
Tumour environment sustains neutrophil survival, activation and programmed death-ligand 1 (PD-L1) expression. (A and B) Dot plots and statistics analysis of annexin V− viable non-apoptotic neutrophils exposed to 50% tumour tissue culture supernatants (TTCS) and 50% non-tumour tissue culture supernatants (NTCS) from autologous gastric cancer (GC) patients (A); or neutrophils to exposed to 20%, 40% or 80% TTCS from GC patients for 16 hours (B) (n=6). (C) Expressions of CD54 and PD-L1 on neutrophils exposed to 50% TTCS and 50% NTCS from autologous GC patients, or to medium control for 12 hours; or exposed to 50% TTCS from patients with GC for 3, 6 or 12 hours. black, isotype control. MFI: mean fluorescence intensity. *p<0.05, **p<0.01 for groups connected by horizontal lines.
Meanwhile, we also hypothesised that GC environments contribute to the activated immunosuppressive phenotype of neutrophils. Consistent with our hypothesis, compared with NTCS-conditioned neutrophils, TCN significantly upregulated CD54 and PD-L1 expression in a time-dependent manner (figure 3C). These findings together imply that GC environment is involved in the survival of neutrophils as well as the activation and PD-L1 expression on neutrophils.
Tumour-infiltrating PD-L1+ neutrophils exhibit immunosuppressive function and are correlated with disease stage and poor patient survival
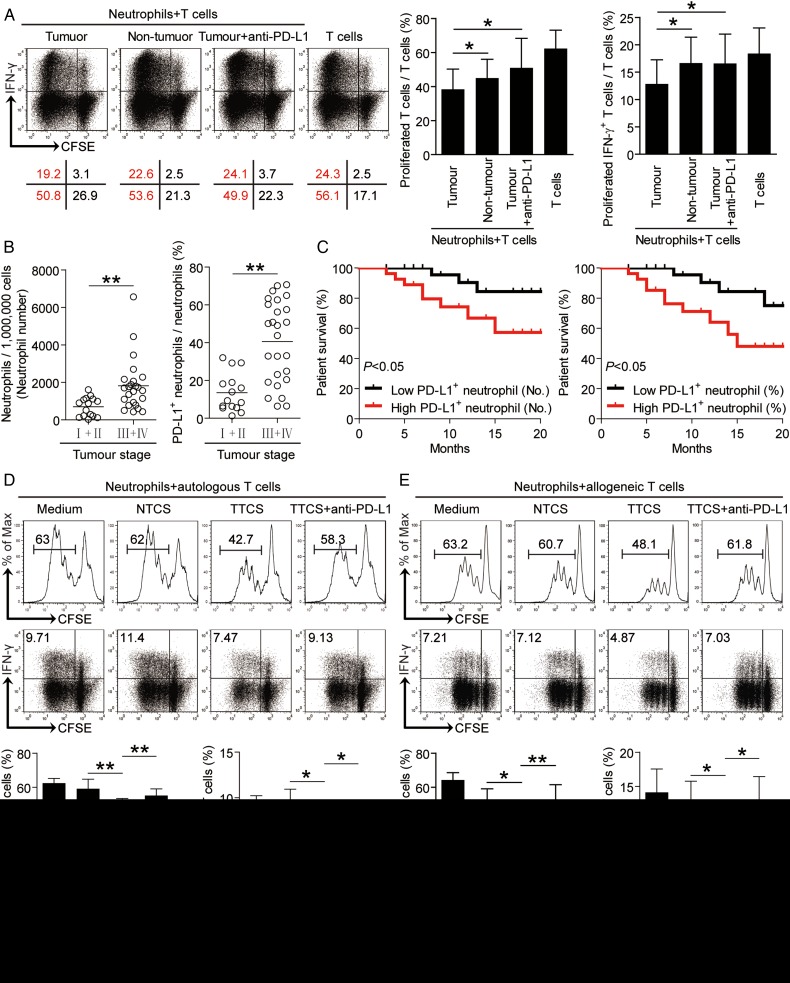
As for the accumulation and poor clinical relevance of neutrophils in human GC, we next studied the function of these cells. The co-localisation of neutrophils and T cells in tumour tissues (see online supplementary figure S3A) suggests that these neutrophils may promote tumour progression by impairing T cell immunity. Neutrophils from tumour and non-tumour tissues of autologous GC patients were therefore isolated and cultured with purified autologous peripheral blood CD3+ T cells. Neutrophil/T-cell co-cultures showed that tumour-infiltrating neutrophils were superior to non-tumour-derived neutrophils in inhibiting T cell proliferation and interferon-γ (IFN-γ) production (figure 4A and see online supplementary figure S3B), suggesting an immunosuppressive function of tumour-infiltrating neutrophils in tumour immunity.
Figure 4.
Tumour-infiltrating and tumour-conditioned neutrophils suppress T cell immunity through programmed death-ligand 1 (PD-L1). (A) Carboxyfluorescein succinimidyl ester (CFSE)-labelled peripheral CD3+ T cells of patients with gastric cancer (GC) were co-cultured for 5 days with autologous neutrophils from non-tumour or tumour tissues with or without anti-PD-L1 antibody. Representative data and statistical analysis of T cell proliferation and interferon (IFN)-γ production were shown (n=5). (B) PD-L1+ neutrophil percentage or PD-L1+ neutrophil number among tumour, node, metastases (TNM) stages was compared. (C) Kaplan-Meier plots for overall survival by median PD-L1+ neutrophil percentage (24%) or median PD-L1+ neutrophil number (1147 per million). (D and E) CFSE-labelled peripheral CD3+ T cells of donors were co-cultured for 5 days with tumour tissue culture supernatants (TTCS)-, or non-tumour tissue culture supernatants (NTCS)-conditioned autologous (D) or allogeneic (E) blood neutrophils with or without anti-PD-L1 antibody. Representative data and statistical analysis of T cell proliferation and IFN-γ production were shown (n=5). The horizontal bars in panel B represent mean values. Each ring in panels B represents one patient. *p<0.05, **p<0.01 for groups connected by horizontal lines.
Our next objective was to determine the role of PD-L1 on tumour-infiltrating neutrophils in T cell suppression. Therefore, we added neutralising antibodies against PD-L1 into our tumorous neutrophil/peripheral T-cell co-culture. Interestingly, blockade of PD-L1 significantly attenuated such T cell suppression mediated by tumour-infiltrating neutrophils (figure 4A and see online supplementary figure S3B). Collectively, these findings show that PD-L1 contributes to tumour-infiltrating neutrophil-mediated suppression in vitro.
We next found that in tumour tissues from patients with advanced GC high levels of PD-L1 were expressed on neutrophils with disease progression (figure 4B), and that there were striking negative associations between the PD-L1+ neutrophil percentage in tumours and overall survival (figure 4C). Similar observations were made when analysing the total number of PD-L1+ neutrophils per million total cells in tumour tissues (figure 4C). These results suggest that an increase of PD-L1+ neutrophils in GC is correlated with progression of GC and poor patient survival.
Neutrophils are activated by tumours to suppress T cell immunity through PD-L1
Given the better ability of tumour-infiltrating neutrophils to suppress T cells when compared with non-tumour neutrophil populations, we hypothesised that the tumour microenvironment itself might play an important role in this process. Purified peripheral CD3+ T cells were co-cultured with TTCS-conditioned or NTCS-conditioned autologous blood neutrophils. Interestingly, TCN suppressed significantly more IFN-γ production and T cell proliferation (figure 4D). To see whether PD-L1 operates in this suppression on T cell immunity, we added neutralising antibodies against PD-L1 in T cell/TTCS-conditioned neutrophil co-culture. Interestingly, antibody blockade of PD-L1 efficiently attenuated such T cell suppression mediated by TCN (figure 4D).
To ensure the suppressive effect is not specific to autologous T cells, we repeated this assay with T cells isolated from allogeneic donors. The results showed that TCN significantly suppressed the proliferation and IFN-γ production of allogeneic T cells in a PD-L1 dependent manner (figure 4E). Similar observations were made when analysing the purified CD4+ or CD8+ T cells (see online supplementary figure S4). These results above indicate that, in the GC environment, neutrophils are activated by tumour-derived soluble factors and acquire ability to suppress T cell function through PD-L1.
Tumour-derived factor GM-CSF activates neutrophils and induces PD-L1 expression on neutrophils via JAK-STAT3 pathway
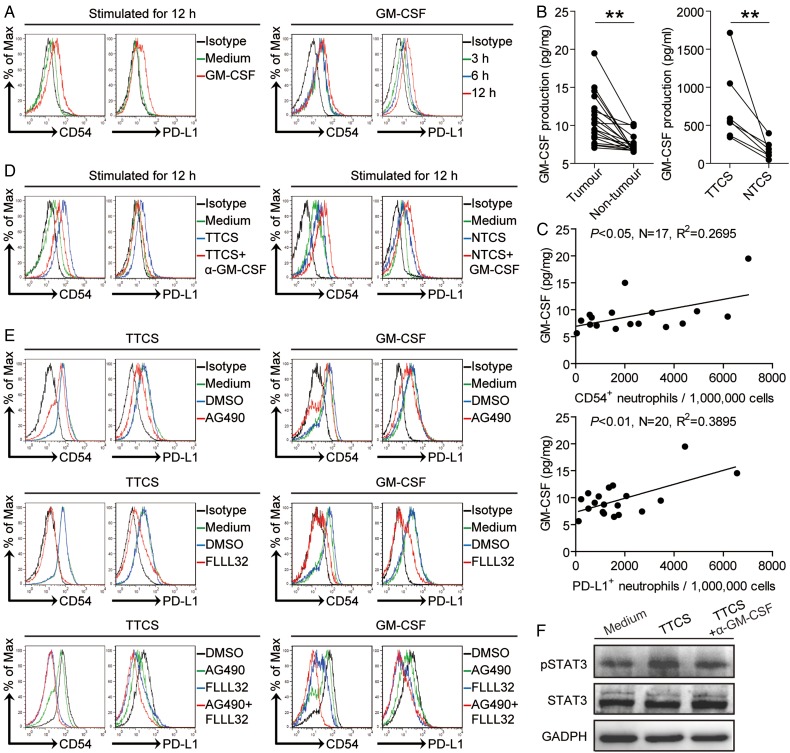
Tumour microenvironment can possess various soluble factors,7 including pro-inflammatory cytokines. To see which cytokines might activate neutrophils and induce PD-L1 expression on neutrophils, we first screened pro-inflammatory cytokines in human GC environments by microarray (see online supplementary figure S5A), and stimulated normal neutrophils with highly expressed cytokines including GM-CSF, M-CSF, G-CSF, IFN-γ, TGF-β, interleukin (IL)-1β, IL-6, IL-17A, IL-23, IL-22, IL-10 and so on. We found that only GM-CSF remarkably upregulated the expression of PD-L1 on neutrophils in a time-dependent manner (figure 5A and see online supplementary figure S5B). Next, we found that the concentrations of GM-CSF in tumour tissues or tumour tissue culture supernatants (TTSC) were significantly increased when compared with that in non-tumour tissues or NTCS (figure 5B). There was clearly a positive correlation between PD-L1+ neutrophil infiltration and GM-CSF production within tumours (figure 5C). Next, to evaluate the potential role of GC tumour-derived GM-CSF in PD-L1 induction on neutrophils, we added neutralising antibody against GM-CSF into TTCS/neutrophil co-culture. Interestingly, antibody blockade of GM-CSF efficiently inhibited the induction of PD-L1 on neutrophils (figure 5D). Consistent with these findings, provision of exogenous GM-CSF into NTCS/neutrophil co-culture significantly increased the PD-L1 expression on neutrophils (figure 5D). Similar observations were made when analysing the induction of neutrophil activation marker CD54 (figure 5A,D). These findings show that tumour-derived GM-CSF plays an essential role in neutrophil activation and PD-L1 induction.
Figure 5.
Tumour-derived GM-CSF induces neutrophil activation and neutrophil programmed death-ligand 1 (PD-L1) expression via Janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) pathway. (A) Expression of CD54 and PD-L1 on neutrophils exposed to GM-CSF for 3, 6, 12 hours. black, isotype control. (B) GM-CSF concentration between autologous tumour and non-tumour tissues (n=16) or between autologous tumour tissue culture supernatants (TTCS) and non-tumour tissue culture supernatants (NTCS) (n=7) was analysed. (C) The correlations between GM-CSF and PD-L1+ neutrophils or CD54+ neutrophils in human tumours were analysed. Results are expressed as the number of PD-L1+ neutrophils or CD54+ neutrophils per million total cells and GM-CSF concentration in tumour tissues. (D) Expression of CD54 and PD-L1 on neutrophils exposed to TTCS with anti-GM-CSF antibody or NTCS with GM-CSF for 12 hours. black, isotype control. (E) Expression of CD54 and PD-L1 on neutrophils exposed to TTCS or GM-CSF with or without JAK signal transduction inhibitor AG490 or STAT3 phosphorylation inhibitor FLLL32 alone or both for 12 hours. (F) STAT3 and p-STAT3 in neutrophils exposed to autologous TTCS, NTCS, or TTCS with anti-GM-CSF antibody were analysed by western blot. Each dot in panel C represents one patient. *p<0.05, **p<0.01 for groups connected by horizontal lines.
To see which signalling pathways might operate in the activation of neutrophils and their PD-L1 induction, we first pretreated neutrophils with corresponding inhibitors and then exposed them to the indicated TTCS. The results showed that only blocking the signal transduction of JAK with inhibitor AG490 and/or abolishing the phosphorylation of STAT3 with inhibitor FLLL32 effectively suppressed CD54 and PD-L1 expression on TCN either alone or in combination (figure 5E and see online supplementary figure S5C). Furthermore, STAT3, a direct JAK-STAT3 pathway downstream substrate, was predominantly phosphorylated in neutrophils after treatment with TTCS, and this phosphorylation was abolished when blocking GM-CSF (figure 5F), implying that activation of JAK-STAT3 signalling pathway is crucial for neutrophil activation and PD-L1 induction by GM-CSF most likely derived from epithelial cell adhesion molecule (EpCam)+ tumour cells (see online supplementary figure S5E) in the GC environments.
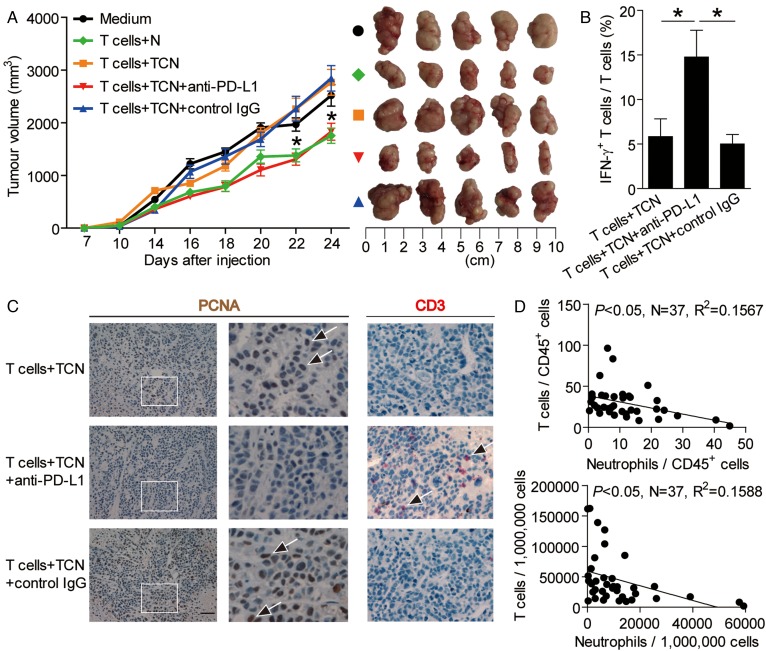
Blockade of neutrophil-associated PD-L1 on T cell immunity inhibits tumour growth and GC progression
To test the suppressive effect of PD-L1+ neutrophils on T cell immunity in vivo, we treated TCN with PD-L1 blocking or control IgG and then injected them together with autologous T cells into our established human NOD/SCID mice bearing SGC-7901-derived GC. As expected, mice without T cell transfusions, mice treated with T cells plus TCN or control IgG-treated TCN, showed tumour growth and disease progression (figure 6A). Consistent with a vital role in assisting tumours of activated PD-L1+ neutrophils in vivo, mice treated with T cells plus PD-L1 blocking antibody-treated TCN showed reduced tumour volumes and disease progression at each measurement time point from day 18 (figure 6A). Moreover, mice treated with T cells plus PD-L1 blocking antibody-treated TCN, also showed an increased CD3+ T cell infiltration and IFN-γ production from T cells, and a decreased tumour cell proliferation (figure 6B,C), as well as an increased production of cytolytic molecules perforin and granzyme B (see online supplementary figure S6), compared with the mice treated with T cells plus TCN or control IgG-treated TCN. Moreover, significant correlations were found between the levels of T cells and neutrophils in human GC tumours analysed (figure 6D). These findings suggest that tumour-activated neutrophils suppress T cell immunity in vivo dependent on PD-L1 and thereby contribute to tumour growth and GC progression.
Figure 6.
Blockade of neutrophil-associated programmed death-ligand 1 (PD-L1) on T cell immunity inhibits tumour growth and gastric cancer (GC) progression in vivo. (A) Mice were injected with human GC cells (SGC)-7901 cells, as described in Materials and methods. The control animals (●) received no further injections. The experimental treatments entailed injections with T cells in combination with untreated neutrophils (N) ( ) or TTCS (tumour tissue culture supernatants)-conditioned neutrophils (TCN) (
) or TTCS (tumour tissue culture supernatants)-conditioned neutrophils (TCN) ( ), or TCN pre-treated with an anti-PD-L1 antibody (
), or TCN pre-treated with an anti-PD-L1 antibody ( ) or a control IgG (
) or a control IgG ( ). The illustrated data represent tumour volumes (5 mice in each group). The day of tumour cell injection was counted as day 0. *p<0.05, for groups injections with TCN pre-treated with an anti-PD-L1 antibody (
). The illustrated data represent tumour volumes (5 mice in each group). The day of tumour cell injection was counted as day 0. *p<0.05, for groups injections with TCN pre-treated with an anti-PD-L1 antibody ( ), compared with groups injections with TCN pre-treated with a control IgG (
), compared with groups injections with TCN pre-treated with a control IgG ( ). The tumours were excised and photographed 24 day after injecting the tumour cells. (B and C) Interferon (IFN)-γ-producing T cell response (B) in spleens and proliferating cell nuclear antigen (PCNA) (brown) expression or CD3+ T cell infiltration (red) (C) in tumours of mice injected with T cells in combination with TCN, or TCN pre-treated with an anti-PD-L1 antibody or a control IgG on day 24 after tumour cell injection were compared. (D) The correlations between T cells and neutrophils in human tumours were analysed. Results are expressed as percentage of T cells and neutrophils in CD45+ cells or the number of T cells and neutrophils per million total cells in tumour tissues of patients with GC. Scale bars: 100 μ. Arrows indicate staining-positive cells. *p<0.05 for groups connected by horizontal lines.
). The tumours were excised and photographed 24 day after injecting the tumour cells. (B and C) Interferon (IFN)-γ-producing T cell response (B) in spleens and proliferating cell nuclear antigen (PCNA) (brown) expression or CD3+ T cell infiltration (red) (C) in tumours of mice injected with T cells in combination with TCN, or TCN pre-treated with an anti-PD-L1 antibody or a control IgG on day 24 after tumour cell injection were compared. (D) The correlations between T cells and neutrophils in human tumours were analysed. Results are expressed as percentage of T cells and neutrophils in CD45+ cells or the number of T cells and neutrophils per million total cells in tumour tissues of patients with GC. Scale bars: 100 μ. Arrows indicate staining-positive cells. *p<0.05 for groups connected by horizontal lines.
Tumour-activated neutrophils specifically suppress T cell immunity by PD-L1-PD-1 interaction
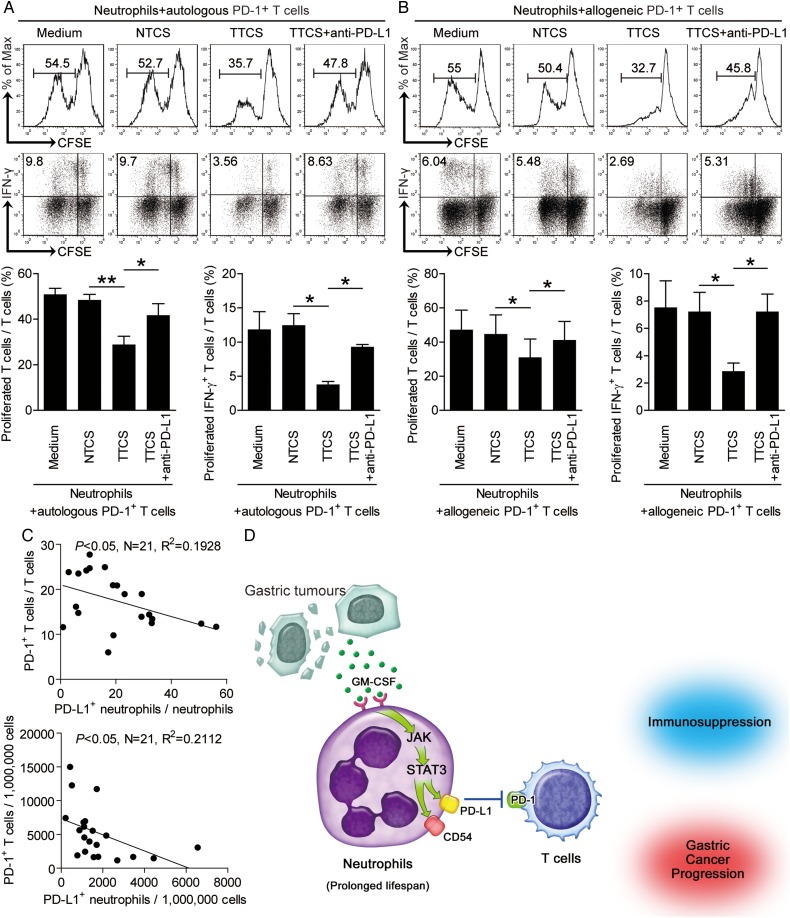
To further see whether PD-L1-PD-1 interaction operates in this suppression on T cell immunity specifically, we sorted PD-1+ or PD-1− T cells and co-cultured with autologous TCN with or without neutralising antibodies against PD-L1 respectively. Interestingly, antibody blockade of PD-L1 efficiently attenuated the suppression of PD-1+ T cells (figure 7A) but not PD-1− T cells (see online supplementary figure S7A) mediated by TCN. To ensure the suppressive effect is not specific to autologous T cells, we repeated this assay with PD-1+ or PD-1− T cells isolated from allogeneic donors. The results showed that TCN also significantly suppressed the proliferation and IFN-γ production of allogeneic PD-1+ T cells (figure 7B) but not PD-1− T cells (see online supplementary figure S7B) in a PD-L1 dependent manner. Moreover, significant correlations were found between the levels of PD-1+ T cells and PD-L1+ neutrophils in human GC analysed (figure 7C), and an increased PD-L1 expression on tumour-infiltrating neutrophils with disease progression were also found (see online supplementary figure S7C). These findings suggest that tumour-activated neutrophils suppress T cell immunity specifically dependent on PD-L1-PD-1 interaction and thereby contribute to immunosuppression and GC progression.
Figure 7.
Tumour-activated neutrophils suppress T cell immunity through programmed death-ligand 1 (PD-L1)-PD-1 interaction, and a proposed model of cross-talk among neutrophils, T cells and tumour cells leading to neutrophil-mediated immunosuppression in gastric cancer (GC). (A and B) carboxyfluorescein succinimidyl ester (CFSE)-labelled fluorescence activating cell sorter (FACS)-sorted peripheral PD-1+ T cells of donors were co-cultured for 5 days with tumour tissue culture supernatants (TTCS)-conditioned or non-tumour tissue culture supernatants (NTCS)-conditioned autologous (A) or allogeneic (B) blood neutrophils with or without anti-PD-L1 antibody. Representative data and statistical analysis of T cell proliferation and interferon (IFN)-γ production were shown (n=3). (C) The correlations between PD-1+ T cells and PD-L1+ neutrophils in human tumours were analysed. Results are expressed as percentage of PD-1+ T cells in T cells and PD-L1+ neutrophils in neutrophils or the number of PD-1+ T cells and PD-L1+ neutrophils per million total cells in tumour tissues of patients with GC. (D) Release of GM-CSF induces the activation of intratumorous neutrophils, a process that is accompanied by the induction of PD-L1 expression on these cells as a result of Janus kinase (JAK)-signal transducer and activator of transcription 3 (STAT3) signalling pathway activation. These activated neutrophils exert their pro-tumour effect, contributing to immunosuppression and GC progression by inhibition T cell immunity in a PD-L1-PD-1-dependent manner.
Discussion
In this study, we have shown that within GC neutrophils play an active role on promoting tumour progression. Although neutrophils have already been described in patients with tumours,8 to our knowledge this is the first demonstration of a statistically significant correlation between prevalent high PD-L1 expressing neutrophils in human tumours and poor tumour prognosis; it is also the first demonstration for tumour-derived GM-CSF to induce immunosuppressive neutrophils which inhibit T-cell immunity connecting mechanistically the pathological role of neutrophils within the tumour microenvironment.
Neutrophils have been identified in tumours recently. However, very little is currently known about tumour-infiltrating neutrophils' phenotype, function and their clinical relevance. In human GC, it has been shown that elevated neutrophils in peripheral blood5 and tumour tissues6 predict poor prognosis of patients with GC. Here, we have significantly expanded upon these previous observations. Our profiling of neutrophils within GC confirms that they are phenotypically and functionally distinct from their peripheral counterparts. As expected, tumour-infiltrating neutrophils exhibited an activated phenotype characterised by the upregulation of CD54, compared with normal activated peripheral cohorts.9 Most interestingly, these activated neutrophils expressed high level immunosuppressive molecule PD-L1, indicating that their main role is likely to be directly modulating effector function.
Immunosuppression has been known as a hallmark of cancer.10 Crosstalk between PD-L1 and PD-1 is one of the main mechanisms contributing to such suppression applied to T cells.11 Neutrophils' PD-L1 upregulation occurs during infection or inflammation, such as HIV-1 infection12 and systemic inflammation.13 Although previous studies have shown that tumour-activated neutrophils could function as G-MDSCs and could inhibit T cell proliferation via arginase-114 and iNOS,15 we now are the first to report, in human GC, that the induced high PD-L1 expression on infiltrating neutrophils exhibits more profound suppressive role on T-cell proliferation and IFN-γ production than that of arginase-1 or iNOS (see online supplementary figure S3D). Our results further emphasise the importance of PD-1-PD-L1 pathway in tumour-related immunosuppression.
In analogy to M1 and M2 macrophage polarisation,16 researchers have reported the existence of N1 and N2 neutrophils.17 N2 neutrophils promote tumour progression mainly through releasing of matrix metalloproteinases18 and angiogenic factors,19 which can be prevented by blocking the pro-inflammatory factors and then intend to display an N1 phenotype.17 However, the neutrophils' tumour-promoting or tumour-alleviating roles have been controversial. In early lung cancer, an activating effect of neutrophils on T cells was reported.20 By contrast, in melanoma, neutrophils promote disease progression.18 Our results are consistent with the latter study showing neutrophils' tumour-promoting effect in GC. Importantly, with an in vivo GC model, our hypothesis that activated PD-L1+ neutrophils effectively suppress T cell immunity and promote GC progression was fully verified. We further demonstrated that such T cell suppression was through PD-L1 on the neutrophils as blocking PD-L1 reversed it. Conversely, it could be speculated that at different tumour progress stages tumour-infiltrating neutrophils may have different functions. During the initial stages of tumour, neutrophils might have a transient tumour-alleviating role; however, as disease progressed, neutrophils could be influenced by tumour-derived factors to acquire tumour-promoting phenotype and function.
GM-CSF is a pluripotent and pro-inflammatory cytokine that regulates haemopoiesis as well as immune response.21 The GM-CSF-secreting tumours, including colorectal cancer22 and lung cancer,23 albeit not very common, are among the most rapidly advancing ones due to a pro-inflammatory cytokine-mediated immunosuppression.24 Here, we identify GM-CSF as a novel pro-inflammatory factor within GC tumour environment, and show that GC tumour-derived GM-CSF effectively induces PD-L1 expression on neutrophils via activation of JAK-STAT3 pathway.
Collectively, based on our in vitro and in vivo data, we propose a model involving the progressive immunosuppression within GC (figure 7D). First, GM-CSF with pro-inflammatory feature is produced within the gastric tumours. Second, released GM-CSF induces the activation of intratumorous neutrophils, a process that is accompanied by the induction of PD-L1 expression on these cells via JAK-STAT3 signalling pathway activation. Third, these activated immunosuppressive neutrophils exert a pro-tumour effect by suppressing T cell function in a PD-L1-PD-1 dependent manner in the GC microenvironment. In this way, neutrophils appear to contribute to tumour progression via GM-CSF-PD-L1 pathway, which is consistent with our observations that advanced tumour staging is associated with significant increase of PD-L1+ neutrophils in GC. In the future, therapeutics aimed at interfering these pathological neutrophils and the GM-CSF-PD-L1 immunosuppressive pathway may be developed to provide novel strategies for GC treatment.
Footnotes
Contributors: Conception and design; data analysis; drafting the manuscript: YZ. Manuscript revision: YZ and WC. Statistical analysis: YZ, T-tW, Y-lZ, L-sP, NC, Y-pL, F-yM, J-yZ, PC, Y-sT, X-lF, P-wY and GG. Obtained funding: YZ, Y-lZ. Technical support: PL and Q-mZ. Final approval of submitted version: YZ.
Funding: This work was supported by the National Key Research and Development Program of China (2016YFC1302200) and grant of National Natural Science Foundation of China (81402355 and 81372560).
Competing interests: None declared.
Patient consent: Obtained.
Ethics approval: The biopsy specimens were obtained under protocols approved by the ethics committees of Southwest Hospital of Third Military Medical University and informed consent was obtained from all patients. All animal experiments were undertaken with approval from the Animal Ethical and Experimental Committee of Third Military Medical University.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Torre LA, Bray F, Siegel RL, et al. . Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87–108. 10.3322/caac.21262 [DOI] [PubMed] [Google Scholar]
- 2.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005;5:263–74. 10.1038/nrc1586 [DOI] [PubMed] [Google Scholar]
- 3.Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med 2013;19:1423–37. 10.1038/nm.3394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wu Y, Zhao Q, Peng C, et al. . Neutrophils promote motility of cancer cells via a hyaluronan-mediated TLR4/PI3K activation loop. J Pathol 2011;225: 438–47. 10.1002/path.2947 [DOI] [PubMed] [Google Scholar]
- 5.Graziosi L, Marino E, De Angelis V, et al. . Prognostic value of preoperative neutrophils to lymphocytes ratio in patients resected for gastric cancer. Am J Surg 2015;209:333–7. 10.1016/j.amjsurg.2014.06.014 [DOI] [PubMed] [Google Scholar]
- 6.Zhao JJ, Pan K, Wang W, et al. . The prognostic value of tumor-infiltrating neutrophils in gastric adenocarcinoma after resection. PLoS One 2012;7:e33655 10.1371/journal.pone.0033655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhuang Y, Peng LS, Zhao YL, et al. . Increased intratumoral IL-22-producing CD4(+) T cells and Th22 cells correlate with gastric cancer progression and predict poor patient survival. Cancer Immunol Immunother 2012;61:1965–75. 10.1007/s00262-012-1241-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li XF, Chen DP, Ouyang FZ, et al. . Increased autophagy sustains the survival and pro-tumourigenic effects of neutrophils in human hepatocellular carcinoma. J Hepatol 2015;62:131–9. 10.1016/j.jhep.2014.08.023 [DOI] [PubMed] [Google Scholar]
- 9.Fortunati E, Kazemier KM, Grutters JC, et al. . Human neutrophils switch to an activated phenotype after homing to the lung irrespective of inflammatory disease. Clin Exp Immunol 2009;155:559–66. 10.1111/j.1365-2249.2008.03791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhuang Y, Peng LS, Zhao YL, et al. . CD8(+) T cells that produce interleukin-17 regulate myeloid-derived suppressor cells and are associated with survival time of patients with gastric cancer. Gastroenterology 2012;143:951–62.e8. 10.1053/j.gastro.2012.06.010 [DOI] [PubMed] [Google Scholar]
- 11.Kuang DM, Zhao Q, Peng C, et al. . Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med 2009;206:1327–37. 10.1084/jem.20082173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowers NL, Helton ES, Huijbregts RP, et al. . Immune suppression by neutrophils in HIV-1 infection: role of PD-L1/PD-1 pathway. PLoS Pathog 2014;10:e1003993 10.1371/journal.ppat.1003993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Kleijn S, Langereis JD, Leentjens J, et al. . IFN-γ-stimulated neutrophils suppress lymphocyte proliferation through expression of PD-L1. PLoS ONE 2013;8: e72249 10.1371/journal.pone.0072249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sippel TR, White J, Nag K, et al. . Neutrophil degranulation and immunosuppression in patients with GBM: restoration of cellular immune function by targeting arginase I. Clin Cancer Res 2011;17:6992–7002. 10.1158/1078-0432.CCR-11-1107 [DOI] [PubMed] [Google Scholar]
- 15.Dufait I, Schwarze JK, Liechtenstein T, et al. . Ex vivo generation of myeloid-derived suppressor cells that model the tumor immunosuppressive environment in colorectal cancer. Oncotarget 2015;6:12369–82. 10.18632/oncotarget.3682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allavena P, Sica A, Garlanda C, et al. . The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev 2008;222:155–61. 10.1111/j.1600-065X.2008.00607.x [DOI] [PubMed] [Google Scholar]
- 17.Fridlender ZG, Sun J, Kim S, et al. . Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009;16:183–94. 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piccard H, Muschel RJ, Opdenakker G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit Rev Oncol Hematol 2012;82:296–309. 10.1016/j.critrevonc.2011.06.004 [DOI] [PubMed] [Google Scholar]
- 19.Shojaei F, Singh M, Thompson JD, et al. . Role of Bv8 in neutrophil-dependent angiogenesis in a transgenic model of cancer progression. Proc Natl Acad Sci USA 2008;105:2640–5. 10.1073/pnas.0712185105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, et al. . Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest 2014;124:5466–80. 10.1172/JCI77053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hercus TR, Broughton SE, Ekert PG, et al. . The GM-CSF receptor family: mechanism of activation and implications for disease. Growth Factors 2012;30:63–75. 10.3109/08977194.2011.649919 [DOI] [PubMed] [Google Scholar]
- 22.Urdinguio RG, Fernandez AF, Moncada-Pazos A, et al. . Immune-dependent and independent antitumor activity of GM-CSF aberrantly expressed by mouse and human colorectal tumors. Cancer Res 2013;73:395–405. 10.1158/0008-5472.CAN-12-0806 [DOI] [PubMed] [Google Scholar]
- 23.Lammel V, Stoeckle C, Padberg B, et al. . Hypereosinophilia driven by GM-CSF in large-cell carcinoma of the lung. Lung Cancer 2012;76:493–5. 10.1016/j.lungcan.2012.02.014 [DOI] [PubMed] [Google Scholar]
- 24.Aliper AM, Frieden-Korovkina VP, Buzdin A, et al. . A role for G-CSF and GM-CSF in nonmyeloid cancers. Cancer Med 2014;3:737–46. 10.1002/cam4.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
gutjnl-2016-313075supp001.pdf (202.7KB, pdf)
gutjnl-2016-313075supp002.pdf (105.3KB, pdf)
gutjnl-2016-313075supp003.pdf (2.1MB, pdf)